IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression
Abstract
1. Introduction
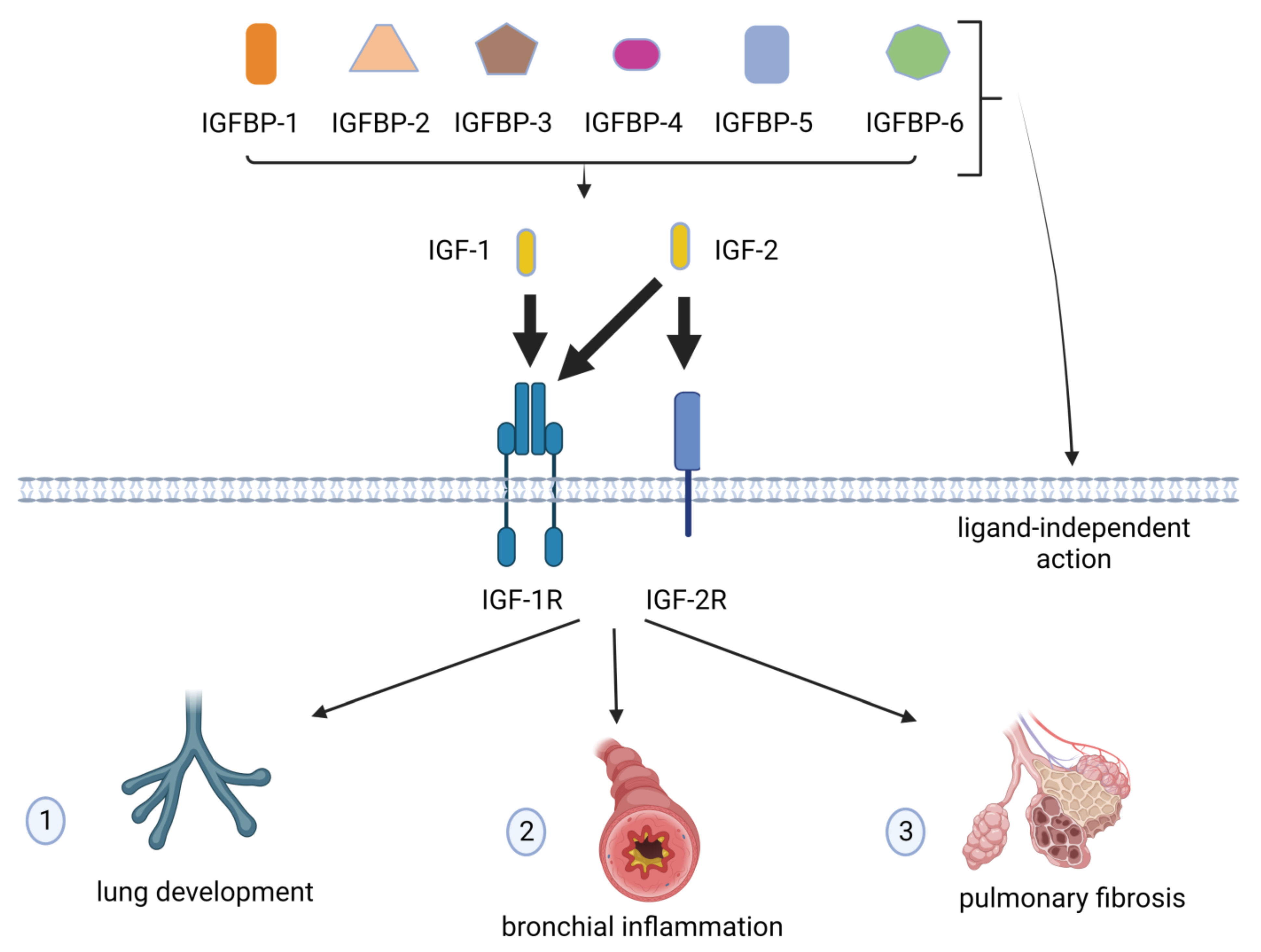
2. IGFs, IGFRs, and IGFBPs
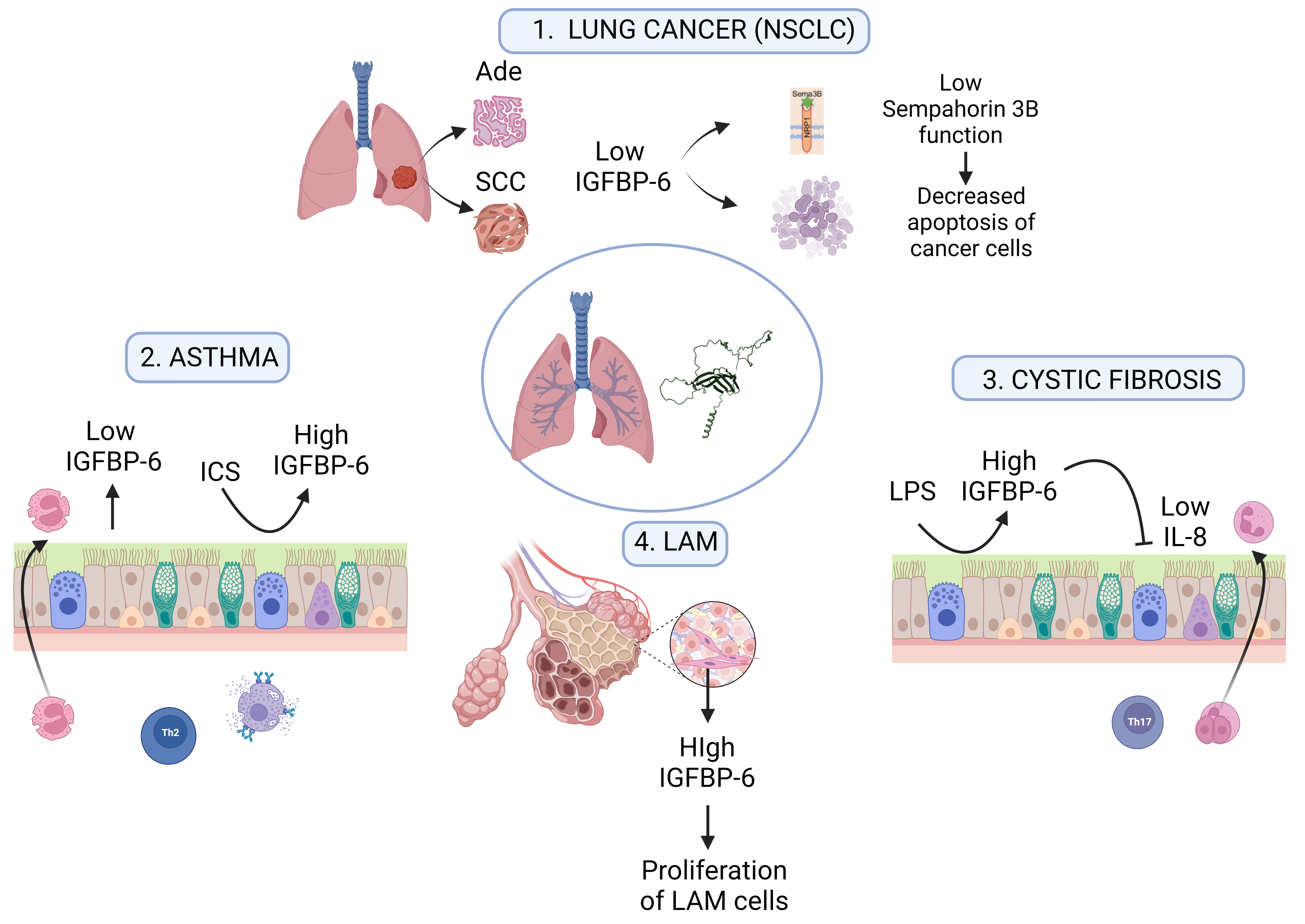
3. IGFBP-6 Expression in the Lung
4. IGFBP-6, Fibrosis and Respiratory Diseases
| Diseases | IGFBP-6 Function | IGFBP-6 Expression/Levels | Ref. |
|---|---|---|---|
| Asthma | Asthma pathophysiology, cell growth, and proliferation | Differentially expressed in two microarrays studies using bronchial tissues of asthmatic and control subjects | [12] |
| Bronchial tissue inflammation | Pathophysiologic process active in the asthmatic lung | Significantly upregulated (4.03-fold change; p = 0.001) in bronchial tissues obtained from asthmatic patients after inhaled corticosteroids | [48] |
| Allergic airway inflammation | Suppression of allergic airway inflammation | Significantly increased in asthmatic mice (1.52-fold change) after anti-allergic treatment | [13] |
| Acute mountain sickness (AMS) | Predicting AMS susceptibility | Significantly lower in AMS-susceptible individuals (37,318.99 ± 23,493.11 pg/mL and 25,665.38 ± 25,691.29 pg/mL, respectively; p = 0.04). | [49] |
| Lymphangioleiomyomatosis (LAM) | Proliferation of LAM cells, associated with of spindle-shaped LAM cells | Upregulated (1.82, biopsies; 1.57, explants; p < 0.05) in patients compared to controls. | [9,50] |
| Cystic fibrosis (CF) | Mediator of airway inflammation | IGFBP-6 mRNA and protein levels are both upregulated in bronchial epithelial F508del-CFTR CFBE cells lines and primary nasal epithelial cells (HNE) from three CF patients bearing the most common CF-causing mutation (F508del) | [14] |
4.1. IGFBP-6 in Asthma Progression
4.2. Idiopathic Pulmonary Fibrosis (IPF) and Lymphangioleiomyomatosis (LAM)
4.3. Cystic Fibrosis (CF)
5. IGFBP-6 Controls the Proliferation of Normal and Neoplastic Epithelial Cells
5.1. IGFBP-6 Role in the Proliferation of Epithelial Cells
5.2. IGFBP-6 Role in Lung Cancer
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- van de Wetering, J.K.; Elfring, R.H.; Oosterlaken-Dijksterhuis, M.A.; Mol, J.A.; Haagsman, H.P.; Batenburg, J.J. Perinatal expression of IGFBPs in rat lung and its hormonal regulation in fetal lung explants. Am. J. Physiol. 1997, 273, L1174–L1181. [Google Scholar] [CrossRef] [PubMed]
- Retsch-Bogart, G.Z.; Moats-Staats, B.M.; Howard, K.; D’Ercole, A.J.; Stiles, A.D. Cellular localization of messenger RNAs for insulin-like growth factors (IGFs), their receptors and binding proteins during fetal rat lung development. Am. J. Respir. Cell Mol. Biol. 1996, 14, 61–69. [Google Scholar] [CrossRef]
- Melnick, M.; Chen, H.; Rich, K.A.; Jaskoll, T. Developmental expression of insulin-like growth factor II receptor (IGF-IIR) in congenic mouse embryonic lungs: Correlation between IGF-IIR mRNA and protein levels and heterochronic lung development. Mol. Reprod. Dev. 1996, 44, 159–170. [Google Scholar] [CrossRef]
- Moats-Staats, B.M.; Price, W.A.; Xu, L.; Jarvis, H.W.; Stiles, A.D. Regulation of the insulin-like growth factor system during normal rat lung development. Am. J. Respir. Cell. Mol. Biol. 1995, 12, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.C.; Matsui, K.; Bondy, C.; Zhou, J.; Rasmussen, A.; Cullen, K.; Yu, Z.X.; Moss, J.; Ferrans, V.J. Distribution and mRNA expression of insulin-like growth factor system in pulmonary lymphangioleiomyomatosis. J. Investig. Med. 2001, 49, 421–433. [Google Scholar] [CrossRef]
- Sueoka, N.; Lee, H.Y.; Walsh, G.L.; Fang, B.; Ji, L.; Roth, J.A.; LaPushin, R.; Hong, W.K.; Cohen, P.; Kurie, J.M. Insulin-like growth factor binding protein-6 inhibits the growth of human bronchial epithelial cells and increases in abundance with all-trans-retinoic acid treatment. Am. J. Respir. Cell. Mol. Biol. 2000, 23, 297–303. [Google Scholar] [CrossRef]
- Allen, J.T.; Bloor, C.A.; Kedia, R.K.; Knight, R.A.; Spiteri, M.A. Expression of growth hormone-releasing factor, growth hormone, insulin-like growth factor-1 and its binding proteins in human lung. Neuropeptides 2000, 34, 98–107. [Google Scholar] [CrossRef]
- Kim, S.D.; Kang, S.A.; Kim, Y.W.; Yu, H.S.; Cho, K.S.; Roh, H.J. Screening and Functional Pathway Analysis of Pulmonary Genes Associated with Suppression of Allergic Airway Inflammation by Adipose Stem Cell-Derived Extracellular Vesicles. Stem Cells Int. 2020, 2020, 5684250. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, V.T.; Bordeleau, M.; Laviolette, M.; Laprise, C. From expression pattern to genetic association in asthma and asthma-related phenotypes. BMC Res. Notes 2012, 5, 630. [Google Scholar] [CrossRef]
- Laselva, O.; Criscione, M.L.; Allegretta, C.; Di Gioia, S.; Liso, A.; Conese, M. Insulin-Like Growth Factor Binding Protein (IGFBP-6) as a Novel Regulator of Inflammatory Response in Cystic Fibrosis Airway Cells. Front. Mol. Biosci. 2022, 9, 905468. [Google Scholar] [CrossRef] [PubMed]
- DeLong, P.A.; Kotloff, R.M. An overview of pulmonary host defense. Semin. Roentgenol. 2000, 35, 118–123. [Google Scholar] [CrossRef]
- Wu, H.; Suzuki, T.; Carey, B.; Trapnell, B.C.; McCormack, F.X. Keratinocyte growth factor augments pulmonary innate immunity through epithelium-driven, GM-CSF-dependent paracrine activation of alveolar macrophages. J. Biol. Chem. 2011, 286, 14932–14940. [Google Scholar] [CrossRef]
- Liso, A.; Capitanio, N.; Gerli, R.; Conese, M. From fever to immunity: A new role for IGFBP-6? J. Cell. Mol. Med. 2018, 22, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.T.; Bloor, C.A.; Knight, R.A.; Spiteri, M.A. Expression of insulin-like growth factor binding proteins in bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. Am. J. Respir. Cell. Mol. Biol. 1998, 19, 250–258. [Google Scholar] [CrossRef]
- Allen, J.T.; Knight, R.A.; Bloor, C.A.; Spiteri, M.A. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am. J. Respir. Cell. Mol. Biol. 1999, 21, 693–700. [Google Scholar] [CrossRef]
- Kasik, J.W.; Lu, C.; Menon, R.K. The expanding insulin family: Structural, genomic, and functional considerations. Pediatr. Diabetes 2000, 1, 169–177. [Google Scholar] [CrossRef]
- Emtage, P.; Vatta, P.; Arterburn, M.; Muller, M.W.; Park, E.; Boyle, B.; Hazell, S.; Polizotto, R.; Funk, W.D.; Tang, Y.T. IGFL: A secreted family with conserved cysteine residues and similarities to the IGF superfamily. Genomics 2006, 88, 513–520. [Google Scholar] [CrossRef]
- LeRoith, D.; Roberts, C.T., Jr. The insulin-like growth factor system and cancer. Cancer Lett. 2003, 195, 127–137. [Google Scholar] [CrossRef]
- Clemmons, D.R. Modifying IGF1 activity: An approach to treat endocrine disorders, atherosclerosis and cancer. Nat. Rev. Drug Discov. 2007, 6, 821–833. [Google Scholar] [CrossRef]
- Smith, T.J. Insulin-like growth factor-I regulation of immune function: A potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 2010, 62, 199–236. [Google Scholar] [CrossRef]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr. Rev. 1999, 20, 761–787. [Google Scholar] [CrossRef] [PubMed]
- Bach, L.A.; Fu, P.; Yang, Z. Insulin-like growth factor-binding protein-6 and cancer. Clin. Sci. 2013, 124, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Lobito, A.A.; Ramani, S.R.; Tom, I.; Bazan, J.F.; Luis, E.; Fairbrother, W.J.; Ouyang, W.; Gonzalez, L.C. Murine insulin growth factor-like (IGFL) and human IGFL1 proteins are induced in inflammatory skin conditions and bind to a novel tumor necrosis factor receptor family member, IGFLR1. J. Biol. Chem. 2011, 286, 18969–18981. [Google Scholar] [CrossRef]
- Siddle, K. Signalling by insulin and IGF receptors: Supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Duan, C.; Xu, Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 2005, 142, 44–52. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, A.; Castro, R.; Wang, T.; Secombes, C.J.; Boudinot, P.; Macqueen, D.J.; Martin, S.A. Cross Talk Between Growth and Immunity: Coupling of the IGF Axis to Conserved Cytokine Pathways in Rainbow Trout. Endocrinology 2016, 157, 1942–1955. [Google Scholar] [CrossRef] [PubMed]
- Benbassat, C.A.; Lazarus, D.D.; Cichy, S.B.; Evans, T.M.; Moldawer, L.L.; Lowry, S.F.; Unterman, T.G. Interleukin-1 alpha (IL-1 alpha) and tumor necrosis factor alpha (TNF alpha) regulate insulin-like growth factor binding protein-1 (IGFBP-1) levels and mRNA abundance in vivo and in vitro. Horm. Metab. Res. 1999, 31, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pooley, N.J.; Tacchi, L.; Secombes, C.J.; Martin, S.A. Inflammatory responses in primary muscle cell cultures in Atlantic salmon (Salmo salar). BMC Genomics 2013, 14, 747. [Google Scholar] [CrossRef]
- Conese, M.; D’Oria, S.; Castellani, S.; Trotta, R.; Montemurro, P.; Liso, A. Insulin-like growth factor-6 (IGFBP-6) stimulates neutrophil oxidative burst, degranulation and chemotaxis. Inflamm. Res. 2018, 67, 107–109. [Google Scholar] [CrossRef]
- Conese, M.; Pace, L.; Pignataro, N.; Catucci, L.; Ambrosi, A.; Di Gioia, S.; Tartaglia, N.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 Is Secreted in Extracellular Vesicles upon Hyperthermia and Oxidative Stress in Dendritic Cells But Not in Monocytes. Int. J. Mol. Sci. 2020, 21, 4428. [Google Scholar] [CrossRef] [PubMed]
- Liso, A.; Castellani, S.; Massenzio, F.; Trotta, R.; Pucciarini, A.; Bigerna, B.; De Luca, P.; Zoppoli, P.; Castiglione, F.; Palumbo, M.C.; et al. Human monocyte-derived dendritic cells exposed to hyperthermia show a distinct gene expression profile and selective upregulation of IGFBP6. Oncotarget 2017, 8, 60826–60840. [Google Scholar] [CrossRef]
- Alunno, A.; Bistoni, O.; Manetti, M.; Cafaro, G.; Valentini, V.; Bartoloni, E.; Gerli, R.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Front. Immunol. 2017, 8, 554. [Google Scholar] [CrossRef]
- Liso, A.; Venuto, S.; Coda, A.R.D.; Giallongo, C.; Palumbo, G.A.; Tibullo, D. IGFBP-6: At the Crossroads of Immunity, Tissue Repair and Fibrosis. Int. J. Mol. Sci. 2022, 23, 4358. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Guo, Q.; Wang, Y.; Ma, L.; Zhang, X. Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases. Biomed Res. Int. 2018, 2018, 6057589. [Google Scholar] [CrossRef]
- Wallen, L.D.; Myint, W.; Nygard, K.; Shimasaki, S.; Clemmons, D.R.; Han, V.K. Cellular distribution of insulin-like growth factor binding protein mRNAs and peptides during rat lung development. J. Endocrinol. 1997, 155, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.G.; Zwarthoff, E.C.; Drop, S.L. Gene expression of the six insulin-like growth factor binding proteins in the mouse conceptus during mid- and late gestation. Endocrinology 1993, 132, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Putzer, P.; Breuer, P.; Gotz, W.; Gross, M.; Kubler, B.; Scharf, J.G.; Schuller, A.G.; Hartmann, H.; Braulke, T. Mouse insulin-like growth factor binding protein-6: Expression, purification, characterization and histochemical localization. Mol. Cell. Endocrinol. 1998, 137, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. In vivo differentiation potential of tracheal basal cells: Evidence for multipotent and unipotent subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L643–L649. [Google Scholar] [CrossRef]
- Ruan, W.; Ying, K. Abnormal expression of IGF-binding proteins, an initiating event in idiopathic pulmonary fibrosis? Pathol. Res. Pract. 2010, 206, 537–543. [Google Scholar] [CrossRef]
- Conover, C.A. Regulation and physiological role of insulin-like growth factor binding proteins. Endocr. J. 1996, 43, S43–S48. [Google Scholar] [CrossRef]
- Laprise, C.; Sladek, R.; Ponton, A.; Bernier, M.C.; Hudson, T.J.; Laviolette, M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genom. 2004, 5, 21. [Google Scholar] [CrossRef]
- Lu, H.; Wang, R.; Li, W.; Xie, H.; Wang, C.; Hao, Y.; Sun, Y.; Jia, Z. Plasma cytokine profiling to predict susceptibility to acute mountain sickness. Eur. Cytokine Netw. 2016, 27, 90–96. [Google Scholar] [CrossRef]
- American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Sathirareuangchai, S.; Shimizu, D.; Vierkoetter, K.R. Pulmonary Lymphangioleiomyomatosis: A Case Report and Literature Review. Hawaii J. Health Soc. Welf. 2020, 79, 224–229. [Google Scholar] [PubMed]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2022. [Google Scholar]
- Pawankar, R.; Canonica, G.W.; Holgate, S.T.; Lockey, R.F. Allergic diseases and asthma: A major global health concern. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.; Lesosky, M.; Garcia-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; El Sony, A.; Ellwood, P.; Marks, G.B.; et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur. Respir. J. 2022, 60. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, I.K.; Yoon, H.K.; Kwon, S.S.; Rhee, C.K.; Lee, S.Y. Inhibitory Effects of Resveratrol on Airway Remodeling by Transforming Growth Factor-beta/Smad Signaling Pathway in Chronic Asthma Model. Allergy Asthma Immunol. Res. 2017, 9, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Huang, M.T. Asthma in the Precision Medicine Era: Biologics and Probiotics. Int. J. Mol. Sci. 2021, 22, 4528. [Google Scholar] [CrossRef] [PubMed]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.U.; Renz, H.; Dufour, J.F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523. [Google Scholar] [CrossRef]
- Wangberg, H.; Woessner, K. Choice of biologics in asthma endotypes. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 79–85. [Google Scholar] [CrossRef]
- Gonzalez-Perez, R.; Poza-Guedes, P.; Mederos-Luis, E.; Sanchez-Machin, I. Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype. Biomedicines 2022, 10, 2635. [Google Scholar] [CrossRef]
- Abreu, S.C.; Xisto, D.G.; de Oliveira, T.B.; Blanco, N.G.; de Castro, L.L.; Kitoko, J.Z.; Olsen, P.C.; Lopes-Pacheco, M.; Morales, M.M.; Weiss, D.J.; et al. Serum from Asthmatic Mice Potentiates the Therapeutic Effects of Mesenchymal Stromal Cells in Experimental Allergic Asthma. Stem Cells Transl. Med. 2019, 8, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Hur, J.; Jeon, S.; Jung, C.K.; Rhee, C.K. Effects of human adipose tissue- and bone marrow-derived mesenchymal stem cells on airway inflammation and remodeling in a murine model of chronic asthma. Sci. Rep. 2022, 12, 12032. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; Madala, S.; Karpati, S.; et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Julian, C.G.; Subudhi, A.W.; Wilson, M.J.; Dimmen, A.C.; Pecha, T.; Roach, R.C. Acute mountain sickness, inflammation, and permeability: New insights from a blood biomarker study. J. Appl. Physiol. 2011, 111, 392–399. [Google Scholar] [CrossRef]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Holgate, S.T. Pathogenesis of asthma. Clin. Exp. Allergy 2008, 38, 872–897. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Ferrans, V.J.; Yu, Z.X.; Nelson, W.K.; Valencia, J.C.; Tatsuguchi, A.; Avila, N.A.; Riemenschn, W.; Matsui, K.; Travis, W.D.; Moss, J. Lymphangioleiomyomatosis (LAM): A review of clinical and morphological features. J. Nippon Med. Sch. 2000, 67, 311–329. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Cuevas-Ocana, S.; Laselva, O.; Avolio, J.; Nenna, R. The era of CFTR modulators: Improvements made and remaining challenges. Breathe 2020, 16, 200016. [Google Scholar] [CrossRef]
- Reiniger, N.; Ichikawa, J.K.; Pier, G.B. Influence of cystic fibrosis transmembrane conductance regulator on gene expression in response to Pseudomonas aeruginosa infection of human bronchial epithelial cells. Infect. Immun. 2005, 73, 6822–6830. [Google Scholar] [CrossRef]
- Nagy, L.; Saydak, M.; Shipley, N.; Lu, S.; Basilion, J.P.; Yan, Z.H.; Syka, P.; Chandraratna, R.A.; Stein, J.P.; Heyman, R.A.; et al. Identification and characterization of a versatile retinoid response element (retinoic acid receptor response element-retinoid X receptor response element) in the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 1996, 271, 4355–4365. [Google Scholar] [CrossRef]
- Ma, Y.; Koza-Taylor, P.H.; DiMattia, D.A.; Hames, L.; Fu, H.; Dragnev, K.H.; Turi, T.; Beebe, J.S.; Freemantle, S.J.; Dmitrovsky, E. Microarray analysis uncovers retinoid targets in human bronchial epithelial cells. Oncogene 2003, 22, 4924–4932. [Google Scholar] [CrossRef] [PubMed]
- Longhitano, L.; Tibullo, D.; Vicario, N.; Giallongo, C.; La Spina, E.; Romano, A.; Lombardo, S.; Moretti, M.; Masia, F.; Coda, A.R.D.; et al. IGFBP-6/sonic hedgehog/TLR4 signalling axis drives bone marrow fibrotic transformation in primary myelofibrosis. Aging 2021, 13, 25055–25071. [Google Scholar] [CrossRef] [PubMed]
- Remsing Rix, L.L.; Sumi, N.J.; Hu, Q.; Desai, B.; Bryant, A.T.; Li, X.; Welsh, E.A.; Fang, B.; Kinose, F.; Kuenzi, B.M.; et al. IGF-binding proteins secreted by cancer-associated fibroblasts induce context-dependent drug sensitization of lung cancer cells. Sci. Signal. 2022, 15, eabj5879. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, F.; Busch, A.M.; Chinyengetere, F.; Ma, T.; Sekula, D.; Memoli, V.A.; Dragnev, K.H.; Liu, F.; Johnson, K.C.; Guo, Y.; et al. Response to inhibition of smoothened in diverse epithelial cancer cells that lack smoothened or patched 1 mutations. Int. J. Oncol. 2012, 41, 1751–1761. [Google Scholar] [CrossRef]
- Cancer (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 23 February 2023).
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Zinn, R.L.; Gardner, E.E.; Marchionni, L.; Murphy, S.C.; Dobromilskaya, I.; Hann, C.L.; Rudin, C.M. ERK phosphorylation is predictive of resistance to IGF-1R inhibition in small cell lung cancer. Mol. Cancer Ther. 2013, 12, 1131–1139. [Google Scholar] [CrossRef]
- Macaulay, V.M. Insulin-like growth factors and cancer. Br. J. Cancer 1992, 65, 311–320. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, S.; Torossian, A.; Speirs, C.K.; Schleicher, S.; Giacalone, N.J.; Carbone, D.P.; Zhao, Z.; Lu, B. Role of insulin-like growth factor-1 signaling pathway in cisplatin-resistant lung cancer cells. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e563–e572. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiu, Y.; Chen, S.; Wang, S.; Yang, R.; Liu, B.; Li, Y.; Deng, J.; Su, Y.; Lin, Z.; et al. Different Roles of the Insulin-like Growth Factor (IGF) Axis in Non-small Cell Lung Cancer. Curr. Pharm. Des. 2022, 28, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Arnedo, E.; Lopez, I.P.; Pineiro-Hermida, S.; Canalejo, M.; Gotera, C.; Sola, J.J.; Roncero, A.; Peces-Barba, G.; Ruiz-Martinez, C.; Pichel, J.G. IGF1R acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene 2022, 41, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, I.; Chaudhary, S.; Perumal, N.K.; Batra, S.; Ganti, A.K. FP12.10 IGF-1R Inhibition in Small Cell Lung Cancer: Role of Brigatinib. J. Thorac. Oncol. 2021, 16. [Google Scholar] [CrossRef]
- Pohlman, A.W.; Moudgalya, H.; Jordano, L.; Lobato, G.C.; Gerard, D.; Liptay, M.J.; Seder, C.W.; Borgia, J.A. The role of IGF-pathway biomarkers in determining risks, screening, and prognosis in lung cancer. Oncotarget 2022, 13, 393–407. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.G.; Li, D.; Xu, J.X.; Zeng, Z.G. Gene expression and prognosis of insulin-like growth factor-binding protein family members in non-small cell lung cancer. Oncol. Rep. 2019, 42, 1981–1995. [Google Scholar] [CrossRef]
- London, S.J.; Yuan, J.M.; Travlos, G.S.; Gao, Y.T.; Wilson, R.E.; Ross, R.K.; Yu, M.C. Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J. Natl. Cancer Inst. 2002, 94, 749–754. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Liang, Z.; Liu, J.; Shi, W.; Bai, P.; Lin, X.; Magaye, R.; Zhao, J. Expression and clinical significance of IGF-1, IGFBP-3, and IGFBP-7 in serum and lung cancer tissues from patients with non-small cell lung cancer. Onco Targets Ther. 2013, 6, 1437–1444. [Google Scholar] [CrossRef]
- Okamura, J.; Huang, Y.; Moon, D.; Brait, M.; Chang, X.; Kim, M.S. Downregulation of insulin-like growth factor-binding protein 7 in cisplatin-resistant non-small cell lung cancer. Cancer Biol. Ther. 2012, 13, 148–155. [Google Scholar] [CrossRef]
- Ibanez de Caceres, I.; Cortes-Sempere, M.; Moratilla, C.; Machado-Pinilla, R.; Rodriguez-Fanjul, V.; Manguan-Garcia, C.; Cejas, P.; Lopez-Rios, F.; Paz-Ares, L.; de CastroCarpeno, J.; et al. IGFBP-3 hypermethylation-derived deficiency mediates cisplatin resistance in non-small-cell lung cancer. Oncogene 2010, 29, 1681–1690. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, T.; Knosel, T.; Yang, L.; Zoller, K.; Petersen, I. IGFBP7 is a p53 target gene inactivated in human lung cancer by DNA hypermethylation. Lung Cancer 2011, 73, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shiraishi, K.; Eguchi, A.; Ikeda, K.; Mori, T.; Yoshimoto, K.; Ohba, Y.; Yamada, T.; Ito, T.; Baba, Y.; et al. Aberrant methylation of LINE-1, SLIT2, MAL and IGFBP7 in non-small cell lung cancer. Oncol. Rep. 2013, 29, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Shersher, D.D.; Vercillo, M.S.; Fhied, C.; Basu, S.; Rouhi, O.; Mahon, B.; Coon, J.S.; Warren, W.H.; Faber, L.P.; Hong, E.; et al. Biomarkers of the insulin-like growth factor pathway predict progression and outcome in lung cancer. Ann. Thorac. Surg. 2011, 92, 1805–1811, discussion 1811. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, J.; Ma, L.; Tan, Q.; Wang, J.; Tan, J.; Zhang, S. IGFBP7 overexpression promotes acquired resistance to AZD9291 in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2021, 571, 38–45. [Google Scholar] [CrossRef]
- Wu, S.G.; Chang, T.H.; Tsai, M.F.; Liu, Y.N.; Hsu, C.L.; Chang, Y.L.; Yu, C.J.; Shih, J.Y. IGFBP7 Drives Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibition in Lung Cancer. Cancers 2019, 11, 36. [Google Scholar] [CrossRef]
- Nur, S.I.; Ozturk, A.; Kavas, M.; Bulut, I.; Alparslan, S.; Aydogan, E.S.; Atinkaya, B.C.; Kolay, M.; Coskun, A. IGFBP-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, S.; Yin, W.; Liu, X.; Hu, Y. IGFBP-4 expression is adversely associated with lung cancer prognosis. Oncol. Lett. 2017, 14, 6876–6880. [Google Scholar] [CrossRef]
- Tang, D.; Yao, R.; Zhao, D.; Zhou, L.; Wu, Y.; Yang, Y.; Sun, Y.; Lu, L.; Gao, W. Trichostatin A reverses the chemoresistance of lung cancer with high IGFBP2 expression through enhancing autophagy. Sci. Rep. 2018, 8, 3917. [Google Scholar] [CrossRef]
- Forbes, B.; Ballard, F.J.; Wallace, J.C. An insulin-like growth factor-binding protein purified from medium conditioned by a human lung fibroblast cell line (He[39]L) has a novel N-terminal sequence. J. Endocrinol. 1990, 126, 497–506. [Google Scholar] [CrossRef]
- Wegmann, B.R.; Schoneberger, H.J.; Kiefer, P.E.; Jaques, G.; Brandscheid, D.; Havemann, K. Molecular cloning of IGFBP-5 from SCLC cell lines and expression of IGFBP-4, IGFBP-5 and IGFBP-6 in lung cancer cell lines and primary tumours. Eur. J. Cancer 1993, 29A, 1578–1584. [Google Scholar] [CrossRef]
- Yao, R.; Wang, Y.; Lubet, R.A.; You, M. Differentially expressed genes associated with mouse lung tumor progression. Oncogene 2002, 21, 5814–5821. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Xu, Y.; Ding, R.; Qiu, K.; Zhang, R.; Wang, H.; Huang, L.; Xie, X.; Yan, H.; Deng, Y.; et al. Extensive serum biomarker analysis in patients with non-small-cell lung carcinoma. Cytokine 2020, 126, 154868. [Google Scholar] [CrossRef] [PubMed]
- Koyama, N.; Zhang, J.; Huqun; Miyazawa, H.; Tanaka, T.; Su, X.; Hagiwara, K. Identification of IGFBP-6 as an effector of the tumor suppressor activity of SEMA3B. Oncogene 2008, 27, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuto, S.; Coda, A.R.D.; González-Pérez, R.; Laselva, O.; Tolomeo, D.; Storlazzi, C.T.; Liso, A.; Conese, M. IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression. Int. J. Mol. Sci. 2023, 24, 4804. https://doi.org/10.3390/ijms24054804
Venuto S, Coda ARD, González-Pérez R, Laselva O, Tolomeo D, Storlazzi CT, Liso A, Conese M. IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression. International Journal of Molecular Sciences. 2023; 24(5):4804. https://doi.org/10.3390/ijms24054804
Chicago/Turabian StyleVenuto, Santina, Anna Rita Daniela Coda, Ruperto González-Pérez, Onofrio Laselva, Doron Tolomeo, Clelia Tiziana Storlazzi, Arcangelo Liso, and Massimo Conese. 2023. "IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression" International Journal of Molecular Sciences 24, no. 5: 4804. https://doi.org/10.3390/ijms24054804
APA StyleVenuto, S., Coda, A. R. D., González-Pérez, R., Laselva, O., Tolomeo, D., Storlazzi, C. T., Liso, A., & Conese, M. (2023). IGFBP-6 Network in Chronic Inflammatory Airway Diseases and Lung Tumor Progression. International Journal of Molecular Sciences, 24(5), 4804. https://doi.org/10.3390/ijms24054804











