Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release
Abstract
1. Introduction
2. Results
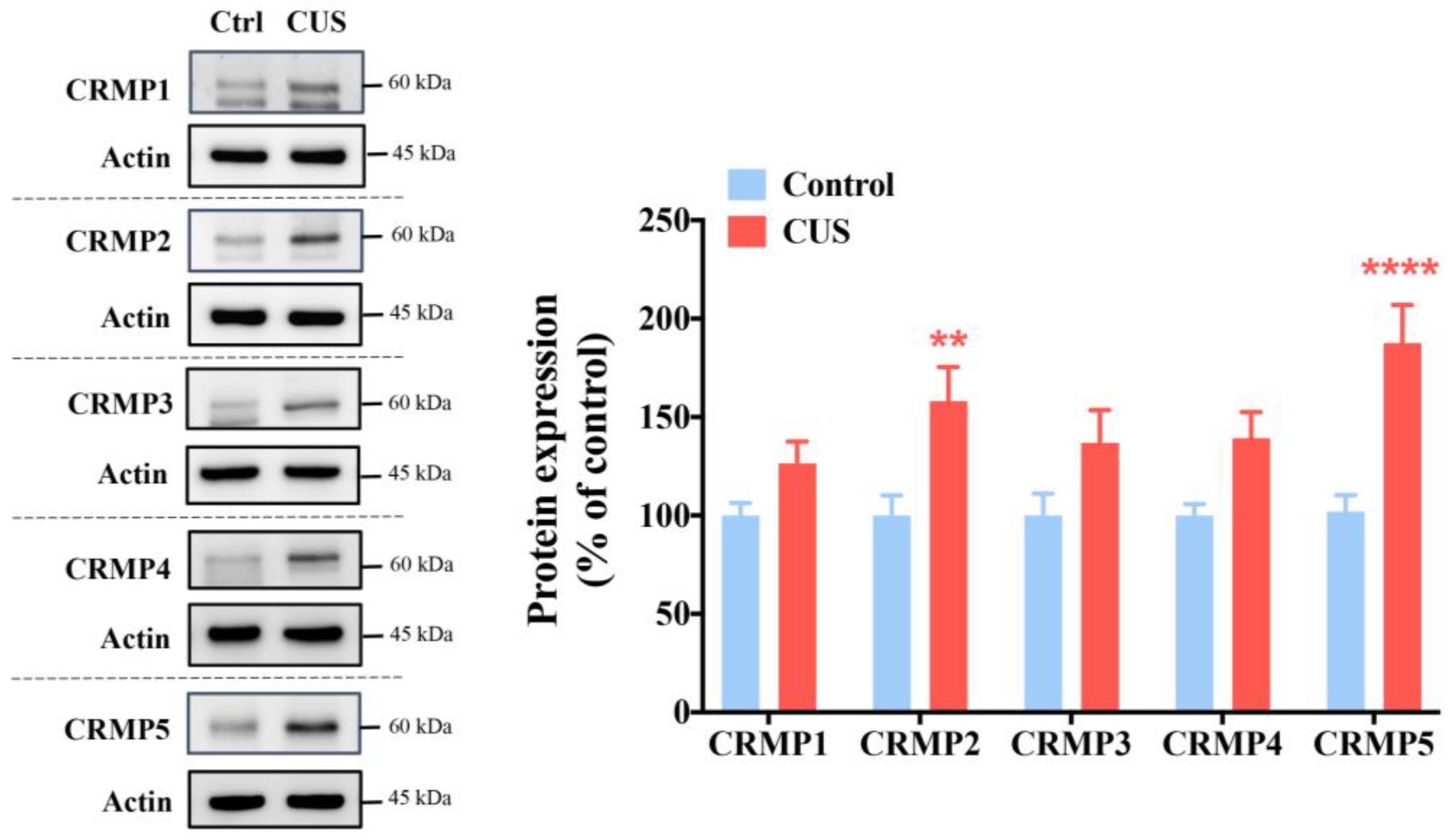
2.1. CRMPs Are Significantly Increased in CUS-Exposed Mice, Especially CRMP2 and CRMP5
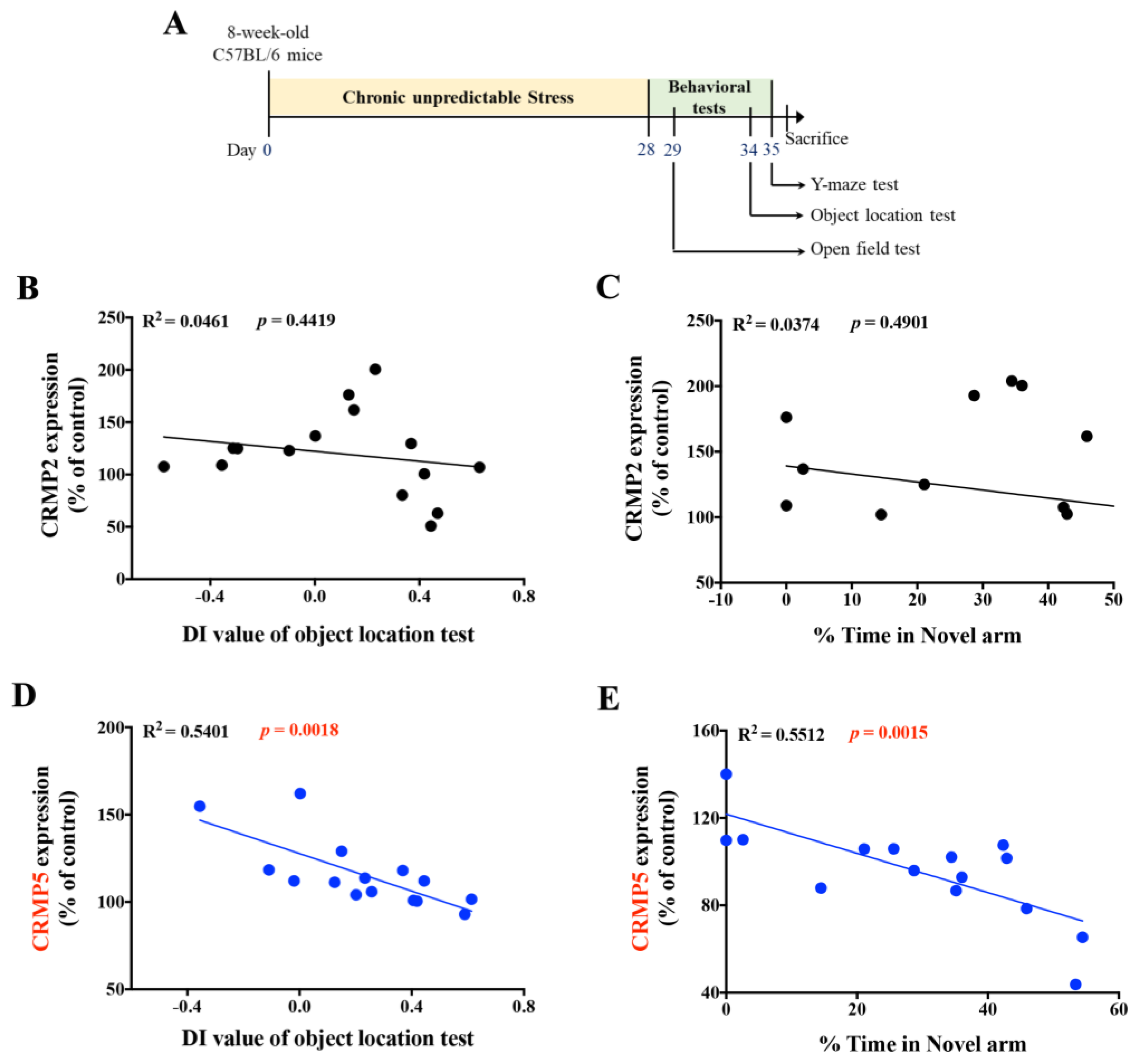
2.2. Hippocampal CRMP5 Levels Are Inversely Correlated with Memory Performance in Mice
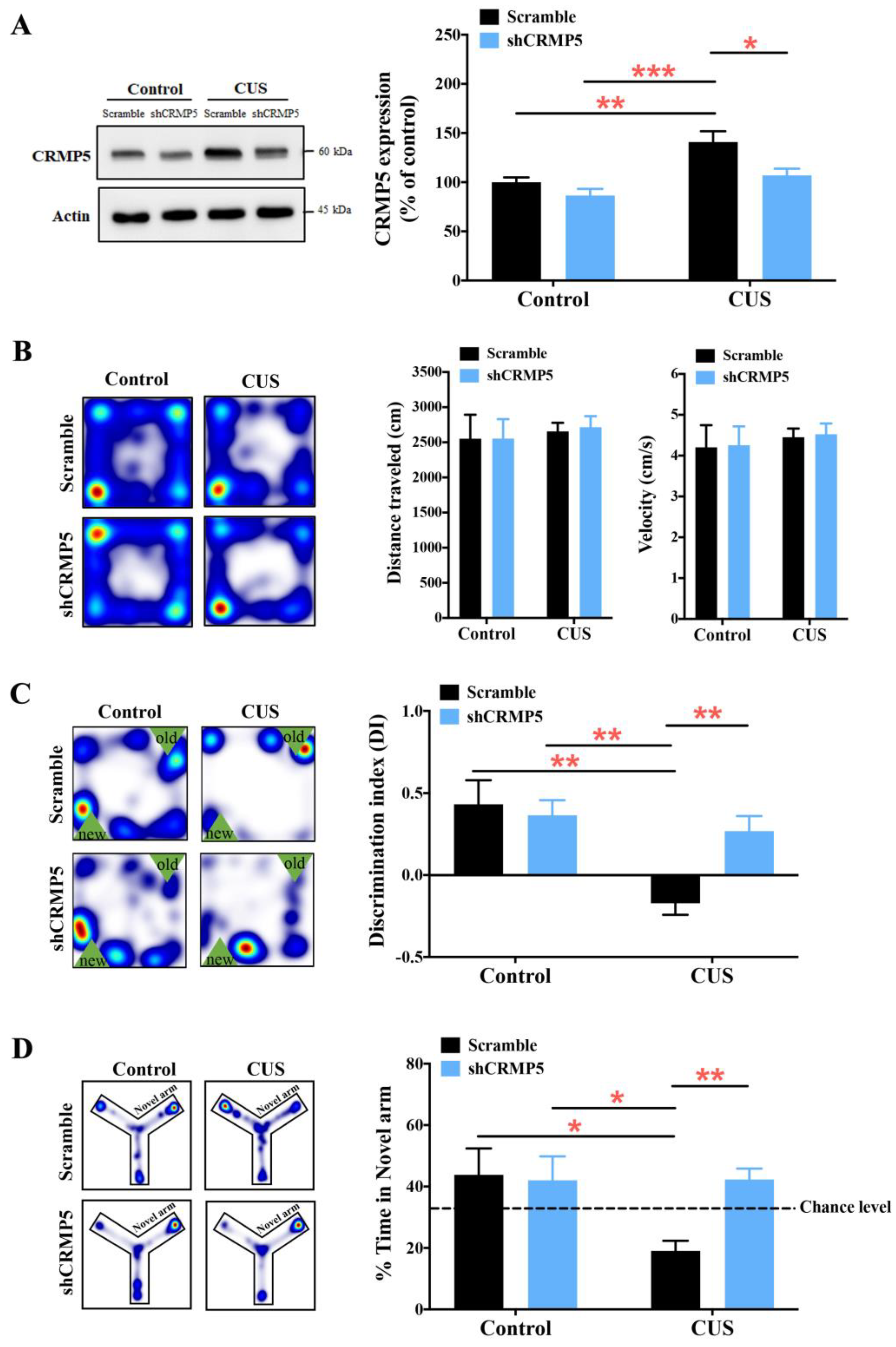
2.3. Decreased Hippocampal CRMP5 Expression, but Not CRMP2 Expression, Ameliorates CUS-Triggered Memory Impairment
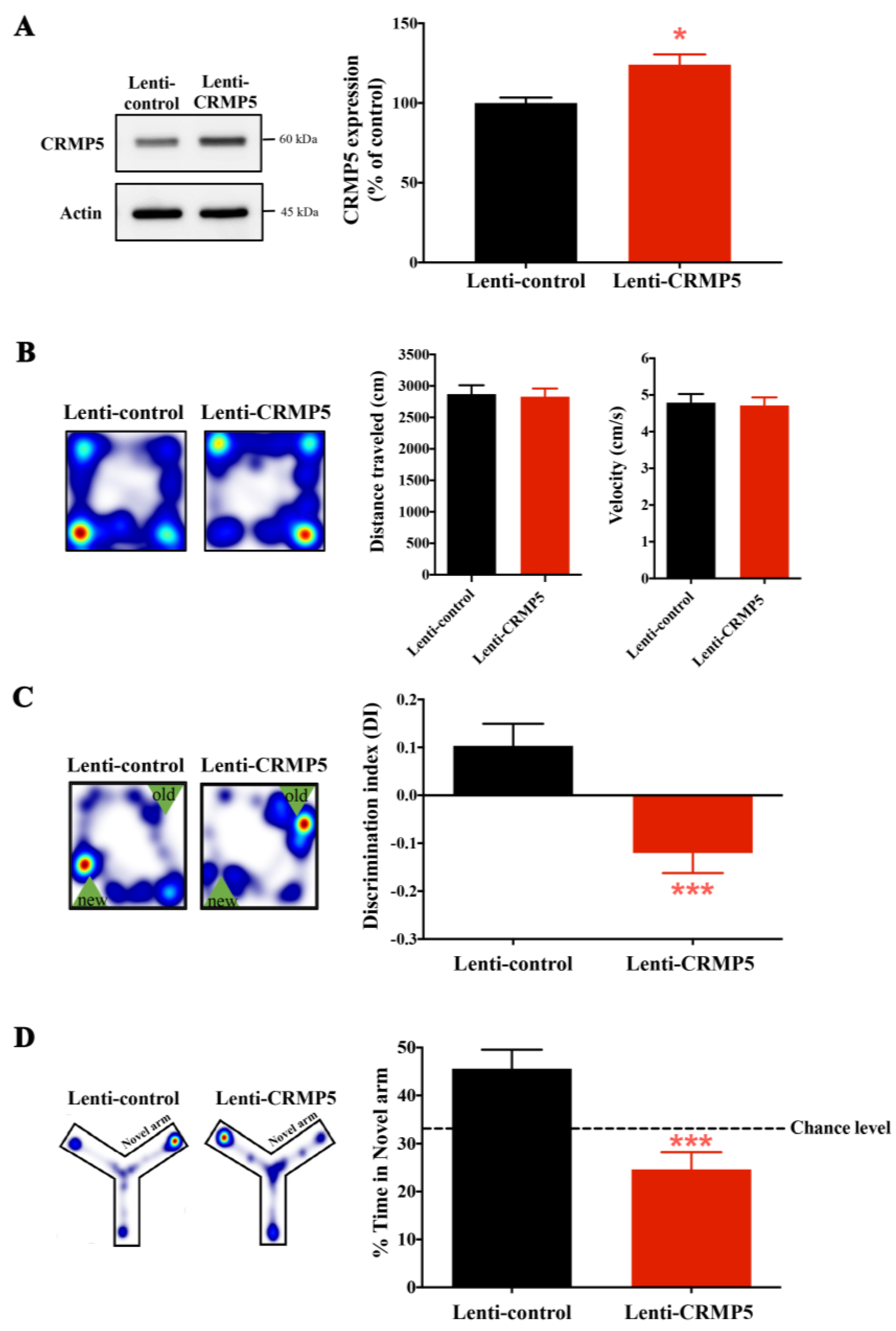
2.4. Enhanced CRMP5 Expression Impairs Memory Performance in Control Nonstressed Mice
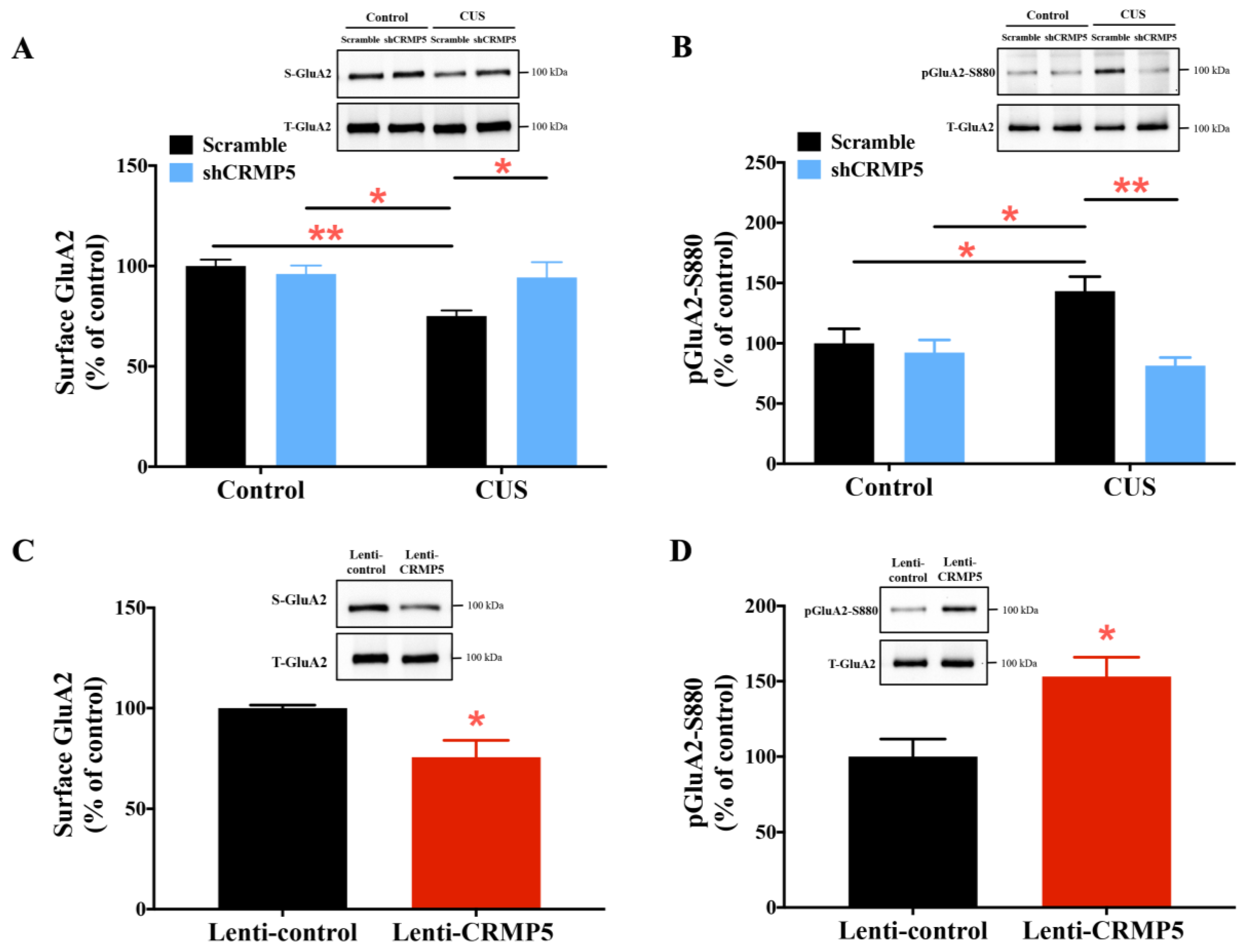
2.5. Inhibiting Hippocampal CRMP5 Alleviates Chronic Stress-Induced Dendritic Atrophy and Spine Loss
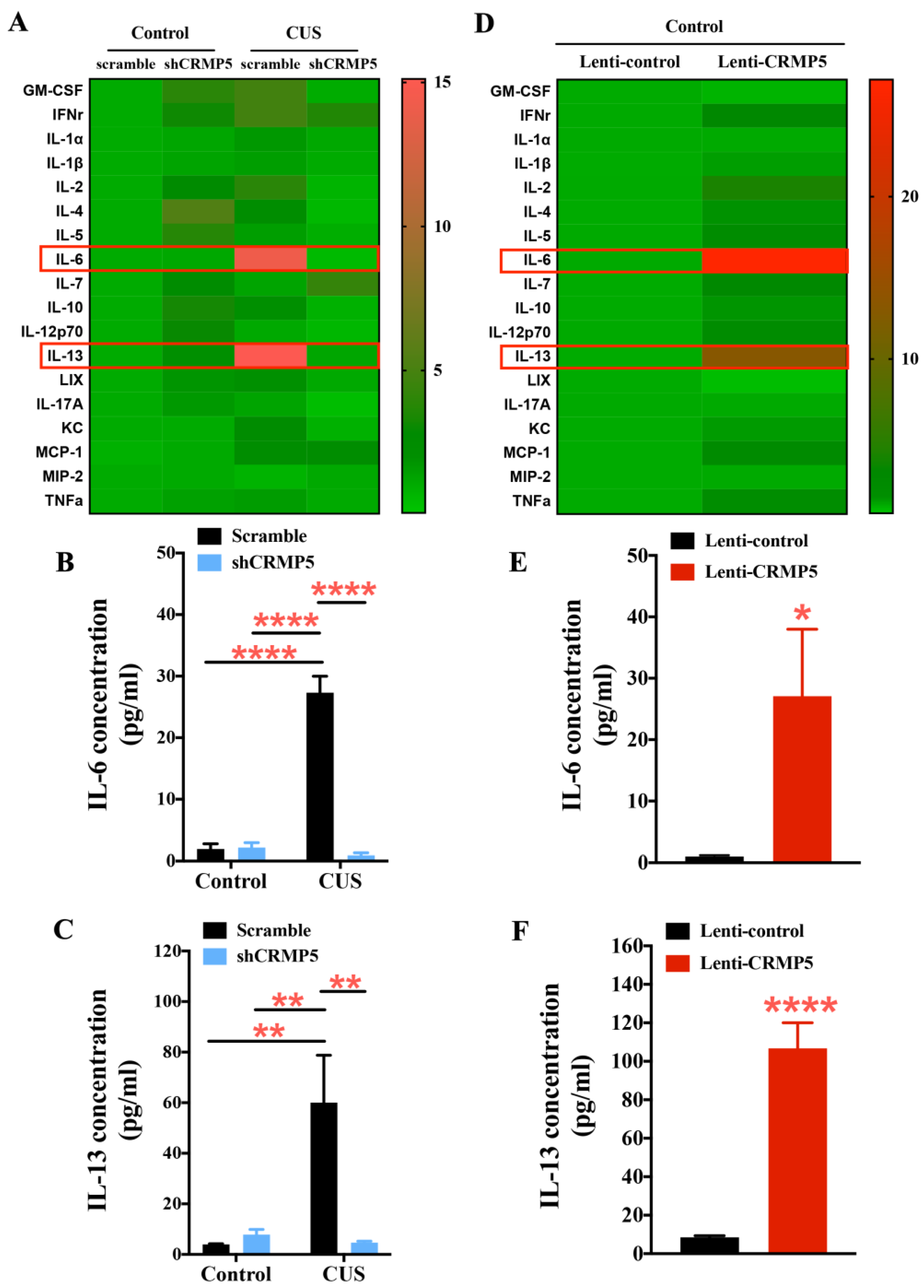
2.6. Hippocampal CRMP5 Suppression Reverses Chronic Stress Triggering the Inflammatory Response
2.7. Chronic Stress Enhances the Phosphorylation of Glucocorticoid Receptors and Increases CRMP5 Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chronic Unpredictable Stress (CUS)
4.3. Subthreshold Social Defeat Stress
4.4. Open Field Test
4.5. Object Location Test
4.6. Y-Maze Test
4.7. Western Blot Analysis
4.8. Golgi–Cox Staining
4.9. Stereotaxic Intrahippocampal Lentivirus Injection
4.10. Multiplex Cytokine Assay
4.11. Chromatin Immunoprecipitation (ChIP) Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Salleh, M.R. Life event, stress and illness. Malays. J. Med. Sci. 2008, 15, 9–18. [Google Scholar]
- De Sio, S.; Buomprisco, G.; Perri, R.; Bruno, G.; Mucci, N.; Nieto, H.A.; Trovato Battagliola, E.; Cedrone, F. Work-related stress risk and preventive measures of mental disorders in the medical environment: An umbrella review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 821–830. [Google Scholar]
- Cahill, L.; Gorski, L.; Le, K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learn. Mem. 2003, 10, 270–274. [Google Scholar] [CrossRef]
- Roozendaal, B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol. Learn. Mem. 2002, 78, 578–595. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, Fso23. [Google Scholar] [CrossRef]
- Keeney, A.; Jessop, D.S.; Harbuz, M.S.; Marsden, C.A.; Hogg, S.; Blackburn-Munro, R.E. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J. Neuroendocrinol. 2006, 18, 330–338. [Google Scholar] [CrossRef]
- Wada, S.; Yanagida, J.; Sasase, H.; Zhang, T.; Li, X.; Kamii, H.; Domoto, M.; Deyama, S.; Hinoi, E.; Yamanaka, A.; et al. Acute restraint stress augments the rewarding memory of cocaine through activation of alpha1 adrenoceptors in the medial prefrontal cortex of mice. Neuropharmacology 2020, 166, 107968. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Y.; Wang, Y.; Liu, L.; Zhang, X.; Li, B.; Cui, R. The Effects of Psychological Stress on Depression. Curr. Neuropharmacol. 2015, 13, 494–504. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Della-Pace, I.D.; de Oliveira Ferreira, A.P.; Funck, V.R.; Pinton, S.; Bobinski, F.; de Oliveira, C.V.; da Silva Fiorin, F.; Duarte, M.M.; Furian, A.F.; et al. Chronic administration of methylmalonate on young rats alters neuroinflammatory markers and spatial memory. Immunobiology 2013, 218, 1175–1183. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Xu, L. How could stress lead to major depressive disorder? IBRO Rep. 2018, 4, 38–43. [Google Scholar] [CrossRef]
- Anand, K.S.; Dhikav, V. Hippocampus in health and disease: An overview. Ann. Indian Acad. Neurol. 2012, 15, 239–246. [Google Scholar]
- Ben-Yakov, A.; Dudai, Y. Constructing realistic engrams: Poststimulus activity of hippocampus and dorsal striatum predicts subsequent episodic memory. J. Neurosci. 2011, 31, 9032–9042. [Google Scholar] [CrossRef]
- Immordino-Yang, M.H.; Singh, V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013, 34, 945–955. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, H.; Tong, L.; Li, Z.; Wang, L.; Zhang, C.; Yang, Q.; Yan, B. Emotion Regulation of Hippocampus Using Real-Time fMRI Neurofeedback in Healthy Human. Front. Hum. Neurosci. 2019, 13, 242. [Google Scholar] [CrossRef]
- Tomar, A.; Polygalov, D.; McHugh, T.J. Differential Impact of Acute and Chronic Stress on CA1 Spatial Coding and Gamma Oscillations. Front. Behav. Neurosci. 2021, 15, 710725. [Google Scholar] [CrossRef]
- Kim, E.J.; Pellman, B.; Kim, J.J. Stress effects on the hippocampus: A critical review. Learn. Mem. 2015, 22, 411–416. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef]
- Goshima, Y.; Nakamura, F.; Strittmatter, P.; Strittmatter, S.M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 1995, 376, 509–514. [Google Scholar] [CrossRef]
- Charrier, E.; Reibel, S.; Rogemond, V.; Aguera, M.; Thomasset, N.; Honnorat, J. Collapsin response mediator proteins (CRMPs): Involvement in nervous system development and adult neurodegenerative disorders. Mol. Neurobiol. 2003, 28, 51–64. [Google Scholar] [CrossRef]
- Fukata, Y.; Itoh, T.J.; Kimura, T.; Ménager, C.; Nishimura, T.; Shiromizu, T.; Watanabe, H.; Inagaki, N.; Iwamatsu, A.; Hotani, H.; et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 2002, 4, 583–591. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohshima, T.; Yamashita, N.; Ogawara, M.; Sasaki, Y.; Nakamura, F.; Goshima, Y. Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. J. Biol. Chem. 2009, 284, 27393–27401. [Google Scholar] [CrossRef]
- Quach, T.T.; Honnorat, J.; Kolattukudy, P.E.; Khanna, R.; Duchemin, A.M. CRMPs: Critical molecules for neurite morphogenesis and neuropsychiatric diseases. Mol. Psychiatry 2015, 20, 1037–1045. [Google Scholar] [CrossRef]
- Quach, T.T.; Moutal, A.; Khanna, R.; Deems, N.P.; Duchemin, A.M.; Barrientos, R.M. Collapsin Response Mediator Proteins: Novel Targets for Alzheimer’s Disease. J. Alzheimers Dis. 2020, 77, 949–960. [Google Scholar] [CrossRef]
- Leung, T.; Ng, Y.; Cheong, A.; Ng, C.H.; Tan, I.; Hall, C.; Lim, L. p80 ROKalpha binding protein is a novel splice variant of CRMP-1 which associates with CRMP-2 and modulates RhoA-induced neuronal morphology. FEBS Lett. 2002, 532, 445–449. [Google Scholar] [CrossRef]
- Nakamura, F.; Ohshima, T.; Goshima, Y. Collapsin Response Mediator Proteins: Their Biological Functions and Pathophysiology in Neuronal Development and Regeneration. Front. Cell Neurosci. 2020, 14, 188. [Google Scholar] [CrossRef]
- Kawano, Y.; Yoshimura, T.; Tsuboi, D.; Kawabata, S.; Kaneko-Kawano, T.; Shirataki, H.; Takenawa, T.; Kaibuchi, K. CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol. Cell Biol. 2005, 25, 9920–9935. [Google Scholar] [CrossRef]
- Rosslenbroich, V.; Dai, L.; Baader, S.L.; Noegel, A.A.; Gieselmann, V.; Kappler, J. Collapsin response mediator protein-4 regulates F-actin bundling. Exp. Cell Res. 2005, 310, 434–444. [Google Scholar] [CrossRef]
- Hotta, A.; Inatome, R.; Yuasa-Kawada, J.; Qin, Q.; Yamamura, H.; Yanagi, S. Critical role of collapsin response mediator protein-associated molecule CRAM for filopodia and growth cone development in neurons. Mol. Biol. Cell 2005, 16, 32–39. [Google Scholar] [CrossRef]
- Inatome, R.; Tsujimura, T.; Hitomi, T.; Mitsui, N.; Hermann, P.; Kuroda, S.; Yamamura, H.; Yanagi, S. Identification of CRAM, a novel unc-33 gene family protein that associates with CRMP3 and protein-tyrosine kinase(s) in the developing rat brain. J. Biol. Chem. 2000, 275, 27291–27302. [Google Scholar] [CrossRef]
- Brot, S.; Rogemond, V.; Perrot, V.; Chounlamountri, N.; Auger, C.; Honnorat, J.; Moradi-Améli, M. CRMP5 interacts with tubulin to inhibit neurite outgrowth, thereby modulating the function of CRMP2. J. Neurosci. 2010, 30, 10639–10654. [Google Scholar] [CrossRef]
- Yamashita, N.; Mosinger, B.; Roy, A.; Miyazaki, M.; Ugajin, K.; Nakamura, F.; Sasaki, Y.; Yamaguchi, K.; Kolattukudy, P.; Goshima, Y. CRMP5 (collapsin response mediator protein 5) regulates dendritic development and synaptic plasticity in the cerebellar Purkinje cells. J. Neurosci. 2011, 31, 1773–1779. [Google Scholar] [CrossRef]
- Tada, S.; Furuta, M.; Fukada, K.; Hirozawa, D.; Matsui, M.; Aoike, F.; Okuno, T.; Sawada, J.; Mochizuki, H.; Hazama, T. Severe parkinsonism associated with anti-CRMP5 antibody-positive paraneoplastic neurological syndrome and abnormal signal intensity in the bilateral basal ganglia. J. Neurol. Neurosurg Psychiatry 2016, 87, 907–910. [Google Scholar] [CrossRef]
- Yap, S.M.; Lynch, T.; MacMahon, P.; Murray, B. Paraneoplastic Atypical Parkinsonism with Anti-CRMP5 Antibodies and Severe Caudate and Putaminal Hypometabolism on 18-Fluorodeoxyglucose Positron Emission Tomography of the Brain. Mov. Disord Clin. Pract. 2017, 4, 263–265. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, Y.F.; Chen, K.C.; Yang, Y.K.; Hsiao, Y.H. Collapsin response mediator protein 5 (CRMP5) causes social deficits and accelerates memory loss in an animal model of Alzheimer’s disease. Neuropharmacology 2019, 157, 107673. [Google Scholar] [CrossRef]
- Lin, Y.F.; Wang, L.Y.; Chen, C.S.; Li, C.C.; Hsiao, Y.H. Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress. Neurobiol. Stress 2021, 15, 100341. [Google Scholar] [CrossRef]
- Khanna, R.; Wilson, S.M.; Brittain, J.M.; Weimer, J.; Sultana, R.; Butterfield, A.; Hensley, K. Opening Pandora’s jar: A primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol. 2012, 7, 749–771. [Google Scholar] [CrossRef]
- Bader, V.; Tomppo, L.; Trossbach, S.V.; Bradshaw, N.J.; Prikulis, I.; Leliveld, S.R.; Lin, C.Y.; Ishizuka, K.; Sawa, A.; Ramos, A.; et al. Proteomic, genomic and translational approaches identify CRMP1 for a role in schizophrenia and its underlying traits. Hum. Mol. Genet. 2012, 21, 4406–4418. [Google Scholar] [CrossRef]
- Tabares-Seisdedos, R.; Rubenstein, J.L. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: Implications for schizophrenia, autism and cancer. Mol. Psychiatry 2009, 14, 563–589. [Google Scholar] [CrossRef]
- Fallin, M.D.; Lasseter, V.K.; Avramopoulos, D.; Nicodemus, K.K.; Wolyniec, P.S.; McGrath, J.A.; Steel, G.; Nestadt, G.; Liang, K.Y.; Huganir, R.L.; et al. Bipolar I disorder and schizophrenia: A 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am. J. Hum. Genet. 2005, 77, 918–936. [Google Scholar] [CrossRef]
- Yang, G.; Pan, F.; Gan, W.B. Stably maintained dendritic spines are associated with lifelong memories. Nature 2009, 462, 920–924. [Google Scholar] [CrossRef]
- Frankfurt, M.; Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm. Behav. 2015, 74, 28–36. [Google Scholar] [CrossRef]
- Asok, A.; Leroy, F.; Rayman, J.B.; Kandel, E.R. Molecular Mechanisms of the Memory Trace. Trends Neurosci. 2019, 42, 14–22. [Google Scholar] [CrossRef]
- Henley, J.M.; Wilkinson, K.A. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin. Neurosci. 2013, 15, 11–27. [Google Scholar] [CrossRef]
- Anggono, V.; Huganir, R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012, 22, 461–469. [Google Scholar] [CrossRef]
- Bassani, S.; Folci, A.; Zapata, J.; Passafaro, M. AMPAR trafficking in synapse maturation and plasticity. Cell Mol. Life Sci. 2013, 70, 4411–4430. [Google Scholar] [CrossRef]
- Seidenman, K.J.; Steinberg, J.P.; Huganir, R.; Malinow, R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J. Neurosci. 2003, 23, 9220–9228. [Google Scholar] [CrossRef]
- Diering, G.H.; Huganir, R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron 2018, 100, 314–329. [Google Scholar] [CrossRef]
- Kino, T.; Ichijo, T.; Amin, N.D.; Kesavapany, S.; Wang, Y.; Kim, N.; Rao, S.; Player, A.; Zheng, Y.L.; Garabedian, M.J.; et al. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: Clinical implications for the nervous system response to glucocorticoids and stress. Mol. Endocrinol. (Baltimore Md.) 2007, 21, 1552–1568. [Google Scholar] [CrossRef]
- Davis, M.T.; Holmes, S.E.; Pietrzak, R.H.; Esterlis, I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress 2017, 1, 2470547017710916. [Google Scholar] [CrossRef] [PubMed]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 2015, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.F.; Lord, C.; Andrews, J.; Juster, R.P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem 2011, 96, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D. A critical review of chronic stress effects on spatial learning and memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 742–755. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.J.; Gomez, J.L.; Baran, S.E.; Conrad, C.D. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res. 2007, 1161, 56–64. [Google Scholar] [CrossRef]
- Lupien, S.J.; Gaudreau, S.; Tchiteya, B.M.; Maheu, F.; Sharma, S.; Nair, N.P.; Hauger, R.L.; McEwen, B.S.; Meaney, M.J. Stress-induced declarative memory impairment in healthy elderly subjects: Relationship to cortisol reactivity. J. Clin. Endocrinol. Metab. 1997, 82, 2070–2075. [Google Scholar] [CrossRef]
- Peavy, G.M.; Salmon, D.P.; Jacobson, M.W.; Hervey, A.; Gamst, A.C.; Wolfson, T.; Patterson, T.L.; Goldman, S.; Mills, P.J.; Khandrika, S.; et al. Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am. J. Psychiatry 2009, 166, 1384–1391. [Google Scholar] [CrossRef]
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic. Neurosci. 2020, 12, 36. [Google Scholar] [CrossRef]
- Dong, Z.; Han, H.; Li, H.; Bai, Y.; Wang, W.; Tu, M.; Peng, Y.; Zhou, L.; He, W.; Wu, X.; et al. Long-term potentiation decay and memory loss are mediated by AMPAR endocytosis. J. Clin. Investig. 2015, 125, 234–247. [Google Scholar] [CrossRef]
- Glanzman, D.L. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr. Biol. 2010, 20, R31–R36. [Google Scholar] [CrossRef]
- Jung, Y.H.; Hong, S.I.; Ma, S.X.; Hwang, J.Y.; Kim, J.S.; Lee, J.H.; Seo, J.Y.; Lee, S.Y.; Jang, C.G. Strain differences in the chronic mild stress animal model of depression and anxiety in mice. Biomol. Ther. 2014, 22, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 2005, 52, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Chen, K.C.; Yang, Y.K.; Hsiao, Y.H. Collapsin response mediator protein 5 (CRMP5) modulates susceptibility to chronic social defeat stress in mice. Mol. Neurobiol. 2021, 58, 3175–3186. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.F.; Li, X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Gawali, N.B.; Bulani, V.D.; Gursahani, M.S.; Deshpande, P.S.; Kothavade, P.S.; Juvekar, A.R. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res. 2017, 1663, 66–77. [Google Scholar] [CrossRef]
- Tatomir, A.; Micu, C.; Crivii, C. The impact of stress and glucocorticoids on memory. Clujul. Med. 2014, 87, 3–6. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef]
- Abraham, Y.; Gerrits, B.; Ludwig, M.G.; Rebhan, M.; Gubser Keller, C. Exploring Glucocorticoid Receptor Agonists Mechanism of Action Through Mass Cytometry and Radial Visualizations. Cytometry B Clin. Cytom. 2017, 92, 42–56. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid effects on cell signalling. Eur. Respir J. 2006, 27, 413–426. [Google Scholar] [CrossRef]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Hou, G.; Li, D.; Yuan, T.F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci. World J. 2014, 2014, 780616. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhuang, K.; Liu, Z.; Huang, C.; Gao, Y.; Chen, G.; Lin, H.; Hu, Y.; Wu, D.; Shi, M.; et al. Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation. Neuron 2018, 100, 551–563.e7. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-F.; Chen, C.-A.; Hsu, F.-Y.; Hsiao, Y.-H. Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release. Int. J. Mol. Sci. 2023, 24, 4898. https://doi.org/10.3390/ijms24054898
Lin Y-F, Chen C-A, Hsu F-Y, Hsiao Y-H. Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release. International Journal of Molecular Sciences. 2023; 24(5):4898. https://doi.org/10.3390/ijms24054898
Chicago/Turabian StyleLin, Yu-Fen, Ching-An Chen, Fang-Yu Hsu, and Ya-Hsin Hsiao. 2023. "Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release" International Journal of Molecular Sciences 24, no. 5: 4898. https://doi.org/10.3390/ijms24054898
APA StyleLin, Y.-F., Chen, C.-A., Hsu, F.-Y., & Hsiao, Y.-H. (2023). Elevated Hippocampal CRMP5 Mediates Chronic Stress-Induced Cognitive Deficits by Disrupting Synaptic Plasticity, Hindering AMPAR Trafficking, and Triggering Cytokine Release. International Journal of Molecular Sciences, 24(5), 4898. https://doi.org/10.3390/ijms24054898





