Autoimmune Regulator Gene Polymorphisms and the Risk of Primary Immune Thrombocytopenic Purpura: A Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of the Studied Cohorts
2.2. AIRE SNPs and ITP Risk
2.3. AIRE SNP Haplotypes and ITP Risk
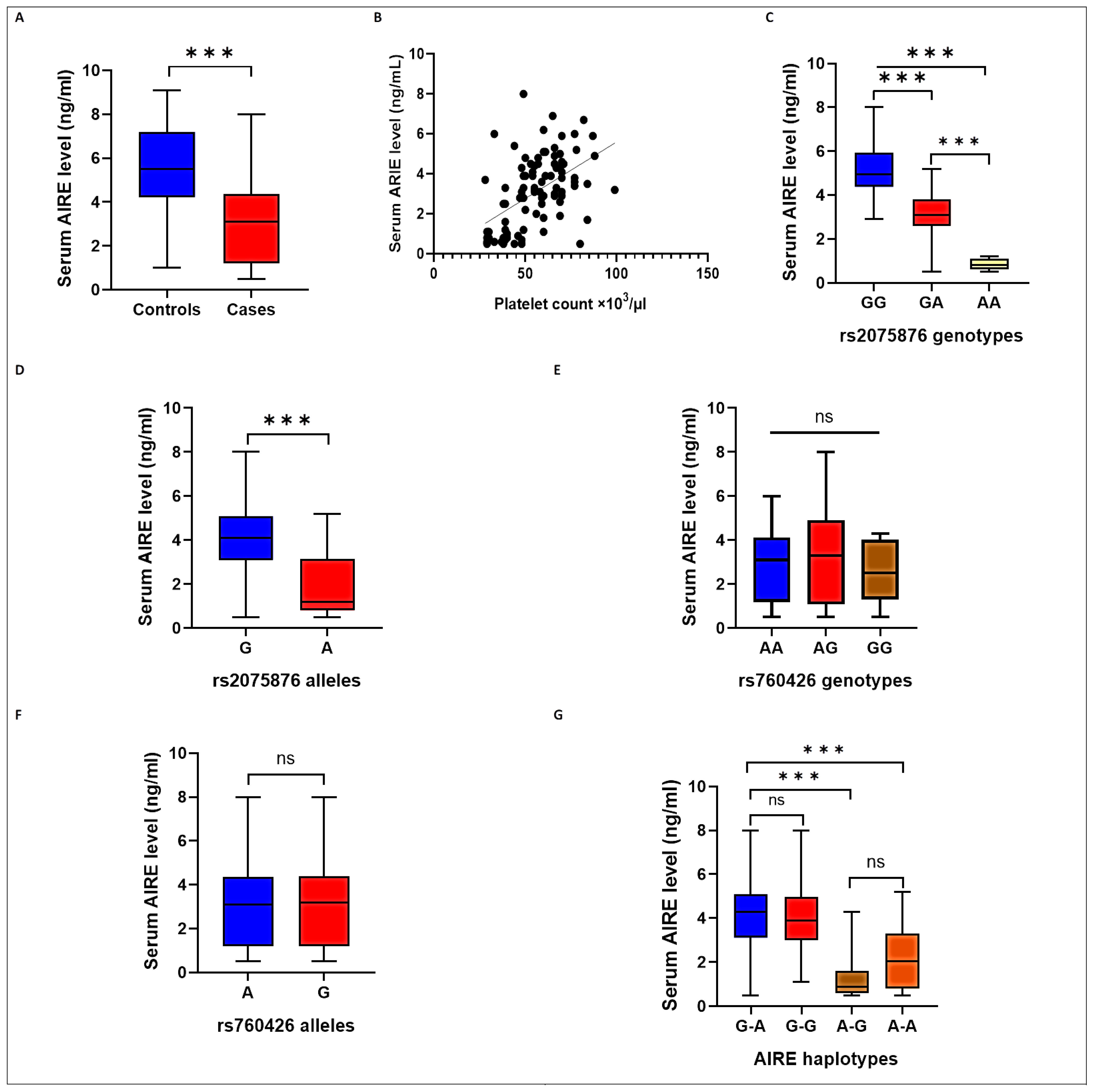
2.4. AIRE SNPs and Haplotypes and Serum AIRE Levels
3. Discussion
4. Materials and Methods
4.1. Study Cohort
4.2. Clinical and Laboratory Assessment
4.3. Sampling
4.4. AIRE SNP Genotyping
4.5. Serum AIRE Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gernsheimer, T. Chronic idiopathic thrombocytopenic purpura: Mechanisms of pathogenesis. Oncologist 2009, 14, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, J.M.; Polfus, L.M.; Flanagan, J.M.; Bennett, C.M.; Lambert, M.P.; Neunert, C.; Kumar, M.; Klaassen, R.J.; Thornburg, C.; Jeng, M.; et al. Genes Influencing the Development and Severity of Chronic ITP Identified through Whole Exome Sequencing. Blood 2015, 126, 73. [Google Scholar] [CrossRef]
- Audia, S.; Mahévas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.G.; Guo, L.; Freedman, J.; Semple, J.W. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br. J. Haematol. 2013, 163, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Feng, Q.; Xu, M.; Li, G.S.; Liu, X.N.; Sheng, Z.; Zhou, H.; Ma, J.; Wei, Y.; Sun, Y.X.; et al. High-dose dexamethasone corrects impaired myeloid-derived suppressor cell function via Ets1 in immune thrombocytopenia. Blood 2016, 127, 1587–1597. [Google Scholar] [CrossRef]
- Ebbo, M.; Audonnet, S.; Grados, A.; Benarous, L.; Mahevas, M.; Godeau, B.; Viallard, J.F.; Piperoglou, C.; Cognet, C.; Farnarier, C.; et al. NK cell compartment in the peripheral blood and spleen in adult patients with primary immune thrombocytopenia. Clin. Immunol. 2017, 177, 18–28. [Google Scholar] [CrossRef]
- Grodzielski, M.; Goette, N.P.; Glembotsky, A.C.; Constanza Baroni Pietto, M.; Méndez-Huergo, S.P.; Pierdominici, M.S.; Montero, V.S.; Rabinovich, G.A.; Molinas, F.C.; Heller, P.G.; et al. Multiple concomitant mechanisms contribute to low platelet count in patients with immune thrombocytopenia. Sci. Rep. 2019, 9, 2208. [Google Scholar] [CrossRef]
- Rizzi, M.; Ferrera, F.; Filaci, G.; Indiveri, F. Disruption of immunological tolerance: Role of AIRE gene in autoimmunity. Autoimmun. Rev. 2006, 5, 145–147. [Google Scholar] [CrossRef]
- Nagamine, K.; Peterson, P.; Scott, H.S.; Kudoh, J.; Minoshima, S.; Heino, M.; Krohn, K.J.; Lalioti, M.D.; Mullis, P.E.; Antonarakis, S.E.; et al. Positional cloning of the APECED gene. Nat. Genet. 1997, 17, 393–398. [Google Scholar] [CrossRef]
- Abramson, J.; Giraud, M.; Benoist, C.; Mathis, D. Aire’s partners in the molecular control of immunological tolerance. Cell 2010, 140, 123–135. [Google Scholar] [CrossRef]
- Poliani, P.L.; Kisand, K.; Marrella, V.; Ravanini, M.; Notarangelo, L.D.; Villa, A.; Peterson, P.; Facchetti, F. Human peripheral lymphoid tissues contain autoimmune regulator-expressing dendritic cells. Am. J. Pathol. 2010, 176, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.M.; Metzger, T.C.; McMahon, E.J.; Au-Yeung, B.B.; Krawisz, A.K.; Lu, W.; Price, J.D.; Johannes, K.P.; Satpathy, A.T.; Murphy, K.M.; et al. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4⁺ T cells. Immunity 2013, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.J.; Zhang, S.L.; Wen, H.F.; Liang, Y. Association of rs2075876 polymorphism of AIRE gene with rheumatoid arthritis risk. Hum. Immunol. 2015, 76, 281–285. [Google Scholar] [CrossRef]
- Terao, C.; Yamada, R.; Ohmura, K.; Takahashi, M.; Kawaguchi, T.; Kochi, Y.; Okada, Y.; Nakamura, Y.; Yamamoto, K.; Melchers, I.; et al. The human AIRE gene at chromosome 21q22 is a genetic determinant for the predisposition to rheumatoid arthritis in Japanese population. Hum. Mol. Genet. 2011, 20, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- NH, A.M.; Mansour, M.F.; Omar, H.H.; Fouad, M.M.; Metwally, L.; El-Abaseri, T.B.; Abdelnaby, M.M. Association of autoimmune regulator gene polymorphism with susceptibility to rheumatoid arthritis in Egyptian population. Immunol. Res. 2020, 68, 90–96. [Google Scholar]
- Alghamdi, S.A.; Kattan, S.W.; Toraih, E.A.; Alrowaili, M.G.; Fawzy, M.S.; Elshazli, R.M. Association of AIRE (rs2075876), but not CTLA4 (rs231775) polymorphisms with systemic lupus erythematosus. Gene 2021, 768, 145270. [Google Scholar] [CrossRef] [PubMed]
- Attia, D.H.; Dorgham, D.A.; El Maghraby, A.A.; Alkaffas, M.; Abdel Kawy, M.A.; Sherif, M.M.; Abdel Halim, R.M. Autoimmune Regulator Gene Polymorphisms in Egyptian Systemic Lupus Erythematosus Patients: Preliminary Results. Int. J. Rheumatol. 2021, 2021, 5546639. [Google Scholar] [CrossRef]
- Ferrera, F.; Rizzi, M.; Sprecacenere, B.; Balestra, P.; Sessarego, M.; Di Carlo, A.; Filaci, G.; Gabrielli, A.; Ravazzolo, R.; Indiveri, F. AIRE gene polymorphisms in systemic sclerosis associated with autoimmune thyroiditis. Clin. Immunol. 2007, 122, 13–17. [Google Scholar] [CrossRef]
- Ströbel, P.; Chuang, W.Y.; Chuvpilo, S.; Zettl, A.; Katzenberger, T.; Kalbacher, H.; Rieckmann, P.; Nix, W.; Schalke, B.; Gold, R.; et al. Common cellular and diverse genetic basis of thymoma-associated myasthenia gravis: Role of MHC class II and AIRE genes and genetic polymorphisms. Ann. N. Y. Acad. Sci. 2008, 1132, 143–156. [Google Scholar] [CrossRef]
- Tazi-Ahnini, R.; McDonagh, A.J.; Wengraf, D.A.; Lovewell, T.R.; Vasilopoulos, Y.; Messenger, A.G.; Cork, M.J.; Gawkrodger, D.J. The autoimmune regulator gene (AIRE) is strongly associated with vitiligo. Br. J. Dermatol. 2008, 159, 591–596. [Google Scholar] [CrossRef]
- Bonner, S.M.; Pietropaolo, S.L.; Fan, Y.; Chang, Y.; Sethupathy, P.; Morran, M.P.; Beems, M.; Giannoukakis, N.; Trucco, G.; Palumbo, M.O.; et al. Sequence variation in promoter of Ica1 gene, which encodes protein implicated in type 1 diabetes, causes transcription factor autoimmune regulator (AIRE) to increase its binding and down-regulate expression. J. Biol. Chem. 2012, 287, 17882–17893. [Google Scholar] [CrossRef] [PubMed]
- Lankisch, T.O.; Mourier, O.; Sokal, E.M.; Habes, D.; Lacaille, F.; Bridoux-Henno, L.; Hermeziu, B.; Lenaerts, C.; Strassburg, C.P.; Jacquemin, E. AIRE gene analysis in children with autoimmune hepatitis type I or II. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Bérczi, B.; Gerencsér, G.; Farkas, N.; Hegyi, P.; Veres, G.; Bajor, J.; Czopf, L.; Alizadeh, H.; Rakonczay, Z.; Vigh, É.; et al. Association between AIRE gene polymorphism and rheumatoid arthritis: A systematic review and meta-analysis of case-control studies. Sci. Rep. 2017, 7, 14096. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, N.; Mitani, T.; Takeda, N.; Ishimaru, N.; Arakaki, R.; Hayashi, Y.; Bando, Y.; Izumi, K.; Takahashi, T.; Nomura, T.; et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J. Immunol. 2005, 174, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Eldershaw, S.A.; Sansom, D.M.; Narendran, P. Expression and function of the autoimmune regulator (Aire) gene in non-thymic tissue. Clin. Exp. Immunol. 2011, 163, 296–308. [Google Scholar] [CrossRef]
- Proekt, I.; Miller, C.N.; Lionakis, M.S.; Anderson, M.S. Insights into immune tolerance from AIRE deficiency. Curr. Opin. Immunol. 2017, 49, 71–78. [Google Scholar] [CrossRef]
- AbdelGhafar, M.T.; El-Kholy, R.A.; Elbedewy, T.A.; Allam, A.A.; Eissa, R.A.E.; Samy, S.M.; El-Khalik, S.R.A.; Rabah, H. Impact of CD40 gene polymorphisms on the risk of immune thrombocytopenic purpura. Gene 2020, 736, 144419. [Google Scholar] [CrossRef]
- Ellithy, H.N.; Yousry, S.M.; Abdel-Aal, A.; Tawadros, L.; Momen, N. Association of CD40 gene polymorphisms and immune thrombocytopenic purpura in the adult Egyptian population. Blood Res. 2022, 57, 229–234. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhang, C.; Feng, Q.; Hou, M.; Peng, J.; Hu, X.; Wang, S. HDAC3 single-nucleotide polymorphism rs2530223 is associated with increased susceptibility and severity of primary immune thrombocytopenia. Int. J. Lab. Hematol. 2022, 44, 875–882. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-mutations and autoimmune disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef]
- Shao, S.; Li, X.R.; Cen, H.; Yin, Z.S. Association of AIRE polymorphisms with genetic susceptibility to rheumatoid arthritis in a Chinese population. Inflammation 2014, 37, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lovewell, T.R.; McDonagh, A.J.; Messenger, A.G.; Azzouz, M.; Tazi-Ahnini, R. The AIRE -230Y Polymorphism Affects AIRE Transcriptional Activity: Potential Influence on AIRE Function in the Thymus. PLoS ONE 2015, 10, e0127476. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.B. Intron-mediated regulation of gene expression. Curr. Top Microbiol. Immunol. 2008, 326, 277–290. [Google Scholar] [PubMed]
- Yanagihara, T.; Sanematsu, F.; Sato, T.; Uruno, T.; Duan, X.; Tomino, T.; Harada, Y.; Watanabe, M.; Wang, Y.; Tanaka, Y.; et al. Intronic regulation of Aire expression by Jmjd6 for self-tolerance induction in the thymus. Nat. Commun. 2015, 6, 8820. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Martínez-Muñoz, L.; Cascio, G.; Lucas, P.; Pablos, J.L.; Rodríguez-Frade, J.M. T Cell Migration in Rheumatoid Arthritis. Front. Immunol. 2015, 6, 384. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, D.P.; Khamashta, M.A.; Hughes, G.R. Systemic lupus erythematosus. Lancet 2007, 369, 587–596. [Google Scholar] [CrossRef]
- Zhang, X.L.; Peng, J.; Sun, J.Z.; Guo, C.S.; Yu, Y.; Wang, Z.G.; Chu, X.X.; Hou, M. Modulation of immune response with cytotoxic T-lymphocyte-associated antigen 4 immunoglobulin-induced anergic T cells in chronic idiopathic thrombocytopenic purpura. J. Thromb. Haemost. 2008, 6, 158–165. [Google Scholar] [CrossRef]
- Shan, N.N.; Ji, X.B.; Wang, X.; Li, Y.; Liu, X.; Zhu, X.J.; Hou, M. In vitro recovery of Th1/Th2 balance in PBMCs from patients with immune thrombocytopenia through the actions of IL-18BPa/Fc. Thromb. Res. 2011, 128, e119–e124. [Google Scholar] [CrossRef]
- Kuwana, M.; Okazaki, Y.; Ikeda, Y. Detection of circulating B cells producing anti-GPIb autoantibodies in patients with immune thrombocytopenia. PLoS ONE 2014, 9, e86943. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Chen, M.; Chai, Y. Association of AIRE gene polymorphisms with susceptibility to rheumatoid arthritis among ethnic Han Chinese from Shaanxi. Zhonghua Yi Xue Yi Chuan Xue Za Zhi = Zhonghua Yixue Yichuanxue Zazhi = Chin. J. Med. Genet. 2016, 33, 373–377. [Google Scholar]
- Salesi, M.; Noormohamadi, E.; Pakzad, B.; Yousefisadr, F.; Salehi, R. Single Nucleotide Polymorphism rs2075876 in AIRE Gene Is a Strong Rheumatoid Arthritis Determinant. Acta Med. Iran. 2021, 59, 259–264. [Google Scholar] [CrossRef]
- Montufar-Robles, I.; Robles-Garnica, J.C.; Cadena-Sandoval, D.; Barbosa-Cobos, R.E.; González-Castillo, D.D.; Romero-Diaz, J.; Sánchez-Muñoz, F.; Saavedra, M.A.; Olivares-Martínez, E.; Miranda-Hernández, D.; et al. The AIRE Ser196Ser synonymous variant is a risk factor for systemic lupus erythematosus. Cell. Immunol. 2019, 346, 103986. [Google Scholar] [CrossRef] [PubMed]
- Wengraf, D.A.; McDonagh, A.J.; Lovewell, T.R.; Vasilopoulos, Y.; Macdonald-Hull, S.P.; Cork, M.J.; Messenger, A.G.; Tazi-Ahnini, R. Genetic analysis of autoimmune regulator haplotypes in alopecia areata. Tissue Antigens 2008, 71, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Stranger, B.E.; Forrest, M.S.; Dunning, M.; Ingle, C.E.; Beazley, C.; Thorne, N.; Redon, R.; Bird, C.P.; de Grassi, A.; Lee, C.; et al. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science 2007, 315, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L., Jr.; Crowther, M.A.; American Society of Hematology. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Case Group (n = 96) | Control Group (n = 100) | p-Value |
|---|---|---|---|
| Age, years, mean ± SD | 44.6 ± 12.6 | 42.3 ± 13.0 | 0.201 |
| Gender Male, n (%) Female, n (%) | 31 (32.3) 65 (67.7) | 40 (40.0) 60 (60.0) | 0.262 |
| Positive family history, n (%) | 23 (24.0) | 6 (6.0%) | <0.001 * |
| Platelets count (×103/μL), median (IQR) | 55.5 (23) | 210.5 (112) | <0.001 * |
| SNPs | Genetic Model | Case Group, n (%) (n = 96) | Control Group, n (%) (n = 100) | χ2 | p | |
|---|---|---|---|---|---|---|
| AIRE (rs2075876 G/A) | Additive | GG | 26 (0.27) | 41 (41.0) | -- | -- |
| GA | 47 (0.49) | 49 (49.0) | 1.645 | 0.200 | ||
| AA | 23 (0.24) | 10 (10.0) | 8.443 | 0.004 * | ||
| HWE p | -- | 0.399 | ||||
| Dominant | GG | 26 (0.27) | 41 (0.41) | 2.216 | 0.040 * | |
| GA/AA | 70 (0.73) | 59 (0.59) | ||||
| Recessive | GG/GA | 73 (0.76) | 90 (0.90) | 6.815 | 0.009 * | |
| AA | 23 (0.24) | 10 (0.10) | ||||
| Multiplicative (Allele) | G | 99 (0.52) | 131 (0.655) | 7.848 | 0.005 * | |
| A | 93 (0.48) | 69 (0.345) | ||||
| AIRE (rs760426 A/G) | Co-dominant | AA | 43 (0.45) | 46 (0.46) | -- | -- |
| AG | 47 (0.49) | 39 (0.39) | 0.703 | 0.402 | ||
| GG | 6 (0.06) | 15 (0.15) | 2.681 | 0.102 | ||
| HWE p | 0.170 | |||||
| Dominant | AA | 43 (0.45) | 46 (0.46) | 0.029 | 0.865 | |
| AG/GG | 53 (0.55) | 54 (0.54) | ||||
| Recessive | AA/AG | 90 (0.94) | 85 (0.85) | 3.920 | 0.054 | |
| GG | 6 (0.06) | 15 (0.15) | ||||
| Multiplicative (Allele) | A | 133 (0.69) | 131 (0.655) | 0.633 | 0.426 | |
| G | 59 (0.31) | 69 (0.345) | ||||
| SNPs | Model | Genotype | Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | p-corr |
|---|---|---|---|---|---|---|---|
| AIRE (rs2075876 G/A) | Additive | GG | Ref | -- | Ref | -- | |
| GA | 1.513 (0.803–2.851) | 0.201 | 1.460 (0.754–2.826) | 0.262 | |||
| AA | 3.627 (1.489–8.835) | 0.005 * | 4.299 (1.650–11.202) | 0.003 * | 0.008 * | ||
| Dominant | GG | Ref | -- | Ref | -- | ||
| GA/AA | 1.871 (1.026–3.413) | 0.041 * | 1.859 (0.994–3.477) | 0.052 | |||
| Recessive | GG/GA | Ref | -- | Ref | -- | ||
| AA | 2.836 (1.269–6.336) | 0.011 * | 3.257 (1.406–7.545) | 0.006 * | 0.005 * | ||
| Multiplicative (Allele) | G | Ref | -- | Ref | -- | ||
| A | 1.783 (1.188–2.678) | 0.005 * | 1.847 (1.209–2.822) | 0.005 * | 0.004 * | ||
| AIRE (rs760426 A/G) | Co-dominant | AA | Ref | -- | Ref | -- | |
| AG | 1.289 (0.712–2.336) | 0.402 | 1.248 (0.675–2.309) | 0.480 | |||
| GG | 0.428 (0.152–1.203) | 0.108 | 0.421 (0.144–1.233) | 0.115 | |||
| Dominant | AA | Ref | -- | Ref | -- | ||
| AG/GG | 0.952 (0.543–1.672) | 0.865 | 0.985 (0.549–1.767) | 0.960 | |||
| Recessive | AA/AG | Ref | -- | Ref | -- | ||
| GG | 0.378 (0.140–1.019) | 0.054 | 0.380 (0.135–1.064) | 0.065 | |||
| Multiplicative (Allele) | A | Ref | -- | Ref | -- | ||
| G | 0.842 (0.552–1.286) | 0.426 | 0.828 (0.533–1.286) | 0.401 |
| AIRE SNP Haplotypes | Case Group (n = 192) | Control Group (n = 200) | Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| G-A | n (%) | 59 (0.307) | 79 (0.395) | --- | --- | --- | --- |
| G-G | n (%) | 40 (0.208) | 52 (0.26) | 0.971 (0.570–1.654) | 0.913 | 1.089 (0.623–1.902) | 0.765 |
| A–G | n (%) | 19 (0.099) | 17 (0.085) | 1.497 (0.717–3.124) | 0.283 | 1.823 (0.836–3.973) | 0.131 |
| A–A | n (%) | 74 (0.385) | 52 (0.23) | 1.905 (1.168–3.109) | 0.010* | 1.821 (1.099–3.017) | 0.020 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Ghafar, M.T.; Elshora, O.A.; Allam, A.A.; Mashaal, R.G.; Hamous, S.A.A.; Abd El-Khalik, S.R.; Abd-Ellatif, R.N.; Mariah, R.A.; Eissa, R.; Mwafy, M.; et al. Autoimmune Regulator Gene Polymorphisms and the Risk of Primary Immune Thrombocytopenic Purpura: A Case-Control Study. Int. J. Mol. Sci. 2023, 24, 5007. https://doi.org/10.3390/ijms24055007
Abdel Ghafar MT, Elshora OA, Allam AA, Mashaal RG, Hamous SAA, Abd El-Khalik SR, Abd-Ellatif RN, Mariah RA, Eissa R, Mwafy M, et al. Autoimmune Regulator Gene Polymorphisms and the Risk of Primary Immune Thrombocytopenic Purpura: A Case-Control Study. International Journal of Molecular Sciences. 2023; 24(5):5007. https://doi.org/10.3390/ijms24055007
Chicago/Turabian StyleAbdel Ghafar, Muhammad T., Ola A. Elshora, Alzahraa A. Allam, Raghda Gabr Mashaal, Shereen Awny Abdelsalam Hamous, Sarah Ragab Abd El-Khalik, Rania Nagi Abd-Ellatif, Reham A. Mariah, Radwa Eissa, Mai Mwafy, and et al. 2023. "Autoimmune Regulator Gene Polymorphisms and the Risk of Primary Immune Thrombocytopenic Purpura: A Case-Control Study" International Journal of Molecular Sciences 24, no. 5: 5007. https://doi.org/10.3390/ijms24055007
APA StyleAbdel Ghafar, M. T., Elshora, O. A., Allam, A. A., Mashaal, R. G., Hamous, S. A. A., Abd El-Khalik, S. R., Abd-Ellatif, R. N., Mariah, R. A., Eissa, R., Mwafy, M., Shalaby, R. E., Nasif, E., & Elkholy, R. A. (2023). Autoimmune Regulator Gene Polymorphisms and the Risk of Primary Immune Thrombocytopenic Purpura: A Case-Control Study. International Journal of Molecular Sciences, 24(5), 5007. https://doi.org/10.3390/ijms24055007






