Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Clinical Question
2.3. Selection Criteria
2.4. Cohen’s Kappa Coefficient
2.5. Risk of Bias Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michelotti, A.; Alstergren, P.; Goulet, J.P.; Lobbezoo, F.; Ohrbach, R.; Peck, C.; Schiffman, E.; List, T. Next steps in development of the diagnostic criteria for temporomandibular disorders (DC/TMD): Recommendations from the International RDC/TMD Consortium Network workshop. J. Oral Rehabil. 2016, 43, 453–467. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International RDC/TMD Consortium Network, International association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Fac. Pain Headache 2014, 28, 6–27. [Google Scholar]
- Crandall, J.A. An Introduction to Orofacial Pain. Dent. Clin. N. Am. 2018, 62, 511–523. [Google Scholar] [CrossRef]
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular disorders: A review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Is the Temporomandibular Joints’ Reciprocal Clicking Related to the Morphology and Position of the Mandible, as Well as to the Sagittal Position of Lower Incisors?—A Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 4994. [Google Scholar] [CrossRef]
- Schiffman, E.L.; Fricton, J.R.; Haley, D.P.; Shapiro, B.L. The prevalence and treatment needs of subjects with temporomandibular disorders. J. Am. Dent. Assoc. 1990, 120, 295–303. [Google Scholar] [CrossRef]
- Liu, F.; Steinkeler, A. Epidemiology, diagnosis, and treatment of temporomandibular disorders. Dent. Clin. N. Am. 2013, 57, 465–479. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Wolford, L.M.; Ellis, E., 3rd; Neff, A. The hierarchy of different treatments for arthrogenous temporomandibular disorders: A network meta-analysis of randomized clinical trials. J. Craniomaxillofac. Surg. 2020, 48, 9–23. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef]
- Ferrillo, M.; Nucci, L.; Giudice, A.; Calafiore, D.; Marotta, N.; Minervini, G.; d’Apuzzo, F.; Ammendolia, A.; Perillo, L.; de Sire, A. Efficacy of conservative approaches on pain relief in patients with temporomandibular joint disorders: A systematic review with network meta-analysis. Cranio 2022, 23, 1–17. [Google Scholar] [CrossRef]
- Ferrillo, M.; Marotta, N.; Giudice, A.; Calafiore, D.; Curci, C.; Fortunato, L.; Ammendolia, A.; de Sire, A. Effects of Occlusal Splints on Spinal Posture in Patients with Temporomandibular Disorders: A Systematic Review. Healthcare 2022, 10, 739. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Orally Administered NSAIDs-General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis—A Narrative Review. Pharmaceuticals 2021, 14, 219. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Han, B.; Wang, C.; Fu, C.; Liu, B.; Chen, L. The antioxidative and immunostimulating properties of D-glucosamine. Int. Immunopharmacol. 2007, 7, 29–35. [Google Scholar]
- Dahmer, S.; Schiller, R.M. Glucosamine. Am. Fam. Physician 2008, 78, 471–476. [Google Scholar]
- Vynios, D.H. Metabolism of cartilage proteoglycans in health and disease. Biomed Res. Int. 2014, 2014, 452315. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; Bruyere, O.; Giacovelli, G.; Henrotin, Y.; Dacre, J.E.; Gossett, C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Bloch, B.; Srinivasan, S.; Mangwani, J. Current Concepts in the Management of Ankle Osteoarthritis: A Systematic Review. J. Foot Ankle Surg. 2015, 54, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; (Updated February 2022); Wiley: Cochrane, AB, Canada, 2022. [Google Scholar]
- Thie, N.M.; Prasad, N.G.; Major, P.W. Evaluation of glucosamine sulfate compared to ibuprofen for the treatment of temporomandibular joint osteoarthritis: A randomized double blind controlled 3 month clinical trial. J. Rheumatol. 2001, 28, 1347–1355. [Google Scholar]
- Haghighat, A.; Behnia, A.; Kaviani, N.; Khorami, B. Evaluation of Glucosamine sulfate and Ibuprofen effects in patients with temporomandibular joint osteoarthritis symptom. J. Res. Pharm. Pract. 2013, 2, 34–39. [Google Scholar] [PubMed]
- Damlar, I.; Esen, E.; Tatli, U. Effects of glucosamine-chondroitin combination on synovial fluid IL-1β, IL-6, TNF-α and PGE2 levels in internal derangements of temporomandibular joint. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e278–e283. [Google Scholar] [CrossRef]
- Nguyen, P.; Mohamed, S.E.; Gardiner, D.; Salinas, T. A randomized double-blind clinical trial of the effect of chondroitin sulfate and glucosamine hydrochloride on temporomandibular joint disorders: A pilot study. Cranio 2001, 19, 130–139. [Google Scholar] [CrossRef]
- Cahlin, B.J.; Dahlström, L. No effect of glucosamine sulfate on osteoarthritis in the temporomandibular joints—A randomized, controlled, short-term study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 760–766. [Google Scholar] [CrossRef]
- Cen, X.; Liu, Y.; Wang, S.; Yang, X.; Shi, Z.; Liang, X. Glucosamine oral administration as an adjunct to hyaluronic acid injection in treating temporomandibular joint osteoarthritis. Oral Dis. 2018, 24, 404–411. [Google Scholar] [CrossRef]
- Yang, W.; Liu, W.; Miao, C.; Sun, H.; Li, L.; Li, C. Oral Glucosamine Hydrochloride Combined with Hyaluronate Sodium Intra-Articular Injection for Temporomandibular Joint Osteoarthritis: A Double-Blind Randomized Controlled Trial. J. Oral Maxillofac. Surg. 2018, 76, 2066–2073. [Google Scholar] [CrossRef]
- Cömert Kılıç, S. Does glucosamine, chondroitin sulfate, and methylsulfonylmethane supplementation improve the outcome of temporomandibular joint osteoarthritis management with arthrocentesis plus intraarticular hyaluronic acid injection. A randomized clinical trial. J. Craniomaxillofac. Surg. 2021, 49, 711–718. [Google Scholar] [CrossRef]
- Miao, Q.; Li, Q.; Tan, W.; Mi, Y.; Ma, B.; Zhang, J.; Guo, Z. Preparation, Anticoagulant and Antioxidant Properties of Glucosamine-Heparin Salt. Mar. Drugs 2022, 20, 646. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Deleonibus, S.; Moretto, P.; Karousou, E.; Viola, M.; Bartolini, B.; Hascall, V.C.; Tammi, M.; De Luca, G.; Passi, A. Role of UDP-N-acetylglucosamine (GlcNAc) and O-GlcNAcylation of hyaluronan synthase 2 in the control of chondroitin sulfate and hyaluronan synthesis. J. Biol. Chem. 2012, 28, 35544–35555. [Google Scholar] [CrossRef] [PubMed]
- Rychel, J.K. Diagnosis and treatment of osteoarthritis. Top. Companion Anim. Med. 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Owens, S.; Wagner, P.; Vangsness, C.T., Jr. Recent advances in glucosamine and chondroitin supplementation. J. Knee Surg. 2004, 17, 185–193. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. PubChem. Glucosamine Sulfate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/115046 (accessed on 31 December 2022).
- National Library of Medicine. PubChem. Glucosamine Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2723635 (accessed on 31 December 2022).
- Persiani, S.; Roda, E.; Rovati, L.C.; Locatelli, M.; Giacovelli, G.; Roda, A. Glucosamine oral bioavailability and plasma pharmacokinetics after increasing doses of crystalline glucosamine sulfate in man. Osteoarthr. Cartil. 2005, 13, 1041–1049. [Google Scholar] [CrossRef]
- Jackson, C.G.; Plaas, A.H.; Sandy, J.D.; Hua, C.; Kim-Rolands, S.; Barnhill, J.G.; Harris, C.L.; Clegg, D.O. The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthr. Cartil. 2010, 18, 297–302. [Google Scholar] [CrossRef]
- Uitterlinden, E.J.; Jahr, H.; Koevoet, J.L.; Jenniskens, Y.M.; Bierma-Zeinstra, S.M.; Degroot, J.; Verhaar, J.A.; Weinans, H.; van Osch, G.J. Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr. Cartil. 2006, 14, 250–257. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Guo, Z.; Yu, H.; Li, C.; Ji, X.; Feng, J.; Li, P. The antioxidant activity of glucosamine hydrochloride in vitro. Bioorg. Med. Chem. 2006, 14, 1706–1709. [Google Scholar] [CrossRef]
- Xing, R.; Liu, S.; Wang, L.; Cai, S.; Yu, H.; Feng, J.; Li, P. The preparation and antioxidant activity of glucosamine sulfate. Chin. J. Ocean Limnol. 2009, 27, 283–287. [Google Scholar] [CrossRef]
- Pharmindex. Available online: https://pharmindex.pl (accessed on 31 December 2022).
- Bruyere, O.; Altman, R.D.; Reginster, J.-Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S12–S17. [Google Scholar] [CrossRef]
- Block, J.A.; Oegema, T.R.; Sandy, J.D.; Plaas, A. The effects of oral glucosamine on joint health: Is a change in research approach needed? Osteoarthr. Cartil. 2010, 18, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Nicolosi, R.J.; Borzelleca, J.F. Glucosamine effects in humans: A review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food Chem. Toxicol. 2005, 43, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Persiani, S.; Rotini, R.; Trisolino, G.; Rovati, L.C.; Locatelli, M.; Paganini, D.; Antonioli, D.; Roda, A. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystalline glucosamine sulphate at therapeutic dose. Osteoarthr. Cartil. 2007, 15, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Setnikar, I.; Rovati, L.C. Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review. Arzneimittelforschung 2001, 51, 699–725. [Google Scholar] [PubMed]
- Salazar, J.; Bello, L.; Chávez, M.; Añez, R.; Rojas, J.; Bermúdez, V. Glucosamine for osteoarthritis: Biological effects, clinical efficacy, and safety on glucose metabolism. Arthritis 2014, 2014, 432463. [Google Scholar] [CrossRef]
- Zahedipour, F.; Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms of anticancer effects of Glucosamine. Biomed. Pharmacother. 2017, 95, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
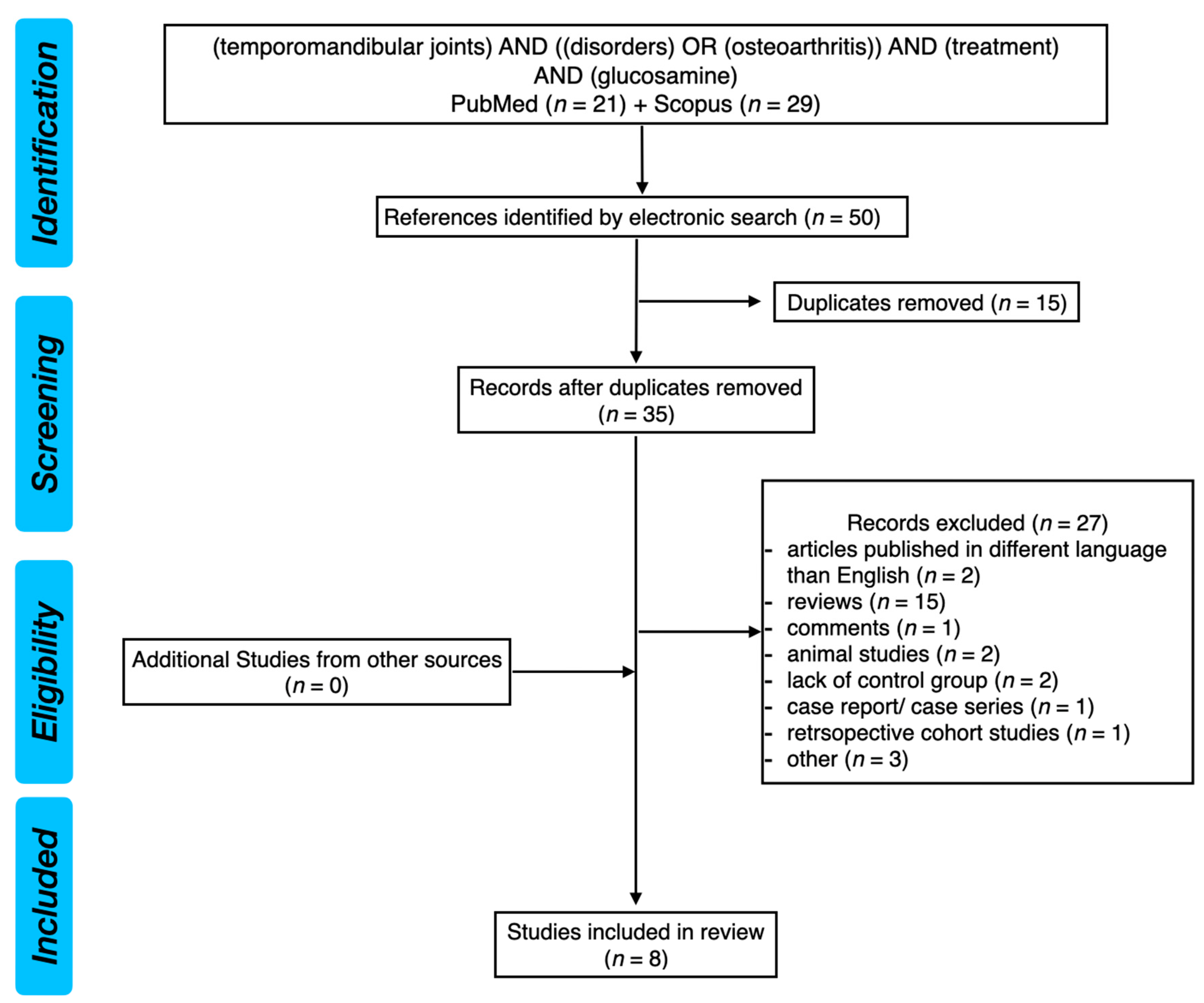
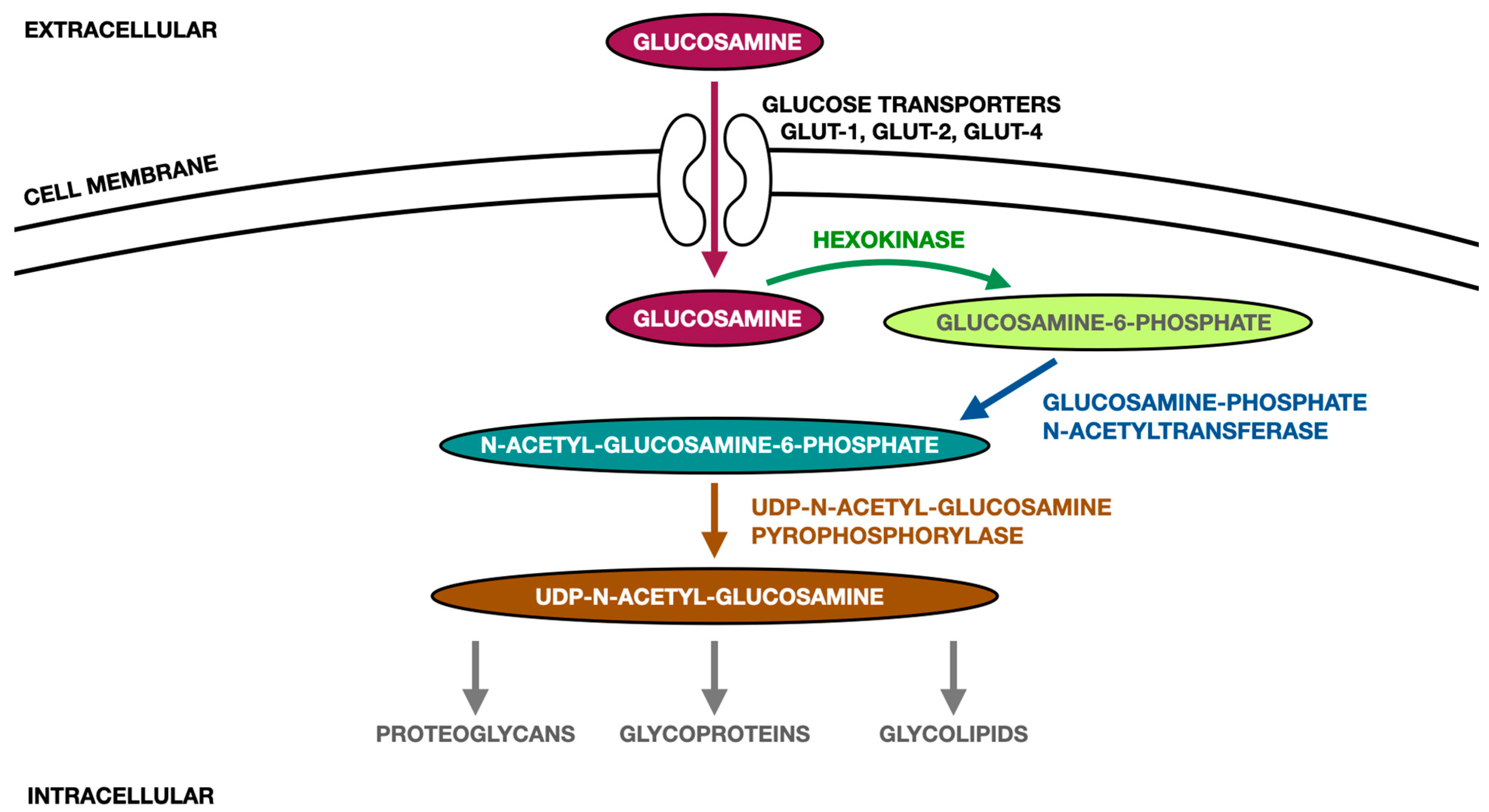
| Reference | Study Design | Participants and Intervention | Endpoint and Results |
|---|---|---|---|
| Thie et al. (2001) [24] | Randomized, double-blind study | 45 patients (40 women, 5 men) qualified for the study, 39 patients completed the study Diagnosis: degenerative joint disease in at least one of the TMJs Imaging of TMJs: CT Study groups: - glucosamine sulfate 500 mg tablets taken q8h (21 patients, mean age: 36.62 ± 10.3 years) - ibuprofen 400 mg tabelts taken q8h (18 patients, mean age: 38.73 ± 13.3 years) - all of the patients were also allowed to take acetaminophen 500 mg for pain breakthrough | Endpoint: 3 months (90 days) Both the ibuprofen and glucosamine led to a significant reduction in pain levels in patients with DJD. Patients who received glucosamine presented statistically significant improvement of functional pain evaluation and an overall pain interference compared to patients who received ibuprofen. |
| Haghighat et al. (2013) [25] | Randomized clinical trial | 60 patients (46 women, 14 men) Diagnosis: painful TMJ, TMJ crepitation, limitation of mouth opening Imaging of TMJs: none Study groups: - glucosamine sulfate 1500 mg daily for 90 days (30 patients, mean age: 26.60 ± 10.32 years) - ibuprofen 400 mg 2×/day for 90 days (30 patients, mean age: 27.12 ± 10.83 years) | Endpoint: 90 days Patients taking oral glucosamine presented significantly lower TMJ pain intensity during all follow-up appointments and significantly increased mandibular opening following the end of the second month of treatment. Oral glucosamine was recommended as a more effective and safer drug compared to ibuprofen for the treatment of TMJ OA. |
| Damlar et al. (2015) [26] | Randomized clinical trial | 34 patients (34 women, mean age: 28.6 ± 6.89 years) Diagnosis: TMJ internal derangement (Wilkes II or III) Imaging of TMJs: MRI Study groups: - study group: 1500 mg glucosamine sulfate and 1200 mg chondroitin sulfate per day (16 patients) - control group: 50 mg tramadol HCl 2× daily peroral for pain relief (15 patients) - all of the superior and inferior joint spaces were rinsed with 2 mL of saline solution (procedure repeated 10 times) | Endpoint: 8 weeks Both groups presented significantly reduced pain levels after the end of the treatment, whereas significantly increased maximum mouth opening was observed only in the study group. Having compared the changes that occurred within both of the examined groups, the study group presented a significantly decreased concentration of IL-1β and IL-6. |
| Nguyen et al. (2001) [27] | Randomized, double-blind study | 34 patients (30 women, 4 men) Diagnosis: cellulitis, disc displacement, disc dislocation, painful osteoarthritis of the TMJ Imaging of TMJs: no information Study groups: - the active medication group (CS-GH): 3 tablets twice a day for three months, containing glucosamine hydrochloride 250 mg and chondroitin sulfate 200 mg (14 patients, mean age 43 ± 14 years) - the inactive medication group (placebo): 3 tablets twice a day for three months, containing placebo (20 patients, mean age 46 ± 15 years) | Endpoint: 3 months Patients CS-GH presented decreased tenderness in the area of TMJs, decreased sounds within TMJs, and needed a decreased number of over-the-counter medications. Patients who received placebo, reported a significantly decreased pain within the TMJs after the end of the treatment. |
| Cahlin et al. (2011) [28] | Randomized, double-blind study | 59 patients (51 women, 8 men) Diagnosis: presence of osteoarthritis in at least one of the TMJs Imaging of TMJs: lack of precise information Study groups: - the glucosamine sulfate group (glucosmaine sulfate 400 mg): 3 capsules a day for six weeks (30 patients, mean age female: 61 ± 16 years, mean age male: 61 ± 9 years) - the placebo group (placebo 400 mg): 3 capsules a day for six weeks (29 patients, mean age female: 59 ± 8 years, mean age male: 49 ± 11 years) - all of the patients were given 15 tablets of paracetamol (at 1000 mg) as a rescue medication (patients could ask for more tablets if needed) | Endpoint: 6 weeks The effectiveness of oral glucosamine sulfate in TMJ pain reduction, as well as in reduction of TMJ osteoarthritis symptoms, was not better than the placebo. |
| Cen et al. (2018) [29] | Randomized, double-blind study | 136 patients (118 women, 18 men) Diagnosis: TMJ osteoarthritis Imaging of TMJs: CBCT Study groups: - the intervention group (GS + HA): 1 mL sodium HA injection into the superior and inferior spaces of TMJ, repeated in total 4 times (once a week for 4 following weeks) + 2 tablets of glucosamine hydrochloride 240 mg taken 3 times a day for 3 months (67 patients, mean age 40.1 ± 15.8 years) - the control group (placebo + HA): 1 mL sodium HA injection into the superior and inferior spaces of TMJ, repeated in total 4 times (once a week for 4 following weeks) + 2 tablets of placebo 240 mg taken 3 times a day for 3 months (69 patients, mean age 36.2 ± 15.8 years) - all of the superior joint spaces were rinsed with 2 mL of saline solution (3 times) prior to HA intra-articular injection | Endpoint: 1 year In the short-term (1 month) observation, both groups presented a statistically significant increase in maximum mouth opening, statistically significant pain reduction, and significantly decreased concetration of IL-1β and IL-6 (no differences between the groups aparat from the concentartion of IL-6, which was significantly lower in intervention group). After 1 year, patients who received oral glucosamine presented significantly increased maximum mouth opening, significantly decreased pain scores, significantly increased concentration of TGF-β and significantly decreased concentration of IL-1β and IL-6 compared to the control group. |
| Yang et al. (2018) [30] | Randomized, double-blind study | 144 patients (120 women, 24 men) Diagnosis: TMJ osteoarthritis Imaging of TMJs: CBCT Study groups: - group A: 4 intraraticular injections of 2 mL sodium hyaluronate 1×/week + 2 tablets of glucosamine hydrochloride 240 mg 3×/day for 3 months (72 patients, mean age: 40.1 ± 15.8 years) - group B: 4 intra-articular injection of 2 mL sodium hyaluronate 1×/week + placebo pills for 3 months (72 patients, mean age: 36.2 ± 15.8 years) - all of the superior and inferior joint spaces were rinsed prior to HA intra-articular injection - all of the patients received Diclofenac (50 mg) as a rescue medication | Endpoint: 1 year Oral glucosamine supplementation, in short term, had no extra effect on TMJ OA; whereas, in the long-term observations, complementary administration of oral glucosamine led to significant TMJ pain reduction and significant increase in maximum mouth opening. |
| Kılıç (2021) [31] | Randomized clinical trial | 26 patients (23 women, 3 men) Diagnosis: TMJ osteoarthritis Imaging of TMJs: CBCT Study groups: - group 1 (control): single-session arthrocentesis + intra-articular injection of HA (14 patients, mean age: 28.71 ± 10.94 years) - group 2 (study): single-session arthrocentesis + intra-articular injection of HA + 3 months of supplementation of 750 mg glucosamine hydrochloride, 600 mg chondroitin sulfate, and 350 mg methylsulfonylmethane (2 × 1 dosage daily) (12 patients, mean age: 27.92 ± 11.20 years) | Endpoint: 1 year The results obtained in both groups were similar. Both methods of treatment presented a similar effectiveness. |
| Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | |
|---|---|---|---|---|---|---|---|
| Thie et al. (2001) [24] | + | + | + | + | + | + | ? |
| Haghighat et al.(2013) [25] | ? | ? | – | – | + | + | + |
| Damlar et al. (2015) [26] | ? | + | + | + | + | + | + |
| Nguyen et al. (2001) [27] | + | + | + | ? | - | + | + |
| Cahlin et al. (2011) [28] | + | + | + | + | + | + | + |
| Cen et al. (2018) [29] | + | + | + | + | + | + | + |
| Yang et al. (2018) [30] | + | + | + | + | + | + | + |
| Kılıç (2021) [31] | + | ? | – | + | + | + | + |
| Variables | Glucosamine Sulfate (GS) | Glucosamine Hydrochloride (GH) | References |
|---|---|---|---|
| Molecular structure | C6H15NO9S | C6H14ClNO5 | [36,37] |
| Molecular weight [unit] | 277.25 | 215.63 | [36,37] |
| Stability and purity | Needs compound stabilizers in the form of salts, usually potassium chloride (KCl) or sodium chloride (NaCl). It has a 74% purity. | Does not need compound stabilizers. It has a 99% purity. | [38,39] |
| Pharmacokinetic parameters when administrated 1500 mg once daily steady-state | Cmax (mean) 1602 ± 425 ng/mL 8.9 ± 2.4 μmol/L T 1/2 (h) 15 | Cmax (mean) 492 ± 161 ng/mL 2.7 ± 0.9 μmol/L T 1/2 (h) 2.51 ± 1.84 | [38,39] |
| Expression of catabolic and anabolic genes | Stronger inhibition | Weaker inhibition | [40] |
| Antioxidant potential | Pronounced reducing power, superoxide/hydroxyl radical scavenging ability, quite weak ferrous ion chelating effect. | Considerable reducing power, superoxide/hydroxyl radical scavenging ability, limited ferrous ion chelating potency. | [41,42] |
| Exemplary Medicinal Product (ex. Brand Name) | Oral Dosage (Only for Adults) | Maximum Daily Dose | Additional Information |
|---|---|---|---|
| Glucosamine sulfate (Arthryl) | 1500 mg once a day | 1500 mg | Glucosamine is not used in children and adolescents under 18 years of age and should not be taken during pregnancy and breast-feeding. Glucosamine can be combined with NSAIDs and analgesics in case of the exacerbation of the symptoms. |
| Glucosamine hydrochloride (Flexove) | 625 mg × 2 once a day | 1250 mg | Glucosamine is not used in children and adolescents under 18 years of age and should not be taken during pregnancy and breast-feeding. It may increase the effect coumarin anticoagulants (i.e., warfarin). |
| Chondroitin sulfate sodium and Glucosamine hydrochloride (Chronada) | 2 capsules 3 times a day 1 capsule contains: 200 mg Chondrotine sulfate + 250 mg Glucosamine hydrochloride | 1200 mg CS + 1500 mg GH | Chronada is not used in children and adolescents under 18 years of age and should not be taken during pregnancy and breast-feeding. It may increase the effect coumarin anticoagulants (i.e., warfarin) or antibiotics (i.e., tetracyclines). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derwich, M.; Górski, B.; Amm, E.; Pawłowska, E. Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4925. https://doi.org/10.3390/ijms24054925
Derwich M, Górski B, Amm E, Pawłowska E. Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(5):4925. https://doi.org/10.3390/ijms24054925
Chicago/Turabian StyleDerwich, Marcin, Bartłomiej Górski, Elie Amm, and Elżbieta Pawłowska. 2023. "Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review" International Journal of Molecular Sciences 24, no. 5: 4925. https://doi.org/10.3390/ijms24054925
APA StyleDerwich, M., Górski, B., Amm, E., & Pawłowska, E. (2023). Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. International Journal of Molecular Sciences, 24(5), 4925. https://doi.org/10.3390/ijms24054925







