Abstract
Cultivated meat (CM) technology has the potential to disrupt the food industry—indeed, it is already an inevitable reality. This new technology is an alternative to solve the environmental, health and ethical issues associated with the demand for meat products. The global market longs for biotechnological improvements for the CM production chain. CM, also known as cultured, cell-based, lab-grown, in vitro or clean meat, is obtained through cellular agriculture, which is based on applying tissue engineering principles. In practice, it is first necessary to choose the best cell source and type, and then to furnish the necessary nutrients, growth factors and signalling molecules via cultivation media. This procedure occurs in a controlled environment that provides the surfaces necessary for anchor-dependent cells and offers microcarriers and scaffolds that favour the three-dimensional (3D) organisation of multiple cell types. In this review, we discuss relevant information to CM production, including the cultivation process, cell sources, medium requirements, the main obstacles to CM production (consumer acceptance, scalability, safety and reproducibility), the technological aspects of 3D models (biomaterials, microcarriers and scaffolds) and assembly methods (cell layering, spinning and 3D bioprinting). We also provide an outlook on the global CM market. Our review brings a broad overview of the CM field, providing an update for everyone interested in the topic, which is especially important because CM is a multidisciplinary technology.
1. Introduction
Cultivated meat (CM), also known as cultured, cell-based, lab-grown, in vitro or clean meat, has gained prominence in recent years due to increasing societal and industrial interest. CM has arisen as an alternative to help solve environmental, health and ethical issues related to meat product demands []. Cellular agriculture is used to produce CM. Its development has been promoted by different concerns: (1) the increase in the global population; (2) the environmental impact of animal agriculture, such as land use, greenhouse gas emissions and impact on biodiversity; (3) animal ethics, including livestock living conditions and slaughter; and (4) the impact of animal agriculture on human health through issues such as animal-borne disease and antibiotic use [].
Instead of slaughtering animals for food, the meat cultivation process usually starts by obtaining a biopsy and isolating cells. Then, these cells are cultivated to grow and differentiate into skeletal muscle cells. This process can begin with different cell types, but stem cells are an obvious choice due to their ability to proliferate and to differentiate into several lineages []. The cells can be obtained from two sources: adult stem cells, which have a limited proliferative capacity, or pluripotent stem cells, which have an indefinite proliferative capacity. The cells usually employed for this application include: (1) muscle satellite cells that are muscle stem cells that can differentiate into myotubes, which is the target cell type; (2) mesenchymal stem/stromal cells (MSCs) that can differentiate into fibroblastic, chondrogenic or adipogenic cell lineages; (3) fibro-adipogenic progenitors (FAPs) that can generate adipocytes and fibroblasts; (4) embryonic stem cells (ESCs); and (5) induced pluripotent stem cells (iPSCs) that can differentiate into any cell type [].
Two different meat products are expected to be introduced to the market. Unstructured products such as burgers, sausages or nuggets will likely be commercialised first, and then structured products such as a chicken breast or a beefsteak will come later []. The manufactured tissue must resemble the in vivo tissue, reproducing morphological and functional characteristics, such as highly aligned muscle fibres and well-distributed fat [].
There have been significant advances in the CM field; however, no product is commercially available on a large scale. There are numerous technological and social challenges to this, as shown in Figure 1 [], that we will address in this review.
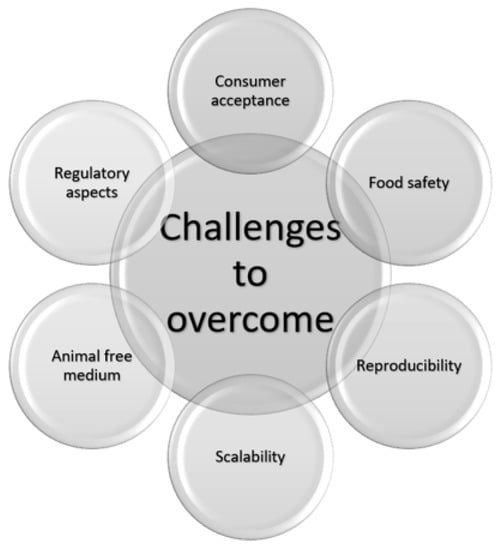
Figure 1.
The technological and social challenges that must be overcome before cultivated meat is available on a large scale.
2. The Cultivated Meat Market
2.1. The Global Market
The CM market has grown exponentially in the last few years, producing millions of dollars throughout the world. This growth and the tremendous potential have drawn the interest of several companies []. The world’s first cultivated beef burger was revealed in 2013 at a packed press conference in London by Mark Post, who founded the Netherlands-based company Mosa Meat in 2016 []. The world’s first CM company was UPSIDE Foods, launched in 2015. Since then, there has been an exponential expansion and dozens of companies in more than 20 countries have been founded. In 2021, at least 21 new companies emerged, which represented significant growth, since until that time there had only been 86 CM companies. Investments have also followed this exponential increase. In 2021, invested capital grew by about 336% from 2020, reaching USD 410 million [].
Some countries have greater prominence in the global CM market. In April 2022, in the United States, UPSIDE Foods (formerly known as Memphis Meats) raised USD 400 million in Series C funding, a distinguished milestone for the industry []. Israel is another important player in this market. In 2021, 36% of CM funding worldwide went to Israeli companies []. In 2022, the world’s largest CM consortium raised USD 18 million in government funding for 14 companies and 10 academic laboratories. Important companies such as SuperMeat, Future Meat and Aleph Farms are located in Israel [,]. In Europe, the Netherlands, the birthplace of CM, announced EUR 60 million in funding in April 2022 to support the creation of a national cellular agriculture ecosystem as part of the country’s National Growth Fund. Another significant player in this scenario is Spain, which invested EUR 5.2 million in a CM project led by BioTech Foods in 2021 [].
Over the past two decades, Brazil has been consolidating its position as a major producer of agricultural commodities and related food products as well as a supplier to international markets. It is the fifth-largest country in the world in terms of area and population and the largest in terms of arable land, and it is among the few countries with the potential to increase its agricultural productivity []. In the current scenario, the CM field is also extremely attractive and favourable to the emergence of new companies. Brazil is currently the home of a few companies that are involved with CM production [,,,].
2.2. Regulatory Aspects
In contrast to the explosion of the CM market, the regulatory scenario has been developing slowly. Singapore was the pioneer and remains the only regulatory authority in the world to allow the commercialisation of CM []. In December 2020, the Singapore Food Agency (SFA) approved the sale of cultivated chicken, in the form of cultivated chicken nuggets (GOOD Meat™), produced by Eat Just. One year later, new products have gained regulatory approval, such as chicken breasts [].
Progress in the CM industry requires overcoming another big challenge: regulatory approval. It is expected that regulatory approvals will come on a country-by-country basis. In the USA, since 2019 the Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) have begun to work together to establish a regulatory framework for the industry []. Recently, the FDA completed its first pre-market consultation for a human food made from cultured animal cells. While this is not an approval process, it represents a major advance within the global regulatory system []. In South Korea, the 2022 National Plan includes official guidance for alternative proteins for the first time. This new guidance could establish the standards for CM []. In the Brazilian regulatory environment, GFI-Brazil recently launched a regulatory study that aims to identify possible adjustments in the current regulatory frameworks, supporting this discourse with scientifically based arguments, as well as mobilising the country’s regulatory agents [].
3. Challenges to Overcome
3.1. Consumer Acceptance
In general, acceptance of CM is more likely in younger, more educated people who eat meat [,,]. The majority are open to trying CM and purchasing it regularly or even using it to replace conventional meat, but only half are open to paying more for it []. Some studies have also shown better acceptance of CM compared with similar food technology innovations, such as genetically modified organisms (GMOs) or insect protein. Nonetheless, to ensure commercial viability, consumer acceptance is essential, and strategies to increase it are necessary. The efforts to achieve this goal focus on lowering costs and reproducing conventional meat characteristics, such as taste, texture and appearance [].
Consumer reluctance to compromise on the organoleptic properties of conventional meat is the principal industry motivation for providing a product similar in terms of appearance, structure, texture, flavour and nutritional composition. The post-mortem process is the transformation of muscle tissue into meat; this complex process interferes directly with meat properties. Unlike conventional meat, the post-mortem metabolism of CM has not yet been fully described, and basic comparative studies vis-à-vis animal-based meat are needed due to its importance to meat quality.
Conventional meat is commonly processed. The added ingredients aim to improve meat quality in terms of texture and modulating flavour, improving product stability, replacing fat or protein and delivering bioactive compounds. The same principle can be applied to CM during the post-culture period to ensure that CM has comparable properties to animal-based meat products [].
3.2. Food Safety
Among the crucial concerns regarding the willingness to consume CM are flavour, nutrition and safety []. Regarding safety, the potential benefits of CM have been highlighted, such as avoiding health issues related to consuming a diet rich in red meat, including zoonotic diseases []. The ongoing COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has raised concerns about the use of meat from livestock as well as unconventional animal meat [,].
Cultivated cell lines are usually not designated for human consumption. Immortal cell lines could express oncogenes through spontaneous immortalisation or genetic engineering. Thus, it must be confirmed that the food products from these cells do not induce tumourigenicity []. Limited evidence suggests that DNA from genetically engineered plant cells can integrate or be transferred into somatic cells or the microflora of the human gastrointestinal tract []. Even after confirming the safety of CM, regular monitoring will be needed to avoid contamination and genetic drift. This process can occur over time due to the accumulation of mutations that eventually cause modifications in phenotypes [].
CM is a disruptive innovation and, as such, is subject to scientific uncertainty. The crucial point lies in finding the optimal timing of market authorisation relative to a better scientific understanding. Although it is worth being cautious to avoid allowing people to consume potentially unsafe products, being overly cautious could impede the benefits of this innovation []. The industry’s success is linked to transparency around CM health and safety [].
3.3. Reproducibility
Science is based on existing knowledge and exploring the unknown in pursuit of a significant discovery or paradigm shift. The scientific method consists of precisely controlled and documented experiments using validated reagents. However, the complexity of biology being explored itself, as well as pressures to publish; the focus on novel, positive and impactful results; the use of suboptimal research practices; and the scarcity of research funding results in an undesirable and irreproducible level of scientific data []. The reproducibility crisis has been discussed for quite some time. In 2016, a study of 1576 researchers found that 90% agree that there is a crisis related to reproducibility, as more than 70% of them had failed to reproduce other scientists’ experiments. In addition, more than half of these researchers could not repeat their own experiments []. Reproducibility is the capacity to replicate an independent finding or the published work’s results using the described data []. To improve reproducibility, all required information must be available, and all methods must be reported in detail and without ambiguity []. Almost all CM research has been carried out by private companies and their intellectual property is not accessible for refinement and development by the general scientific community []. In this way, each company develops their own technology and cultivation process []. An alternative to publishing relevant data in this field is funding academic scientific work with a more democratic disclosure of the findings. The lack of publicly available data is the reason why this review contains fewer references than one would typically expect.
3.4. Scalability
The ability to scale up CM is crucial for its development. The production process requires an average of 1 × 1012–1 × 1013 cells to generate ~10–100 kg of CM. Scaling up and scaling out are necessary when a large number of cells is required in biotechnological and bioprocessing industries. Scale-up systems consist of making a component bigger or faster so that it can handle a greater load. Thus, for these systems, the surface area/culture volume is increased progressively as the number of cells is increased. On the other hand, scale-out systems are based on adding more components in parallel to spread out a load. These systems use multiple culture vessels/bioreactors working in parallel.
The cells used for CM production are dependent on anchorage: they must adhere to a surface to remain viable and proliferate. Improving the production process is essential to alleviate the surface-to-volume ratio, to more tightly control critical growth parameters, to optimise dissociation from the growth surface and to ensure an efficient final cell harvest. The strategies to enable a better surface-to-volume balance are: (1) adapt the cells to grow in suspension (anchorage independent) and (2) use suspension culture systems (such as microcarriers), where cells are attached to and proliferate upon carriers that are constantly agitated to remain in suspension [].
A bioreactor is an essential piece of technology used to scale up the production of cells. This device contains a vessel that supports a biologically active environment, allowing cell growth and development []. A bioreactor allocates a significant volume to proliferation, maximises nutrient diffusion and provides mechanical stimulation. The advantages are of great value: it enables large-scale cell culture while also simplifying medium recycling and replacement throughout the proliferation stage. It is possible to control the biological conditions, therefore guaranteeing optimal culture conditions. Oxygen can be introduced by aeration through sparges or upstream to ensure the media is saturated with dissolved oxygen. Sensors monitor the carbon dioxide concentration to maintain the pH at 7.2–7.4. The nutritional support strategy used is usually the fed-batch system, where one or more nutrients can be supplied during the culture period [].
Some features of a bioreactor need to be accounted for. Mechanical mixing can generate turbulent flow and, consequently, cause cell damage or early apoptosis. The use of primary cells will require a bioreactor with a surface for cells to adhere to or which supports a scaffold. Finally, it is also essential to consider whether a bioreactor could co-culture multiple cell types in the production process. Of course, constant optimisation of bioreactor systems will be necessary for large-scale production to meet industry requirements [].
3.5. Animal-Free Medium
Culture media is crucial for meat cultivation, but it also represents substantial obstacles to CM production. These obstacles mainly consist of cost and ethics, two critical factors for developing and selling a future product. First, the medium represents more than 99% of the expenses in CM production []. Currently, the culture media used for CM is the same used to culture cells in the laboratory; it is composed of ingredients of pharmacological grade (high cost). It would be beneficial if the culture medium could be composed of food-grade ingredients (potentially lower cost). Second, cell culture growth is strongly associated with foetal bovine serum (FBS), which is extracted from bovine foetuses. FBS is expensive and, because it is derived from animals, its use is inconsistent with the proposed development of animal-free CM []. FBS contains cell attachment factors, micronutrients, trace elements, growth factors and hormones that promote rapid cell growth. A more detailed view of FBS composition is shown in Table 1. Although it originated from an in vivo source, the serum composition can vary dramatically between different batches and also carries the risk of virus or prion contamination for the culture [,,].

Table 1.
Constituents of foetal bovine serum.
Thus, there is an incessant search for the development of a cheap medium, free of animal components and which is capable of sustaining the proliferation and differentiation of bovine satellite cells. Below, we discuss the advantages and limitations of some of these media.
There are some newly developed commercial products that can serve as an FBS substitute. Ultroser G® (Gottingen, Germany) is one of them; although its exact composition is protected by commercial copyright, it contains adhesion and growth factors, vitamins, minerals and hormones []. Moreover, the reproducibility of its composition, both quantitatively and qualitatively, guarantees consistent biological activity from batch to batch []. Although these FBS substitutes guarantee reproducibility between batches and are easy to use and add to culture media, their use has some disadvantages. They are still relatively expensive, they are not available for sale in several countries and there is no consensus on whether they would allow for culturing any isolated cell type. While [] reported that Ultroser G® is not a good substitute for FBS for the in vitro culture of bovine embryos, Ref. [] used it to cultivate bovine satellite cells. However, to date, there does not appear to be a single commercial compound that can replace this serum for all cell types.
Another approach to serum-free media, and the most used and most effective to date, is the development of defined media. Generally, they are designed for specific purposes, to culture a target cell type, and are developed by independent research groups. Here, we highlight four that are relevant for the development of CM.
The first is the Essential 8™ (E8) medium, first described by [] for the cultivation of human iPSCs cells and, later, commercialised by Thermo Fisher Scientific (Waltham, MA, USA) as a serum-free medium for the cultivation of human stem cells []. This medium consists of a DMEM/F12 base in a 1:1 ratio, L-ascorbic acid-2-phosphate (64 mg/L), sodium selenium (14 µg/L), FGF-2 (100 µg/L), insulin (19.4 mg/L), NaHCO3 (543 mg/L) and transferrin (10.7 mg/L), TGF-β1 (2 µg/L) or NODAL (100 µg/L). These components will also be found in other serum-free media, making this perhaps a base for the development of other media. The E8 medium has been used to cultivate bovine myoblasts []. The authors reported consistently sustained proliferation of myoblasts; however, the number of cells did not reach the level of the control used, DMEM with 20% FBS and 10% horse serum. The authors also studied this medium with the addition of the growth factors EGF and IGF-1, and, unexpectedly, there was no effect on the proliferation, even though this medium contained FGF-2 and insulin, which are known to induce the proliferation of myoblasts [].
The second medium is an optimisation of the B8 medium [], which had already been able to reduce the serum requirement in bovine satellite cell cultures by 87.5% []. The so-called Beefy-9 or B9 medium seems to perform even better. It consists of a 1:1 DMEM/F12 base with HEPES, insulin (20 µg/mL), L-ascorbic acid-2-phosphate (200 µg/mL), transferrin (20 µg/mL), sodium selenite (20 ng/mL), FGF-2 (40 ng/mL), TGF-β3 (0.1 ng/mL), NRG1 (0.1 ng/mL) and human albumin (800 µg/mL). It could maintain short-term growth comparable to serum-containing medium as well as bovine satellite cell morphology in vitro []. The B9 medium has also been evaluated for its ability to maintain the proliferative property of bovine satellite cells. For this, the satellite cells were expanded to confluence in B9 medium and then differentiated in a serum-free differentiation medium, which we discuss later, allowing for visualisation of the formation of multinucleated myotubes []. Thus, the B9 medium seems to be promising for culturing bovine satellite cells under serum-free conditions, directly aimed at the development of CM.
The third medium is capable of differentiating bovine satellite cells in a serum-free condition together with the B9 medium. This medium was initially described for the establishment of a system by which mature myotubes could be routinely formed from adult rat satellite cells []. It presents as a simple composition of 1:1 Neurobasal/L15 medium with the addition of EGF (0.1 mg/mL) and IGF (0.01 mg/mL). [] used this to differentiate bovine satellite cells into myotubes in serum-free conditions.
The fourth medium is also aimed at bovine satellite cell differentiation []. It is based on the E8 medium, but with the addition of some ligands for receptors identified as upregulated during the initial phase of differentiation of these cells. It has a base of 1:1 DMEM/F12 as well as EGF-1 (10 ng/mL), human albumin (0.5 mg/mL), L-ascorbic acid-2-phosphate (40 mM), sodium selenite (80 nM), NaHCO3 (6.5 mM), MEM amino acid solution (0.5%), insulin (1.8 µM), transferrin (135 nM), lysophosphatidic acid (1 µM) and acetylcholine (10 µM). This medium aims to mimic the conditions of serum starvation, a technique used to induce the differentiation of satellite cells, reducing the concentration of serum in the medium to 2%. When compared, both the decrease in serum and the addition of the serum-free medium showed similar results for the gene and protein expression of canonical myogenic markers, indicating that the serum-free differentiation medium induces a myogenic phenotype similar to the technique using serum [].
The development of defined media for muscle cell proliferation or differentiation has shown promising results in recent years and appears to be the preferred approach. However, there is still another strategy that is promising, less expensive and simpler: the search for natural products of non-animal origin that can replace foetal serum.
Biftek, a Turkish startup, is working on a bacteria-based supplement to replace the use of foetal serum for muscle stem cell growth. Registered under patent US20220098546A1, the substitute is a biological supplement that includes microbiota-derived post-biotics obtained from a fermentation medium of a beneficial microorganism isolated from natural sources. It was able to maintain cell proliferation at rates similar to a conventional medium with FBS []. Biftek also highlights that it has achieved a reduction in costs, as its product costs USD 10 per litre to produce [].
Another research group at Nanyang Technological University in Singapore is working on a substitute for FBS based on okara, a protein- and fibre-rich residue from the production process of certain soy-based products, such as soy milk and tofu []. This residue is fermented and a protein hydrolysate is extracted from it, which also contains certain plant growth hormones. This hydrolysate could maintain the proliferation of HEK293 and HepG2 cells and reduced the need for serum in the culture of C2C12 muscle cells. It is currently being tested by startups in Singapore [,]. It is also a low-cost product, with a production cost of USD 2 per litre [].
Benjaminson, Gilchriest and Lorenz [], in collaboration with the National Aeronautics and Space Administration (NASA), sought substitutes for FBS in the culture medium for the growth of muscle cell explants from goldfish (Carassius auratus). They obtained good results with the crude extract from maitake (Grifola frondosa), an edible mushroom widely cultivated in Japan, as a source of amino acids, carbohydrates and minerals []. They showed a 13.1% increase in the area of fish primary cell explants when using this extract, compared with 13.8% using a standard serum-containing medium [].
Another product, already used for decades in microbiology for the growth of bacteria, is yeast extract. Ref. [] investigated it as a serum substitute for bovine skeletal muscle cell culture; it could recover cell growth (metabolism and proliferation) under reduced and serum-free conditions. However, Ref. [] evaluated this medium in long-term culture and found a significantly lower yield of Vero cells incubated with yeast extract compared with 10% serum.
Based on our current knowledge, we can replace almost all animal-derived substances with recombinant proteins, plant derivatives, fermentation derivatives or synthetic compounds []. The culture media that use these techniques are presented in Table 2. Even at this stage of our knowledge, there is still no single strategy for formulating a serum-free medium that works efficiently for every cell type in every situation []. Therefore, the development of an animal-free medium is still a challenge.

Table 2.
Serum-free media: composition and uses.
4. Three-Dimensional Models for Cultivated Meat Production: Technological Aspects
Initially, in vitro studies maintained the cells in a two-dimensional (2D) culture, which allowed for an understanding of the biological mechanisms underlying cell functions, such as migration and differentiation. Over time, interdisciplinary improvements were made, and the application of biomaterials in culture was essential for the creation of a three-dimensional (3D) environment. It is possible to simulate variable and complex topographies in which cells can more closely mimic the behaviors of their in vivo environments [,]. Figure 2 shows some technological aspects that must be considered for CM.
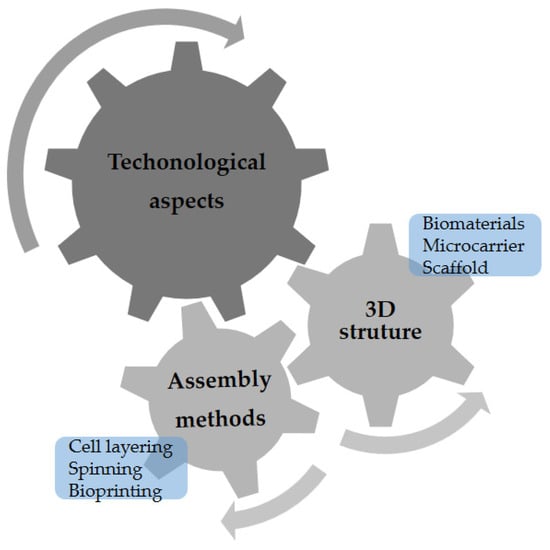
Figure 2.
The technological aspects that must be considered for cultivated meat production.
Tissue construction aiming at successful recreation must mimic an extracellular matrix (ECM), including the composition, the physical properties and the technique for building a structure, all of which will affect the mechanical characteristics of the generated tissue []. It is also important to bear in mind that each tissue has a characteristic ECM, and different types of scaffolds can provide different cell targeting and differentiation outcomes []. Different scaffold architectures can be achieved with existing techniques like porogen leaching, gas foaming, freeze-drying, electrospinning, 3D printing [], sol-gel transition of gelatine [] and 3D bioprinting (3DP) [], among others.
CM is obtained through a process called cellular agriculture, which is based on tissue engineering principles []. Tissue engineering requires three technical components: cells, signals and scaffolds. In practice, it is first necessary to choose the best cell source and type. Second, a biocompatible tissue scaffold should be selected to provide structural support to those cells so they can proliferate and differentiate. Finally, but equally important, the necessary nutrients and small molecules must be provided; they will serve as external signals necessary for cell development (Figure 3).
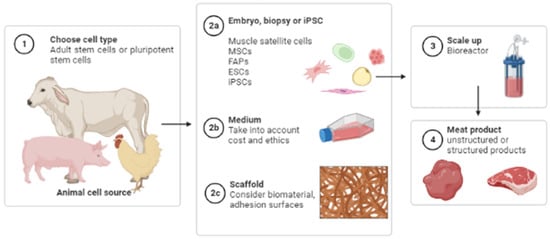
Figure 3.
A general workflow for cultivated meat production.
4.1. Biomaterials
The biomaterials used in tissue engineering are commonly classified according to their origin as natural or synthetic. The natural biomaterials include chitosan, hyaluronic acid, fibrin, alginate, elastin, keratin, poly(hydroxybutyrate) (PHB) and decellularised extracellular matrix (dECM), among others. Some synthetic materials include polyglycolic acid (PGA), polylactic acid (PLA), poly DL-lactic co-glycolic acid (PLGA), polycaprolactone (PCL) and polyethylene glycol (PEG) [,]. Table 3 presents some examples of scaffolds with different compositions generated via different techniques.

Table 3.
Chemical compositions and techniques used to construct scaffolds.
The ideal material should be unlimited, biocompatible—to which cells can bind and interact with the extracellular proteins necessary to form the tissue—non-toxic and edible, which is one of the biggest challenges. Collagen is an animal-derived material used in a mixture with Matrigel and can be considered as a matrix or support. It enables differentiating myoblasts to align, compact and form a muscle fibre []. Plant-derived or synthetic polymers are an alternative to avoid using proteins from animal sources. The main difference between decellularised plant material and material of animal origin is the presence of ECM proteins. These proteins represent a mix of functional molecules such as collagen, fibronectin, glycosaminoglycans and a variety of growth factors that can influence cell development [,]. The absence of these molecules influences cell fixation and proliferation. Due to its biocompatibility, cellulose has arisen as a promising candidate for cell adhesion improvement []. However, other options are also viable: [] showed successful myoblast cultivation in agarose, gellan and a xanthan–locust bean gum blend (XLB) as support materials with pea and soy protein additives.
As CM is designed for human consumption, compounds accepted by the FDA are favoured. The supply of these natural sources can guarantee availability for large-scale production. Among those already known are alginate, chitins and cellulose, which are widely used in food applications. Table 4 shows possible alternative substances to be used in the construction of potential scaffolds, using some FDA-accepted and listed substances that are generally already used in the food industry as additives [,,]. Note that agarose, gellan, xanthan gum, locust bean gum and pea and soy protein have already been used to construct scaffolds. Hence, they could be useful for CM production.

Table 4.
Examples of substances recognised by the Food and Drug Administration (FDA) as safe for food industry use that have been used to construct scaffolds.
4.2. Microcarriers
Microcarriers have been used for quite some time in animal cell culture and they should be able to adapt to CM without major obstacles []. Although cell attachment is often a complex and empirical function, the interdependent factors are hydrophilicity, surface topography, net surface charge, charged group density, curvature and shear rates [].
Cells attach and grow by apposition in microcarriers, which are beads ordinarily having a diameter of 100–200 μm []. Microcarriers differ in their physical properties such as size, porosity, rigidity, density and surface chemistry []. They can be made of synthetic or natural polymers. As mentioned, these microcarriers are applied to large-scale bioprocesses allowing for efficient proliferation of anchor-dependent cells. The advantages of this method are: easy production of large quantities of material and compatibility with various bioreactors and efficient proliferation of adherent cells. The disadvantages are the costs and potential inedibility []. For those that cannot become an integrated part of final product, the cells can be harvested from microcarriers by changing temperature, or through electronically induced shape change []. There is usually a significant cell/tissue yield lost independent of the dissociation process because the cell detachment is incomplete; this loss directly impacts the production efficiency and costs []. On a smaller scale, other alternatives to microcarrier cultures are being considered [], such as spheroids [,], organoids [] or single-cell suspension cultures [].
4.3. Scaffolds
Scaffolds are 3D structures made to resemble the in vivo environment. These porous materials provide mechanical support and allow for an integrated network. Scaffolds are usually used to aid the differentiation step because they enable cells to adhere and mature into an edible meat product. Depending on the model type, scaffolds can grant potential vascularisation and spatial heterogeneity, essential features that the improve texture and structure of the final product, making it more like conventional meat [].
Because CM is an edible product, the tissue scaffolds should be biodegradable and non-toxic. However, in some cases, they may be designed to be degraded or removed before consumption []. The scaffolds may also have appropriate mechanical properties, including strength, thickness, stiffness, pore size, texture and architecture []. For example, porosity directly influences media perfusion, and tissue maturation for a similarity with conventional meat scaffolds also need to support tissue maturation beyond a thickness of 1 cm []. Other properties such as nutritional value, thermal stability, non-allergenicity, non-toxicity and the ability to improve organoleptic properties are important items to consider when choosing the best scaffold depending on the final desired product [].
Currently, there is no commercially available scaffold for CM that is free of animal biomaterials, although a scaffold could be derived from natural, synthetic or composite biomaterials. Natural materials can be derived from animal sources, such as collagen, fibrin and hyaluronic acid, or from plant origin, like alginate, decellularised plant materials and cellulose. Other sources of scaffold materials include chitosan from crustaceans, yeast or fungi and fungal mycelium. Synthetic polymers include a range of polyesters (polyamide and polyethylene). Scaffolds made of these materials can be food safe. Synthetic polymers such as PLA and PLGA are degraded by chemical hydrolysis to generate products like lactic acid and glycolic acid, which are considered safe in food [].
Textured soy protein (TSP) is an important potential scaffold to consider. It is a porous, food-grade, inexpensive by-product of soybean oil processing that has great nutritional value and high protein content in addition to improving the final texture of the product. Due to its characteristics, it is commonly used in plant-based meat substitutes and does not require major modifications; hence, it is highly applicable for mass production. TSP can be adapted to various sizes and shapes, thus facilitating implementation in cultivation processes—for example, in bioreactors [].
Other materials have characteristics that have attracted attention for scaffold production. A group in Korea fabricated microspheres of gelatine (GMS) to use as a scaffold with a high surface-area-to-volume ratio for CM. Gelatine is an edible material capable of promoting cell adhesion through its tripeptide Arg-Gly-Asp (RGD) sequence. In addition to being a collagen-derived natural polymer, its other advantages include high biocompatibility, biodegradability and processability []. Meat analogues with a suitable scaffold made of gelatine fibres can be produced through dry-jet wet-spinning, producing 3D aligned tissues. Aortic smooth muscle cells and skeletal muscle myoblasts have been cultivated in gelatine fibre scaffolds, which are safe and edible materials [].
5. Assembly Methods
Functional tissues require characteristics such as mechanical stiffness and chemical and surface properties for desirable cellular interactions to trigger cell responses. Because scaffolds are important for mimicking the complex spatiotemporal distribution of in vivo tissue, some assembly methods used in tissue engineering to manufacture such structures have been developed [].
5.1. Cell Layering or Self-Assembly
Layer-by-layer (LbL) assembly is a highly adjustable and simple multilayer self-assembly technique. It is possible to produce multilayer coatings with a specific architecture and composition from an extensive catalogue of available materials for several biomedical applications []. This production process is fast and scalable, and it can manufacture highly dense, multicellular and textured tissues in normal culture plates without a bioreactor. A bioreactor is necessary if the final product needs to be thicker [].
There are three basic methods for cell layering: stacking cell sheets, rolling a cohesive tissue sheet and in situ deposition of cell-laden biomaterials. The first one uses a temperature-responsive polymer-coated culture dish to form a multi-layered tissue. The second method consists of wrapping a whole piece of a thin tissue sheet around a tubular support and culturing until tissue fusion. For the third approach, a handheld apparatus is used to deposit cell-laden biomaterials [].
Biomaterials are used to promote or prevent cell adhesion, to maintain or direct cellular phenotypes, and to provide 3D structures for cell culture or co-culture. The range of biomaterials applied for LbL assembly include biomolecules, polyelectrolytes, particles and colloids, among others []. The successful co-culture of myoblasts and preadipocytes has already demonstrated the feasibility of this approach for building meat-like tissues of any size and thickness. Scaffolds do not need to be used because the cells produce their own ECM that is preserved and makes robust sheets [].
5.2. Spinning
Spinning refers to a manufacturing process for creating fibre-shaped materials and can be applied in various manufacturing fields, including tissue engineering []. This material is a potential choice for in vitro tissue production because it produces highly aligned structures with long lengths and flexibility, features that produce functional and morphological characteristics. Among the spinning methods, wet spinning and electrospinning are suitable for this []. Wet spinning is commonly used to produce fibres with micron diameters by using polymers dissolved in non-volatile or heat-unstable solvents. Electrospinning enables the production of nanofibers that can meet the functional requirements of tolerating high temperatures and demonstrating strong absorption for filtration [].
Wet-spun fibres allow for cell adhesion and proliferation into highly oriented porous structures. For this approach, diverse biomaterials can be used as polymers, such as PLGA, chitosan and alginate []. Cells can be mixed with biopolymers and laden or encapsulated within the polymer through microfluidics devices, forming cell-laden fibres. It is possible to assemble larger scaffolds or tissues with this methodology. The main advantage of this method for CM production is the ability to modulate the thickness, shape and mechanical rigidity of the fibres through microfluidic channels [].
Electrospinning is based on the use of electrical forces to produce fibres, which provide a large surface-area-to-volume ratio, thus improving cellular development. Biopolymers for cell electrospinning can also be natural or synthetic polymers []. Electrospun scaffolds are also very interesting for CM production because this technique generates alignment cues that guide the uniaxial alignment of seeded cells. Ref. [] demonstrated that even after electrospinning, myoblasts on fibrin scaffolds exhibited a uniform distribution, and they continued to proliferate and differentiate.
5.3. Bioprinting
3DP is a promising tissue engineering technique for simulating the structural characteristics of meat. The advantages of 3D bioprinting are the ability to control the structure and composition of a product in addition to its potential scalability (Kang et al. 2021). 3DP is an advanced additive manufacturing platform that allows for the pre-defined deposition of cells, biomaterials and growth factors. It is based on computer-aided design and manufacturing (CAD/CAM), which customises the layer-by-layer printing process with a high level of flexibility and reproducibility. 3DP is an emerging technology that has attracted increased attention in the last few years in the food field due to its applicability for sustainably manufacturing customised products with intricate shapes and textures []. It also allows for improving the nutritional profile and sensorial values of the product [].
The 3DP production process allows for the deposition of materials or inks in a layer-by-layer fashion to generate complex 3D structures that resemble laboratory cultured cells []. The most-used 3DP methods are extrusion, inkjet printing, binder jetting and bioprinting. For meat product fabrication, the extrusion method is commonly used to print the 3D structures [].
Bio-inks consist of cells, biomaterials and other molecules such as growth factors. The medium for the cell suspension contains polymer crosslinkers, such as CaCl2, thrombin, salt (NaCl), gelatine and fibrinogen. Biomaterials, such as melt-cure polymers, hydrogels or dECM, are utilised as scaffolds in bio-inks to provide an appropriate microenvironment for cell adhesion, migration and differentiation [,,]. Therefore, 3DP provides the possibility for reshaping the structure of conventional meat: the structure can be designed in such a way that raw materials can be thoroughly mixed and organised. With this process, it is possible to fabricate flexible artificial vessels and to control the graininess and toughness of the final product, to ensure that it is similar to conventional meat [].
Printed constructs enable nutrient diffusion and enhance porosity. Researchers have described successful printing of engineered tissues, including skeletal muscle [,,]. Obviously, 3D-printed CM has benefited from advances made in the tissue engineering field. Although it does not need a complex vascular system like in natural tissue, more research is required to improve printable, non-animal materials and potentially edible scaffold compositions.
Ianovici et al. (2022) [] tested two 3D-printed scaffolding compositions, not derived from animal biomaterials and enriched with plant proteins, for bovine satellite cell cultivation. They evaluated mixtures of pea protein isolate (PPI) and soy protein isolate (SPI) with RGD-modified alginate suitable for flexible 3DP and cell cultivation. They observed bovine satellite cell attachment, spreading, maturation and differentiation. They applied extrusion using an edible, removable agar support bath. PPI-enriched bio-inks allowed for cellular bioprinting.
Kang et al. (2021) [] produced the first whole-cut CM-like tissue. It was composed of three types of primary bovine cells (satellite cells, adipose-derived stem cells and endothelial cells). The authors used tendon-gel-integrated bioprinting (TIP) to fabricate cell fibres. They then modelled the subsequent cell differentiation into the structure of real meat. When assembled, it mimicked the histological structures of a real steak. Despite its good appearance, the meat-like tissue was very small and not edible, indicating that more research is needed for improvement. Although 3DP is likely to achieve a final product with a thickness close to that of real meat, it might be less amenable to the scaling required to achieve CM production.
6. Conclusions
Meat is exsanguinated and aged musculoskeletal tissue—comprising skeletal muscle, connective tissues, bone, blood vessels and nerves—that is derived from biochemical reactions triggered by lack of oxygen following the slaughter of the animal [,]. CM is an emergent and disruptive technology in cellular agriculture that aims to reproduce, ultimately, all of the organoleptic properties of meat. Currently, most CM is muscle protein made solely from muscle fibres []. An imitation of the complexity of conventional meat needs to have a 3D structure and multiple cell types, but the technology for this assembly process has not yet been developed (Table 5). The expectation is that, initially, unstructured products (burgers, sausages and nuggets) will be produced and marketed.

Table 5.
The outlook of cultivated meat (CM) production in recent publications.
The future of CM is uncertain, but it already has the potential to become a significant part of the meat industry in the coming years. Many see it as a sustainable alternative to conventionally produced meat. Since 1931, when Winston Churchill made the remarkable prediction that it would be possible to grow chicken breasts and wings without the ‘absurdity of growing a whole chicken’ [], tissue engineering has increased tremendously and in the future will play more and more of a role in the food industry. However, there are still technical and economic challenges to overcome, such as scalability, cost and regulatory approval, before it can be produced at scale and sold at prices competitive with conventional meat. Additionally, there may be cultural hurdles to overcome before CM is widely accepted by consumers. Overall, the future of CM is likely to be shaped by a combination of scientific and technological progress, changing consumer preferences and regulatory developments. The industry is expected to grow in the coming years: investments and partnerships from major food companies and startups should shorten the wait time for CM to hit market shelves.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: A.C.A.S. and S.S.M.-E.; data curation: A.C.A.S., D.E.M.C. and G.R.R.; original draft preparation: A.C.A.S.; writing review & editing: A.C.A.S. and S.S.M.-E. Supervision: V.A.N., F.S.C., M.A.T. ands S.S.M.-E.; project administration and funding acquisition: S.S.M.-E. All authors have read and agreed to the published version of the manuscript.
Funding
GOSTOSISSIMI COMÉRCIO DE ALIMENTOS LTDA. Foundation: FUSP and CNPq Fellowship 309440/2021-1.
Data Availability Statement
The research data described in the manuscript is available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ianovici, I.; Zagury, Y.; Redenski, I.; Lavon, N.; Levenberg, S. 3D-printable plant protein-enriched scaffolds for cultivated meat development. Biomaterials 2022, 284, 121487. [Google Scholar] [CrossRef]
- Stephens, N.; Ellis, M. Cellular agriculture in the UK: A review. Wellcome Open Res. 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.N.; Cosenza, Z.A.; Baar, K.; Block, D.E. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr. Rev. Food Sci. Food Saf. 2021, 20, 686–709. [Google Scholar] [CrossRef]
- Jo, B.; Nie, M.; Takeuchi, S. Manufacturing of animal products by the assembly of microfabricated tissues. Essays Biochem. 2021, 65, 611–623. [Google Scholar] [CrossRef]
- Schätzlein, E.; Blaeser, A. Recent trends in bioartificial muscle engineering and their applications in cultured meat, biorobotic systems and biohybrid implants. Commun. Biol. 2022, 5, 737. [Google Scholar] [CrossRef]
- Corsato, B.; Gallon, V. Setor de Proteínas Alternativas Recebeu Investimento Recorde de $5 Bilhões em 2021, 60% a Mais do que em 2020. Available online: https://gfi.org.br/2022/03/21/setor-de-proteinas-alternativas-recebeu-investimento-recorde-de-5-bilhoes-em-2021-60-a-mais-do-que-em-2020/ (accessed on 24 April 2022).
- Mosa Meat. Our Progress so Far; Mosa Meat: San Leandro, CA, USA, 2022. [Google Scholar]
- GFI. 2021 State of the Industry Report. Cultivated Meat and Seafood. 2022. Available online: https://gfi.org/resource/cultivated-meat-eggs-and-dairy-state-of-the-industry-report/ (accessed on 6 October 2022).
- Food Ingridedients First. Upside Foods Raises US$400M to Commercialize Cell-Based Meat at Scale. 2022. Available online: https://www.foodingredientsfirst.com/news/upside-foods-raises-us400m-to-commercialize-cell-based-meat-at-scale.html (accessed on 19 September 2022).
- Vegconomist. Israeli Government Grants $18M to Cultivated Meat Consortium in ‘Unprecedented’ Move. 2022. Available online: https://vegconomist.com/cultivated-cell-cultured-biotechnology/cultivated-meat/cultivated-meat-consortium/ (accessed on 19 September 2022).
- Green Queen. Israel’s Cultivated Meat Consortium Gets Green Light and $18 Million From Government. 2022. Available online: https://www.greenqueen.com.hk/israels-cultivated-meat-consortium-given-green-light/ (accessed on 19 September 2022).
- Vegaconomist. Cultured Meat in Europe: Which Country Is Leading the Race? 2022. Available online: https://vegconomist.com/cultivated-cell-cultured-biotechnology/cultured-meat-in-europe-which-country-is-leading-the-race/ (accessed on 19 September 2022).
- Valdes, C. Brazil’s Momentum as a Global Agricultural Supplier Faces Headwinds. In Amber Waves: The Economics of Food, Farming, Natural Resources, and Rural America 2022; AgEcon: St. Paul, MN, USA, 2022. [Google Scholar] [CrossRef]
- Abras. JBS Investe Pesado Em Carne de Laboratório. 2022. Available online: https://www.abras.com.br/clipping/carnes-peixes/110871/jbs-investe-pesado-em-carne-de-laboratorio (accessed on 24 November 2022).
- Food Connection. Carne Cultivada Em Laboratório Deve Chegar Ao Mercado Brasileiro Em 2024. 2022. Available online: https://www.foodconnection.com.br/ingredientes/carne-cultivada-em-laboratorio-deve-chegar-ao-mercado-brasileiro-em-2024 (accessed on 24 November 2022).
- Ambi Real Food. Somos a Primeira Start up de Carne Cultivada Brasileira! 2022. Available online: https://ambirealfood.com/ (accessed on 25 April 2022).
- Sustineri PiscisSustineri Piscis Aquicultura Celular. 2022. Available online: https://sustineripiscis.com/#home (accessed on 24 November 2022).
- Green Queen. A Year After Historic Cultivated Meat Approval, Singapore Regulator Greenlights More Eat Just Products. 2021. Available online: https://www.greenqueen.com.hk/eat-just-cultivated-meat-regulatory-approval-singapore/ (accessed on 19 September 2022).
- Smart Brief Cultured Meat Could Come further out of the Lab in 2022. 2022. Available online: https://corp.smartbrief.com/original/2022/05/cultured-meat-could-come-further-out-of-the-lab-in-2022 (accessed on 19 September 2022).
- FDA. FDA Completes First Pre-Market Consultation for Human Food Made Using Animal Cell Culture Technology; FDA: Silver Spring, MD, USA, 2022.
- Vegaconomist. South Korea’s National Plan 2022 to Include Cultivated Meat Regulatory Approval Guidance. 2022. Available online: https://vegconomist.com/politics-law/south-koreas-national-plan-alt-protein/ (accessed on 19 September 2022).
- GFI Brasil. Estudo Regulatório Sobre Proteínas Alternativas No Brasil—Proteínas Vegetais. 2022. Available online: http://gfi.org.br/resources/estudo-regulatorio-sobre-proteinas-alternativas-no-brasil-proteinas-vegetais-2/ (accessed on 5 October 2022).
- Szejda, K.; Bryant, C.J.; Urbanovich, T. US and UK Consumer Adoption of Cultivated Meat: A Segmentation Study. Foods 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Barnett, J. Consumer Acceptance of Cultured Meat: An Updated Review (2018–2020). Appl. Sci. 2020, 10, 5201. [Google Scholar] [CrossRef]
- Valente, J.d.P.S.; Fiedler, R.A.; Sucha Heidemann, M.; Molento, C.F.M. First glimpse on attitudes of highly educated consumers towards cell-based meat and related issues in Brazil. PLoS ONE 2019, 14, e0221129. [Google Scholar] [CrossRef]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef]
- Soice, E.; Johnston, J. Immortalizing Cells for Human Consumption. Int. J. Mol. Sci. 2021, 22, 11660. [Google Scholar] [CrossRef] [PubMed]
- Treich, N. Cultured Meat: Promises and Challenges. Environ. Resour. Econ. 2021, 79, 33–61. [Google Scholar] [CrossRef] [PubMed]
- Pajčin, I.; Knežić, T.; Savic Azoulay, I.; Vlajkov, V.; Djisalov, M.; Janjušević, L.; Grahovac, J.; Gadjanski, I. Bioengineering Outlook on Cultivated Meat Production. Micromachines 2022, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Mesnage, R.; Tsatsakis, A.M.; Golokhvast, K.S.; Yang, S.H.; Antoniou, M.N.; Chung, G. Addressing concerns over the fate of DNA derived from genetically modified food in the human body: A review. Food Chem. Toxicol. 2019, 124, 423–430. [Google Scholar] [CrossRef]
- Knudtson, K.L.; Carnahan, R.H.; Hegstad-Davies, R.L.; Fisher, N.C.; Hicks, B.; Lopez, P.A.; Meyn, S.M.; Mische, S.M.; Weis-Garcia, F.; White, L.D.; et al. Survey on Scientific Shared Resource Rigor and Reproducibility. J. Biomol. Tech. 2019, 30, 36–44. [Google Scholar] [CrossRef]
- Baker, M. 1500 scientists lift the lid on reproducibility. Nature 2016, 533, 452–454. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Prenoveau, J.M. Rigor and reproducibility for data analysis and design in the behavioral sciences. Behav. Res. Ther. 2020, 126, 103552. [Google Scholar] [CrossRef]
- Merz, K.M.; Amaro, R.; Cournia, Z.; Rarey, M.; Soares, T.; Tropsha, A.; Wahab, H.A.; Wang, R. Editorial: Method and Data Sharing and Reproducibility of Scientific Results. J. Chem. Inf. Model. 2020, 60, 5868–5869. [Google Scholar] [CrossRef]
- Ng, E.T.; Singh, S.; Yap, W.S.; Tay, S.H.; Choudhury, D. Cultured meat—A patentometric analysis. Crit. Rev. Food Sci. Nutr. 2021, 1–11. [Google Scholar] [CrossRef]
- Bellani, C.F.; Ajeian, J.; Duffy, L.; Miotto, M.; Groenewegen, L.; Connon, C.J. Scale-Up Technologies for the Manufacture of Adherent Cells. Front. Nutr. 2020, 7, 575146. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef]
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.; Bryant, C.J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Gaydhane, M.K.; Mahanta, U.; Sharma, C.S.; Khandelwal, M.; Ramakrishna, S. Cultured meat: State of the art and future. Biomanuf. Rev. 2018, 3, 1. [Google Scholar] [CrossRef]
- Park, Y.H.; Gong, S.P.; Kim, H.Y.; Kim, G.A.; Choi, J.H.; Ahn, J.Y.; Lim, J.M. Development of a serum-free defined system employing growth factors for preantral follicle culture. Mol. Reprod. Dev. 2013, 80, 725–733. [Google Scholar] [CrossRef]
- Freshney, R.I. Defined Media and Supplements. In Culture of Animal Cells; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Brunner, D.; Frank, J.; Appl, H.; Schöffl, H.; Pfaller, W.; Gstraunthaler, G. Serum-free Cell Culture: The Serum-free Media Interactive Online Database. ALTEX 2010, 27, 53–62. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.-D.; Joo, S.T.; Choi, I.; et al. Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat. Food Sci. Anim. Resour. 2022, 42, 775–799. [Google Scholar] [CrossRef]
- Kadim, I.T.; Mahgoub, O.; Baqir, S.; Faye, B.; Purchas, R. Cultured meat from muscle stem cells: A review of challenges and prospects. J. Integr. Agric. 2015, 14, 222–233. [Google Scholar] [CrossRef]
- Duque, P.; Gómez, E.; Díaz, E.; Facal, N.; Hidalgo, C.; Díez, C. Use of two replacements of serum during bovine embryo culture in vitro. Theriogenology 2003, 59, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Messmer, T.; Klevernic, I.; Furquim, C.; Ovchinnikova, E.; Dogan, A.; Cruz, H.; Post, M.J.; Flack, J.E. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food 2022, 3, 74–85. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Essential 8TM Medium; Thermo Fisher Scientific: Waltham, MA, USA, 2022.
- Kolkmann, A.M.; Post, M.J.; Rutjens, M.A.M.; van Essen, A.L.M.; Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020, 72, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-H.; Gao, X.; DeKeyser, J.-M.; Fetterman, K.A.; Pinheiro, E.A.; Weddle, C.J.; Fonoudi, H.; Orman, M.V.; Romero-Tejeda, M.; Jouni, M.; et al. Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Rep. 2020, 14, 256–270. [Google Scholar] [CrossRef]
- McAleer, C.W.; Rumsey, J.W.; Stancescu, M.; Hickman, J.J. Functional myotube formation from adult rat satellite cells in a defined serum-free system. Biotechnol. Prog. 2015, 31, 997–1003. [Google Scholar] [CrossRef]
- Can, A.K.; Erikci, E.; Kiran, F. Microbiota-Derived Postbiotics: Alternative Supplement to Fetal Bovine Serum for Cultured Meat. U.S. Patent US20220098546A1, 28 September 2021. [Google Scholar]
- FiGlobal. New Growth Medium Supplement for ‘Clean Meat. 2021. Available online: https://insights.figlobal.com/startups/new-growth-medium-supplement-clean-meat (accessed on 21 August 2022).
- Gupta, S.; Lee, J.J.L.; Chen, W.N. Analysis of Improved Nutritional Composition of Potential Functional Food (Okara) after Probiotic Solid-State Fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar] [CrossRef]
- Teng, T.S. Development of Serum Alternative with Fermented Soybean Residue (Okara): Towards Cultured Meat Production Application; Nanyang Technological University: Singapore, 2022. [Google Scholar]
- Straits Times. Saving Soya Pulp for Novel Foods and Aquaculture. 2021. Available online: https://www.straitstimes.com/singapore/environment/saving-soya-pulp-for-novel-foods-and-aquaculture (accessed on 18 October 2022).
- Benjaminson, M.A.; Gilchriest, J.A.; Lorenz, M. In vitro edible muscle protein production system (mpps): Stage 1, fish. Acta Astronaut. 2002, 51, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Siu, K.-C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Andreassen, R.; Pedersen, M.; Kristoffersen, K.; Rønning, S. Screening of by-products from the food industry as growth promoting agents in serum-free media for skeletal muscle cell culture. Food Funct. 2020, 11, 2477–2488. [Google Scholar] [CrossRef]
- Posung, M.; Promkhatkaew, D.; Borg, J.; Tongta, A. Development of a modified serum-free medium for Vero cell cultures: Effects of protein hydrolysates, l-glutamine and SITE liquid media supplement on cell growth. Cytotechnology 2021, 73, 683–695. [Google Scholar] [CrossRef]
- Merten, O.-W. Safety issues of animal products used in serum-free media. Dev. Biol. Stand. 1999, 99, 167–180. [Google Scholar]
- Zhang, Z.; He, Q.; Deng, W.; Chen, Q.; Hu, X.; Gong, A.; Cao, X.; Yu, J.; Xu, X. Nasal ectomesenchymal stem cells: Multi-lineage differentiation and transformation effects on fibrin gels. Biomaterials 2015, 49, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Burla, F.; Mulla, Y.; Vos, B.E.; Aufderhorst-Roberts, A.; Koenderink, G.H. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys. 2019, 1, 249–263. [Google Scholar] [CrossRef]
- Yu, D.; Cai, Z.; Li, D.; Zhang, Y.; He, M.; Yang, Y.; Liu, D.; Xie, W.; Li, Y.; Xiao, W. Myogenic Differentiation of Stem Cells for Skeletal Muscle Regeneration. Stem Cells Int. 2021, 2021, 8884283. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Gyawali, D.; Nair, P.; Kim, H.K.W.; Yang, J. Citrate-based biodegradable injectable hydrogel composites for orthopedic applications. Biomater. Sci. 2013, 1, 52–64. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Varkey, M.; Gorkun, A.; Clouse, C.; Xu, L.; Chou, Z.; Murphy, S.V.; Molnar, J.; Lee, S.J.; Yoo, J.J.; et al. Bioprinted Skin Recapitulates Normal Collagen Remodeling in Full-Thickness Wounds. Tissue Eng. Part A 2020, 26, 512–526. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Bone Scaffolds from Electrospun Fiber Mats of Poly(3-Hydroxybutyrate), Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Their Blend. Polymer 2007, 48, 1419–1427. [Google Scholar] [CrossRef]
- Depan, D.; Surya, P.V.; Girase, B.; Misra, R. Organic/Inorganic Hybrid Network Structure Nanocomposite Scaffolds Based on Grafted Chitosan for Tissue Engineering. Acta Biomater. 2011, 7, 2163–2175. [Google Scholar] [CrossRef]
- Page, R.L.; Malcuit, C.; Vilner, L.; Vojtic, I.; Shaw, S.; Hedblom, E.; Hu, J.; Pins, G.D.; Rolle, M.W.; Dominko, T. Restoration of Skeletal Muscle Defects with Adult Human Cells Delivered on Fibrin Microthreads. Tissue Eng. Part A 2011, 17, 2629–2640. [Google Scholar] [CrossRef]
- Dugan, J.M.; Collins, R.F.; Gough, J.E.; Eichhorn, S.J. Oriented Surfaces of Adsorbed Cellulose Nanowhiskers Promote Skeletal Muscle Myogenesis. Acta Biomater. 2013, 9, 4707–4715. [Google Scholar] [CrossRef]
- Lu, H.; Lv, L.; Dai, Y.; Wu, G.; Zhao, H.; Zhang, F. Porous Chitosan Scaffolds with Embedded Hyaluronic Acid/Chitosan/Plasmid-DNA Nanoparticles Encoding TGF-Β1 Induce DNA Controlled Release, Transfected Chondrocytes, and Promoted Cell Proliferation. PLoS ONE 2013, 8, e69950. [Google Scholar] [CrossRef]
- Wang, L.; Cao, L.; Shansky, J.; Wang, Z.; Mooney, D.; Vandenburgh, H. Minimally Invasive Approach to the Repair of Injured Skeletal Muscle With a Shape-Memory Scaffold. Mol. Ther. 2014, 22, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Gualandi, C.; Zucchelli, A.; Boyle, L.A.; Kao, A.P.; Reilly, G.C.; Tozzi, G.; Cristofolini, L.; Focarete, M.L. Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic Acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties. Sci. Rep. 2018, 8, 17167. [Google Scholar] [CrossRef]
- Barros, D.; Conde-Sousa, E.; Gonçalves, A.M.; Han, W.M.; García, A.J.; Amaral, I.F.; Pêgo, A.P. Engineering Hydrogels with Affinity-Bound Laminin as 3D Neural Stem Cell Culture Systems. Biomater. Sci. 2019, 7, 5338–5349. [Google Scholar] [CrossRef]
- Wei, Q.; Young, J.; Holle, A.; Li, J.; Bieback, K.; Inman, G.; Spatz, J.P.; Cavalcanti-Adam, E.A. Soft Hydrogels for Balancing Cell Proliferation and Differentiation. ACS Biomater. Sci. Eng. 2020, 6, 4687–4701. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Wright, M.E.E.; Cheng, N.; Oh, H.H.; Wang, Y.; Datu, A.K.; Santerre, J.P.; Amini-Nik, S.; Jeschke, M.G. Electrospun Polyurethane–Gelatin Composite: A New Tissue-Engineered Scaffold for Application in Skin Regeneration and Repair of Complex Wounds. ACS Biomater. Sci. Eng. 2020, 6, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Stadler, F.J.; Fu, M. Biomimetic Electrospun Tubular PLLA/Gelatin Nanofiber Scaffold Promoting Regeneration of Sciatic Nerve Transection in SD Rat. Mater. Sci. Eng. C 2021, 121, 111858. [Google Scholar] [CrossRef]
- Post, M.J.; Hocquette, J.-F. New Sources of Animal Proteins: Cultured Meat. In New Aspects of Meat Quality; Elsevier: Amsterdam, The Netherlands, 2017; pp. 425–441. [Google Scholar]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-Based Scaffolds in Tissue Engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Wollschlaeger, J.O.; Maatz, R.; Albrecht, F.B.; Klatt, A.; Heine, S.; Blaeser, A.; Kluger, P.J. Scaffolds for Cultured Meat on the Basis of Polysaccharide Hydrogels Enriched with Plant-Based Proteins. Gels 2022, 8, 94. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Substances Added to Food (Formerly EAFUS); FDA: Silver Spring, MD, USA, 2018.
- FAO. Dietary Carbohydrate Composition. 2022. Available online: https://www.fao.org/3/W8079E/w8079e0h.htm (accessed on 21 October 2022).
- ODEX STAN 247. General Standard for Fruit Juices and Nectars (CODEX STAN 247-2005). 2005. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B247-2005%252FCXS_247e.pdf (accessed on 21 October 2022).
- Nwe, N.; Furuike, T.; Tamura, H. The Mechanical and BiologicalProperties of Chitosan Scaffolds for Tissue Regeneration Templates Are SignificantlyEnhanced by Chitosan from Gongronella Butleri. Materials 2009, 2, 374–398. [Google Scholar] [CrossRef]
- Basha, R.Y.; Kumar, T.S.S.; Doble, M. Electrospun Nanofibers of Curdlan (β-1,3 Glucan) Blend as a Potential Skin Scaffold Material. Macromol. Mater. Eng. 2017, 302, 1600417. [Google Scholar] [CrossRef]
- Prasopdee, T.; Sinthuvanich, C.; Chollakup, R.; Uttayarat, P.; Smitthipong, W. The Albumin/Starch Scaffold and Its Biocompatibility with Living Cells. Mater. Today Commun. 2021, 27, 102164. [Google Scholar] [CrossRef]
- Poddar, S.; Agarwal, P.S.; Sahi, A.K.; Vajanthri, K.Y.; Pallawi; Singh, K.N.; Mahto, S.K. Fabrication and Cytocompatibility Evaluation of Psyllium Husk (Isabgol)/Gelatin Composite Scaffolds. Appl. Biochem. Biotechnol. 2019, 188, 750–768. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed Cellulose Scaffolds Derived from Green Macroalgae for Tissue Engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef] [PubMed]
- Indurkar, A.; Bangde, P.; Gore, M.; Agrawal, A.K.; Jain, R.; Dandekar, P. Fabrication of Guar Gum-Gelatin Scaffold for Soft Tissue Engineering. Carbohydr. Polym. Technol. Appl. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Li, N.; Xue, F.; Zhang, H.; Sanyour, H.J.; Rickel, A.P.; Uttecht, A.; Fanta, B.; Hu, J.; Hong, Z. Fabrication and Characterization of Pectin Hydrogel Nanofiber Scaffolds for Differentiation of Mesenchymal Stem Cells into Vascular Cells. ACS Biomater. Sci. Amp; Eng. 2019, 5, 6511–6519. [Google Scholar] [CrossRef]
- Bektas, E.I.; Pekozer, G.G.; Kök, F.N.; Kose, G.T. Evaluation of Natural Gum-Based Cryogels for Soft Tissue Engineering. Carbohydr. Polym. 2021, 271, 118407. [Google Scholar] [CrossRef]
- Feroz, S.; Dias, G. Hydroxypropylmethyl Cellulose (HPMC) Crosslinked Keratin/Hydroxyapatite (HA) Scaffold Fabrication, Characterization and in Vitro Biocompatibility Assessment as a Bone Graft for Alveolar Bone Regeneration. Heliyon 2021, 7, e08294. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Razak, S.I.A.; Ansari, M.N.M.; Zulkifli, R.M.; Zawawi, N.A.; Arshad, M. Development of Biodegradable Bio-Based Composite for Bone Tissue Engineering: Synthesis, Characterization and In Vitro Biocompatible Evaluation. Polymers 2021, 13, 3611. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; García-San-Martín, N.; Molina-Mateo, J.; Cabanilles, C.T.; Quiles, V.D.; Serrano-Aroca, A.; Serra, R.S. Electroactive Calcium- Alginate/Polycaprolactone/Reduced Graphene Oxide Nanohybrid Hydrogels for Skeletal Muscle Tissue Engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef] [PubMed]
- Rekulapally, R.; Udayachandrika, K.; Hamlipur, S.; Nair, A.S.; Pal, B.; Singh, S. Tissue Engineering of Collagen Scaffolds Crosslinked with Plant Based Polysaccharides. Prog. Biomater. 2021, 10, 29–41. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Yao, L.; Li, Y.; Weng, Y.; Qiu, D. Fabrication and Characterization of Gelatin/Polyvinyl Alcohol Composite Scaffold. Polymers 2022, 14, 400. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Questions and Answers on Dietary Fiber; FDA: Silver Spring, MD, USA, 2021.
- Food Standards Australia & New Zealand. Application A1077—Fungal Hitosan as a Processing Aid; Food Standards Australia & New Zealand: Barton, Australia, 2013.
- CODEX STAN 192-1995; General Standard for Food Additives. CODEX Alimentarius International Food Standards: Rome, Italy, 1995.
- FDA. Food Additive Status List; FDA: Silver Spring, MD, USA, 2022.
- AgriFiber Solutions, LLC. Safety Evaluation Dossier Supporting a Generally Recognized as Safe (Gras) Conclusion for the Use of Corn Bran Arabinoxylan in Conventional Foods—GRAS Notice (GRN) No. 998; AgriFiber Solutions, LLC.: Mundelein, IL, USA, 2021. [Google Scholar]
- Zhang, G.; Zhao, X.; Li, X.; Du, G.; Zhou, J.; Chen, J. Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci. Technol. 2020, 97, 443–450. [Google Scholar] [CrossRef]
- Moritz, M.S.M.; Verbruggen, S.E.L.; Post, M.J. Alternatives for large-scale production of cultured beef: A review. J. Integr. Agric. 2015, 14, 208–216. [Google Scholar] [CrossRef]
- Andreassen, R.C.; Rønning, S.B.; Solberg, N.T.; Grønlien, K.G.; Kristoffersen, K.A.; Høst, V.; Kolset, S.O.; Pedersen, M.E. Production of food-grade microcarriers based on by-products from the food industry to facilitate the expansion of bovine skeletal muscle satellite cells for cultured meat production. Biomaterials 2022, 286, 121602. [Google Scholar] [CrossRef] [PubMed]
- Bomkamp, C.; Skaalure, S.C.; Fernando, G.F.; Ben-Arye, T.; Swartz, E.W.; Specht, E.A. Scaffolding Biomaterials for 3D Cultivated Meat: Prospects and Challenges. Adv. Sci. 2022, 9, 2102908. [Google Scholar] [CrossRef]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. A rapid biofabrication technique for self-assembled collagen-based multicellular and heterogeneous 3D tissue constructs. Acta Biomater. 2019, 92, 172–183. [Google Scholar] [CrossRef]
- Koudan, E.V.; Gryadunova, A.A.; Karalkin, P.A.; Korneva, J.V.; Meteleva, N.Y.; Babichenko, I.I.; Volkov, A.V.; Rodionov, S.A.; Parfenov, V.A.; Pereira, F.D.A.S.; et al. Multiparametric Analysis of Tissue Spheroids Fabricated from Different Types of Cells. Biotechnol. J. 2020, 15, 1900217. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Todhunter, M.E.; LaBarge, M.A.; Gartner, Z.J. Opportunities for organoids as new models of aging. J. Cell Biol. 2018, 217, 39–50. [Google Scholar] [CrossRef]
- Milián, E.; Julien, T.; Biaggio, R.; Venereo-Sanchez, A.; Montes, J.; Manceur, A.P.; Ansorge, S.; Petiot, E.; Rosa-Calatrava, M.; Kamen, A. Accelerated mass production of influenza virus seed stocks in HEK-293 suspension cell cultures by reverse genetics. Vaccine 2017, 35, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials 2022, 280, 121274. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Park, S.; Jung, S.; Choi, M.; Lee, M.; Choi, B.; Koh, W.-G.; Lee, S.; Hong, J. Gelatin MAGIC powder as nutrient-delivering 3D spacer for growing cell sheets into cost-effective cultured meat. Biomaterials 2021, 278, 121155. [Google Scholar] [CrossRef] [PubMed]
- Shahin-Shamsabadi, A.; Selvaganapathy, P.R. Engineering Murine Adipocytes and Skeletal Muscle Cells in Meat-like Constructs Using Self-Assembled Layer-by-Layer Biofabrication: A Platform for Development of Cultivated Meat. Cells Tissues Organs 2022, 211, 304–312. [Google Scholar] [CrossRef]
- Zhang, S.; Xing, M.; Li, B. Biomimetic Layer-by-Layer Self-Assembly of Nanofilms, Nanocoatings, and 3D Scaffolds for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1641. [Google Scholar] [CrossRef] [PubMed]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Isotropic and Anisotropic Scaffolds for Tissue Engineering: Collagen, Conventional, and Textile Fabrication Technologies and Properties. Int. J. Mol. Sci. 2021, 22, 9561. [Google Scholar] [CrossRef]
- Jin, S.; Chen, Z.; Xin, B.; Xi, T.; Meng, N. An investigation on the comparison of wet spinning and electrospinning: Experimentation and simulation. Fibers Polym. 2017, 18, 1160–1170. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gilbert-Honick, J.; Somers, S.M.; Mao, H.-Q.; Grayson, W.L. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Ramachandraiah, K. Potential development of sustainable 3d-printed meat analogues: A review. Sustainability 2021, 13, 938. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Liu, W.; Pushparaj, K.; Park, S. The Epic of In Vitro Meat Production—A Fiction into Reality. Foods 2021, 10, 1395. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Święszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Kim, J.H.; Seol, Y.-J.; Ko, I.K.; Kang, H.-W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, H.; Kim, G.H. Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration. Biofabrication 2016, 8, 035021. [Google Scholar] [CrossRef]
- Kang, D.-H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Jairath, G.; Mal, G.; Gopinath, D.; Singh, B. A holistic approach to access the viability of cultured meat: A review. Trends Food Sci. Technol. 2021, 110, 700–710. [Google Scholar] [CrossRef]
- Chriki, S.; Payet, V.; Pflanzer, S.B.; Ellies-Oury, M.-P.; Liu, J.; Hocquette, É.; Rezende-de-Souza, J.H.; Hocquette, J.-F. Brazilian Consumers’ Attitudes towards So-Called ‘Cell-Based Meat’. Foods 2021, 10, 2588. [Google Scholar] [CrossRef] [PubMed]
- Churchill, W. Fifty Years Hence. 1931. Available online: https://www.nationalchurchillmuseum.org/fifty-years-hence.html (accessed on 6 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).