Impacts of Environmental Pollution on Brain Tumorigenesis
Abstract
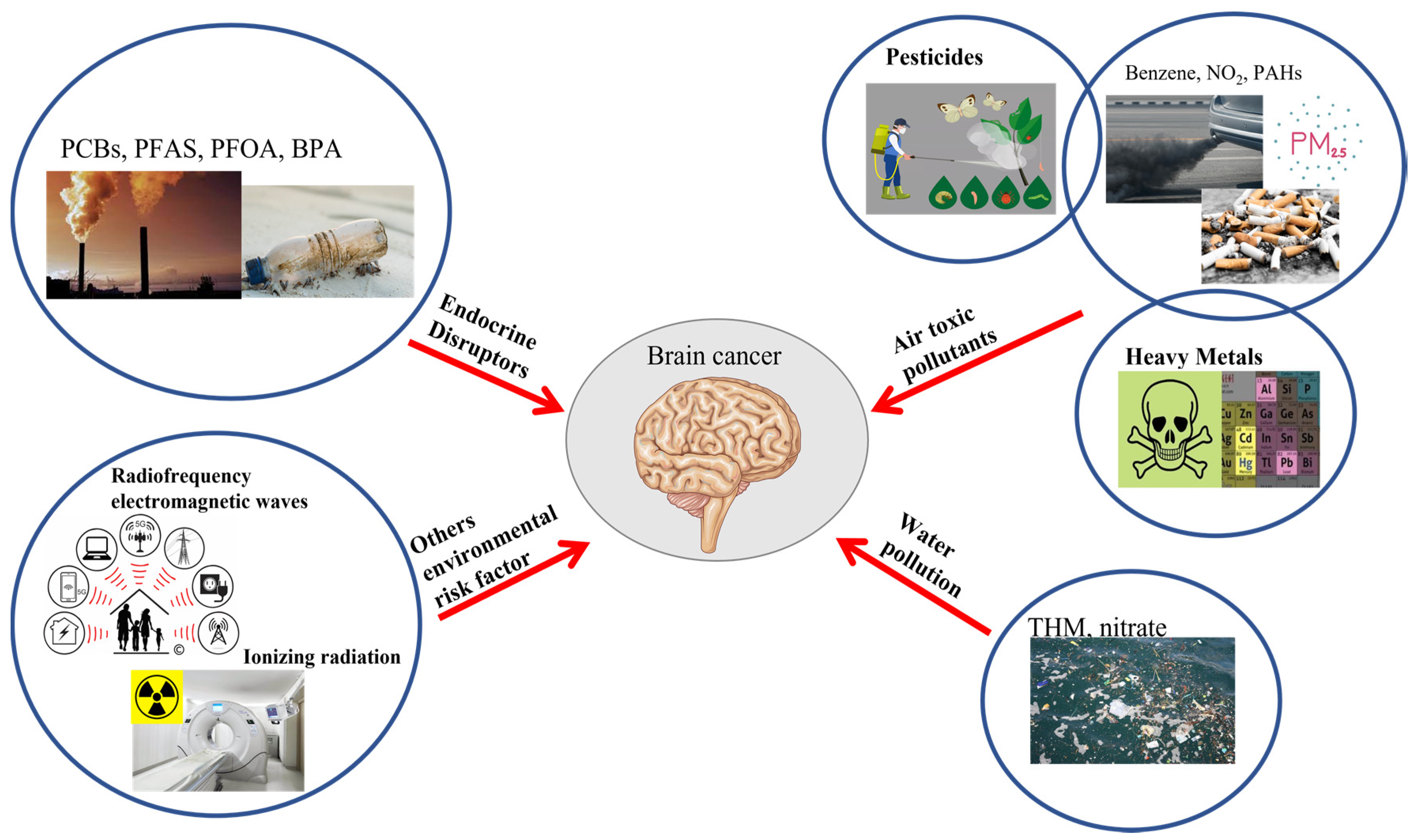
1. Introduction
2. Endocrine Disruptors
3. Air Toxic Pollutants
3.1. Pesticides
3.2. Heavy Metals
3.3. Chemicals Pollutants
3.4. Particulate Matter (PM)
4. Water Pollution
5. Others Environmental Risk Factors
5.1. Ionizing Radiation
5.2. Radiofrequency Electromagnetic Waves
6. Study of Genotoxic and Non-Genotoxic Carcinogens
| Association between Environmental Pollutants and Brain Tumor | ||
|---|---|---|
| Pollutants | Tumor | References |
| EDs: PCBs, PFAS, PFOA, BPA | Meningioma, glioma neuroendocrine tumors | [26,32,47,54]. |
| Pesticide | Glioblastoma multiforme, meningioma | [70,71,74]. |
| THM, nitrate | Neuroepithelial brain tumor, brain tumor | [93]. |
| Benzene, NO2, butadiene, acetaldehyde, chloroform, perchlorethylene, trichloroethylene, PAHs | Neuroectodermal tumor, medulloblastoma, astrocytoma | [85,86]. |
| PMs | Glioma, glioblastoma multiforme | [87,89]. |
| HM: copper, arsenic, lead, nickel, cadmium, zinc | Glioma | [81,82]. |
| RAD | Glioma, meningioma, nerve sheath tumors, | [70,97]. |
| RF | Glioma | [100,101]. |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPA | bisphenol A |
| CAT | catalase |
| CECs | contaminants of emerging concern |
| CECs | contaminants of emerging concern |
| CNS | central nervous system |
| CT | computed tomography |
| EDs | endocrine disruptors |
| EPs | emerging pollutants |
| RAD | ionizing radiation |
| lncRNA | long non-coding RNA |
| MDA | malonyl dialdehyde |
| PAH | polycyclic aromatic hydrocarbons |
| PFAS | per- and polyfluoroalkyl substances |
| PFOA | perfluorooctanoic acid |
| PM | particular matter |
| PM | particulate matter |
| PNET | primitive neuroectodermal tumor |
| RF | radio frequency |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| THM | trihalomethanes |
| TTR | transport protein transthyretin |
References
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. The incidence of major subtypes of primary brain tumors in adults in England 1995–2017. Neuro Oncol. 2021, 23, 1371–1382. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 5, 23. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J.L.; Buffler, P.A.; Omenn, G.S. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu. Rev. Public Health 2013, 34, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M. Mechanisms of chemical carcinogenesis and application to human cancer risk assessment. Toxicology 2001, 166, 3–10. [Google Scholar] [CrossRef]
- Nohmi, T. Thresholds of Genotoxic and Non-Genotoxic Carcinogens. Toxicol. Res. 2018, 34, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.G.; van Steeg, H.; Luijten, M.; van Benthem, J. Mechanisms of non-genotoxic carcinogens and importance of a weight of evidence approach. Mutat. Res./Rev. Mutat. Res. 2009, 682, 94–109. [Google Scholar] [CrossRef]
- Hemminki, K. Environmental Carcinogens. In Chemical Carcinogenesis and Mutagenesis I; Part of the Handbook of Experimental Pharmacology; Cooper, C.S., Grover, P.L., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 94/1. [Google Scholar] [CrossRef]
- Bhat, S.A.; Hassan, T.; Majid, S.; Ashraf, R.; Kuchy, S. Environmental Pollution as Causative Agent for Cancer—A Review. Cancer Clin. Res. 2017, 1, 003. [Google Scholar]
- Arsenic and arsenic compounds. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; The International Agency for Research on Cancer: Lyon, France, 1980; Volume 23, pp. 39–141.
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/news-room/feature-stories/detail/what-are-the-who-air-quality-guidelines (accessed on 15 December 2022).
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Outdoor air pollution; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2016; Volume 109. [Google Scholar]
- Andersen, Z.J.; Pedersen, M.; Weinmayr, G.; Stafoggia, M.; Galassi, C.; Jørgensen, J.T.; Sommar, J.N.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-term exposure to ambient air pollution and incidence of brain tumor: The European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro-Oncology 2018, 20, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, M.; Rahimi, S.; Rezaiemanesh, M.R.; Yektay, S. Association between water and sanitation, air and emission pollution and climate change and neurological disease distribution: A study based on GBD data. Chemosphere 2021, 285, 131522. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.R. N-nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur. J. Cancer Prev. Off. J. Eur. Canc. Prev. Org. 1997, 6, 226–268. [Google Scholar] [CrossRef]
- Sadeghi, H.; Nasseri, S.; Yunesian, M.; Mahvi, A.H.; Nabizadeh, R.; Alimohammadi, M. Trihalomethanes in urban drinking water: Measuring exposures and assessing carcinogenic risk. J. Environ. Health Sci. Eng. 2019, 17, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Ron, E. Ionizing radiation and cancer risk: Evidence from epidemiology. Radiat. Res. 1998, 150, S30–S41. [Google Scholar] [CrossRef] [PubMed]
- Braganza, M.Z.; Kitahara, C.M.; Berrington de González, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing radiation and the risk of brain and central nervous system tumors: A systematic review. Neuro Oncol. 2012, 14, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acién, A.; Pollán, M.; Gustavsson, P.; Plato, N. Occupation, exposure to chemicals and risk of gliomas and meningiomas in Sweden. Am. J. Ind. Med. 2002, 42, 214–227. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as riskfactor for child-hood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef]
- Tan, S.Y.; Praveena, S.M.; Abidin, E.Z.; Cheema, M.S. A review of heavy metals in indoor dust and its human health-risk implications. Rev. Environ. Health 2016, 31, 447–456. [Google Scholar] [CrossRef]
- Morakinyo, O.M.; Mukhola, M.S.; Mokgobu, M.I. Health Risk Analysis of Elemental Components of an Industrially Emitted Respirable Particulate Matter in an Urban Area. Int. J. Environ. Res. Public Health 2021, 18, 3653. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Answer, F.; Chaurasia, S.; Khan, A. A Hormonally active agents in the environment: A state-of-the-art review. Rev. Environ. Health 2016, 31, 415–433. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Franco Bernardes, M.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. Int. 2015, 22, 13800–13823. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, L.; Zhang, Y.; Zhao, Y.; Liu, Y. Air pollution: A culprit of lung cancer. J. Hazard. Mater. 2022, 434, 128937. [Google Scholar] [CrossRef] [PubMed]
- Nikmanesh, Y.; Mohammadi, M.; Yousefi, H.; Mansourimoghadam, S.; Taherian, M. The effect of long-term exposure to toxic air pollutants on the increased risk of malignant brain tumors. Rev. Environ. Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Mesnil, M.; Defamie, N.; Naus, C.; Sarrouilhe, D. Brain Disorders and Chemical Pollutants: A Gap Junction Link? Biomolecules 2020, 11, 51. [Google Scholar] [CrossRef]
- Jankowska-Kieltyka, M.; Roman, A.; Nalepa, I. The Air We Breathe: Air Pollution as a Prevalent Proinflammatory Stimulus Contributing to Neurodegeneration. Front. Cell. Neurosci. 2021, 15, 647643. [Google Scholar] [CrossRef]
- Pessah, I.N.; Lein, P.J.; Seegal, R.F.; Sagiv, S.K. Neurotoxicity of polychlorinated biphenyls and related orga-nohalogens. Acta Neuropathol. 2019, 138, 363–387. [Google Scholar] [CrossRef]
- Calderon Calderón-Garcidueñas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henríquez-Roldán, C.; Schoonhoven, R.; Acuña-Ayala, H.; Villarreal-Calderón, A.; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol. Pathol. 2003, 31, 524–538. [Google Scholar] [CrossRef]
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Eco-Toxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef]
- Gilani, S.R.; Zaidi, S.R.; Batool, M.; Bhatti, A.A.; Durrani, A.I.; Mahmood, Z. Report: Central nervous system (CNS) toxicity caused by metal poisoning: Brain as a target organ. Pak. J. Pharm. Sci. 2015, 28, 1417–1423. [Google Scholar] [PubMed]
- Available online: https://www.nrdc.org/stories/air-pollution-everything-you-need-know (accessed on 15 December 2022).
- Available online: https://thesustainabilityproject.life/blog/2018/10/07/ecobricks-plastic-solved (accessed on 15 December 2022).
- Available online: https://www.targatocn.it/2021/09/23/leggino-tizia/argomenti/attualita/articolo/misure-anti-smog-il-comune-di-cuneo-chiedera-una-sospensione-temporanea-della-normativa.html (accessed on 15 December 2022).
- Available online: https://www.ilgiornale.it/news/salute/smettere-fumare-variante-genetica-favorisce-ricadute-1586365.html (accessed on 15 December 2022).
- Available online: https://www.tiredearth.com/news/water-pollution-causes-effects-and-solutions (accessed on 15 December 2022).
- Available online: https://www.shutterstock.com/it/search/pesticide (accessed on 15 December 2022).
- Available online: https://www.my-personaltrainer.it/salute-benessere/tac-contrasto.html (accessed on 15 December 2022).
- Available online: https://alamoemfsolutions.com/ (accessed on 15 December 2022).
- Available online: https://www.slideshare.net/RahatKhan97/toxicity-of-heavy-metals-140475298 (accessed on 15 December 2022).
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on hu-man health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- He, Q.L.; Zhang, L.; Liu, S.Z. Effects of Polychlorinated Biphenyls on Animal Reproductive Systems and Epige-netic Modifications. Bull. Environ. Contam. Toxicol. 2021, 107, 398–405. [Google Scholar] [CrossRef]
- Available online: https://www.atsdr.cdc.gov/csem/polychlorinated-biphenyls/standards.html (accessed on 15 December 2022).
- Seelbach, M.; Chen, L.; Powell, A.; Choi, Y.J.; Zhang, B.; Hennig, B.; Toborek, M. Polychlorinated biphenyls disrupt blood–brain barrier integrity and promote brain metastasis formation. Environ. Health Perspect. 2010, 118, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html (accessed on 15 December 2022).
- Available online: https://cen.acs.org/environment/persistent-pollutants/EU-agency-sets-limit-PFAS/98/web/2020/09 (accessed on 15 December 2022).
- Kar, S.; Sepúlveda, M.S.; Roy, K.; Leszczynski, J. Endocrine-disrupting activity of per- and polyfluoroalkyl sub-stances: Exploring combined approaches of ligand and structure-based modeling. Chemosphere 2017, 184, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ng, C. Absorption, distribution, and toxicity of per- and polyfluoroalkyl substances (PFAS) in the brain: A review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640. [Google Scholar] [CrossRef]
- Xie, M.Y.; Lin, Z.Y.; Liu, L.Y.; Wu, C.C.; Liu, Y.W.; Huang, G.L.; Zeng, E.Y. Use of glioma to assess the distribution patterns of perfluoroalkyl and polyfluoroalkyl substances in human brain. Environ. Res. 2022, 204, 112011. [Google Scholar] [CrossRef]
- Xie, M.Y.; Sun, X.F.; Wu, C.C.; Huang, G.L.; Wang, P.; Lin, Z.Y.; Liu, Y.W.; Liu, L.Y.; Zeng, E.Y. Glioma is associated with exposure to legacy and alternative per- and polyfluoroalkyl substances. J. Hazard. Mater. 2023, 441, 129819. [Google Scholar] [CrossRef]
- Available online: https://www.consilium.europa.eu/en/press/press-releases/2022/06/21/council-and-parliament-agree-to-reduce-limit-values-for-the-presence-of-persistent-organic-pollutants-in-waste/#:~:text=The%20maximum%20limit%20value%20was,set%20at%205%20%CE%BCg%2Fkg (accessed on 13 December 2022).
- Kirk, A.B.; Michelsen-Correa, S.; Rosen, C.; Martin, C.F.; Blumberg, B. PFAS and Potential Adverse Effects on Bone and Adipose Tissue through Interactions with PPARγ. Endocrinology 2021, 162, bqab194. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef] [PubMed]
- Chludzińska, S.; Modzelewska, P.; Koda, M.; Lewko, J.; Reszeć, J. The role of bisphenol A in the carcinogenesis process. Med. Stud. 2018, 34, 246–251. [Google Scholar] [CrossRef]
- Duan, B.; Hu, X.; Zhao, H.; Qin, J.; Luo, J. The relationship between urinary bisphenol A levels and meningioma in Chinese adults. Int. J. Clin. Oncol. 2013, 18, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Komarowska, M.; Chrzanowski, R.; Tylicka, M.; Rutkowski, R.; Mariak, Z.; Zelazowska-Rutkowska, B.; Lyson, T.; Hermanowicz, A. Plasma concentration of Bisphenol A and leptin in patients with meningioma and glioma: A pilot study. Adv. Med. Sci. 2022, 67, 229–233. [Google Scholar] [CrossRef]
- Effatpanah, M.; Effatpanah, H.; Jalali, S.; Parseh, I.; Goudarzi, G.; Barzegar, G.; Geravandi, S.; Darabi, F.; Ghasemian, N.; Mohammadi, M.J. Hospital admission of exposure to air pollution in Ahvaz megacity during 2010–2013. Clin. Epidemiol. Glob. Health 2020, 8, 550–556. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air pollution: Mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- What is a Pesticide? US EPA 2022. Available online: https://www.epa.gov/minimum-risk-pesticides/what-pesticide (accessed on 15 December 2022).
- Rusiecki, J.A.; De Roos, A.; Lee, W.J.; Dosemeci, M.; Lubin, J.H.; Hoppin, J.A.; Blair, A.; Alavanja, M.C. Cancer incidence among pesticide applicators exposed to atrazine in the Agricultural Health Study. J. Natl. Cancer Inst. 2004, 96, 1375–1382. [Google Scholar] [CrossRef]
- De Roos, A.J.; Blair, A.; Rusiecki, J.A.; Hoppin, J.A.; Svec, M.; Dosemeci, M.; Sandler, D.P.; Alavanja, M.C. Cancer incidence among glyphosate-exposed pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2005, 113, 49–54. [Google Scholar] [CrossRef]
- Li, X.; He, S.; Xiao, H.; He, T.T.; Zhang, J.D.; Luo, Z.R.; Ma, J.Z.; Yin, Y.L.; Luo, L.; Cao, L.Y. Neonico-tinoid insecticides promote breast cancer progression via G protein-coupled estrogen receptor: In vivo, in vitro and in silico studies. Environ. Int. 2022, 170, 107568. [Google Scholar] [CrossRef]
- Vienne-Jumeau, A.; Tafani, C.; Ricard, D. Environmental risk factors of primary brain tumors: A review. Rev. Neurol. 2019, 175, 664–678. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epi-demiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Khuder, S.A.; Mutgi, A.B.; Schaub, E.A. Meta-analyses of brain cancer and farming. Am. J. Ind. Med. 1998, 34, 252–260. [Google Scholar] [CrossRef]
- Samanic, C.M.; De Roos, A.J.; Stewart, P.A.; Rajaraman, P.; Waters, M.A.; Inskip, P.D. Occupational expo-sure to pesticides and risk of adult brain tumors. Am. J. Epidemiol. 2008, 167, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Ogata, P.; Lytle, B. Farming, Pesticides, and Brain Cancer: A 20-Year Updated Systematic Litera-ture Review and Meta-Analysis. Cancers 2021, 13, 4477. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, S.; Wang, Y.; Yao, Y.; Wang, G.; Jin, Y.; Wu, Y. Exposure to pyrethroid pesticides and the risk of childhood brain tumors in East China. Environ. Pollut. 2016, 218, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; David, A.; Freire, C.; Fernández, M.F.; D’Cruz, S.C.; Reina-Pérez, I.; Fini, J.B.; Blaha, L. Pyre-throids and developmental neurotoxicity—A critical review of epidemiological studies and supporting mechanistic evidence. Environ. Res. 2022, 214, 113935. [Google Scholar] [CrossRef]
- Kunkle, B.; Bae, S.; Singh, K.P.; Roy, D. Increased Risk of Childhood Brain Tumors among Children Whose Parents had Farm-Related Pesticide Exposures during Pregnancy. JP J. Biostat. 2014, 11, 89–101. [Google Scholar]
- Mulware, S.J. Comparative Trace Elemental Analysis in Cancerous and Noncancerous Human Tissues Using PIXE. J. Biophys. 2013, 2013, 192026. [Google Scholar] [CrossRef]
- Cilliers, K.; Muller, C.J.F.; Page, B.J. Trace Element Concentration Changes in Brain Tumors: A Review. Anat. Rec. 2020, 303, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Popov, B.; Gadjeva, V.; Valkanov, P.; Popova, S.; Tolekova, A. Lipid peroxidation, superoxide dismutase and catalase activities in brain tumor;tissues. Arch. Physiol. Biochem. 2003, 111, 455–459. [Google Scholar] [CrossRef]
- Arslan, M.; Demir, H.; Arslan, H.; Gokalp, A.S.; Demir, C. Trace elements, heavy metals, and other biochemical parameters in malignant glioma patients. Asian Pac. J. Cancer Prev. 2011, 12, 447–451. [Google Scholar] [PubMed]
- Inskip, P.D.; Linet, M.S.; Heineman, E.F. Etiology of brain tumors in adults. Epidemiol. Rev. 1995, 17, 382–414. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, E.; Dosemeci, M. Brain cancer mortality and potential occupational exposure to lead: Findings from the National Longitudinal Mortality Study, 1979–1989. Int. J. Cancer 2006, 119, 1136–1144. [Google Scholar] [CrossRef]
- Wu, A.H.; Wu, J.; Tseng, C.; Yang, J.; Shariff-Marco, S.; Fruin, S.; Larson, T.; Setiawan, V.W.; Masri, S.; Porcel, J.; et al. Association Between Outdoor Air Pollution and Risk of Malignant and Benign Brain Tumors: The Multiethnic Cohort Study. JNCI Cancer Spectr. 2020, 4, pkz107. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Heck, J.E.; Park, A.S.; Cockburn, M.; Escobedo, L.; Ritz, B. In Utero and Early Life Exposure to Ambient Air Toxics and Childhood Brain Tumors: A Population-Based Case-Control Study in California, USA. Environ. Health Perspect. 2016, 124, 1093–1099. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kundu, U.; Desai, D.; Pillai, P.P. Particulate Matters Affecting lncRNA Dysregulation and Glio-blastoma Inva-siveness: In Silico Applications and Current Insights. J. Mol. Neurosci. 2022, 72, 2188–2206. [Google Scholar] [CrossRef]
- Santibáñez-Andrade, M.; Chirino, Y.I.; González-Ramírez, I.; Sánchez-Pérez, Y.; García-Cuellar, C.M.D. Deci-phering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks. Int. J. Mol. Sci. 2019, 21, 136. [Google Scholar] [CrossRef]
- Harbo Poulsen, A.; Arthur Hvidtfeldt, U.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Christensen, J.H.; Geels, C.; Raaschou-Nielsen, O. Components of particulate matter air-pollution and brain tumors. Environ. Int. 2020, 144, 106046. [Google Scholar] [CrossRef]
- Zhang, Y.; West, J.J.; Marthur, R.; Xing, J.; Hongrefe, C.; Roselle, S.J.; Bash, J.O.; Pleim, J.E.; Gan, C.M.; Wong, D.C. Long-term trends in the ambient PM2.5- and O3-related mortality burdens in the United States under emission reductions from (1990) to 2010. Atmos. Chem. Phys. 2018, 18, 15003–15016. [Google Scholar] [CrossRef]
- Weichenthal, S.; Olaniyan, T.; Christidis, T.; Lavigne, E.; Hatzopoulou, M.; Van Ryswyk, K.; Tjepkema, M.; Burnett, R. With-in-city Spatial Variations in Ambient Ultrafine Particle Concentrations and Incident Brain Tumors in Adults. Epidemiology 2020, 31, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Zumel-Marne, A.; Castaño-Vinyals, G.; Alguacil, J.; Villanueva, C.M.; Maule, M.; Gracia-Lavedan, E.; Momoli, F.; Krewski, D.; Mohipp, C.; Petridou, E.; et al. Exposure to drinking water trihalomethanes and nitrate and the risk of brain tumors in young people. Environ. Res. 2021, 200, 111392. [Google Scholar] [CrossRef] [PubMed]
- Bilzer, T.; Reifenberger, G.; Wechsler, W. Chemical induction of brain tumors in rats by nitrosoureas: Molecu-lar biology and neuropathology. Neurotoxicology Teratol. 1989, 11, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.A.; Nielsen, S.S.; Preston-Martin, S.; Holly, E.A.; Cordier, S.; Filippini, G.; Peris-Bonet, R.; Choi, N.W. Household water source and the risk of childhood brain tumors: Results of the SEARCH International Brain Tumor Study. Int. J. Epidemiol. 2004, 33, 1209–1216. [Google Scholar] [CrossRef]
- Mueller, B.A.; Newton, K.; Holly, E.A.; Preston-Martin, S. Residential water source and the risk of childhood brain tumors. Environ. Health Perspect. 2001, 109, 551–556. [Google Scholar] [CrossRef]
- Meet, P.; Kuldeepsinh, V.; Rushil, P.; Dixita, P.; Patel, P.; Upadhyay, U. A review on brain tumor. World J. Pharm. Res. 2021, 10, 867–892. [Google Scholar] [CrossRef]
- Available online: https://www.bfs.de/EN/topics/ion/radiation-protection/limit-values/limitvalues_node.html (accessed on 21 December 2022).
- Available online: https://www.cancer.org/healthy/cancer-causes/radiation-exposure/cellular-phones.html (accessed on 21 December 2022).
- Melnick, R. Commentary on the utility of the National Toxicology Program study on cell phone radiofrequen-cy radiation data for assessing human health risks despite unfounded criticisms aimed at minimizing the findings of adverse health effects. Environ. Res. 2019, 168, 1–6. [Google Scholar] [CrossRef]
- Miller, A.B.; Sears, M.E.; Morgan, L.L.; Davis, D.L.; Hardell, L.; Oremus, M.; Soskolne, C.L. Risks to Health and Well-Being from Radio-Frequency Radiation Emitted by Cell Phones and Other Wireless Devices. Front. Public Health. 2019, 7, 223. [Google Scholar] [CrossRef]
- Morgan, K.M.; Riedlinger, G.M.; Rosenfeld, J.; Ganesan, S.; Pine, S.R. Patient-Derived Xenograft Models of Non-Small Cell Lung Cancer and Their Potential Utility in Personalized Medicine. Front. Oncol. 2017, 7, 2. [Google Scholar] [CrossRef]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, J.; Ge, J.; Yang, J.; Song, X.; Wang, C.; Mao, J.; Zhang, Y.; Zou, Y.; Liu, Y.; et al. Particulate Matter Facilitates C6 Glioma Cells Activation and the Release of Inflammatory Factors Through MAPK and JAK2/STAT3 Pathways. Neurochem. Res. 2016, 41, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pacheco, M.; Hidalgo-Miranda, A.; Romero-Córdoba, S.; Valverde, M.; Rojas, E. MRNA and miRNA expression patterns associated with pathways linked to metal mixture health effects. Gene 2014, 533, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ljubimova, J.Y.; Braubach, O.; Patil, R.; Chiechi, A.; Tang, J.; Galstyan, A.; Shatalova, E.S.; Kleinman, M.T.; Black, K.L.; Holler, E. Coarse particulate matter (PM2.5–10) in Los Angeles Basin air induces expression of inflammation and cancer biomarkers in rat brains. Sci. Rep. 2018, 8, 5708. [Google Scholar] [CrossRef]
- Ljubimova, J.Y.; Kleinman, M.T.; Karabalin, N.M.; Inoue, S.; Konda, B.; Gangalum, P.; Markman, J.L.; Ljubimov, A.V.; Black, K.L. Gene expression changes in rat brain after short and long exposures to particulate matter in Los Angeles basin air: Comparison with human brain tumors. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2013, 65, 1063–1071. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as a Mainstream Model for In Vivo Systems Pharmacology and Toxicology. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 43–64. [Google Scholar] [CrossRef]
- Bourdineaud, J.P.; Rossignol, R.; Brèthes, D. Zebrafish: A model animal for analyzing the impact of environmental pollutants on muscle and brain mitochondrial bioenergetics. Int. J. Biochem. Cell Biol. 2013, 45, 16–22. [Google Scholar] [CrossRef]
- Azevedo, R.D.S.; Falcão, K.V.G.; Assis, C.R.D.; Martins, R.M.G.; Araújo, M.C.; Yogui, G.T.; Neves, J.L.; Seabra, G.M.; Maia, M.B.S.; Amaral, I.P.G.; et al. Effects of pyriproxyfen on zebrafish brain mitochondria and acetylcholinesterase. Chemosphere 2021, 263, 128029. [Google Scholar] [CrossRef]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Geels, C.; Christensen, J.H.; Raaschou-Nielsen, O. Intracranial tumors of the central nervous system and air pollution—A nationwide case–control study from Denmark. Environ. Health 2020, 19, 81. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Johansen, M.S.; Ravnskjær, L.; Andersen, K.K.; Bräuner, E.V.; Loft, S.; Ketzel, M.; Becker, T.; Brandt, J.; Hertel, O.; et al. Long-term exposure to ambient air pollution and incidence of brain tumors: The Danish Nurse Cohort. Neurotoxicology 2016, 55, 122–130. [Google Scholar] [CrossRef]
- Plant, N. Can systems toxicology identify common biomarkers of non-genotoxic carcinogenesis? Toxicology 2008, 254, 164–169. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.; Navarra, G.; Coppola, L.; Savarese, B.; Avilia, G.; Giarra, A.; Pagano, G.; Marano, A.; Trifuoggi, M.; Bifulco, M.; et al. Impacts of Environmental Pollution on Brain Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 5045. https://doi.org/10.3390/ijms24055045
Pagano C, Navarra G, Coppola L, Savarese B, Avilia G, Giarra A, Pagano G, Marano A, Trifuoggi M, Bifulco M, et al. Impacts of Environmental Pollution on Brain Tumorigenesis. International Journal of Molecular Sciences. 2023; 24(5):5045. https://doi.org/10.3390/ijms24055045
Chicago/Turabian StylePagano, Cristina, Giovanna Navarra, Laura Coppola, Beatrice Savarese, Giorgio Avilia, Antonella Giarra, Giovanni Pagano, Alessandra Marano, Marco Trifuoggi, Maurizio Bifulco, and et al. 2023. "Impacts of Environmental Pollution on Brain Tumorigenesis" International Journal of Molecular Sciences 24, no. 5: 5045. https://doi.org/10.3390/ijms24055045
APA StylePagano, C., Navarra, G., Coppola, L., Savarese, B., Avilia, G., Giarra, A., Pagano, G., Marano, A., Trifuoggi, M., Bifulco, M., & Laezza, C. (2023). Impacts of Environmental Pollution on Brain Tumorigenesis. International Journal of Molecular Sciences, 24(5), 5045. https://doi.org/10.3390/ijms24055045











