Usefulness and Limitations of Multiple Ligation-Dependent Probe Amplification in Antithrombin Deficiency
Abstract
1. Introduction
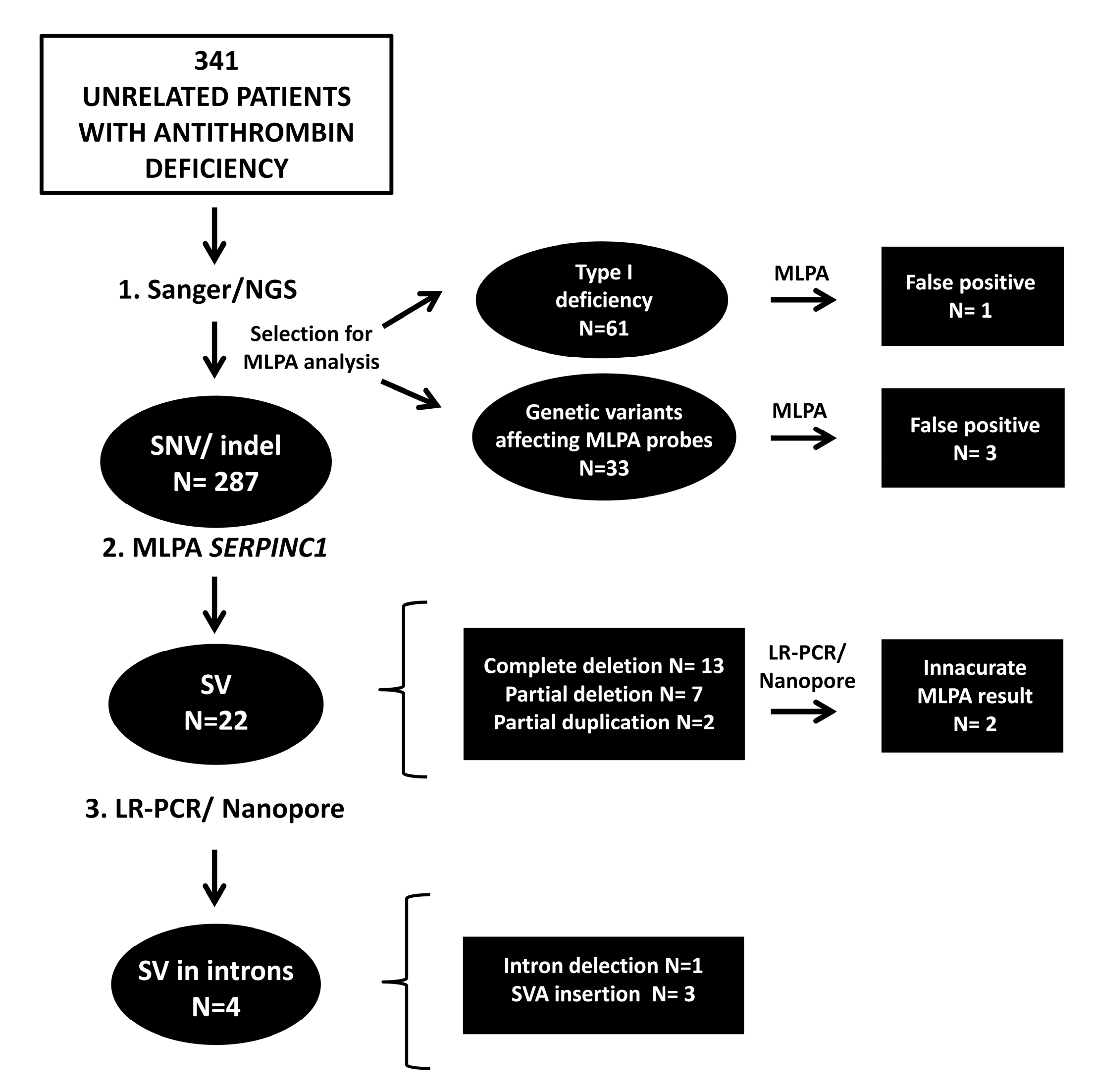
2. Results
2.1. Antithrombin Deficiency-Causing SVs Detected by MLPA
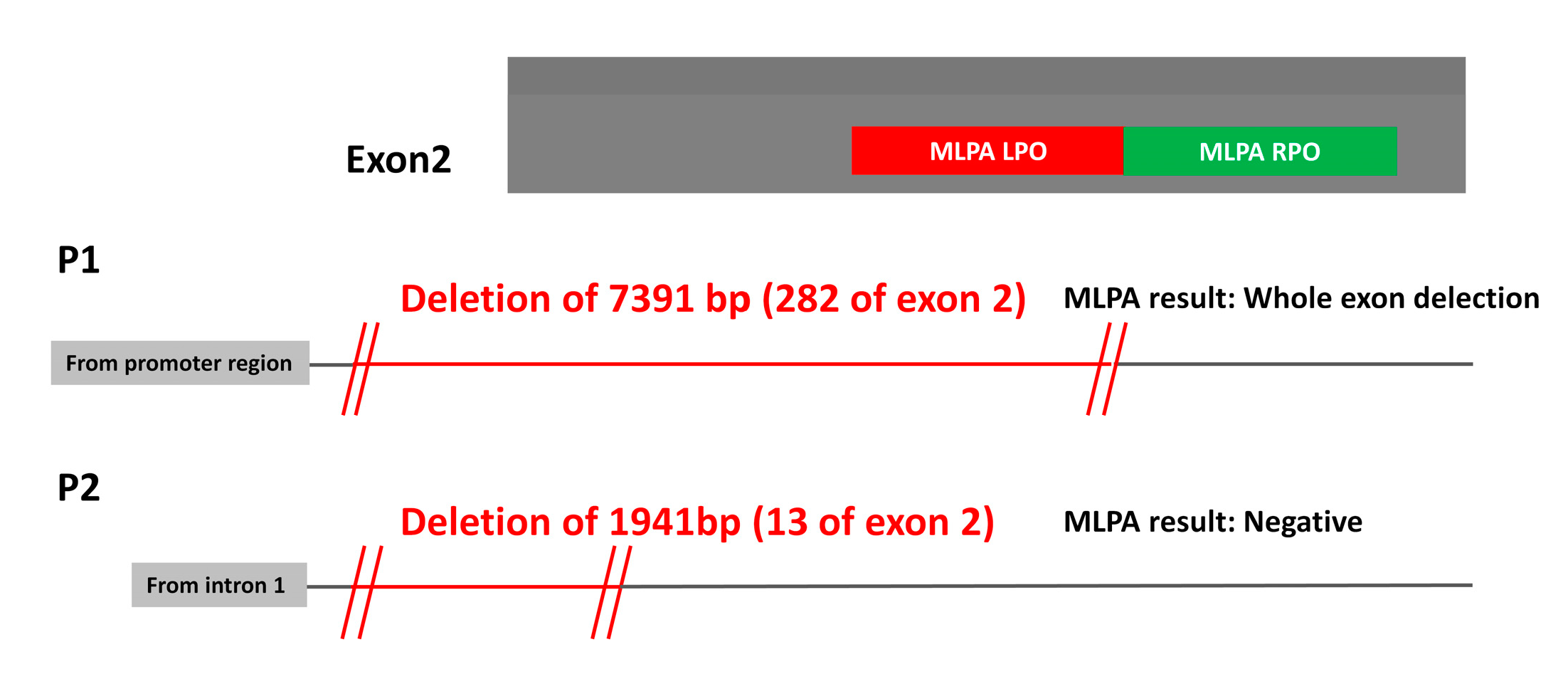
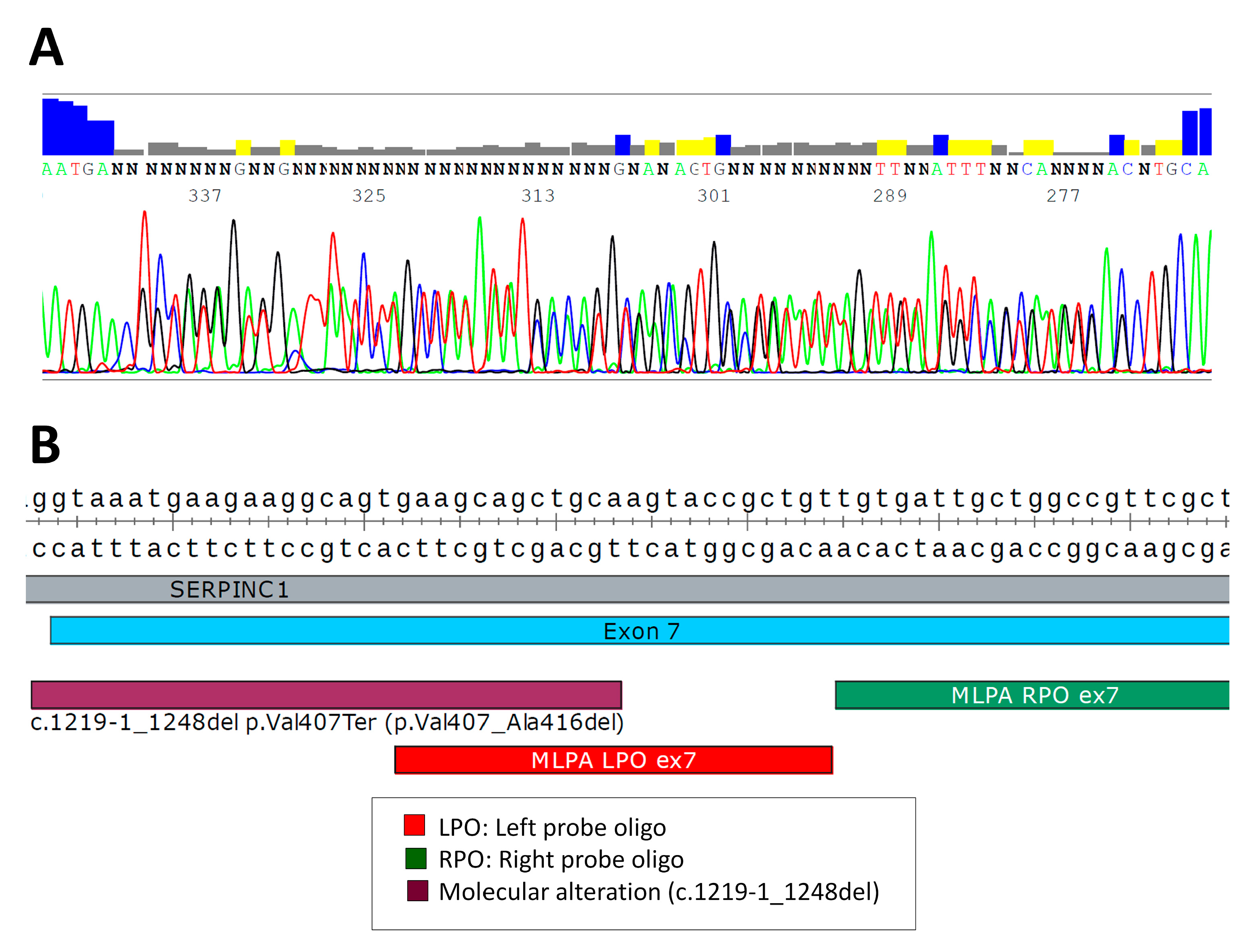
2.2. Analysis of SVs by Alternative Techniques to MLPA
2.3. Search for Hidden SVs
2.4. MLPA False Positives
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Characterization of Antithrombin Deficiency
4.3. Molecular Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feuk, L.; Carson, A.R.; Scherer, S.W. Structural Variation in the Human Genome. Nat. Rev. Genet. 2006, 7, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Escaramís, G.; Docampo, E.; Rabionet, R. A Decade of Structural Variants: Description, History and Methods to Detect Structural Variation. Brief. Funct. Genom. 2015, 14, 305–314. [Google Scholar] [CrossRef]
- Bucciarelli, P.; Passamonti, S.M.; Biguzzi, E.; Gianniello, F.; Franchi, F.; Mannucci, P.M.; Martinelli, I. Low Borderline Plasma Levels of Antithrombin, Protein C and Protein S Are Risk Factors for Venous Thromboembolism. J. Thromb. Haemost. 2012, 10, 1783–1791. [Google Scholar] [CrossRef]
- Corral, J.; de la Morena-Barrio, M.E.; Vicente, V. The Genetics of Antithrombin. Thromb. Res. 2018, 169, 23–29. [Google Scholar] [CrossRef]
- Bravo-Pérez, C.; Vicente, V.; Corral, J. Management of Antithrombin Deficiency: An Update for Clinicians. Expert Rev. Hematol. 2019, 12, 397–405. [Google Scholar] [CrossRef]
- Van Cott, E.M.; Orlando, C.; Moore, G.W.; Cooper, P.C.; Meijer, P.; Marlar, R. Recommendations for Clinical Laboratory Testing for Antithrombin Deficiency; Communication from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 17–22. [Google Scholar] [CrossRef]
- Luxembourg, B.; Delev, D.; Geisen, C.; Spannagl, M.; Krause, M.; Miesbach, W.; Heller, C.; Bergmann, F.; Schmeink, U.; Grossmann, R.; et al. Molecular Basis of Antithrombin Deficiency. Thromb. Haemost. 2011, 105, 635–646. [Google Scholar] [CrossRef] [PubMed]
- De la Morena-Barrio, B.; Borràs, N.; Rodríguez-Alén, A.; de la Morena-Barrio, M.E.; García-Hernández, J.L.; Padilla, J.; Bravo-Pérez, C.; Miñano, A.; Rollón, N.; Corral, J.; et al. Identification of the First Large Intronic Deletion Responsible of Type I Antithrombin Deficiency Not Detected by Routine Molecular Diagnostic Methods. Br. J. Haematol. 2019, 186, e82–e86. [Google Scholar] [CrossRef]
- de la Morena-Barrio, B.; Stephens, J.; de la Morena-Barrio, M.E.; Stefanucci, L.; Padilla, J.; Miñano, A.; Gleadall, N.; García, J.L.; López-Fernández, M.F.; Morange, P.-E.; et al. Long-Read Sequencing Identifies the First Retrotransposon Insertion and Resolves Structural Variants Causing Antithrombin Deficiency. Thromb. Haemost. 2022, 122, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- de la Morena-Barrio, B.; de la Morena-Barrio, M.; Padilla, J.; Teruel-Montoya, R.; Asenjo, S.; Wypasek, E.; Undas, A.; Miñano, A.; Vicente, V.; Corral, J. Identification of a New Mechanism of Antithrombin Deficiency Hardly Detected by Current Methods: Duplication of SERPINC1 Exon 6. Thromb. Haemost. 2018, 118, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Olds, R.J.; Lane, D.A.; Chowdhury, V.; De Stefano, V.; Leone, G.; Thein, S.L. Complete Nucleotide Sequence of the Antithrombin Gene: Evidence for Homologous Recombination Causing Thrombophilia. Biochemistry 1993, 32, 4216–4224. [Google Scholar] [CrossRef] [PubMed]
- de la Morena-Barrio, B.; Orlando, C.; Sanchis-Juan, A.; García, J.L.; Padilla, J.; de la Morena-Barrio, M.E.; Puruunen, M.; Stouffs, K.; Cifuentes, R.; Borràs, N.; et al. Molecular Dissection of Structural Variations Involved in Antithrombin Deficiency. J. Mol. Diagn. 2022, 24, 462–475. [Google Scholar] [CrossRef] [PubMed]
- de la Morena-Barrio, M.E.; Antón, A.I.; Martínez-Martínez, I.; Padilla, J.; Miñano, A.; Navarro-Fernández, J.; Águila, S.; López, M.F.; Fontcuberta, J.; Vicente, V.; et al. Regulatory Regions of SERPINC1 Gene: Identification of the First Mutation Associated with Antithrombin Deficiency. Thromb. Haemost. 2012, 107, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Toderici, M.; de la Morena-Barrio, M.E.; Padilla, J.; Miñano, A.; Antón, A.I.; Iniesta, J.A.; Herranz, M.T.; Fernández, N.; Vicente, V.; Corral, J. Identification of Regulatory Mutations in SERPINC1 Affecting Vitamin D Response Elements Associated with Antithrombin Deficiency. PLoS ONE 2016, 11, e0152159. [Google Scholar] [CrossRef]
- Martinez-Martinez, I.; Johnson, D.J.; Yamasaki, M.; Navarro-Fernandez, J.; Ordonez, A.; Vicente, V.; Huntington, J.A.; Corral, J. Type II Antithrombin Deficiency Caused by a Large In-Frame Insertion. Structural, Functional and Pathological Relevance. J. Thromb. Haemost. 2012, 10, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.L.; Abraham, A.; LaBella, A.L.; Abbot, P.; Rokas, A.; Capra, J.A. The Influence of Evolutionary History on Human Health and Disease. Nat. Rev. Genet. 2021, 22, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Anguela, X.M.; High, K.A. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef] [PubMed]
- McCombie, W.R.; McPherson, J.D.; Mardis, E.R. Next-Generation Sequencing Technologies. Cold Spring Harb. Perspect. Med. 2019, 9, a036798. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Genomic Variation Prediction: A Summary From Different Views. Front. cell Dev. Biol. 2021, 9, 795883. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Antonucci, I.; Palka, G.; Gatta, V. Use of the MLPA Assay in the Molecular Diagnosis of Gene Copy Number Alterations in Human Genetic Diseases. Int. J. Mol. Sci. 2012, 13, 3245–3276. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, R.; Padilla, J.; de la Morena-Barrio, M.E.; de la Morena-Barrio, B.; Bravo-Pérez, C.; Garrido-Rodríguez, P.; Llamas, M.; Miñano, A.; Vicente, V.; Lozano, M.L.; et al. Usefulness and Limitations of Multiple Ligation-Dependent Probe Amplification in Antithrombin Deficiency. Int. J. Mol. Sci. 2023, 24, 5023. https://doi.org/10.3390/ijms24055023
Cifuentes R, Padilla J, de la Morena-Barrio ME, de la Morena-Barrio B, Bravo-Pérez C, Garrido-Rodríguez P, Llamas M, Miñano A, Vicente V, Lozano ML, et al. Usefulness and Limitations of Multiple Ligation-Dependent Probe Amplification in Antithrombin Deficiency. International Journal of Molecular Sciences. 2023; 24(5):5023. https://doi.org/10.3390/ijms24055023
Chicago/Turabian StyleCifuentes, Rosa, José Padilla, María Eugenia de la Morena-Barrio, Belén de la Morena-Barrio, Carlos Bravo-Pérez, Pedro Garrido-Rodríguez, María Llamas, Antonia Miñano, Vicente Vicente, María Luisa Lozano, and et al. 2023. "Usefulness and Limitations of Multiple Ligation-Dependent Probe Amplification in Antithrombin Deficiency" International Journal of Molecular Sciences 24, no. 5: 5023. https://doi.org/10.3390/ijms24055023
APA StyleCifuentes, R., Padilla, J., de la Morena-Barrio, M. E., de la Morena-Barrio, B., Bravo-Pérez, C., Garrido-Rodríguez, P., Llamas, M., Miñano, A., Vicente, V., Lozano, M. L., & Corral, J. (2023). Usefulness and Limitations of Multiple Ligation-Dependent Probe Amplification in Antithrombin Deficiency. International Journal of Molecular Sciences, 24(5), 5023. https://doi.org/10.3390/ijms24055023





