Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characteristics of Cocoons and Degummed Silk
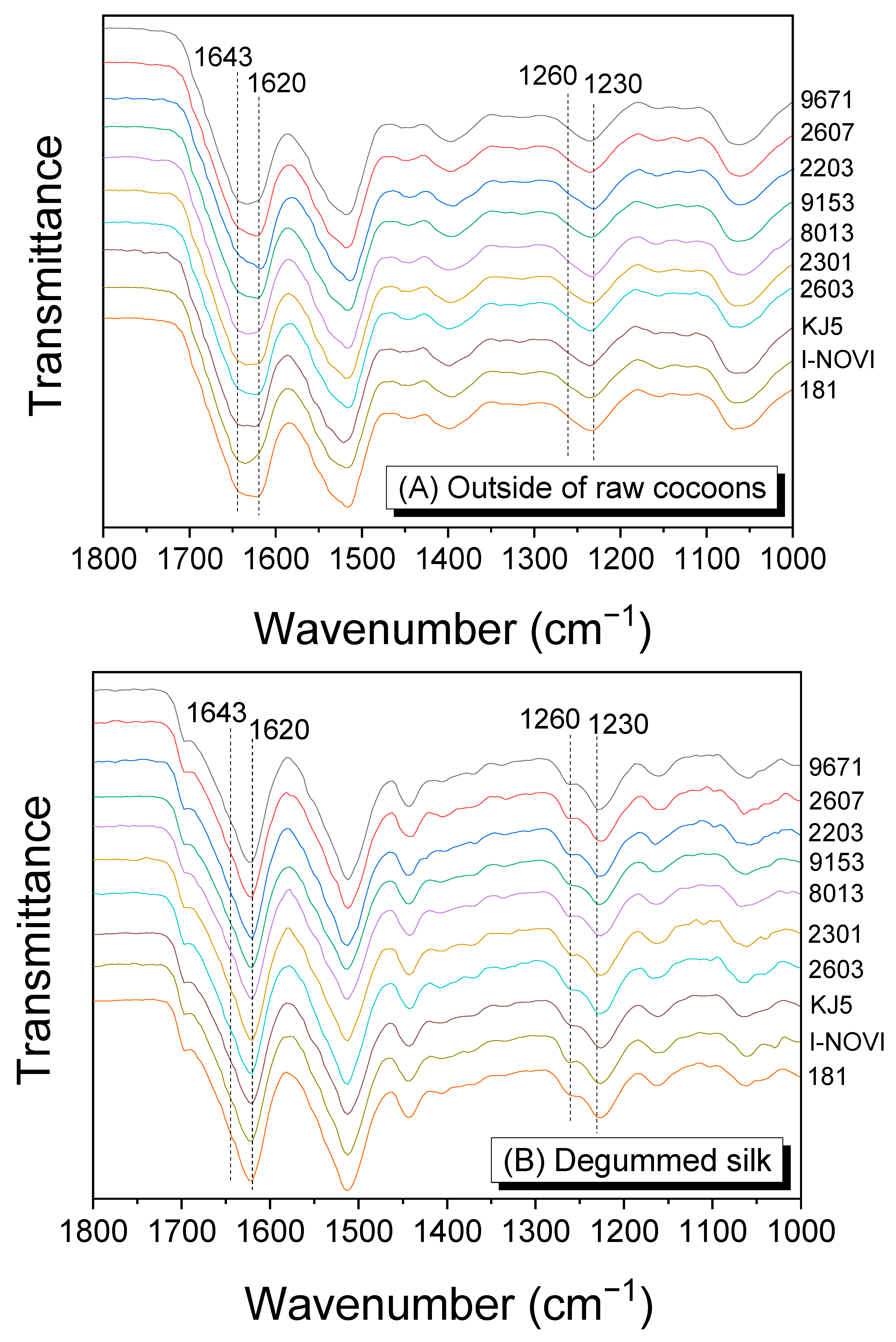
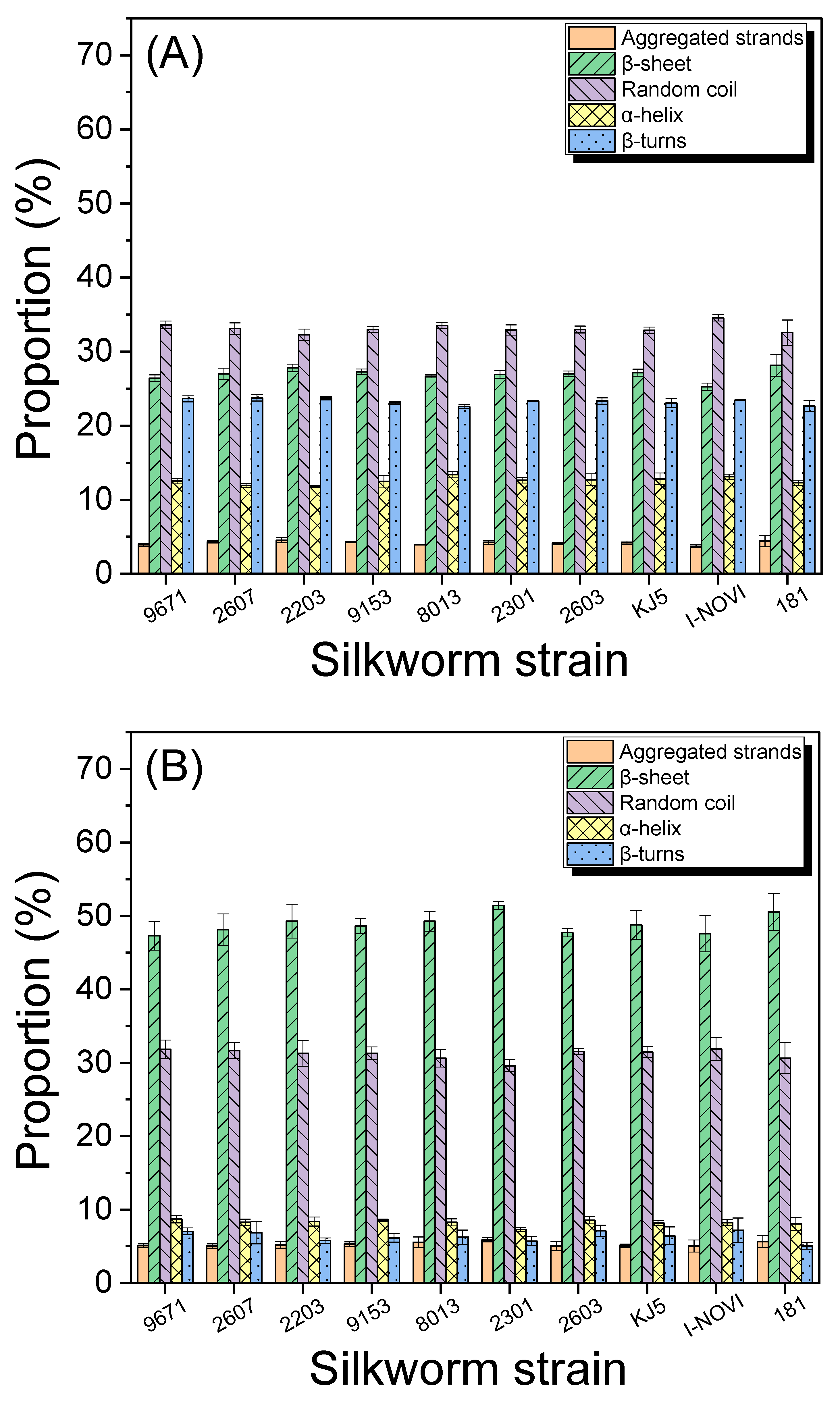
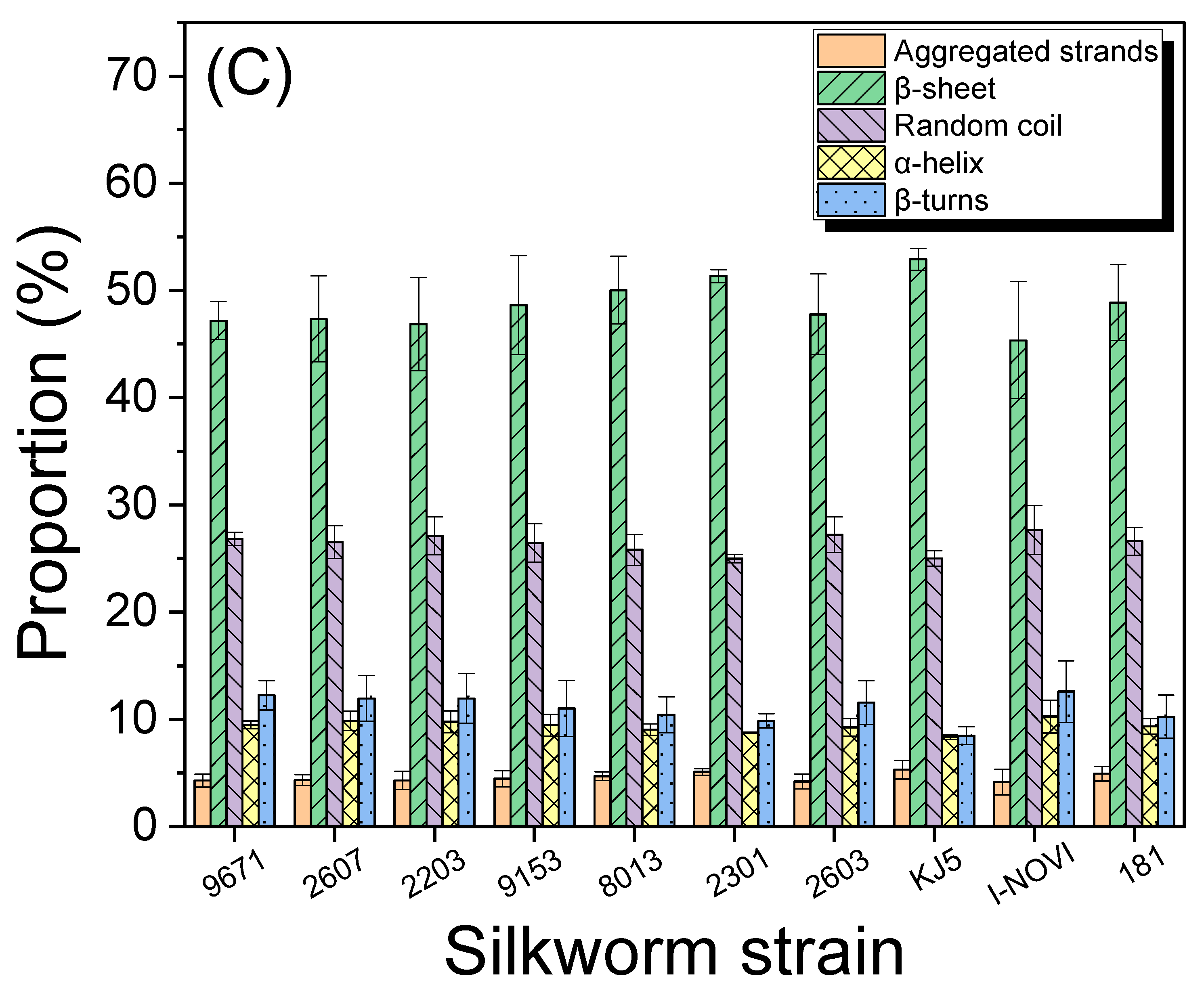
2.2. Degumming and Molecular Conformation of Silks
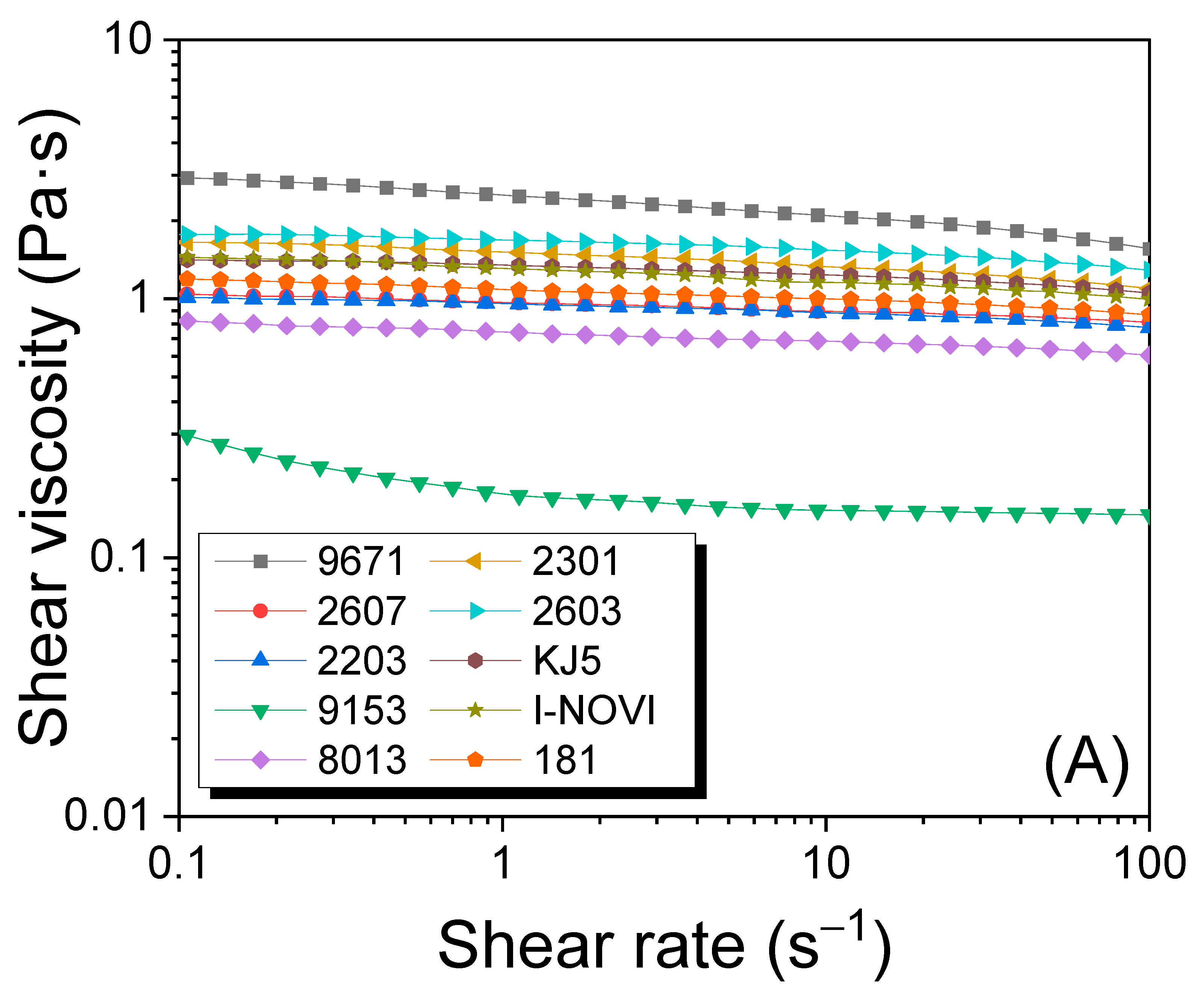
2.3. Solution Viscosity of Regenerated SF
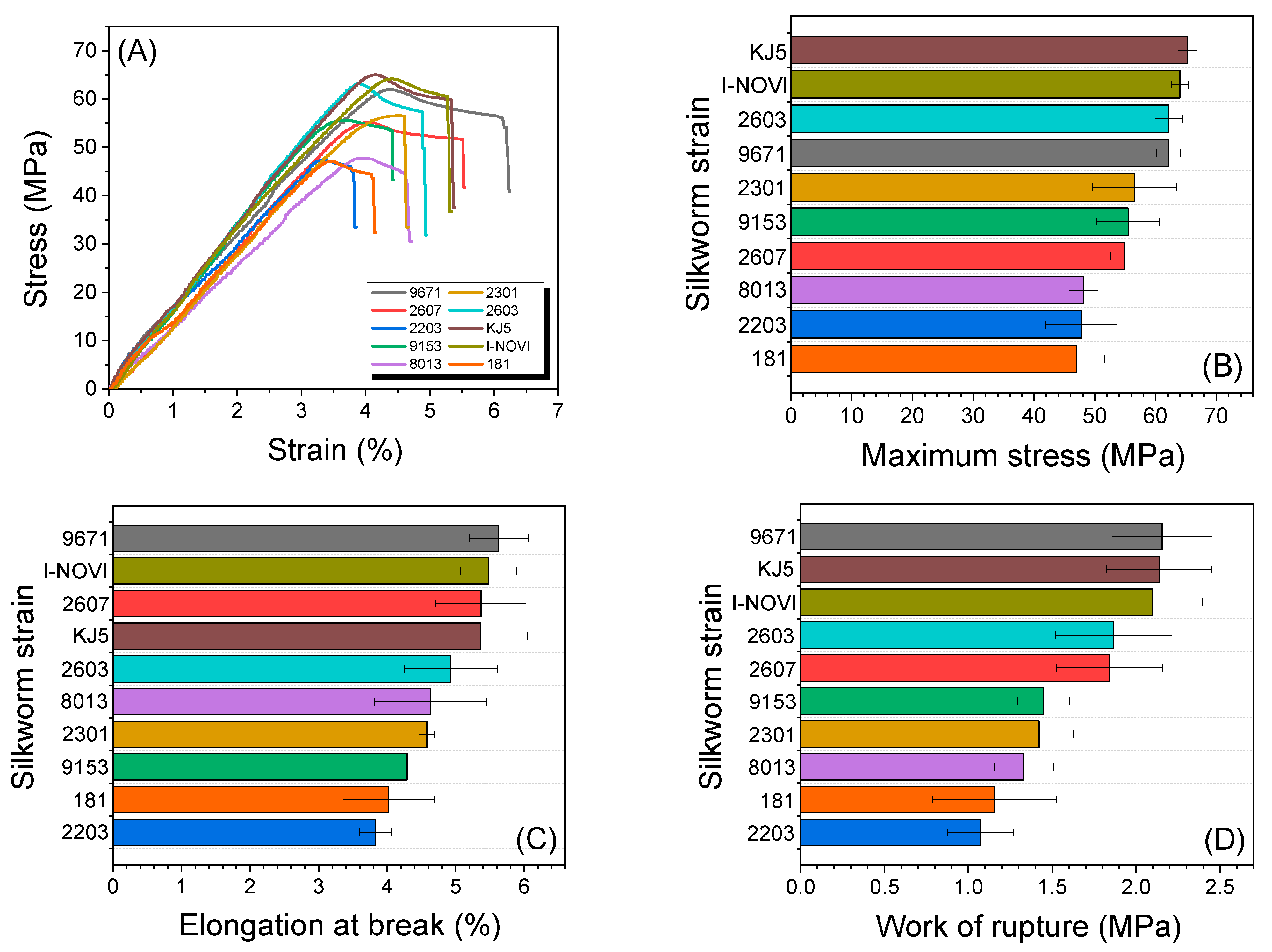
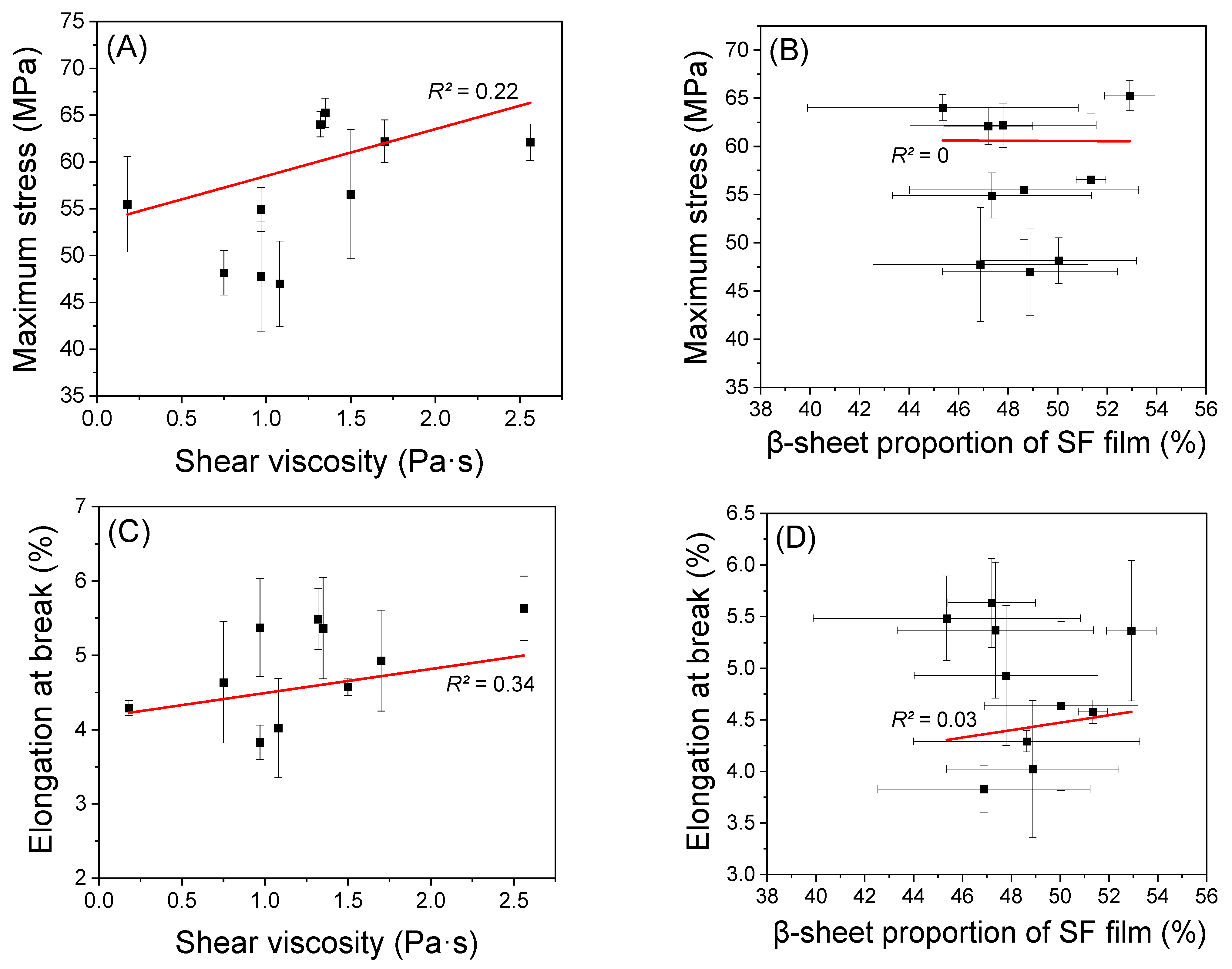
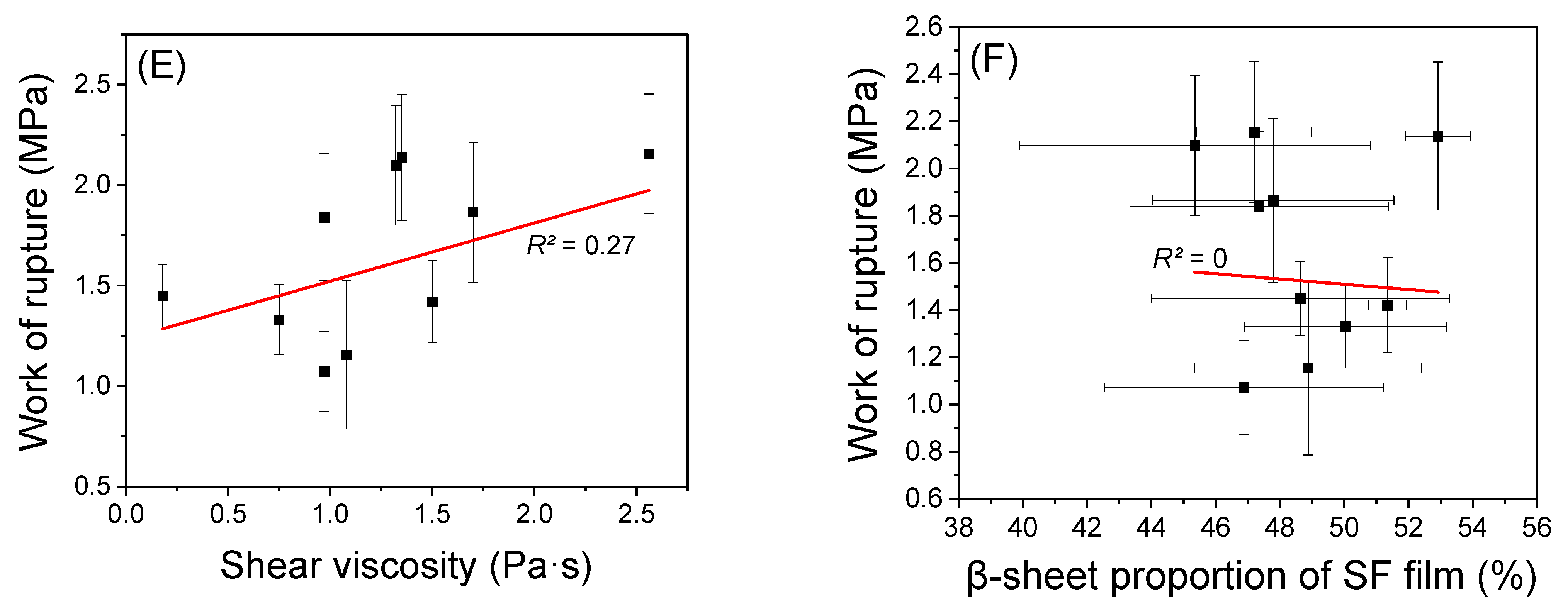
2.4. Mechanical Properties of Regenerated SF Film
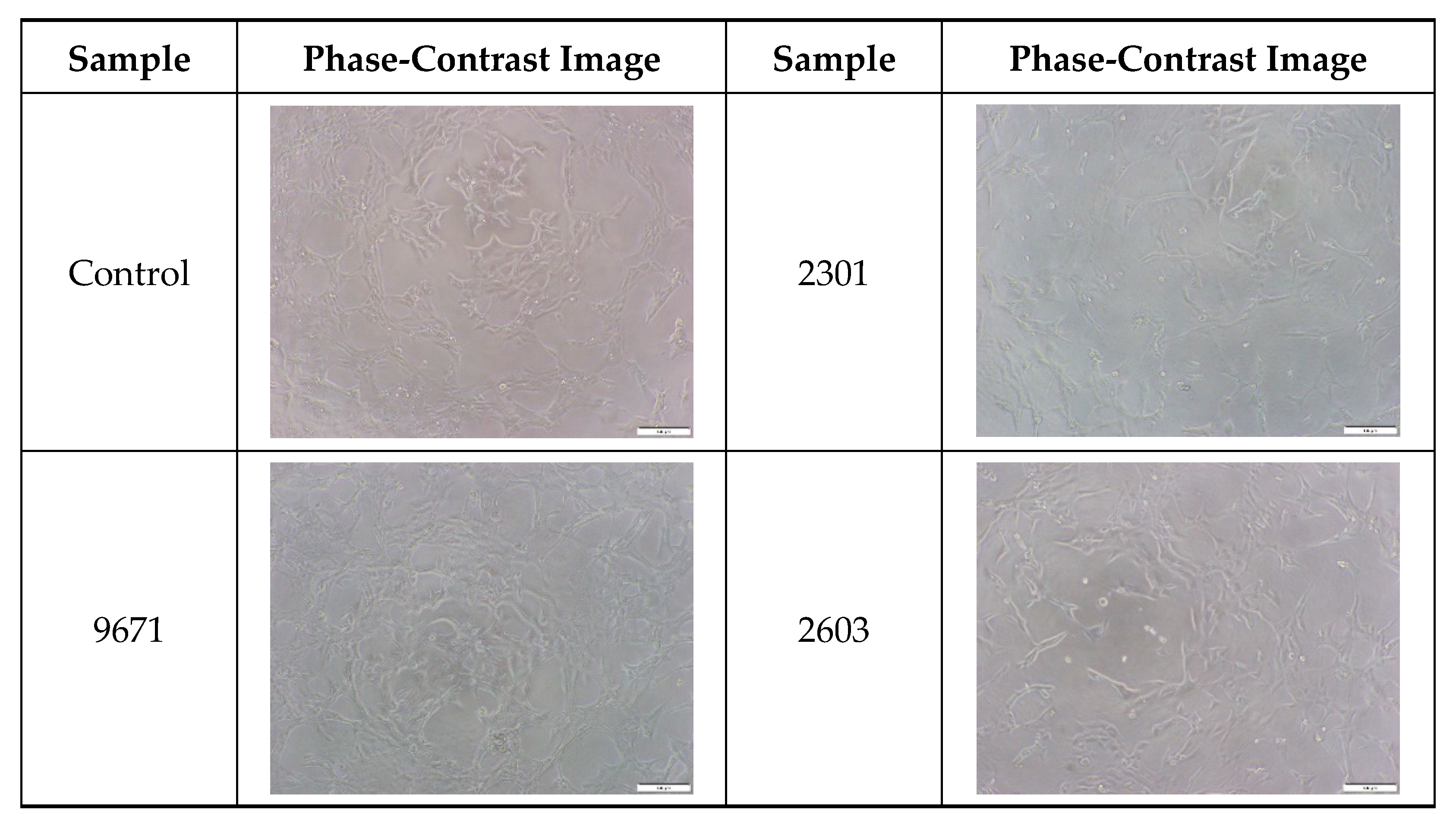
2.5. Cytocompatibility of the Cocoon
3. Materials and Methods
3.1. Materials
3.2. Preparation of Regenerated SF Solution and Films
3.3. Measurement and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakabe, H.; Ito, H.; Miyamoto, T.; Noishiki, Y.; Ha, W.S. In vivo blood compatibility of regenerated silk fibroin. Sen’i Gakkaishi 1989, 45, 487–490. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Hwang, C.M.; Min, B.G.; Park, Y.H. Structural characteristics and properties of silk fibro-in/polyurethane blend films. Int. J. Indust. Entomol. 2002, 5, 163–170. [Google Scholar]
- Chen, Z.; Zhang, Q.; Li, H.; Wei, Q.; Zhao, X.; Chen, F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact. Mater. 2021, 6, 589–601. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation of Bombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2004, 91, 2383–2390. [Google Scholar] [CrossRef]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef]
- Guo, F.; Liu, C.; Han, R.; Lu, Q.; Bai, Y.; Yang, R.; Niu, D.; Zhang, X. Bio-inspired anisotropic polymeric heart valves exhibiting valve-like mechanical and hemodynamic behavior. Sci. China Mater. 2020, 63, 629–643. [Google Scholar] [CrossRef]
- Park, S.Y.; Ki, C.S.; Park, Y.H.; Lee, K.G.; Kang, S.W.; Kweon, H.Y.; Kim, H.J. Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J. Tissue Eng. Regen. Med. 2015, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.-K.; Park, H.S.; Jeong, J.Y.; Yeon, Y.K.; Kumar, V.; Bae, S.H.; Lee, J.M.; Moon, B.M.; Park, C.H. A prospective cohort study of the silk fibroin patch in chronic tympanic membrane perforation. Laryngoscope 2016, 126, 2798–2803. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.H.; Park, C.H.; Seo, J.-N.; Kweon, H.Y.; Kang, S.W.; Lee, K.G. Comparison of methods for the repair of acute tympanic membrane perforations: Silk patch vs. paper patch. Wound Repair Regen. 2010, 18, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.-J.; Seok, H. Silk Protein-Based Membrane for Guided Bone Regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef]
- Song, J.-Y.; Kim, S.-G.; Lee, J.-W.; Chae, W.-S.; Kweon, H.; Jo, Y.-Y.; Lee, K.-G.; Lee, Y.-C.; Choi, J.-Y.; Kim, J.-Y. Accelerated healing with the use of a silk fibroin membrane for the guided bone regeneration technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e26–e33. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, B. Development and performance study of a natural silk fiber facial mask paper. J. Eng. Fibers Fabr. 2020, 15, 1558925020975756. [Google Scholar] [CrossRef]
- Um, I.C.; Ki, C.S.; Kweon, H.; Lee, K.G.; Ihm, D.W.; Park, Y.H. Wet spinning of silk polymer: II. Effect of drawing on the structural characteristics and properties of filament. Int. J. Biol. Macromol. 2004, 34, 107–119. [Google Scholar] [CrossRef]
- Minoura, N.; Tsukada, M.; Nagura, M. Physico-chemical properties of silk fibroin membrane as a biomaterial. Biomaterials 1990, 11, 430–434. [Google Scholar] [CrossRef]
- Um, I.C.; Kweon, H.Y.; Park, Y.H.; Hudson, S. Structural characteristics and properties of the regenerated silk fibroin prepared from formic acid. Int. J. Biol. Macromol. 2001, 29, 91–97. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, D.W.; Park, Y.H.; Um, I.C. Effect of residual sericin on the structural characteristics and properties of regen-erated silk films. Int. J. Biol. Macromol. 2016, 89, 273–278. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers 2021, 13, 2479. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effects of electric field on the maximum electro-spinning rate of silk fibroin solutions. Int. J. Biol. Macromol. 2017, 95, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bae, Y.S.; Kim, S.J.; Song, D.W.; Park, Y.H.; Bae, D.G.; Choi, J.H.; Um, I.C. Preparation of new natural silk non-woven fabrics by using adhesion characteristics of sericin and their characterization. Int. J. Biol. Macromol. 2018, 106, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Jang, M.J.; Um, I.C. Silk/Rayon Webs and Nonwoven Fabrics: Fabrication, Structural Characteristics, and Properties. Int. J. Mol. Sci. 2022, 23, 7511. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef]
- Park, C.J.; Ryoo, J.; Ki, C.S.; Kim, J.W.; Kim, I.S.; Bae, D.G.; Um, I.C. Effect of molecular weight on the structure and mechanical properties of silk sericin gel, film, and sponge. Int. J. Biol. Macromol. 2018, 119, 821–832. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, K.H. Polyethylenimine-functionalized silk sericin beads for high-performance remediation of hexavalent chromium from aqueous solution. Chemosphere 2018, 207, 507–516. [Google Scholar] [CrossRef]
- Kang, G.D.; Nahm, J.H.; Park, J.S.; Moon, J.Y.; Cho, C.S.; Yeo, J.H. Effects of poloxamer on the gelation of silk fibroin. Macromol. Rapid Commun. 2000, 21, 788–791. [Google Scholar] [CrossRef]
- Kim, H.H.; Song, D.W.; Kim, M.J.; Ryu, S.J.; Um, I.C.; Ki, C.S.; Park, Y.H. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 2016, 90, 26–33. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef]
- Kweon, H.; Ha, H.C.; Um, I.C.; Park, Y.H. Physical properties of silk fibroin/chitosan blend films. J. Appl. Polym. Sci. 2001, 80, 928–934. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Crighton, J.S. Structure and compatibility of poly(vinyl alcohol)-silk fibroin (PVA/SA) blend films. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 243–248. [Google Scholar] [CrossRef]
- Jin, H.; Park, J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Biomaterial films of Bombyx mori silk fibroin with poly(ethylene oxide). Biomacromolecules 2004, 5, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Park, Y.H. The effect of casting solvent on the structural characteristics and miscibility of regenerated silk fibroin/Poly(vinyl alcohol) blends. Fiber. Polym. 2007, 8, 579–585. [Google Scholar] [CrossRef]
- Jo, Y.N.; Um, I.C. Effects of solvent on the solution properties, structural characteristics and properties of silk sericin. Int. J. Biol. Macromol. 2015, 78, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.K.; Lee, K.H.; Nho, S.K.; Han, M.S.; Um, I.C. Effect of degumming methods on structural characteristics and properties of regenerated silk. Int. J. Biol. Macromol. 2017, 104, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Ki, C.S.; Um, I.C. Effect of Sericin Content on the Structural Characteristics and Properties of Electro-spun Regenerated Silk. Fibers Polym. 2018, 19, 507–514. [Google Scholar] [CrossRef]
- Ko, J.S.; Yoon, K.; Ki, C.S.; Kim, H.J.; Bae, D.G.; Lee, K.H.; Park, Y.H.; Um, I.C. Effect of degumming condition on the solution properties and electrospinnablity of regenerated silk solution. Int. J. Biol. Macromol. 2013, 55, 161–168. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of molecular weight on electro-spinning performance of regenerated silk. Int. J. Biol. Macromol. 2018, 106, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.E.; Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fiber. Polym. 2014, 15, 153–160. [Google Scholar] [CrossRef]
- Kim, H.J.; Um, I.C. Effect of degumming ratio on wet spinning and post drawing performance of regenerated silk. Int. J. Biol. Macromol. 2014, 67, 387–393. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, B.K.; Um, I.C. Preparation of highly crystalline silk nanofibrils and their use in the improvement of the me-chanical properties of silk films. Int. J. Mol. Sci. 2022, 23, 11344. [Google Scholar] [CrossRef]
- Chung, D.E.; Kim, H.H.; Kim, M.K.; Lee, K.H.; Park, Y.H.; Um, I.C. Effects of different Bombyx mori silkworm varieties on the structural characteristics and properties of silk. Int. J. Biol. Macromol. 2015, 79, 943–951. [Google Scholar] [CrossRef]
- Chung, D.E.; Lee, J.H.; Kweon, H.; Lee, K.-G.; Um, I.C. Structure and properties of silk sericin obtained from different silkworm varieties. Int. J. Ind. Èntomol. 2015, 30, 81–85. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of Korean Bombyx mori variety on electro-spinning performance of regenerated silk fibroin. Fiber. Polym. 2015, 16, 1935–1940. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Effect of Silkworm Variety on Characteristics of Raw Sericin in Silk. Fiber. Polym. 2019, 20, 271–279. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, I.C. Preparation, Structural Characterization, and Properties of Natural Silk Non-woven Fabrics from Different Silkworm Varieties. Fibers Polym. 2022, 23, 1130–1141. [Google Scholar] [CrossRef]
- Choi, H.J.; Noh, S.K.; Um, I.C. Morphology, molecular conformation and moisture regain of cocoons of different silkworm varieties. Int. J. Indust. Entomol. 2020, 40, 6–15. [Google Scholar]
- Bae, Y.J.; Noh, S.K.; Um, I.C. Crystallinity change of silkworm variety cocoons by heat treatment. Int. J. Indust. Entomol. 2021, 42, 7–13. [Google Scholar]
- Um, I.C.; Kweon, H.Y.; Lee, K.G.; Park, Y.H. The role of formic acid in solution stability and crystallization of silk protein polymer. Int. J. Biol. Macromol. 2003, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ki, C.S.; Oh, H.; Lee, K.H.; Um, I.C. Molecular weight distribution and solution properties of silk fibroins with dif-ferent dissolution conditions. Int. J. Biol. Macromol. 2012, 51, 336–341. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoo, Y.J.; Kim, J.W.; Park, Y.H.; Bae, D.G.; Um, I.C. Effect of molecular weight and storage time on the wet- and electro-spinning of regenerated silk fibroin. Polym. Degrad. Stab. 2012, 97, 1060–1066. [Google Scholar] [CrossRef]
- Oh, H.; Lee, J.Y.; Kim, M.K.; Um, I.C.; Lee, K.H. Refining hot-water extracted silk sericin by ethanol-induced precipitation. Int. J. Biol. Macromol. 2011, 48, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Yang, Y.; Shao, Z. Effect of Various Dissolution Systems on the Molecular Weight of Regenerated Silk Fibroin. Biomacromolecules 2012, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, J.W.; Kim, S.B.; Yu, J.H.; Hong, J.W.; Kang, S.K.; Kim, N.S.; Kim, K.Y. A new breed of silkworm variety, Juhwangjam, for light pink cocoon. Int. J. Indust. Entomol. 2020, 40, 51–55. [Google Scholar]
- Bae, Y.-S.; Um, I.-C. Effects of Fabrication Conditions on Structure and Properties of Mechanically Prepared Natural Silk Web and Non-Woven Fabrics. Polymers 2021, 13, 1578. [Google Scholar] [CrossRef]
- Jang, M.J.; Um, I.C. Effect of sericin concentration and ethanol content on gelation behavior, rheological properties, and sponge characteristics of silk sericin. Eur. Polym. J. 2017, 93, 761–774. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, H.N.; Ki, C.S.; Fang, D.; Hsiao, B.S.; Chu, B.; Um, I.C. Effects of degumming conditions on electro-spinning rate of regenerated silk. Int. J. Biol. Macromol. 2013, 61, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.N.; Park, B.-D.; Um, I.C. Effect of storage and drying temperature on the gelation behavior and structural characteristics of sericin. Int. J. Biol. Macromol. 2015, 81, 936–941. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spec-troscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Bae, Y.S.; Um, I.C. Preparation, Structural Characteristics, and Properties of Airlaid Nonwoven Silk Fabric. Polym. Korea 2020, 44, 809–816. [Google Scholar] [CrossRef]




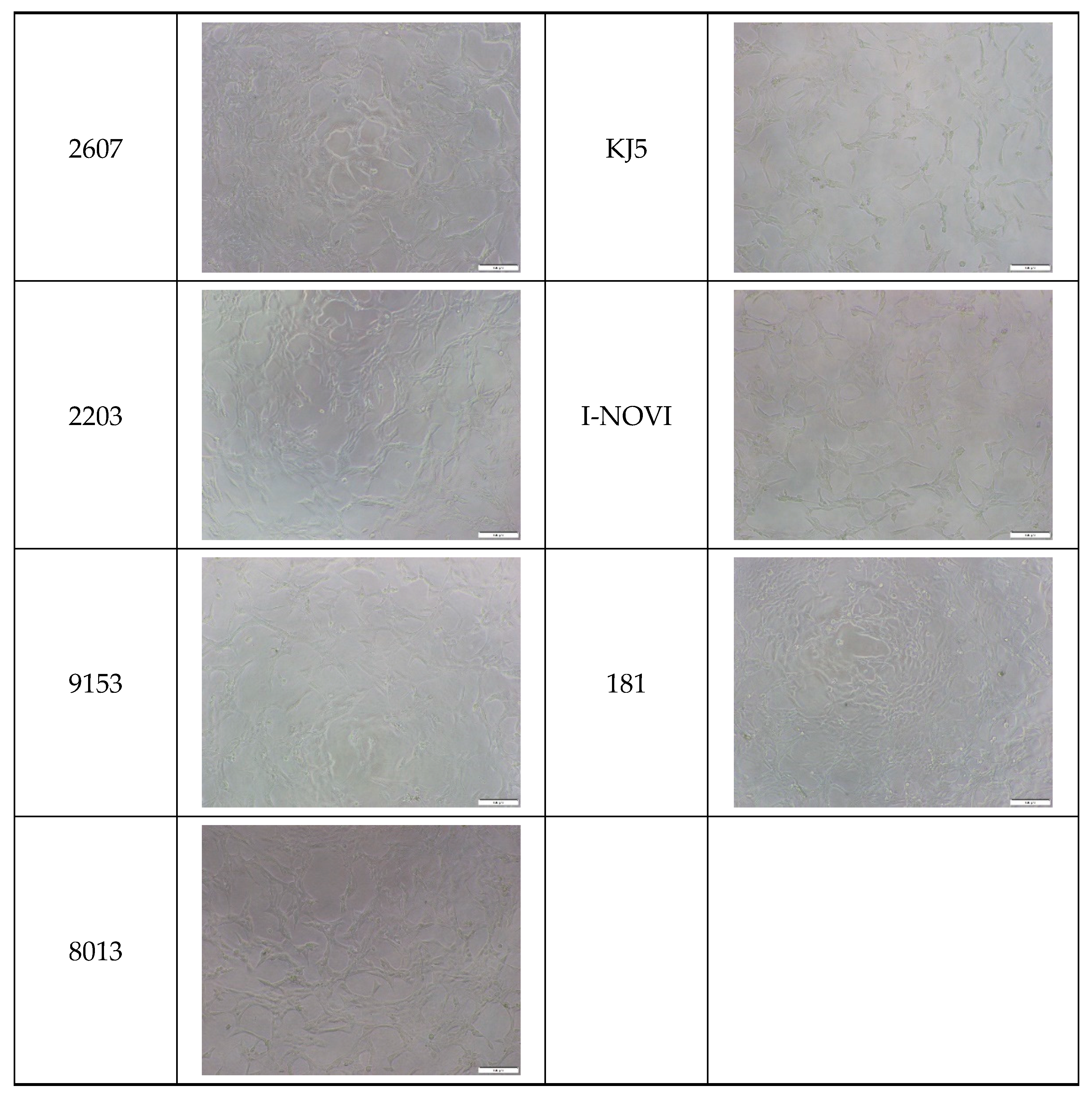
| Silkworm Strain | External Features of Cocoons | SEM Image | Filament Diameter (µm, Mean ± SD, n = 30) | Thickness of Cocoons (mm, Mean ± SD, n = 20) | Weight of the Silk Cocoons (Excluding the Pupae, G, Mean ± SD, n = 3) | ||
|---|---|---|---|---|---|---|---|
| Outside of Cocoon | Degummed Silk | Outside of Cocoon | Degummed Silk | ||||
| 9671 |  |  |  | 29.1 ± 2.7 | 15.3 ± 1.6 | 1.15 ± 0.17 | 0.49 ± 0.02 |
| 2607 |  |  |  | 28.9 ± 3.0 | 14.5 ± 1.1 | 1.01 ± 0.14 | 0.45 ± 0.04 |
| 2203 |  |  | 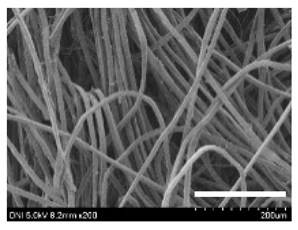 | 27.7 ± 2.7 | 14.1 ± 1.3 | 0.98 ± 0.11 | 0.41± 0.02 |
| 9153 |  |  |  | 28.8 ± 2.6 | 14.5 ± 1.5 | 1.16 ± 0.10 | 0.44 ± 0.04 |
| 8013 |  |  |  | 27.2 ± 2.6 | 13.7 ± 1.3 | 1.09 ± 0.20 | 0.39 ± 0.02 |
| 2301 |  |  | 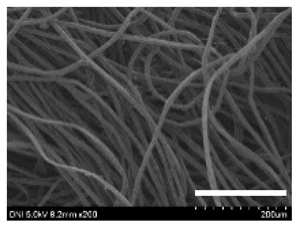 | 28.9 ± 2.4 | 14.7 ± 1.8 | 1.10 ± 0.17 | 0.40 ± 0.01 |
| 2603 |  |  |  | 28.1 ± 2.7 | 13.1 ± 1.4 | 1.19 ± 0.12 | 0.43 ± 0.05 |
| KJ5 |  |  | 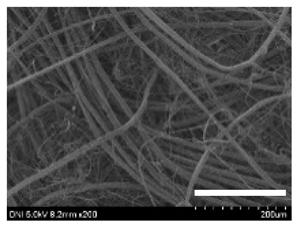 | 29.4 ± 2.5 | 13.2 ± 1.9 | 1.21 ± 0.16 | 0.44 ± 0.01 |
| I-NOVI |  |  |  | 27.1 ± 2.1 | 13.9 ± 1.6 | 0.52 ± 0.09 | 0.20 ± 0.01 |
| 181 |  |  | 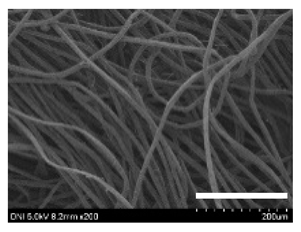 | 26.5 ± 2.4 | 12.4 ± 1.4 | 0.73 ± 0.19 | 0.13 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Kim, S.W.; Kim, K.Y.; Ki, C.S.; Um, I.C. Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains. Int. J. Mol. Sci. 2023, 24, 4965. https://doi.org/10.3390/ijms24054965
Kim YJ, Kim SW, Kim KY, Ki CS, Um IC. Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains. International Journal of Molecular Sciences. 2023; 24(5):4965. https://doi.org/10.3390/ijms24054965
Chicago/Turabian StyleKim, Yeon Jin, Seong Wan Kim, Kee Young Kim, Chang Seok Ki, and In Chul Um. 2023. "Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains" International Journal of Molecular Sciences 24, no. 5: 4965. https://doi.org/10.3390/ijms24054965
APA StyleKim, Y. J., Kim, S. W., Kim, K. Y., Ki, C. S., & Um, I. C. (2023). Structural Characteristics and Properties of Cocoon and Regenerated Silk Fibroin from Different Silkworm Strains. International Journal of Molecular Sciences, 24(5), 4965. https://doi.org/10.3390/ijms24054965







