Abstract
Numerous studies have shown that oxidative stress resulting from an imbalance between the production of free radicals and their neutralization by antioxidant enzymes is one of the major pathological disorders underlying the development and progression of type 2 diabetes (T2D). The present review summarizes the current state of the art advances in understanding the role of abnormal redox homeostasis in the molecular mechanisms of T2D and provides comprehensive information on the characteristics and biological functions of antioxidant and oxidative enzymes, as well as discusses genetic studies conducted so far in order to investigate the contribution of polymorphisms in genes encoding redox state-regulating enzymes to the disease pathogenesis.
1. Introduction
Diabetes mellitus is one of the most common metabolic diseases, affecting every tenth person on the planet [1]. According to the latest edition of the IDF Diabetes Atlas, there are more than 537 million people who suffer from diabetes in the world [2]. It has been recently predicted that 643 million people will have diabetes by 2030 (11.3% of the population) [3]. If trends continue, this number will jump to a staggering 783 million (12.2%) by 2045 [2]. A great majority of diabetics in the world suffer from type 2 diabetes mellitus, or non-insulin-dependent diabetes mellitus, a serious chronic disease that develops when the body does not produce enough insulin or is unable to use it effectively [4,5,6].
Epidemiological studies of T2D conducted over the past decades indicate that T2D is a heterogeneous disease determined by genetic, epigenetic, and environmental risk factors that closely interact with each other [7,8,9]. A huge number of genetic studies have been conducted to elucidate the molecular mechanisms of T2D, including beta-cell dysfunction, insulin resistance, imbalance in redox homeostasis, and impairment of incretin signaling; from these studies, multiple disease-associated gene polymorphisms have been identified [10,11,12,13,14]. Nonetheless, many aspects of the molecular mechanisms of disease pathogenesis remain poorly characterized. In particular, numerous studies have shown that oxidative stress (the imbalance caused by excess ROS or oxidants over the cell’s ability to realize an effective antioxidant response) resulting from an imbalance between the production of free radicals and their neutralization by antioxidant enzymes is one of the major pathological disorders underlying the development and progression of type 2 diabetes.
However, the literature data on the molecular genetic mechanisms underlying abnormal redox homeostasis in type 2 diabetes have not so far been reviewed.
The present review summarizes current state-of-the art advances in understanding the role of abnormal redox homeostasis in the molecular mechanisms of T2D and provides comprehensive information on the characteristics and biological functions of antioxidant and oxidative enzymes, as well as discusses genetic studies conducted so far in order to investigate the contribution of polymorphisms in genes encoding redox state-regulating enzymes in disease pathogenesis. The following internet resources were used in preparing this review: Entrez gene [15], UniProt Knowledgebase [16], BRENDA Enzyme Database [17], GENATLAS [18], HUGO Gene Nomenclature Committee [19], GeneCards [20], BioGPS [21], Genotype-Tissue Expression (GTEx) Portal [22], eQTLGen Consortium [23], GWAS Catalog [24], and DisGeNET [25].
2. General Pathological Alterations in Type 2 Diabetes
It is widely agreed that chronic hyperglycemia, the primary diagnostic indicator of T2D, results from pancreatic beta-cell failure, which manifests by a gradient reduction in beta-cell mass and insulin production in response to glucose [26,27,28]. With the loss of metabolic flexibility, beta-cells begin to oxidize fatty acids instead of glucose, which results in the production of harmful byproducts (peroxides) and decreased insulin secretion [29,30]. It is noteworthy that the damage of the endocrine part of the pancreas is caused by at least three biological phenomena: apoptosis of beta-cells, their dedifferentiation, and transformation into glucagon-producing alpha cells [31,32,33,34]. A number of studies have shown a decrease in the expression of key transcription factors (PDX1, NKX6.1, and MAFA) regulating the function of beta-cells, and discussed the subsequent disorders that are accompanied by a loss of insulin-producing ability in T2D [33,35].
The islets of Langerhans have been found to contain bihormonal cells that produce both glucagon and insulin after the conversion of beta-cells into alpha cells, as has been demonstrated by experimental studies on transgenic mice [33,36]. A shift in the phenotype of pancreatic beta-cells may serve as a mechanism of cell mass conservation [37] since alpha cells are more resistant to metabolic stress induced by excessive nutrition. Lim and co-authors observed that changes in insulin secretion in T2D are potentially reversible due to beta-cell re-differentiation: complete recovery of the first phase of secretion was observed by the eighth week of a hypocaloric diet in 87% of T2D patients with less than four years of disease duration [38]. There is definitely a “point of no return” in the progression of the disease beyond which the lost ability to produce insulin cannot be restored due to irreversible apoptotic changes in the pancreatic islets.
The development of insulin resistance, another phenomenon accompanying T2D pathogenesis, involves supra- and post-receptor mechanisms. The former are associated with hypertrophic extracellular matrix signaling, a decrease in capillary filling, and thus deterioration of local blood flow, limiting the availability of insulin and glucose in muscles and other tissues [39,40,41]. Insulin resistance is exacerbated by an increase in the expression of collagen and other extracellular matrix proteins, including their integrin receptors, which are in direct contact with skeletal muscle capillaries [42]. It is worth noting that the formation of insulin resistance in adipose tissue is triggered by the accumulation of extracellular matrix and fibrosis [43,44]. Given that insulin-like growth factor 1 (IGF1) is a powerful stimulator of collagen expression, hyperinsulinemia can promote collagen synthesis by adipose tissue fibroblasts, leading to insulin resistance by activating IGF1 receptors or affecting IGF1 binding protein [45]. Post-receptor mechanisms underlying insulin resistance are associated with a decrease in intracellular glucose metabolism activity, including de novo fatty acid synthesis in adipose tissue [46,47,48]. These effects are associated with decreased expression of the lipogenic transcription factors ChREBP-α and ChREBP-β, which are linked to GLUT4 inactivation [49,50,51]. The importance of controlling the metabolism of acetyl-CoA is also related to the fact that this substrate is necessary for the acetylation of proteins, including histones [52,53,54].
Insulin resistance and Langerhans islet dysfunction appear early in the pathogenesis of T2D, as shown in observational studies of Pima Indians, whose natural course of disease progressed over time from euglycemia to impaired glucose tolerance and T2D [55,56]. There are two points of view regarding the sequence of development of insulin resistance and beta-cell dysfunction, which is often manifested as hyperinsulinemia. The first explanation states that insulin resistance is the primary cause of hyperinsulinemia. An experimental study on mice has shown that insulin resistance appears as a result of induced disorders of the insulin signaling pathway in the liver, skeletal muscle, and adipose tissue and causes hyperinsulinemia, thereby leading to the development of T2D [57,58]. In humans, mutations in genes encoding insulin signaling proteins are also manifested by an increase in circulating insulin levels in the blood and are the causes of hereditary forms of diabetes [59,60]. A decrease in the effectiveness of insulin in relation to glycemic control is associated with the activation of the FOXO1 transcription factor in the liver [61,62] and impaired translocation of the glucose transporter GLUT4 into the membranes of skeletal myocytes [63,64]. Consequently, FOXO1 increases the expression of key enzymes of gluconeogenesis, causing the liver to produce more glucose. A decrease in GLUT4 in the membrane of skeletal myocytes reduces the uptake of glucose from the bloodstream into the cells. In the liver, insulin normally causes phosphorylation and suppression of FOXO1 by protein kinase B (Akt), which keeps FOXO1 in the cytoplasm, where this transcription factor is inactive [65,66,67]. However, FOXO1 expression was found to be increased in the liver of obese mice, and this transcription factor loses its sensitivity to insulin regulation [68,69,70]. Impact of overnutrition on the FOXO1 dysregulation is currently being extensively researched [71,72] to better understand the primary mechanism of uncontrolled gluconeogenesis in obesity [73,74,75]. It is thought that hyperglycemia caused by uncontrolled FOXO1 activation, combined with chronic hyperinsulinemia, can remove insulin’s inhibitory effect on lipolysis in adipose tissue [69]. Activated lipolysis in abdominal adipocytes in turn increases the flow to the liver of its products—free fatty acids, which are allosteric activators of pyruvate carboxylase, a key enzyme of gluconeogenesis, and glycerol, a substrate of the same pathway [76]. Thus, a high-calorie diet induces the production of glucose by the liver under the influence of FOXO1, which is additionally stimulated by the activation of lipolysis in adipose tissue. In this context, functional beta-cell deficiency results in an inability to secrete enough insulin to compensate for the effects of FOXO1, resulting in diabetes [77,78].
An alternative hypothesis of T2D pathogenesis implies that hyperinsulinemia is the primary cause of insulin resistance. In obese people without diabetes, fasting hyperinsulinemia is found without detectable hyperglycemia, which is theoretically necessary to stimulate additional insulin secretion by beta-cells. This apparent disparity between blood glucose and insulin levels led to the hypothesis that hyperinsulinemia is the initial, primary effect of overeating and obesity [79,80] and is caused by stimulation of insulin secretion [81,82] and suppression of its degradation [83]. Insulin resistance in the liver is caused by primary hyperinsulinemia, which is associated with the suppression of signal transmission from the insulin receptor to Akt. Activation of the Akt protein appears to be sufficient to activate the mTORC1 kinase complex and the SREBP-1c transcription factor, both of which promote de novo fatty acid synthesis [84]. Furthermore, hyperinsulinemia has been found to cause activation of inflammatory signaling pathways in humans [85,86,87] and rats [88], which can impair insulin sensitivity in the target tissues [89]. A similar increase in glucose tolerance was observed in the knockout mice with impaired insulin secretion [90]. Thus, both beta-cell dysfunction and insulin resistance are disorders that can occur at the same time and are not mutually exclusive.
3. Environmental Risk Factors of Type 2 Diabetes
Type 2 diabetes is a multifactorial disease that develops as a result of interactions between environmental and genetic factors [91,92]. It has been estimated that the majority of the burden of type 2 diabetes is related to environmental exposures and modifiable risk factors, such as lifestyle [93,94]. High-calorie diets, high consumption of fatty foods and refined carbohydrates, hypodynamia, and smoking are modifiable risk factors for T2D, whereas gender, age, and family history of diabetes are non-modifiable risk factors.
The leading causes underlying the epidemics of T2D and obesity include urbanization, sedentary lifestyles, and unhealthy diets [26]. Undoubtedly, nutritional status is one of the most important environmental factors influencing the risk of T2D [26]. Food quality is more significant than overall food intake, as evaluated by naturally occurring polyunsaturated fatty acid content, the lack of trans-unsaturated fatty acids, and meals with a high glycemic index. An increase in dietary fat intake raises insulin levels in contrast to normal glycemia [95]. Carter P. et al. [96] observed that daily eating of fresh fruits and vegetables reduces the risk of disease by 14%. According to a study by Cooper A.J. and co-authors [97], a diet with a greater quantity of vegetables and a greater variety of both fruits and vegetables in diet is associated with a reduced risk of type 2 diabetes. It has been found that there is an inverse relationship between consumption of plant food and the risk of disease [98,99]. It is estimated that consuming even four servings of fruit each day can lower the incidence of T2D by 7% [99]. Increased consumption of fresh, plant-based foods and whole-meal breads, as well as decreased consumption of sugary drinks, high-calorie, fried foods, and white bread, are among the nutritional recommendations made by the WHO for the prevention of T2D [100]. Numerous studies have demonstrated that the Mediterranean diet lowers the incidence of T2D [101,102]. The fast-food boom is one of the factors that’s caused the pandemic spike in T2D incidence in Asia (China and South Korea) over the past two decades [103]. Clinical studies, such as the Diabetes Prevention Program in the USA, the Finnish Diabetes Prevention Studies in Finland, the Da Qing IGT, and the Diabetes Study in China, have demonstrated the benefits of changing lifestyle, particularly through a healthy diet and increased physical activity.
Physical inactivity is associated with poor glucose tolerance, hyperinsulinemia, and insulin resistance, which dramatically raises the risk of T2D. The prevalence of type 2 diabetes was found to be two times higher among those who have a sedentary lifestyle than those who engage in strenuous physical activity [104,105,106]. Prospective studies have demonstrated a positive relationship between aerobic and power loads and the risk of T2D [107,108]. Ekelund et al. [109] calculated that the reduction in T2D risk caused by moderate and vigorous physical activity depends only on the length of training and does not depend on the amount of time spent in an inactive state. Rockette-Wagner [110] showed that long-term sedentary work can neutralize the positive effect of physical activity in terms of the T2D risk. Numerous tissues, including skeletal and cardiac muscle, the liver, and brain cells, have been found to be involved in the regulation of redox homeostasis by physical exercise [111]. A body of evidence shows that disruption in exercise-induced redox homeostasis serves as an upstream signal for transcription factor activation, followed by gene expression stimulation, increased antioxidant defense, and decreased age-related oxidative stress [112,113].
Tobacco smoking is also an environmental risk factor for T2D [114]. The number of cigarettes smoked per day was found to be dose-dependently correlated with the risk of T2D, which was found to be 45% higher in smokers than nonsmokers [115]. There is evidence that passive smoking also increases the incidence of T2D [116]. Smoking is an independent factor that worsens tissue insulin sensitivity. Tobacco use has been shown in studies to be toxic to the pancreas and to reduce insulin release by beta-cells [117].
Alcohol drinking patterns have been found to be associated with the risk of type 2 diabetes [118]. Okamura T. and colleagues discovered that heavy alcohol consumers (>280 g/week) with fatty liver disease (FLD) had a higher risk of developing type 2 diabetes than the other groups. Moderate alcohol consumers (140–280 g/week) without FLD had a significantly higher risk for type 2 diabetes, compared with minimal (<40 g/week) and light (40–140 g/week) alcohol consumers without FLD. In contrast, there was no apparent difference in the risk for incident type 2 diabetes between non-drinkers and minimal, light, or moderate alcohol consumers with FLD. Furthermore, no significant difference in the risk for T2D was found between moderate and heavy alcohol drinkers without FLD and non-drinkers or minimal, light, or moderate alcohol consumers with FLD [119].
The prevalence of type 2 diabetes, like many other multifactorial diseases, is linked to cumulative exposure to lipophilic and hydrophilic environmental pollutants such as POPs (persistent organic pollutants), exudates produced by common plastics, air pollutants, and some medicines [120,121]. Prospective studies investigating the dose-response relationship between environmental pollutants and T2D prevalence are needed to substantiate the causal role of chemical factors in disease susceptibility.
4. Genetic Factors of Type 2 Diabetes
Studies in multiethnic populations with varying T2D rates suggest that some ethnic groups may have a genetic predisposition to developing insulin resistance and diabetes when exposed to adverse conditions [122]. It is thought that between 35% and 70% of type 2 diabetes cases are genetically predisposed [123]. The genetic contribution to diabetes is demonstrated by familial aggregation [124] and a high concordance rate for T2D in monozygotic twins. In particular, the relative risk of T2D is 35–39% if one parent suffers from the disease; the risk is 60–70% if both parents have T2D; the risk of T2D in monozygotic twins is 58–65%; and the risk in heterozygotic twins is 16–30% [125,126].
Genome-wide association studies (GWAS), a hypothesis-free method created to test hundreds of thousands of genetic variants across many genomes to find those statistically associated with a specific trait or disease, have made significant progress in the last two decades in identifying the genes that cause diabetes susceptibility [127]. Numerous GWASs have identified multiple genetic loci that are linked to T2D and disease-related phenotypes [9,10,128]. According to the GWAS Catalog (https://www.ebi.ac.uk/gwas/, accessed on 25 February 2023), there have been 222 genome-wide studies on type 2 diabetes and 5348 SNP-disease associations have been detected. Furthermore, large meta-analyses of GWAS were performed [10,129] and identified 2284 SNPs associated with T2D, but only 183 and 38 loci were successfully replicated as T2D susceptibility markers once and twice, respectively [130]. Thus, a large portion of the originally discovered SNP-disease associations were poorly replicated by independent studies [131,132,133].
TCF7L2, HHEX, KCNJ11, CENTD2, ARAP1, FTO, HNF1B, PPARG, IGF2BP2, CDKAL1, VEGFA, SLC30A8, CDKN2A, CDKN2B, KCNQ1, UBE2E2, ANK1, ABCC8, IRS1, and GLP2R are the genes whose polymorphic variants showed strong associations with susceptibility to T2D and related phenotypes [134,135,136]. The variants most strongly associated with disease risk affect gene expression in the islets of Langerhans as well as insulin-sensitive tissues and organs such as adipose, muscle, and liver. Transcriptome studies [137,138] showed that some differentially expressed genes (DEGs) are associated with a decrease in insulin secretion (ZMIZ1, MTNR1B, ADCY5, GIPR, C2CDC4A, CDKAL1, GCK, TCF7L2, GLIS3, THADA, and IGF2BP2), while other DEGs are associated with insulin resistance (PPARG, KLF14, and IRS1) and a decrease in the incretin response (TCF7L2, KCNQ1, GIPR, THADA, WFS1, and MTNR1B). However, a larger portion of the identified genes are not associated with either production and/or action of insulin or glucose metabolism, and their role in disease pathogenesis remains unknown. These genes produce proteins that are involved in signaling pathways (ACHE, ADCY5, ARAP1, ARL15, ATP8A1, BECN1, BRAF, CAMKK2, CMIP, DAAM1, DCDC2C, GATAD2A, GPSM1, GRK5, IGF2BP2, MAEA, MCC, PLEKHA1, RGS7, SHB, SUGP1, SYK, and TMEM132D), antigens and receptors (GABRA4, GIPR, GLP2R, IL17REL, IL23R, LRP12, MTNR1B, NOTCH2, NRXN3, PPARG, PTPRD, PVRL2, RASGRP1, SSR1, TGFBR3, and THADA), transport proteins (ATP8A1, EXOC6, KCNJ11, KCNQ1, SLC16A11, SLC16A13, SLC9B2, SLCO4C1, VPS26A, VPS33B, WFS1, and YKT6), proteins regulating cell cycle, mitosis and apoptosis (BCL2, CENPW, FAM58A, FAM60A, MPHOSPH9, NDUFAF6, RCCD1, RHOU, and ZZEF1) and other proteins (ANK1, FSCN3, INTS8, MACF1, SGCD, SGCG, LAMA1, LIMS2, and ST6GAL1). SNP rs7903146 at the TCF7L2 (transcription factor 7 like 2) gene is a genetic marker, the most strongly associated with T2D susceptibility, and it has been successfully replicated in twenty-two studies in various populations of the world [139,140,141,142,143,144]. Polymorphisms of TCF7L2, IGF2BP2, CDKAL1, KCNQ1, and PPARG genes showed a strong contribution to type 2 diabetes.
The rs7903146 polymorphism of TCF7L2, one of the most strongly T2D-associated loci, has been convincingly linked to diminished incretin responsiveness, elevated hepatic glucose production, and impaired insulin secretion [145,146]. It has been experimentally shown that turning off the TCF7L2 gene in mouse beta-cells impairs glucose tolerance and reduces beta-cell mass [147]. Functional studies have confirmed the essential role of the gene in the control of glucose homeostasis through the regulation of beta-cell mass. TCF7L2 is a dual-acting transcription factor that participates in the Wnt signaling pathway, operating as an activator when CTNNB1 beta-catenin is present and as a repressor when it is not [148]. TCF7L2 also inhibits adipogenesis via NLK (Nemo-like kinase) in adipose tissue and inhibits gluconeogenesis in the liver [149].
The rs11927381 variant of the IGF2BP2 gene is a marker that has been shown to be strongly associated with T2D susceptibility [150,151]. IGF2BP2 encodes a protein that binds insulin-like growth factor 2 (IGF2) mRNA and was found to be associated with both beta-cell dysfunction and decreased sensitivity of peripheral tissues to its effects [152]. Associations of six polymorphisms of the IGF2BP2 gene such as rs4402960, rs1470579, rs7640539, rs71320321, rs1374910, and rs6769511 with type 2 diabetes have been discovered and subsequently replicated by independent studies [134]. Dai and co-authors have revealed that an increase in IGF2BP2 expression can cause a deficit of mitochondrial uncoupler protein 1 (UCP1) and hyperproduction of IGF2 because the IGF2BP2 protein facilitates the translation of insulin-like growth factor 2 (IGF2) mRNA and inhibits the synthesis of UCP1 [152]. Mice knocked out of the IGF2BP2 gene showed that a decrease in UCP1 synthesis worsens tissue sensitivity to insulin and contributes to the development of alimentary obesity [152]. Additionally, a study [153] discovered an inverse correlation between the level of IGF2BP2 gene expression in the blood and serum insulin concentration in T2D patients. Experiments with transgenic mice have shown that an increased level of IGF2 leads to insulin resistance, hyperglycemia, and diabetes in 30% of the animals [154]. An increase in IGF2 levels was found to cause disruption of pancreatic islets, whereas a decrease in IGF2 expression in human liver, muscles, and beta-cells reduced the risk of T2D [155].
CDKAL1 (cyclin dependent kinase 5 regulatory subunit associated protein 1 like 1) is another gene whose polymorphisms (rs4712524, rs6931514) are linked to beta-cell dysfunction [156]. This enzyme regulates cyclin-dependent kinase 5, an activator of insulin production in pancreatic beta-cells [157,158]. As part of the formation of ms2t6A37, the enzyme transfers the thiomethyl group from the S-adenosylmethionine molecule to the N6-threonylcarbamoyladenosine at position 37 of the cytosolic tRNALys (t6A37), which is required for the proper reading of lysine codons during translation of the proinsulin mRNA [159]. The accurate recognition of the AAA and AAG lysine codons during preproinsulin mRNA translation is made possible by CDKAL1-catalyzed tRNA modification [160]. The lysine residue is located at the cleavage site of proinsulin into the A-chain and C-peptide; therefore, translational errors can disrupt the formation and secretion of insulin in pancreatic beta-cells. Loss-of-function SNPs in the introns of the CDKAL1 gene were found to be associated with defects in insulin secretion but not with obesity or insulin resistance [161,162].
The first gene described in candidate gene studies and subsequently replicated in GWAS was the PPARG (peroxisome proliferator activated receptor gamma) gene [163]. The gene encodes a nuclear transcription factor involved in the regulation of hundreds of genes for carbohydrate and lipid metabolism. PPARG controls the expression of lipoprotein lipase and CD36 fatty acid transporter [128]. Adipocytes with activated expression of PPARG secrete leptin and adiponectin in a balanced manner, which mediates the effects of insulin on peripheral organs. It has been shown that polymorphisms in the PPARG gene are involved in the development of both insulin resistance and impaired insulin secretion by pancreatic beta-cells [164]. In particular, the rs11709077 polymorphism of PPARG is associated with T2D, obesity, and cardiovascular diseases [165].
The KCNQ1 gene encodes the potassium channel protein of beta-cells and is directly involved in the process of glucose-stimulated insulin secretion [166]. Polymorphism rs2237896 of the KCNQ1 gene was found to be associated with dysfunction of beta-cells and decreased incretin response [167].
Thus, genome-wide association studies have discovered hundreds of SNPs linked to T2D. The discovered disease-associated alleles influence functional properties of pancreatic beta-cells and sensitivity of insulin-dependent tissues. However, the molecular processes by which many susceptibility genes are implicated in the pathogenesis of T2D are yet recognized and are the subject of ongoing research [168,169]. The polygenic nature of this complex disease, epistatic interactions between genes, effects of environmental factors, variable penetrance (between 10 and 40%), and high frequency of disease-related alleles with weak or moderate effects on disease phenotype make it difficult to understand the primary molecular mechanisms underlying T2D [170].
5. Glutathione Metabolism and Oxidative Enzymes as the Core of Redox Homeostasis
Cellular redox homeostasis is a crucial dynamic process that maintains the balance of reducing and oxidizing reactions within cells and governs a wide range of biological responses and events. Reactive oxygen species are required for life and play a role in practically all biological activities. Excess ROS are neutralized by antioxidant enzymes that catalyze the conversion of reactive oxygen species and their byproducts into stable harmless compounds. Redox homeostasis controls a wide range of biological responses and events by ensuring the balance between oxidation and reduction reactions within the cell [171]. The roles of reactive oxygen species in redox homeostasis and cellular signaling were reviewed in detail [172,173]. Increased levels of reactive oxygen species (ROS) damage macromolecules, impair redox signaling, and were found to be associated with beta-cell dysfunction and insulin resistance [174,175,176]. To reduce the harm of free radicals, cells use both enzymatic and non-enzymatic antioxidant mechanisms. Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione S-transferases (GST) comprise the enzymatic part of antioxidant defense, while vitamin A, ascorbic acid (vitamin C), and alpha-tocopherol (vitamin E) represent non-enzymatic antioxidants [177,178]. Supplementary Table S1 summarizes the biological functions and tissue-specific gene expression of antioxidant enzymes that fight against oxidative stress by multiple mechanisms, including the conversion of superoxide anion into hydrogen peroxide (SOD), breakdown of hydrogen peroxide (CAT, GPX), reduction of disulfide bonds into dithiol-containing ones (thioredoxin reductase), biosynthesis of glutathione (glutamate-cysteine ligase, glutathione synthase, glutathione reductase), and conjugation of xenobiotic substrates with glutathione (GST).
Glutathione-dependent enzymes constitute a crucial antioxidant system and control thiol-disulfide equilibrium. Thiol-dependent peroxidases require electrons to deactivate free radicals with the help of reduced glutathione (GSH). Glutathione is the master antioxidant, capable of protecting cellular components from reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals; it also participates in DNA repair, immunological response, and controls cell death [179]. Furthermore, glutathione determines the activity of several enzymes, acts as a mediator of signaling pathways, regulates the cellular life cycle and division, and promotes protein folding as well as acts as a cysteine depot and a source of vitamins C and E [174,180]. Glutathione is converted by free radical scavenging into the oxidized form known as the GSSG dimer, which is involved in glutathionylation, post-translational protein modification, and epigenetic control of gene expression [181,182,183].
The main metabolic pathway for glutathione biosynthesis is the gamma-glutamyl cycle, which begins with the ATP-dependent formation of gamma-glutamylcysteine from glutamate and cysteine (https://www.kegg.jp/pathway/map00480, accessed on 22 January 2022). The reaction is catalyzed by the enzyme glutamate cysteine ligase (GCL), consisting of modifying (GCLM) and catalytic (GCLC) subunits [184]. In the second step, glutathione synthetase (GSS) is used to add glycine to the cysteine residue of the γ-Glu-Cys dipeptide. GSH is then released from the cell. Since the concentration of glutathione in blood plasma is 1000 times lower than that in a cell’s cytosol, the current gradient acts as a driving force for glutathione transport. Once GSH has left the cell, gamma-glutamyl transferase (GGT) replaces the glutathione-Cys-Gly moiety with an alternative amino acid (phenylalanine, leucine, or lysine). Following the hydrolysis of the dipeptide Cys-Gly by dipeptidase into cysteine and glycine, these amino acids are delivered into the cell via specialized membrane transporters [185]. Gamma-glutamyl-amino acid, a product of the GGT reaction, is also transported into the cell, where it is converted into an amino acid and oxoproline by gamma-glutamyl cyclotransferase (GGCT). With the use of a mole of ATP, oxoproline is transformed into glutamic acid by the enzyme oxoprolinase. Therefore, the cell contains all three of the amino acids required to replenish GSH. In order to assure the formation of GSH and to supply some positively charged (lysine) and non-polar (phenylalanine, leucine) amino acids into the cell, one cycle turn requires the consumption of three ATP molecules [186].
The ChaC-family of proteins [187], which catalyzes a process similar to that of the GGCT enzyme but uses glutathione itself as a substrate, is one of the new enzymes implicated in glutathione catabolism that have been discovered in recent years. This fact has led to a considerable revision of theories regarding GSH degradation and the roles of GGT and GGCT in glutathione metabolism. GGT catalyzes the hydrolysis of GSH into glutamate and cysteinylglycine (1): transpeptidation of amino acids (2):
GSH + H2O → Glu + Cys-Gly
GSH + AA → γ-Glu-AA + Cys-Gly
Several experimental studies showed that there is no connection between GGT activity and the transport of amino acids into the cell. Glutathione is transported by membrane glutathione transporters, including the MRP protein (multidrug resistance protein) and the ABC (ATP binding cassette) family transporter [180]. As it turns out, the main function of gamma-glutamyl transferase is the reutilization of GSSG or GSH conjugates, which are released from the cell into plasma, where they undergo conversion into glutamate and Cys-Gly or dimer (Cys-Gly)2 by GGT. The resulting peptides can be transported into the cell by appropriate proteins or can be hydrolyzed into cysteine and glycine, which are taken up by the cells using specific transporters [188]. Thus, glutathione enters the cell “in parts”—in the form of its individual amino acids or dipeptides, which are subsequently used for de novo synthesis of GSH.
Gamma-glutamyl cyclotransferase was first described by Meister as one of the most important enzymes of the γ-glutamyl cycle [185]. GGCT also regulates de novo glutathione synthesis through its activity against glutamylcysteine, which is also a substrate of glutathione synthase [189]. Glutamate cysteine ligase normally generates a product that is targeted toward the production of GSH, in contrast to GGCT, which has a lesser affinity for γ–glutamylcysteine [190]. Finally, under conditions of Cys deficiency, GCL can catalyze the condensation of Glu with a non-Cys amino acid, forming γ-glutamyl-AA, a potential GGCT substrate converted to the amino acid and 5-oxoproline.
The second source of GSH formation is the enzyme glutathione reductase, which catalyzes the reduction of the GSSG dimer into a functionally active monomer according to the equation:
GSSG + NADPH H+ → 2GSH + NADP+
It is well known that viability of the cell depends on maintaining the optimal ratio between reduced to oxidized glutathione (GSH:GSSG) [191,192,193]. A decrease in the activity of any enzyme involved in the metabolism of glutathione may shift the balance between reduced and oxidized glutathione and encourage the buildup of free radicals in tissues. In addition, an increase in the activity of oxidative enzymes generating ROS may cause glutathione depletion.
The main source of ROS in the cell is the mitochondrial electron transport chain, along with NADPH oxidase and nitric oxide synthase [194,195]. ROS are also produced during the folding of proteins, a process of disulfide bond formation in the endoplasmic reticulum, as well as a result of the activity of cytochromes P450 in the metabolism of xenobiotics [196]. Phagocytic cells such as neutrophils and monocytes utilize free radicals to destroy internalized microorganisms, whereas in non-phagocytic cells, ROS act as signaling molecules regulating a variety of biological processes such as cell division, regeneration, cell differentiation, their apoptosis, and cytoskeleton organization [197]. The combination of increased free radical compound production and inefficient antioxidant defense enzyme function creates the foundation for the development of oxidative stress, a pathological condition thought to be responsible for the development of complications in type 2 diabetes [198,199,200].
The biological functions and tissue-specific gene expression of oxidant enzymes producing superoxide anion (NADPH oxidases), hydrogen peroxide (dual oxidases, monoamine oxidases, lysyl oxidase), and hypochlorous acid (myeloperoxidase) are also summarized in Supplemental Table S1.
As mentioned above, NADPH oxidase is the main oxidant enzyme responsible for the generation of superoxide anion radicals:
NADPH·H+ + 2O2 = 2O2·− + NADP+ + H+
As a result of the dismutation reaction, O2 turns into H2O2.
The NADPH oxidase consists of transmembrane catalytic core proteins such as CYBA and CYBB, four cytosolic subunits—NCF1, NCF2, and NCF4 as well as small GTP-ases RAC1/RAC2 [201,202]. Noteworthy is the fact that only CYBB, out of all the NADPH oxidase subunits, has binding domains for the cofactors NADPH, FAD, and two heme molecules. This means that CYBB is directly involved in the formation of superoxide anion.
Nitric oxide synthases (NOS1, NOS2, and NOS3) and myeloperoxidase (MPO), which have been implicated in the development of type 2 diabetes, are also important ROS-generating enzyme families [203,204,205,206]. The three isoforms of the nitric oxide synthase family members catalyze the generation of NO from L-arginine: neuronal (nNOS/NOS1), inducible (iNOS/NOS2), and endothelial (eNOS/NOS3). Impairment in NO generation is known to be associated with endothelial dysfunction, insulin resistance, and diabetes. Several studies have revealed that single nucleotide polymorphisms (SNP) such as rs2297518 of NOS2 and rs1799983 of NOS3 are linked to the onset of T2D and/or its microvascular complications [206,207,208,209].
In addition, decreased antioxidant defense is a common finding in type 2 diabetes, which may be partially attributed to the spontaneous glycosylation of key antioxidant enzymes such as catalase, superoxide dismutase, glutathione-S-transferases, glutathione peroxidase, glutathione reductase, and others, as well as depletion of the intracellular pool of NADPH·H+ [85,210]. It is observed that enhanced conversion of sorbitol to fructose under the action of NAD+-dependent sorbitol dehydrogenase reduces the ratio of NAD+/NADH·H+ in the cell, leading to inhibition of glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), accumulation of glyceraldehyde-3-phosphate and its conversion to diacylglycerol, a known allosteric activator of protein kinase C [211]. GAPDH, along with advanced glycation end products (AGEs), serves as a potent inducer of NADPH oxidase [212].
Reactive oxygen and nitrogen species and carbonyl compounds are respectively generated under oxidative, nitrosative, and carbonyl stress conditions, which have been found to play a role in the development and progression of type 2 diabetes [85]. Dysregulation of redox homeostasis in T2D was demonstrated at both genetic and biochemical levels [204].
6. Endogenous Deficiency of Glutathione in Type 2 Diabetes
Numerous environmental factors have been identified as risk factors for type 2 diabetes, and many of these same factors are responsible for the depletion of the endogenous glutathione pool, highlighting the importance of glutathione and redox homeostasis in the development of type 2 diabetes. Glutathione is the most prevalent non-protein thiol in cells that functions as the primary reducing agent and provides antioxidant defense against oxidative cell damage [213]. The millimolar level of reduced glutathione is maintained within cells, illustrating its essential biological functions that extend beyond antioxidant defense [214]. Glutathione is required for the detoxification of xenobiotics and endogenous toxic substances, the maintenance of mitochondrial redox balance, direct antiviral defense, immune response, vitamin C and E regeneration, control of cell proliferation, apoptosis, and protein folding [213,214,215]. Given the wide range of important biological functions of reduced glutathione, it is predicted that a GSH deficit plays a substantial role in the onset of a number of disorders [216,217]. It is observed that individuals with type 2 diabetes exhibit impaired redox homeostasis and oxidative stress attributed to, on the one hand, a deficiency in the production of endogenous antioxidants and, on the other hand, an excess of free radicals [218]. Decreased antioxidant defense and oxidative stress are linked to key pathophysiological abnormalities underlying type 2 diabetes mellitus, such as pancreatic beta-cell dysfunction and insulin resistance [219,220,221]. Lutchmansingh and colleagues discovered that T2D patients have lower levels of the reduced form of glutathione and a slower rate of its synthesis in erythrocytes than healthy individuals [193]. Furthermore, several studies [193,222,223] have revealed that depletion of the endogenous glutathione pool correlated to increased blood glucose levels and contributed to disease complications. An experimental study by Stumvoll and colleagues observed that glutathione appears to have the ability to prevent beta-cell insufficiency and reduce glucose tolerance in rats fed a long-term high-glucose diet [223].
Reduced glutathione synthesis in T2D is thought to be caused by a lack of the amino acid precursors of GSH, cysteine, and glycine [224], whereas replenishing glutathione deficiency in diabetic patients leads to improvements in metabolic disorders and disease symptoms. In particular, intravenous glutathione infusions were found to improve tissue insulin resistance [225]. The induction of GSH biosynthesis by activation of the PI3K/Akt/p70S6K signaling pathway was found to significantly improve the therapeutic effect of insulin therapy in patients with T2D [226]. A study by Murakami and colleagues found a decreased activity of key enzymes responsible for glutathione biosynthesis, such as glutamate-cysteine ligase and glutathione reductase, along with decreased levels of reduced glutathione and increased levels of oxidized glutathione in the erythrocytes of type 2 diabetics [227]. In addition, some studies showed decreased rates in the membrane transport of glutathione disulfide in diabetics [228,229]. Inhibiting glutamate-cysteine ligase activity resulted in increased hydrogen peroxide accumulation and decreased insulin transcript levels in pancreatic beta-cells [230], suggesting an important role for glutathione in the regulation of the transcriptional activity of insulin.
The ability of cells in different tissues and organs to synthesize glutathione varies, and many tissues with a limited capacity to synthesize GSH require amino acid precursors from extracellular glutathione synthesized in the liver to enter the cell. The liver, skeletal muscles, and kidneys are examples of tissues and organs with significant metabolic activity and glutathione synthesis, whereas the pancreas, despite increased activity of biosynthetic processes, has a considerably lower potential to produce glutathione [231,232]. In the pancreas, expression of GGT1, GGT6, and ANPEP enzymes, which catabolize extracellular glutathione, is far more abundant than glutathione biosynthesis enzymes such as GCLC, GCLM, GSS, GSR, and GGCT (Supplementary Table S1), meaning that the pancreatic tissues need glutathione transported from the liver [233]. It is critically important that gamma-glutamyl transferase and aminopeptidase N (ANPEP), membrane-associated enzymes that catalyze the breakdown of extracellular GSH into its individual amino acids and promote its uptake into the cell, are responsible for controlling this process [234,235]. This means the pancreas is dependent on the activity of membrane-associated enzymes that catabolize GSH and provide amino acid precursors for the synthesis of glutathione in the cell. Notably, patients with T2D and prediabetes had significantly higher plasma levels of gamma-glutamyl transferase [236,237] and mRNA levels of ANPEP in pancreatic islets than non-diabetics [238,239]. We recently hypothesized that increased gamma-glutamyltransferase and aminopeptidase N levels reflect a cell’s adaptive response to intracellular glutathione deficiency found in T2D, and that an increase in these enzyme levels is required to promote extracellular peptide degradation, providing amino acid precursors for de novo GSH biosynthesis [240]. We also suggested that glutathione deficiency may contribute to the disruption of protein folding in the endoplasmic reticulum [240], since glutathione is required for promoting the formation of disulfide bonds in the tertiary structure of proteins and correcting non-naive formed S-S-bonds [241,242]. Impaired proinsulin folding, observed in type 2 diabetes by several studies [242,243,244,245,246], may be attributed to reduced glutathione level in pancreatic beta-cells. The accumulation of unfolded or misfolded proinsulin molecules may trigger endoplasmic reticulum stress and the unfolded protein response—abnormalities culminating in beta-cell apoptosis in type 2 diabetes [245,247,248]. Deeper genetic and biochemical studies of the redox homeostasis system using cell lines and animal models will shed light on the mechanisms by which glutathione deficiency triggers the development and progression of type 2 diabetes mellitus.
7. Genes Encoding Antioxidant Defense Enzymes and the Risk of Type 2 Diabetes
Variation in genes encoding antioxidant and oxidative enzymes may affect their amount or function, shifting redox homeostasis towards oxidative stress and increasing the risk of type 2 diabetes mellitus. Several genetic association studies have shown that single nucleotide polymorphisms (SNP) in genes for redox state-regulating enzymes are linked with T2D susceptibility. Table 1 summarizes the results of genetic studies that identified associations between these genes and the risk of type 2 diabetes. In particular, the following polymorphisms at genes encoding antioxidant defense enzymes were found to be associated with the risk of type 2 diabetes: del/del of GSTM1 [249,250,251,252,253,254], del/+ GSTT1 [249,250,251], rs1695 [251,252,255] and rs1138272 [249] of GSTP1, rs12524494 of GCLC, rs3827715 and rs41303970 of GCLM [256], rs13041792 of GSS [240], rs2551715 of GSR [257], rs11546155 and rs6119534 of GGT7 [240], rs4270 of GGCT [258], rs1050450 of GPX1 [259], rs4902346 of GPX2 [260], rs769217 of CAT [261], rs2234694 of SOD1 [261], rs4880 of SOD2 [212], and rs2536512 of SOD3 [262]. However, much of the genetic research has focused on polymorphisms in the glutathione S-transferases M1, T1, and P1 (enzymes that used reduced glutathione for xenobiotic conjugation reactions); loss-of-function variants of these genes have been linked to an increased risk of type 2 diabetes. A limited number of studies looked into the link between T2D and genetic variations in hydrogen peroxide-metabolizing enzymes such as superoxide dismutases types 1, 2, and 3, glutathione peroxidases types 1 and 2, and catalase. The genes encoding enzymes directly involved in the metabolism of glutathione have received less attention in diabetes research. The great majority of the investigations were undertaken by our research team to explore for a link between polymorphisms at genes for glutathione metabolism and T2D susceptibility. Below, we describe the results of genetic studies that observed the associations between polymorphisms of genes for antioxidant enzymes and type 2 diabetes risk.
Glutamate cysteine ligase (GCL), also known as gamma-glutamylcysteine synthetase, is the first rate-limiting enzyme in glutathione biosynthesis from L-cysteine and L-glutamate. The enzyme is composed of two subunits: a heavy catalytic subunit (GCLC) and a light regulatory subunit (GCLM). GCLC gene is highly expressed in the liver whereas GCLM has the highest expression in pancreatic islets (Supplementary Table S1). Our recent study showed that polymorphisms such as rs12524494 in the GCLC gene and rs41303970 in the 5’-flanking region of the GCLM gene confer protection against T2D in nonsmokers [256]. The minor allele rs3827715-C GCLM also showed an association with a decreased risk of T2D. In addition, rs2301022 GCLM was associated with decreased levels of ROS, while SNPs rs7517826 and rs41303970 of the gene were associated with increased levels of total GSH in the plasma of T2D patients. These findings are supported by the eQTL analysis, which showed that the rs41303970-A allele was associated with a decreased level of GCLM gene expression in various tissues, including the pancreas. It is known that the rs41303970-A allele GCLM suppresses oxidant-induced gene expression and is associated with lower plasma levels of reduced glutathione and a risk of myocardial infarction [263]. A study by Ma et al. showed that the rs41303970-A allele GCLM was associated with liver damage in patients with viral hepatitis B [264]. Chromatin immunoprecipitation and high-throughput sequencing data from the Roadmap Epigenomics Consortium project show that rs41303970 was located in the region of epigenetic regulation of GCLM gene expression in numerous tissues, including pancreatic islets. In particular, SNP rs41303970 is located at the transcription start site and was associated with an epigenetic modification in the region of the H3K4me3 promoter [265]. Moreover, rs41303970 is located in the region of the histone modifier H3K27ac [266]. In addition, rs41303970 falls into the region of the H3K9ac epigenetic modification (acetylation at the 9th lysine residue of the H3 histone protein) [267]. Thus, the observed protective effect of the rs41303970-A allele against the risk of T2D is apparently attributed to its positive effect on the GCLM gene expression, thereby increasing glutathione production in the cell.
Glutathione synthetase (GSS) is the enzyme responsible for the second step in glutathione biosynthesis. GSS is highly expressed in the liver and pancreatic islets (Supplementary Table S1). Although the GSS gene is polymorphic, no studies investigating the relationship between its polymorphisms and diseases have been conducted so far. The rs13041792 polymorphism of the GSS gene is associated with the level of protein C in blood plasma [268]. Our recent study showed that carriers of the rs13041792-G/A genotype possess an increased risk of type 2 diabetes [240]. The GTEx portal’s data show that the rs13041792 polymorphism has no effect on expression of the GSS gene in the pancreas and other tissues. Instead, the rs13041792-A allele is associated with increased pancreatic expression of other genes such as EDEM2, PROCR, and MYH7B. We also observed that GSS gene polymorphisms correlated with redox homeostasis parameters, such as the levels of glutathione and ROS, as well as with fasting blood glucose [240]. The relationship between the variation at the GSS gene and susceptibility to T2D is also supported by the finding that the rs6088660-C and rs13041792-A alleles are associated with increased levels of fasting blood glucose [240]. Taken together, these findings may indicate that the GSS gene polymorphisms are functionally significant variants with the potential to affect glutathione metabolism. Functional effects of the SNPs might be attributed to linkage disequilibrium with variants of neighboring genes such as EDEM2, MYH7B, and PROCR. rs13041792 is located in the 5’-untranslated region 1.4 kb from the transcription start of the GSS gene and is in strong linkage disequilibrium (D’ ≥ 0.91) with SNPs in nearby or adjacent genes such as MYH7B (rs6120772, rs7268266, rs6120788, rs3746436, and rs3746435), MIR499A (rs3746444), and TRPC4AP (rs752075). A SNP rs3746444 of MIR499A is one of the polymorphisms strongly linked to the rs13041792 variant of the GSS gene.
Gamma-glutamyl transpeptidase type 1 (GGT1) is an enzyme localized on the surface of almost all types of epithelial cells. It plays a critical role in the regulation of levels of reactive oxygen species (ROS) by maintaining the balance between reduced glutathione and its oxidized form [269]. The GGT1 gene is expressed at a high level in the pancreas; however, the highest expression of GGT1 is detected in the liver (Supplementary Table S1). It is noteworthy that an increase in plasma level of gamma-glutamyltransferase is a common finding in T2D [270,271]. A few studies have been done to assess the relationships between the SNPs of GGT1 gene and the risk of type 2 diabetes. Lee with colleagues observed a weak association between the rs4820599 SNP and the risk of type 2 diabetes [272]. Jinnouchi et al. did not find an association between SNP rs4820599 and disease risk, but it was revealed that this polymorphism was associated with the risk of diabetic retinopathy [273]. Studies of Diergaarde B. with colleagues [274] and Brand with colleagues [275] have identified an association of the rs4820599 polymorphism with the risk of pancreatic cancer and chronic pancreatitis, respectively. Two large studies have established the effect of rs4820599 polymorphism on the level of gamma-glutamyltransferase in blood [276,277]. The rs5751909-A allele is known to be associated with increased levels of GGT1 mRNA in the pancreas and decreased levels in the blood (Supplementary Table S1).
Gamma-glutamyl transpeptidase type 5 (GGT5) is an enzyme which cleaves the gamma-glutamyl peptide bond of glutathione and glutathione S-conjugates such as leukotriene C4 [278]. It is known that GGT5 converts C4 leukotriene to D4 leukotriene, which increases smooth muscle tone and promotes plasma exudation in tissues [279]. The GGT5 gene is expressed in almost all tissues and organs, but to the greatest extent in adipose tissue, kidneys, thyroid gland, peripheral nerves, arteries, and cardiac muscle (Supplementary Table S1). A few studies investigated the association of polymorphisms of the GGT5 gene with human traits or diseases. Astle with colleagues found that the rs2275984-C allele was associated with an increase in granulocytes and monocytes in the white blood cells [280]. In whole blood, the rs2275984-C allele is related with lower expression of glutathione metabolism genes GGT5, GGT1, and GSTT1, as well as UPB1, DDT, SUSD2, and SPECC1L genes, and higher expression of the ADORA2A gene (Supplementary Table S2). All the above genes are located in the 22q11.23 chromosomal segment, and their co-expression seems to be under the control of common cis-regulatory elements-enhancers located in this region, as can be seen from the GeneHancer data available at https://www.genecards.org (accessed on 2 December 2022).
Gamma-glutamyl transpeptidase type 6 (GGT6) is a membrane-bound extracellular enzyme which cleaves gamma-glutamyl peptide bonds in glutathione and transfers it to gamma-glutamyl acceptors [184,185]. Like other gamma-glutamyltransferases, GGT6 is a key regulator of glutathione homeostasis by providing cells with amino acid substrates for GSH biosynthesis [281]. To date, no genetic association studies have been undertaken to investigate the association of GGT6 gene polymorphisms and susceptibility to type 2 diabetes and other diseases.
Gamma-glutamyl transpeptidase 7 (GGT7) is the extracellular membrane-bound enzyme, also known as gamma-glutamyl transferase 7, that breaks gamma-glutamyl peptide bonds in the GSH molecule and transfers gamma-glutamyl compounds to acceptors [281]. GGT7, like other gamma-glutamyltransferases, is essential for maintaining glutathione homeostasis by supplying substrates for glutathione synthesis, particularly in tissues like the pancreas that produce glutathione at a low rate. However, the enzyme mitigates oxidative damage by degrading extracellular glutathione [282]. This pathway, known as the gamma-glutamyl cycle, allows cells to use the released amino acids for de novo GSH synthesis [184]. No genetic association studies have been done to assess the association of GGT7 gene polymorphisms with the risk of any disease. We found in our recent study [240] that the rs6119534-T allele of the GGT7 gene is strongly associated with a decreased risk of T2D, especially among individuals without T2D-related risk factors such as physical inactivity, smoking, stress, insufficient consumption of fresh fruits/vegetables and proteins, excessive consumption of carbohydrates, and low-fiber foods. The rs6119534-T allele is known to be associated with an increased level of the GGT7 and GSS genes in skeletal muscles (Supplementary Table S2). Surprisingly, none of the SNPs are associated with the level of GGT7 gene expression in the pancreas. Like GSS, the GGT7 gene is expressed at a low level in the pancreas compared to other organs and tissues [283,284].

Table 1.
Genetic association studies of enzymes involved in the regulation of redox homeostasis in type 2 diabetes mellitus.
Table 1.
Genetic association studies of enzymes involved in the regulation of redox homeostasis in type 2 diabetes mellitus.
| Gene | Gene Name | Polymorphism/ SNP ID | Genotype/ Allele | Odds Ration [95% CI] | Cofactor | Population | Reference |
|---|---|---|---|---|---|---|---|
| GSTM1 | glutathione S-transferase M1 | Deletion | del/del | 1.99 [1.30–3.05] | males | European (Russians) | [282] |
| del/del | 1.99 [1.46–2.71] | - | Chinese Turkish Japanese Indians Taiwanese Iranians Egyptians | [283] | |||
| del/del | 2.90 [1.76–4.78] | - | Indians | [284] | |||
| del/del | 2.042 [1.254–3.325] | - | Indians | [285] | |||
| del/del | 3.841 [2.280–6.469] | - | Turkish | [286] | |||
| del/del | 1.74 [1.13–2.69] | - | Iranians | [287] | |||
| GSTT1 | glutathione S-transferase T1 | Deletion | del/del | 2.23 [1.22–4.09] | males | European (Russians) | [282] |
| del/del | 1.61 [1.19–2.17] | - | Chinese Turkish Japanese Indians Taiwanese Iranians Egyptians | [283] | |||
| del/del | 2.90 [1.76–4.78] | - | Indians | [284] | |||
| GSTP1 | glutathione S-transferase P1 | rs1138272 | 114A/V | 2.85 [1.44–5.62] | males | European (Russians) | [282] |
| rs1695 | 105I/V | 1.99 [1.20–3.32] | - | Romanian | [288] | ||
| rs1695 | 105I/V | 2.56 [1.47–4.48] | - | Indians | [284] | ||
| rs1695 | 105I/V | 0.397 [0.225–0.701] | - | Indians | [285] | ||
| GCLC | glutamate cysteine ligase catalytic subunit | rs12524494 | G | 0.62 [0.41–0.93] | Nonsmokers | European (Russians) | [289] |
| GCLM | glutamate cysteine ligase modifier subunit | rs3827715 | C | 0.86 [0.75–0.99] | - | European (Russians) | [289] |
| rs41303970 | A | 0.77 [0.63–0.93] | Nonsmokers | European (Russians) | [289] | ||
| GSS | glutathione synthetase | rs13041792 | A | 1.14 [1.01–1.29] | - | European (Russians) | [178] |
| GSR | glutathione reductase | rs2551715 | T/T | 0.33 [0.13–0.82] | BMI < 25 kg/m2 Daily consumption of fresh fruits and vegetables | European (Russians) | [290] |
| GGT7 | gamma-glutamyl transferase 7 | rs11546155 | A/A | 0.42 [0.22–0.80] | - | European (Russians) | [178] |
| rs6119534 | T | 0.85 [0.76–0.95] | - | European (Russians) | [178] | ||
| GGCT | gamma-glutamyl cyclotransferase | rs4270 | T/C-C/C | 0.71 [0.54–0.93] | Nonsmokers; Daily consumption of fresh fruits and vegetables | European (Russians) | [291] |
| GPX1 | glutathione peroxidase 1 | rs1050450 | T/T | 1.76 [1.011–3.066] | - | South Indian | [292] |
| GPX2 | glutathione peroxidase 2 | rs4902346 | G/G | 1.41 [1.02–1.96] | Males | European (Russians) | [293] |
| CAT | catalase | rs769217 | T | 2.94 [1.66–5.23] | - | Egyptians | [294] |
| SOD1 | Superoxide dismutase 1 | rs2234694 | C | 2.9 [1.84–4.6] | - | Egyptians | [294] |
| SOD2 | superoxide dismutase 2 | rs4880 | C | 2.434 [1.413–4.191] | - | North Indian | [145] |
| SOD3 | superoxide dismutase 3 | rs2536512 | GA-AA | 1.64 [1.16–2.33] | - | Chinese | [295] |
| RAC1 | Rac family small GTPase 1 | rs7784465 | T/C | 1.40 [1.20–1.65] | Dietary deficit of fresh fruits and vegetables; Excess of carbohydrates in food; High calorie diet; Psychological stress; Sedentary lifestyle | European (Russians) | [296] |
| CYBA | cytochrome b-245 alpha chain | rs4673 | A/A | 1.60 [1.04–2.46] | Females | European (Russians) | [297] |
| T/T | 1.74 [1.15–2.64] | - | Asians | [298] | |||
| T | 1.30 [1.04–1.61] | - | Non-Asians | ||||
| CYBB | cytochrome b-245 beta chain | rs5963327 | T | 1.7 [1.06–2.75] | Males | European (Russians) | [299] |
| rs6610650 | A | 1.71 [CI 1.05–2.78] | Males | European (Russians) | [299] | ||
| rs5963327 | T/T | 1.35 [1.05–1.73] | Females | European (Russians) | [299] | ||
| rs6610650 | A/A | 1.34 [1.05–1.72] | Females | European (Russians) | [299] | ||
| NCF2 | neutrophil cytosolic factor 2 | rs17849502 | G/T | 1.42 [1.08–1.87] | BMI > 25 kg/m2 | European (Russians) | [300] |
| MPO | Myeloperoxidase | rs2107545 | T/C | 1.563 [1.166–2.096] | - | Han Chinese | [301] |
Gamma-glutamyl cyclotransferase (GGCT) is an enzyme catalyzing the formation of 5-oxoproline from gamma-glutamyl dipeptides. GGCT also causes the release of cytochrome c from mitochondria with subsequent induction of apoptosis [285]. No genetic association studies investigated the contribution of GGCT gene polymorphisms to the development of diabetes. Yu. A. Bocharova found that SNP rs6462210 was associated with a decreased risk of ischemic stroke [286]. In our recent study [258], carriage of the rs4270 T/C-C/C genotypes was associated with a decreased risk of T2D, but this association occurred only in those individuals who were non-smokers and consumed a sufficient amount (approximately 400 g daily) of fresh vegetables and fruits.
Glutathione S-transferase pi 1 (GSTP1) is an enzyme conjugating a reduced glutathione with a large number of exogenous and endogenous hydrophobic electrophilic compounds [287,288] and is involved in the formation of glutathione conjugates of prostaglandins A2 and J2 [289]. The rs1695 (Ile105Val) polymorphism is the most studied variant of the GSTP1 gene in a variety of human diseases such as type 2 diabetes [255], acute lymphoblastic leukemia [290], Hodgkin’s lymphoma [291], breast cancer [292], lung cancer [293], acute pancreatitis [294], Alzheimer’s disease [295], and bronchial asthma [296]. It is also known that another well-studied polymorphism rs1138272 (Ala114Val) of the GSTP1 gene is associated with the risk of T2D [249], bronchial asthma [297], cancers [298], and Parkinson’s disease [299].
Glutathione peroxidase 1 (GPX1) is a member of the glutathione peroxidase family, which catalyzes the reduction of organic hydroperoxides and hydrogen peroxide (H2O2) by glutathione, protecting cells from oxidative damage [300]. A decrease in GPX activity in T2D was first noted in rats with streptozotocin-induced diabetes [300,301]. The literature on associations between SNPs in glutathione peroxidase genes and T2D risk is limited and contradictory. In particular, Tanaka [302] showed that an increase in GPX expression protected beta-cells from the damaging effects of oxidative stress during hyperglycemia, while McClung [303] reported that insulin resistance correlated with increased GPX1 expression. The role of glutathione peroxidase isoforms other than GPX1 in the development of diabetes remains unknown. Huang et al. [304] showed that knockout and overexpression of Gpx1 in mice may induce types 1 and 2 diabetes-like phenotypes. Another study [305] discovered that GPX1 gene polymorphisms protected the kidneys from oxidative damage in type 1 diabetic patients. Liu D. et al. [306] discovered that the GPX1 polymorphism rs1050450 was associated with an increased risk of carotid plaques in T2D patients. In ApoE/mice, a lack of functional Gpx1 accelerated diabetes-associated atherosclerosis by upregulating proinflammatory and profibrotic pathways [307].
Glutathione peroxidase 2 (GPX2) is a member of the glutathione peroxidase family, which encodes a selenium-dependent enzyme responsible for the majority of the glutathione-dependent hydrogen peroxide-reducing activity in the epithelial cells of the gastrointestinal tract. GPX2 is characterized by the highest level of expression in the pancreas according to the data from the BioGPS project [308]. GPX2 is active in the cytosol and mitochondrial matrix where the enzyme protects all parts of the cell from the damaging effects of ROS. Substrates for GPX2 include not only hydrogen peroxide and lipid hydroperoxides, but also peroxynitrite. We observed an association between the rs4902346 polymorphism in the intron of the GPX2 gene and an increased risk of T2D [260]. The minor allele rs4902346-G of GPX2 is known to be associated with a decrease in the expression of the GPX2 gene in the liver, the small intestine, subcutaneous and visceral adipose tissues, nervous tissue and skeletal muscle [309].
8. Genes for ROS-Generating Enzymes and Susceptibility to Type 2 Diabetes
Reactive oxygen and nitrogen (RNS) species, formerly believed to be by-products of cellular metabolism, are known to play important regulatory roles in a number of vital physiological processes, including cell survival, proliferation, differentiation, migration, and adhesion [310]. Superoxide anion and hydrogen peroxide produced by NADPH oxidase (NOX) play a significant role in numerous cellular signaling networks [311]. Currently, seven NOX isoenzymes are known, with tissue-specific expression patterns: NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. The main subunits of NADPH oxidase are cytochrome b-245 light chain (CYBA), cytochrome b-245 heavy chain (CYBB), neutrophil cytosolic factor 1 (NCF1), neutrophil cytosolic factor 2 (NCF2), neutrophil cytosolic factor 4 (NCF4), and small GTPases (RAC1/RAC2). Cytosolic protomers such as NCF1, NCF2, and NCF4 provide activation of two transmembrane domains CYBA and CYBB, which form the catalytic core of the multienzyme complex of NADPH oxidase [311]. This complex catalyzes the one-electron reduction of molecular oxygen to superoxide, which is essential for oxidizing pathogens [312]. All ROS-producing NOX isoforms may transport electrons across membranes and produce superoxide anion and/or hydrogen peroxide, which are signaling molecules impacting on all facets of cell activity [313]. As can be seen from Table 1, several genetic studies showed that polymorphic genes encoding oxidative enzymes are associated with the risk of T2D. These polymorphisms are rs7784465 of RAC1 [253], rs4673 of CYBA [314,315], rs5963327 and rs6610650 of CYBB [316], rs17849502 of NCF2 [317], and rs2107545 of MPO [306]. Below, we describe the results of studies that observed associations between polymorphic genes for oxidative enzymes and T2D susceptibility.
Cytochrome b-245 alpha chain (CYBA) is a critical component of the membrane-bound phagocyte oxidase, which, by binding to CYBB, forms a functionally active NADPH oxidase. Several SNPs were identified in the CYBA gene; for instance, –242C>T (rs4673) in the fourth exon, resulting in amino acid substitution His72Tyr, 640A>G (rs1049255) in the 3-prime-untranslated region, and –930A>G (rs9932581) located in the promoter gene region at the binding site for transcription factor CEBP, affecting transcriptional activity of the CYBA gene [318]. There is experimental evidence that the 242C>T and 640A>G polymorphisms of the CYBA gene affect the binding affinity of the p22phox and gp91phox protein subunits, modulating the activity of NADPH oxidase and the generation of superoxide anions [319,320]. A study by Meijles and colleagues showed that the 242C>T polymorphism caused structural changes in p22phox that inhibited the activation of endothelial NOX2 and the oxidative response to tumor necrosis factor alpha or glucose stimulation [321]. It is known from the literature that the rs4673C allele (polymorphism C242T) is associated with endothelial dysfunction in patients with type 2 diabetes [311], the risk of coronary artery disease [322], T2D [315], peripheral neuropathy in type 1 diabetes mellitus [323], hypertension in patients with type 2 diabetes [324], lung cancer [325], bronchial asthma [326], and some other diseases. It has also been found that the rs4673-A/G genotype, compared to the wild-type G/G genotype, of the CYBA gene is associated with more pronounced liver damage in patients with viral hepatitis B [264]. Our recent study showed a sex-specific association of the CYBA rs4673 polymorphism with the risk of type 2 diabetes in women [314]. It has been suggested that the rs9932581 variant of CYBA modulates the transcriptional activity of the gene promoter through allele-specific binding to CEBP (the −930G allele increases the affinity for this transcription factor to interact with the gene promoter) [327]. In particular, functional studies showed that the promoter with the −930G allele variant had a 30% higher expression of the CYBA gene than the promoter with the −930A allele [318]. It was also found that the −930G allele was associated with increased ROS production [327,328]. SNP rs9932581 is associated with premature development of coronary atherosclerosis [329] and hypertension [318,328]. In addition, SNP rs9932581 is associated with an increased risk of renal complications in patients with type 1 diabetes [330].
Cytochrome b-245 beta chain (CYBB) is an essential component of membrane-bound phagocyte oxidase that produces the superoxide anion. CYBB is the terminal component of the respiratory chain that transfers electrons from the cytoplasmic portion of NADPH oxidase across the plasma membrane to molecular oxygen outside. In addition, CYBB functions as a voltage-gated proton channel that transmits H+ fluxes in inactive phagocytes and is involved in the regulation of cellular pH. CYBB expression is regulated by the nuclear transcription factor NF-κB [331]. The CYBB gene is located at chromosome X. According to the DisGeNET database, polymorphisms in the CYBB gene have been the subject for genetic association studies in hematological and infectious diseases. In particular, the rs6610650A allele of the CYBB gene is associated with a decreased risk of tuberculosis in male smokers [332]. Our study revealed sex-specific associations of rs6610650 and rs5963327 with fasting hyperglycemia and increased risk of T2D in males in the presence of risk factors such as cigarette smoking and diets with excess calories and sugar [316]. Furthermore, the above-mentioned SNPs were related with a predisposition to T2D in females, whereas rs5917471 of CYBB was associated with fasting hyperglycemia in males [316] but not with T2D.
Neutrophil cytosolic factor 2 (NCF2) is a neutrophil cytosolic factor (also known as p67phox), which is part of the NADPH oxidase enzyme complex and is responsible for its activation by binding to the CYBA and CYBB subunits [320]. Few studies have been done to investigate the association of NCF2 gene polymorphisms with human diseases. It is known that rs10911363 and rs17849502 NCF2 are associated with the risk of systemic seropositive rheumatic disease [333], rheumatoid arthritis [334], and arthritis in patients with systemic lupus erythematosus [335]. Our recent study showed that SNP rs17849502 of NCF2 was associated with an increased risk of T2D in overweight and obese patients [317].
Neutrophil cytosolic factor 4 (NCF4) is also known as p40phox and together with other proteins (NCF4, CYBA, and CYBB) is involved in activation of NADPH oxidase [336]. SNP rs4821544 NCF4 is associated with the risk of rheumatoid arthritis [337] and Crohn’s disease [338], whereas rs5995355 is associated with the risk of colorectal cancer [339]. Xing and colleagues [340] found an increase in NCF4 expression in the neutrophils of patients with latent adult autoimmune diabetes (LADA), which contributed to oxidative damage to pancreatic beta-cells. Our recent study showed no significant associations of NCF4 polymorphisms such as rs5995355, rs5995357, rs1883112, rs4821544, rs760519, rs729749, rs2075938, and rs2075939 with the risk of T2D [341]. Nevertheless, we revealed associations of the rs4821544-C/C and rs5995357-A/A genotypes with increased risk of coronary artery disease in females with T2D [341]. In addition, carriers of the rs4821544-C/C genotype had significantly higher levels of glycated hemoglobin and oxidized glutathione.
NADPH oxidase 1 activator (NOXA1) is a functional homologue of p67phox for the activation of NADPH oxidase in vascular smooth muscle cells (SMCs) and plays an important role in atherogenesis [291,342]. NOXA1 is capable of activating CYBB/gp91phox and NOX3 [343]. There is no data on the association of NOXA1 gene variants with T2D risk.
NADPH oxidase 1 (NOX1) is a homologue of the catalytic subunit of the superoxide-generating phagocyte NADPH oxidase, gp91phox. The oxidase activity of the enzyme is regulated by NOXA1 and NOXO1 [344]. As can be seen from the DisGeNET database, association studies of NOX1 gene polymorphisms were carried out on cardiovascular diseases [345].
NADPH oxidase 4 (NOX4) was originally identified as a homologue of NADPH oxidase highly expressed in the kidney [346]. In addition to the kidneys, NOX4 is expressed in smooth muscle cells and vascular endothelium, has protective properties through the modulation of endothelial nitric oxide synthase, and is also characterized by anti-inflammatory and anti-apoptotic effects [347]. It has been established that polymorphisms rs3913535, rs10765219, and rs11018670 of the NOX4 gene are associated with the risk of severe diabetic retinopathy [348]. The rs2164521 variant of NOX4 is associated with a decreased risk of hepatopulmonary syndrome [349].
NADPH oxidase 5 (NOX5) from the NOX family is unique in that it does not require NADPH oxidase subunits for its activation [350]. NOX5 is localized in the regions of the perinuclear and endoplasmic reticulum of cells and, after activation, is directed to the cell membrane. Vasoactive substances, growth factors, and pro-inflammatory cytokines all activate NOX5 and modulate its activity by a variety of post-translational changes [350]. NOX5 hyperactivation is linked to the onset of cardiovascular disease, kidney damage, and cancer. However, the exact pathophysiological functions of NOX5 are still unknown [350]. NOX5 was found to be activated in human diabetic nephropathy and affects the function of the filtration barrier and blood pressure through excessive formation of ROS [351]. Expression of human NOX5 in vascular smooth muscle cells and mesangial cells in mice has been shown to induce oxidative stress, glomerulosclerosis, mesangial expansion, inflammation, and fibrosis in the kidneys: processes that are known to accelerate the progression of renal failure in diabetes [352]. It is known from the literature that the NOX5 gene is also associated with Hirschsprung’s disease [353].
Rac family small GTPase 1 (RAC1) belongs to the Rho family of GTPases that regulates the redox state of the cell by controlling enzymes generating and converting reactive oxygen and nitrogen species [310]. RAC1 is an integral part of the NOX2 holoenzyme. Activation of RAC1 is required for the subsequent generation of ROS. Indeed, RAC1 is directly involved in the assembly and activation of NADPH oxidases (NOX1/2/3), which generate superoxide anion radicals, which in turn activate nuclear factor-κB (NFκB), a transcription factor regulating the expression of redox homeostasis genes such as NOX2 and superoxide dismutase 2 [310]. While NOX2 increases ROS generation, SOD2 converts highly reactive oxygen radicals into less reactive hydrogen peroxide. When redox homeostasis is shifted to the reducing state, ROS activate RAC1 and RhoA, whereas under oxidative stress, these GTPases become inactive [310]. The depletion of intracellular glutathione leads to an increase in active forms of RAC1, replenishing the GSH deficit in the cell by the antioxidant N-acetylcysteine blocks RAC1 activation [354]. Notably, acylation of RAC1 at the amino acid residue Cys178 is a critical process for RAC1-mediated remodeling of the actin cytoskeleton [355], which is important in the regulation of transmembrane glucose transport [356] and insulin secretion in pancreatic beta-cells in T2D [357]. The suppression of RAC1 protects β-cells from the damaging effects of glucose, fatty acids, and pro-inflammatory cytokines [358,359,360]. Interestingly, one of the main stages in the development of metabolic dysregulation of islet beta-cells in type 2 diabetes is thought to be sustained activation of RAC1 [361]. It is important to note that chronic activation of RAC1 has been found in many diseases, including cancer, neurodegenerative diseases (Parkinson’s and Alzheimer’s diseases), and cardiometabolic disorders, including type 2 diabetes [362,363].
In our recent study, we discovered that the RAC1 gene polymorphism rs7784465 was associated with the risk of T2D in individuals with a lower daily intake of fresh fruits and vegetables, as well as those who experienced psycho-emotional stress and physical inactivity [314]. rs10951982, another RAC1 gene polymorphism, was found to be protective against T2D risk in subjects who did not abuse food with excessive amount of carbohydrates. It was also revealed that another SNP, rs836478 of RAC1, was associated with elevated levels of glycated hemoglobin and fasting blood glucose. In addition, the RAC1 gene polymorphisms were associated with increased plasma levels of hydrogen peroxide and uric acid in T2D patients [364]. Few studies have been undertaken to assess the association between RAC1 gene polymorphisms and other human diseases. A meta-analysis of genome-wide association studies showed that SNP rs7784465 was associated with body mass index [365].
Rac family small GTPase 2 (RAC2), like RAC1, belongs to the family of Rho GTPases that regulates cellular redox homeostasis. RAC2 is involved in the activation of NADPH oxidase (NOX2), increasing ROS production [366]. The active form of RAC2 binds to various effector proteins that regulate a variety of cellular processes, such as secretion, phagocytosis of apoptotic cells, and polarization of epithelial cells [367]. RAC2 interacts with inducible NO synthase, producing nitric oxide [368]. According to the DisGeNET database, a limited number of genetic association studies have been done to explore the association between RAC2 gene polymorphisms and the risk of oncological, inflammatory, and hematological diseases.
In summarizing the findings of genetic studies, it should be noted that a significant proportion of established associations between redox homeostasis gene polymorphisms and type 2 diabetes have weak or moderate effects on disease risk, and thus these findings require confirmation in independent populations as well as experimental functional annotation of disease-associated alleles.
9. Conclusions
Numerous studies have demonstrated that individuals with type 2 diabetes exhibit impaired redox homeostasis and oxidative stress attributed to, on the one hand, a deficiency in the production of endogenous antioxidants, mainly glutathione, and, on the other hand, an excess of free radicals. In type 2 diabetes, increased levels of reactive oxygen species and oxidative stress were found to be associated with pathological conditions, such as beta-cell dysfunction and insulin resistance. Patients with T2D have lower levels of reduced glutathione and a slower rate of its biosynthesis than healthy individuals, and the decreased level of glutathione correlated with increased blood glucose levels and disease progression. Glutathione has also been demonstrated to inhibit beta-cell dedifferentiation and failure caused by chronic oscillating glucose consumption [34].
Polymorphisms at genes encoding antioxidant defense and oxidative enzymes have the potential to impact redox homeostasis and therefore they represent attractive markers for testing the genetic susceptibility to type 2 diabetes. Several studies have been done to evaluate whether polymorphisms in genes for glutathione S-transferases, superoxide dismutases, glutathione peroxidases, cytochrome b-245 alpha chain, and myeloperoxidase are associated with T2D susceptibility. These studies showed that variants at the oxidative stress-related genes were significantly associated with diabetes risk. Meanwhile, a limited number of studies, a significant proportion of which were carried out by our research team, looked into the link between T2D and genetic variations in glutathione-metabolizing enzymes such as glutamate cysteine ligase, glutathione synthetase, glutathione reductase, gamma-glutamyl cyclotransferase, and gamma-glutamyltransferases. Loss of function variants at genes encoding glutathione metabolizing enzymes, together with environmental factors, contribute to an intracellular glutathione deficiency in type 2 diabetes. The mechanism by which genes encoding enzymes involved in the regulation of redox homeostasis contribute to the development of type 2 diabetes mellitus is summarized in Figure 1.
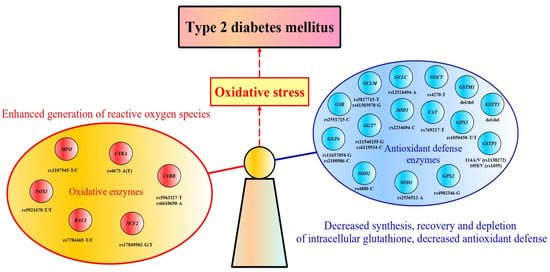
Figure 1.
The relationship between genes encoding enzymes involved in the regulation of redox homeostasis and the development of type 2 diabetes. The scheme reflects the genetic causes responsible for oxidative stress in type 2 diabetes as a result of an imbalance between the generation of free radicals and their neutralization, which is attributed to the impact of polymorphisms in the genes encoding oxidative and antioxidant defense enzymes. Genotypes and alleles of the increased disease risk are shown along the genes. GSTM1, glutathione S-transferase M1; GSTT1, glutathione S-transferase T1; GSTP1, glutathione S-transferase P1; GCLC, glutamate cysteine ligase catalytic subunit; GCLM, glutamate cysteine ligase modifier subunit; GSS, glutathione synthetase; GSR, glutathione reductase; GGT6, gamma-glutamyl transferase 6; GGT7, gamma-glutamyl transferase 7; GGCT, gamma-glutamyl cyclotransferase; GPX1, glutathione peroxidase 1; GPX2, glutathione peroxidase 2; CAT, catalase; SOD1, Superoxide dismutase; SOD2, superoxide dismutase 2; SOD3, superoxide dismutase 3; RAC1, Rac family small GTPase 1; CYBA, cytochrome b-245 alpha chain; CYBB, cytochrome b-245 beta chain; NCF2, neutrophil cytosolic factor 2; NOX1, NADPH oxidase1; MPO, Myeloperoxidase.
The most significant contribution to T2D predisposition is attributed to polymorphisms of genes such as GCLM, GSS, GGT1, GGT7, GSTM1, GSTT1, GSTP1, GPX2, RAC1, CYBA, CYBB, and NCF2. The effects of each gene polymorphism on redox homeostasis and disease risk may vary significantly across populations, and this variation is attributed to interpopulation differences in minor allele frequencies and linkage disequilibrium between SNPs, as recently demonstrated in a number of studies [240,369,370]. The link between polymorphic genes involved in redox homeostasis, such as GCLM (rs2301022), GGT7 (rs6119534, rs11546155), GSTM1 (+/del), GSTP1 (rs1695, rs1138272), RAC1 (rs7784465), and CYBB (rs5917471), and type 2 diabetes differs in overweight and normal-weight individuals. The impact of genetic variants of these enzymes on disease risk is triggered by environmental factors such as insufficient intake of proteins, fresh vegetables and fruits, as well as smoking and physical inactivity. Further research is needed to uncover the molecular mechanisms by which interactions between genetic and environmental factors contribute to type 2 diabetes pathogenesis via alterations in redox homeostasis. In particular, the pathophysiological relationship between glutathione deficiency and T2D development could be explained not only by the development of oxidative stress but also by other mechanisms. Since T2D-associated alleles of genes for glutathione metabolism (e.g., GSS and GGT7) correlate with expression levels of genes involved in the unfolded protein response pathway and regulation of proteostasis [240], it can be hypothesized that glutathione deficiency in beta-cells of pancreatic islets may contribute to the impaired folding of proinsulin identified in type 2 diabetes.
Understanding the importance of redox homeostasis in the pathogenesis of type 2 diabetes substantiates the need for antioxidant treatment focusing on replenishing the endogenous glutathione pool, and several clinical studies have already shown the efficacy and safety of this approach in diabetics [371,372,373]. However, this type of therapy for patients with type 2 diabetes has not been implemented into endocrinological practice or included in any country’s national clinical recommendations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24054738/s1.
Author Contributions
Conceptualization, A.P.; writing—original draft preparation, I.A. and A.P.; writing—review and editing, I.A., A.P. and E.K.; visualization, A.P.; supervision, A.P.; funding acquisition, I.A., A.P. and E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation, project number 20-15-00227.
Acknowledgments
Illustration in the text was created with yEd Graph Editor, version 3.22.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2021, 183, 109119. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2021, 183, 109118. [Google Scholar] [CrossRef]
- Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; 86p.
- IDF Diabetes Atlas [Internet], 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021.
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Haggard, S.; Jeon, S.; Ranard, K.; Tao, D.; Pan, Y.X. Early-life Programming of Type 2 Diabetes Mellitus: Understanding the Association between Epigenetics/Genetics and Environmental Factors. Curr. Genom. 2019, 20, 453–463. [Google Scholar] [CrossRef]
- Beulens, J.W.J.; Pinho, M.G.M.; Abreu, T.C.; Braver, N.R.D.; Lam, T.M.; Huss, A.; Vlaanderen, J.; Sonnenschein, T.; Siddiqui, N.Z.; Yuan, Z.; et al. Environmental risk factors of type 2 diabetes—An exposome approach. Diabetologia 2021, 65, 263–274. [Google Scholar] [CrossRef]
- Laakso, M.; Silva, L.F. Genetics of Type 2 Diabetes: Past, Present, and Future. Nutrients 2022, 14, 3201. [Google Scholar] [CrossRef]
- Fuchsberger, C.; Flannick, J.; Teslovich, T.M.; Mahajan, A.; Agarwala, V.; Gaulton, K.J.; Ma, C.; Fontanillas, P.; Moutsianas, L.; McCarthy, D.J.; et al. The genetic architecture of type 2 diabetes. Nature 2016, 536, 41–47. [Google Scholar] [CrossRef]
- Scott, R.A.; Scott, L.J.; Mägi, R.; Marullo, L.; Gaulton, K.J.; Kaakinen, M.; Pervjakova, N.; Pers, T.H.; Johnson, A.D.; Eicher, J.D.; et al. An Expanded Genome-Wide Association Study of Type 2 Diabetes in Europeans. Diabetes 2017, 66, 2888–2902. [Google Scholar] [CrossRef]
- Yahaya, T.O. A Review of Type 2 Diabetes Mellitus Predisposing Genes. Curr. Diabetes Rev. 2019, 16, 52–61. [Google Scholar] [CrossRef]
- Chen, J.; Spracklen, C.N.; Marenne, G.; Varshney, A.; Corbin, L.J.; Luan, J.; Willems, S.M.; Wu, Y.; Zhang, X.; Horikoshi, M.; et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 2021, 53, 840–860. [Google Scholar] [CrossRef]
- DeForest, N.; Majithia, A.R. Genetics of Type 2 Diabetes: Implications from Large-Scale Studies. Curr. Diabetes Rep. 2022, 22, 227–235. [Google Scholar] [CrossRef]
- Maglott, D.; Ostell, J.; Pruitt, K.; Tatusova, T. Entrez Gene: Gene-centered information at NCBI. Nucleic Acids Res. 2004, 33, D54–D58. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Placzek, S.; Schomburg, I.; Chang, A.; Jeske, L.; Ulbrich, M.; Tillack, J.; Schomburg, D. BRENDA in 2017: New perspectives and new tools in BRENDA. Nucleic Acids Res. 2016, 45, D380–D388. [Google Scholar] [CrossRef]
- Roux-Rouquie, M.; Chauvet, M.-L.; Munnich, A.; Frezal, J. Human Genes Involved in Chromatin Remodeling in Transcription Initiation, and Associated Diseases: An Overview Using the GENATLAS Database. Mol. Genet. Metab. 1999, 67, 261–277. [Google Scholar] [CrossRef]
- Braschi, B.; Denny, P.; Gray, K. Genenames.org: The HGNC and VGNC resources in 2019. Nucleic Acids Res. 2019, 47, D786–D792. [Google Scholar] [CrossRef]
- Barshir, R.; Fishilevich, S.; Iny-Stein, T.; Zelig, O.; Mazor, Y.; Guan-Golan, Y.; Safran, M.; Lancet, D. GeneCaRNA: A Comprehensive Gene-centric Database of Human Non-coding RNAs in the GeneCards Suite. J. Mol. Biol. 2021, 433, 166913. [Google Scholar] [CrossRef]
- Wu, C.; Jin, X.; Tsueng, G. BioGPS: Building your own mash-up of gene annotations and expression profiles. Nucleic Acids Res. 2016, 44, D313–D316. [Google Scholar] [CrossRef]
- Carithers, L.J.; Carithers, L.J.; Moore, H.M. The genotype-tissue expression (GTEx) project. Biopreservat. Biobank. 2015, 13, 307–308. [Google Scholar] [CrossRef]
- Võsa, U.; Claringbould, A.; Westra, H.J. Unraveling the polygenic architecture of complex traits using blood eQTL metaanalysis. BioRxiv 2018, 2, 447367. [Google Scholar] [CrossRef]
- MacArthur, J.; Bowler, E.; Cerezo, M. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017, 45, D896–D901. [Google Scholar] [CrossRef]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Kahn, S. The importance of the β-cell in the pathogenesis of type 2 diabetes mellitus. Am. J. Med. 2000, 108, 2–8. [Google Scholar] [CrossRef]
- Batista, T.M.; Haider, N.; Kahn, C.R. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia 2021, 64, 994–1006. [Google Scholar] [CrossRef]
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.-P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005, 2, 153–163. [Google Scholar] [CrossRef]
- Kaneto, H.; Kimura, T.; Shimoda, M.; Obata, A.; Sanada, J.; Fushimi, Y.; Matsuoka, T.-A.; Kaku, K. Molecular Mechanism of Pancreatic β-Cell Failure in Type 2 Diabetes Mellitus. Biomedicines 2022, 10, 818. [Google Scholar] [CrossRef]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.-C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes, Obes. Metab. 2008, 10, 32–42. [Google Scholar] [CrossRef]
- Marselli, L.; Suleiman, M.; Masini, M.; Campani, D.; Bugliani, M.; Syed, F.; Martino, L.; Focosi, D.; Scatena, F.; Olimpico, F.; et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 2013, 57, 362–365. [Google Scholar] [CrossRef]
- Brereton, M.F.; Iberl, M.; Shimomura, K.; Zhang, Q.; Adriaenssens, A.E.; Proks, P.; Spiliotis, I.I.; Dace, W.; Mattis, K.K.; Ramracheya, R.; et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat. Commun. 2014, 5, 4639. [Google Scholar] [CrossRef]
- Khin, P.-P.; Lee, J.-H.; Jun, H.-S. A Brief Review of the Mechanisms of β-Cell Dedifferentiation in Type 2 Diabetes. Nutrients 2021, 13, 1593. [Google Scholar] [CrossRef]
- Spijker, H.S.; Song, H.; Ellenbroek, J.H.; Roefs, M.M.; Engelse, M.A.; Bos, E.; Koster, A.J.; Rabelink, T.J.; Hansen, B.C.; Clark, A.; et al. Loss of β-Cell Identity Occurs in Type 2 Diabetes and Is Associated With Islet Amyloid Deposits. Diabetes 2015, 64, 2928–2938. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β Cell Dedifferentiation as a Mechanism of Diabetic β Cell Failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Marroqui, L.; Masini, M.; Merino, B.; Grieco, F.A.; Millard, I.; Dubois, C.; Quesada, I.; Marchetti, P.; Cnop, M.; Eizirik, D.L. Pancreatic α Cells are Resistant to Metabolic Stress-induced Apoptosis in Type 2 Diabetes. Ebiomedicine 2015, 2, 378–385. [Google Scholar] [CrossRef]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Marangoni, R.G.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef]
- Ahmad, K.; Lee, E.J.; Moon, J.S.; Park, S.-Y.; Choi, I. Multifaceted Interweaving Between Extracellular Matrix, Insulin Resistance, and Skeletal Muscle. Cells 2018, 7, 148. [Google Scholar] [CrossRef]
- Kang, L.; Ayala, J.E.; Lee-Young, R.S.; Zhang, Z.; James, F.D.; Neufer, P.D.; Pozzi, A.; Zutter, M.M.; Wasserman, D.H. Diet-Induced Muscle Insulin Resistance Is Associated With Extracellular Matrix Remodeling and Interaction With Integrin α2β1 in Mice. Diabetes 2011, 60, 416–426. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.-L.; Torcivia, A.; Sasso, M.; et al. Association of Adipose Tissue and Liver Fibrosis With Tissue Stiffness in Morbid Obesity: Links With Diabetes and BMI Loss After Gastric Bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Gurav, K.; Chouinard, M.; Straubhaar, J.; Thompson, M.; Malkani, S.; Hartigan, C.; Corvera, S. Control of Adipose Tissue Expandability in Response to High Fat Diet by the Insulin-like Growth Factor-binding Protein-4. J. Biol. Chem. 2014, 289, 18327–18338. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wallace, M.; Sanchez-Gurmaches, J.; Hsiao, W.-Y.; Li, H.; Lee, P.L.; Vernia, S.; Metallo, C.M.; Guertin, D.A. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun. 2016, 7, 11365. [Google Scholar] [CrossRef]
- Eldakhakhny, B.M.; Al Sadoun, H.; Choudhry, H.; Mobashir, M. In-Silico Study of Immune System Associated Genes in Case of Type-2 Diabetes With Insulin Action and Resistance, and/or Obesity. Front. Endocrinol. 2021, 12, 641888. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schön, M.R.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef]
- Baraille, F.; Planchais, J.; Dentin, R.; Guilmeau, S.; Postic, C. Integration of ChREBP-Mediated Glucose Sensing into Whole Body Metabolism. Physiology 2015, 30, 428–437. [Google Scholar] [CrossRef]
- Song, Z.; Yang, H.; Zhou, L.; Yang, F. Glucose-Sensing Transcription Factor MondoA/ChREBP as Targets for Type 2 Diabetes: Opportunities and Challenges. Int. J. Mol. Sci. 2019, 20, 5132. [Google Scholar] [CrossRef]
- Dutta, A.; Abmayr, S.M.; Workman, J.L. Diverse Activities of Histone Acylations Connect Metabolism to Chromatin Function. Mol. Cell 2016, 63, 547–552. [Google Scholar] [CrossRef]
- McDonnell, E.; Crown, S.B.; Fox, D.B.; Kitir, B.; Ilkayeva, O.R.; Olsen, C.A.; Grimsrud, P.A.; Hirschey, M.D. Lipids Reprogram Metabolism to Become a Major Carbon Source for Histone Acetylation. Cell Rep. 2016, 17, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, S.; Shahidian, L.Z.; Schneider, R. Histone acylations and chromatin dynamics: Concepts, challenges, and links to metabolism. EMBO Rep. 2021, 22, e52774. [Google Scholar] [CrossRef] [PubMed]
- Staimez, L.R.; Deepa, M.; Ali, M.; Mohan, V.; Hanson, R.L.; Narayan, K.V. Tale of two Indians: Heterogeneity in type 2 diabetes pathophysiology. Diabetes/Metab. Res. Rev. 2019, 35, e3192. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.M.V.; Kondal, D.; Kobes, S.; Staimez, L.R.; Mohan, D.; Gujral, U.P.; Patel, S.; Anjana, R.M.; Shivashankar, R.; Ali, M.K.; et al. Incidence of diabetes in South Asian young adults compared to Pima Indians. BMJ Open Diabetes Res. Care 2021, 9, e001988. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- White, M.F.; Kahn, C.R. Insulin action at a molecular level–100 years of progress. Mol. Metab. 2021, 52, 101304. [Google Scholar] [CrossRef]
- Parker, V.E.R.; Savage, D.B.; O’Rahilly, S.; Semple, R.K. Mechanistic insights into insulin resistance in the genetic era. Diabet. Med. 2011, 28, 1476–1486. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Krafczyk, N.; Klotz, L. FOXO transcription factors in antioxidant defense. IUBMB Life 2021, 74, 53–61. [Google Scholar] [CrossRef]
- Jeffrey, W.R.; Ryder, J.W.; Gilbert, M.; Zierath, J.R. Skeletal muscle and insulin sensitivity: Pathophysiological alterations. Front. Biosci. 2001, 6, D154–D163. [Google Scholar] [CrossRef]
- Wasserman, D.H. Insulin, Muscle Glucose Uptake, and Hexokinase: Revisiting the Road Not Taken. Physiology 2022, 37, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Barr, V.; Accili, D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 2000, 19, 989–996. [Google Scholar] [CrossRef]
- Gross, D.N.; Wan, M.; Birnbaum, M.J. The role of FOXO in the regulation of metabolism. Curr. Diabetes Rep. 2009, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Kim, D.H.; Lee, J.S. FoxO Transcription Factors: Applicability as a Novel Immune Cell Regulators and Therapeutic Targets in Oxidative Stress-Related Diseases. Int. J. Mol. Sci. 2022, 23, 11877. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Altomonte, J.; Perdomo, G.; He, J.; Fan, Y.; Kamagate, A.; Meseck, M.; Dong, H.H. Aberrant Forkhead Box O1 Function Is Associated with Impaired Hepatic Metabolism. Endocrinology 2006, 147, 5641–5652. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef]
- Bergman, R.N.; Iyer, M.S. Indirect Regulation of Endogenous Glucose Production by Insulin: The Single Gateway Hypothesis Revisited. Diabetes 2017, 66, 1742–1747. [Google Scholar] [CrossRef]
- Ozcan, L.; Wong, C.C.L.; Li, G.; Xu, T.; Pajvani, U.; Park, S.K.R.; Wronska, A.; Chen, B.-X.; Marks, A.R.; Fukamizu, A.; et al. Calcium Signaling through CaMKII Regulates Hepatic Glucose Production in Fasting and Obesity. Cell Metab. 2012, 15, 739–751. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Yan, H.; Zhang, K.; Guo, X.; Xu, Z.; Yang, W.; Qi, Y.; Guo, C.A.; Hornsby, C.; et al. Novel Mechanism of Foxo1 Phosphorylation in Glucagon Signaling in Control of Glucose Homeostasis. Diabetes 2018, 67, 2167–2182. [Google Scholar] [CrossRef]
- Zhang, W.; Patil, S.; Chauhan, B.; Guo, S.; Powell, D.R.; Le, J.; Klotsas, A.; Matika, R.; Xiao, X.; Franks, R.; et al. FoxO1 Regulates Multiple Metabolic Pathways in the Liver: Effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 2006, 281, 10105–10117. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Camporez, J.-P.G.; Kursawe, R.; Titchenell, P.M.; Zhang, D.; Perry, C.J.; Jurczak, M.J.; Abudukadier, A.; Han, M.S.; Zhang, X.-M.; et al. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell 2015, 160, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Casteras, S.; Abdul-Wahed, A.; Soty, M.; Vulin, F.; Guillou, H.; Campana, M.; Le Stunff, H.; Pirola, L.; Rajas, F.; Mithieux, G.; et al. The suppression of hepatic glucose production improves metabolism and insulin sensitivity in subcutaneous adipose tissue in mice. Diabetologia 2016, 59, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Schormann, N.; Hayden, K.L.; Lee, P.; Banerjee, S.; Chattopadhyay, D. An overview of structure, function, and regulation of pyruvate kinases. Protein Sci. 2019, 28, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, H. The role of glycogen synthase in the development of hyperglycemia in type 2 diabetes-‘To store or not to store glucose, that’s the question’. Diabetes/Metab. Res. Rev. 2012, 28, 635–644. [Google Scholar] [CrossRef]
- Nolan, C.J.; Ruderman, N.B.; Kahn, S.E.; Pedersen, O.; Prentki, M. Insulin Resistance as a Physiological Defense Against Metabolic Stress: Implications for the Management of Subsets of Type 2 Diabetes. Diabetes 2015, 64, 673–686. [Google Scholar] [CrossRef]
- Pories, W.J.; Dohm, G.L. Diabetes: Have we got it all wrong? Hyperinsulinism as the culprit: Surgery provides the evidence? Diabetes Care 2012, 35, 2438–2442. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, T.; Wang, S. Progression from prediabetes to type 2 diabetes mellitus induced by overnutrition. Hormones 2022, 21, 591–597. [Google Scholar] [CrossRef]
- Erion, K.A.; Berdan, C.A.; Burritt, N.E.; Corkey, B.E.; Deeney, J.T. Chronic Exposure to Excess Nutrients Left-shifts the Concentration Dependence of Glucose-stimulated Insulin Secretion in Pancreatic β-Cells. J. Biol. Chem. 2015, 290, 16191–16201. [Google Scholar] [CrossRef]
- Corkey, B.E.; Deeney, J.T.; Merrins, M.J. What Regulates Basal Insulin Secretion and Causes Hyperinsulinemia? Diabetes 2021, 70, 2174–2182. [Google Scholar] [CrossRef]
- Merino, B.; Fernández-Díaz, C.M.; Parrado-Fernández, C.; González-Casimiro, C.M.; Postigo-Casado, T.; Lobatón, C.D.; Leissring, M.A.; Cózar-Castellano, I.; Perdomo, G. Hepatic insulin-degrading enzyme regulates glucose and insulin homeostasis in diet-induced obese mice. Metabolism 2020, 113, 154352. [Google Scholar] [CrossRef]
- Caron, A.; Richard, D.; Laplante, M. The Roles of mTOR Complexes in Lipid Metabolism. Annu. Rev. Nutr. 2015, 35, 321–348. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Janssen, J. Hyperinsulinemia and Its Pivotal Role in Aging, Obesity, Type 2 Diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Safhi, M.M.; Anwer, T.; Khan, G.; Siddiqui, R.; Sivakumar, S.M.; Alam, M.F. The combination of canagliflozin and omega-3 fatty acid ameliorates insulin resistance and cardiac biomarkers via modulation of inflammatory cytokines in type 2 diabetic rats. Korean J. Physiol. Pharmacol. 2018, 22, 493–501. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2015, 12, 15–28. [Google Scholar] [CrossRef]
- Flach, R.J.R.; Danai, L.V.; DiStefano, M.T.; Kelly, M.; Menendez, L.G.; Jurczyk, A.; Sharma, R.B.; Jung, D.Y.; Kim, J.H.; Kim, J.K.; et al. Protein Kinase Mitogen-activated Protein Kinase Kinase Kinase Kinase 4 (MAP4K4) Promotes Obesity-induced Hyperinsulinemia. J. Biol. Chem. 2016, 291, 16221–16230. [Google Scholar] [CrossRef]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100, 153952. [Google Scholar] [CrossRef]
- Pearson, E.R. Type 2 diabetes: A multifaceted disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, X.F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020, 63, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.A.; Luben, R.N.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. A Prospective Study of the Association Between Quantity and Variety of Fruit and Vegetable Intake and Incident Type 2 Diabetes. Diabetes Care 2012, 35, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Muraki, I.; Imamura, F.; Manson, J.; Hu, F.B.; Willett, W.C.; van Dam, R.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001, Erratum in BMJ 2013, 347, f6935. [Google Scholar] [CrossRef]
- Yingli, F.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and vegetable intake and risk of type 2 diabetes mellitus: Meta-analysis of prospective cohort studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Brasili, C.; Sandri, E. Trends in dietary patterns and compliance with World Health Organization recommendations: A cross-country analysis. Public Health Nutr. 2007, 11, 535–540. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10, Erratum in Ann. Intern. Med. 2018, 169, 271–272. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet for type 2 diabetes: Cardiometabolic benefits. Endocrine 2017, 56, 27–32. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.J.B.J.; Yap, R.W.K.; Loy, S.L.; Norris, S.A.; Biesma, R.; Aagaard-Hansen, J. Prevalence and Determinants of Overweight, Obesity, and Type 2 Diabetes Mellitus in Adults in Malaysia. Asia Pac. J. Public Health 2014, 27, 123–135. [Google Scholar] [CrossRef]
- Hussein, Z.; Taher, S.W.; Singh, H.K.G.; Swee, W.C.S. Diabetes Care in Malaysia: Problems, New Models, and Solutions. Ann. Glob. Health 2016, 81, 851–862. [Google Scholar] [CrossRef]
- Tee, E.-S.; Yap, R.W.K. Type 2 diabetes mellitus in Malaysia: Current trends and risk factors. Eur. J. Clin. Nutr. 2017, 71, 844–849. [Google Scholar] [CrossRef]
- Aj, S.J.; Lc, G. Effects of an educational program focused on self-care and concurrent physical training on glycemia and drug treatment of patients with diabetes mellitus. Diabetes Updat. 2018, 5, 1–7. [Google Scholar] [CrossRef]
- Winding, K.M.; Munch, G.W.; Iepsen, U.W.; van Hall, G.; Pedersen, B.K.; Mortensen, S.P. The effect on glycaemic control of low-volume high-intensity interval training versus endurance training in individuals with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 1131–1139, Erratum in Diabetes Obes. Metab. 2019, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Ekelund, U.; Brage, S.; Griffin, S.J.; Wareham, N.J.; the ProActive UK Research Group. Objectively Measured Moderate- and Vigorous-Intensity Physical Activity but Not Sedentary Time Predicts Insulin Resistance in High-Risk Individuals. Diabetes Care 2009, 32, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Rockette-Wagner, B.; Edelstein, S.; Venditti, E.M.; Reddy, D.; Bray, G.A.; Carrion-Petersen, M.L.; Dabelea, D.; Delahanty, L.M.; Florez, H.; Franks, P.W.; et al. The impact of lifestyle intervention on sedentary time in individuals at high risk of diabetes. Diabetologia 2015, 58, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative stress in biological systems and its relation with pathophysiological functions: The effect of physical activity on cellular redox homeostasis. Free Radic. Res. 2019, 53, 497–521. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Paronetto, M.P.; Caporossi, D. Exercise, redox homeostasis and the epigenetic landscape. Redox Biol. 2020, 35, 101477. [Google Scholar] [CrossRef]
- Matta, L.; Fonseca, T.S.; Faria, C.C.; Lima-Junior, N.C.; De Oliveira, D.F.; Maciel, L.; Boa, L.F.; Pierucci, A.P.T.R.; Ferreira, A.C.F.; Nascimento, J.H.M.; et al. The Effect of Acute Aerobic Exercise on Redox Homeostasis and Mitochondrial Function of Rat White Adipose Tissue. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- The InterAct Consortium; Spijkerman, A. M.; van der Asijsc, D.L.; Nilsson, P.M.; Ardanaz, E.; Gavrila, D.; Agudo, A.; Arriola, L.; Balkau, B.; Beulens, J.W.; et al. Smoking and Long-Term Risk of Type 2 Diabetes: The EPIC-InterAct Study in European Populations. Diabetes Care 2014, 37, 3164–3171. [Google Scholar] [CrossRef] [PubMed]
- Willi, C.; Bodenmann, P.; Ghali, W.A.; Faris, P.D.; Cornuz, J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2007, 298, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diabetes Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, D.O.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Hamaguchi, M.; Obora, A.; Kojima, T.; Fukui, M. Effect of alcohol consumption and the presence of fatty liver on the risk for incident type 2 diabetes: A population-based longitudinal study. BMJ Open Diabetes Res. Care 2020, 8, e001629. [Google Scholar] [CrossRef]
- Zeliger, H.I. Lipophilic chemical exposure as a cause of type 2 diabetes (T2D). Rev. Environ. Health 2013, 28, 9–20. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental Chemicals and Type 2 Diabetes: An Updated Systematic Review of the Epidemiologic Evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef]
- Carulli, N.; Rondinella, S.; Lombardini, S.; Canedi, I.; Loria, P.; Carulli, L. Review article: Diabetes, genetics and ethnicity. Aliment. Pharmacol. Ther. 2005, 22, 16–19. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Oellgaard, J.; Gæde, P.; Rossing, P.; Rørth, R.; Køber, L.; Parving, H.-H.; Pedersen, O. Reduced risk of heart failure with intensified multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: 21 years of follow-up in the randomised Steno-2 study. Diabetologia 2018, 61, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.I.; Zeggini, E. Genetics of type 2 diabetes. Curr. Diabetes Rep. 2006, 6, 147–154. [Google Scholar] [CrossRef]
- Kaul, N.; Ali, S. Genes, Genetics, and Environment in Type 2 Diabetes: Implication in Personalized Medicine. DNA Cell Biol. 2016, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Qin, S.; Jin, X.; Jin, L.; Gu, W.; Mu, Y. Insights into Genome-Wide Association Study for Diabetes: A Bibliometric and Visual Analysis From 2001 to 2021. Front. Endocrinol. 2022, 13, 817620. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J.; Florez, J.F.J.C. Type 2 diabetes: Genetic data sharing to advance complex disease research. Nat. Rev. Genet. 2016, 17, 535–549. [Google Scholar] [CrossRef]
- Zhao, W.; Rasheed, A.; Tikkanen, E.; Lee, J.-J.; Butterworth, A.S.; Howson, J.M.M.; Assimes, T.L.; Chowdhury, R.; Orho-Melander, M.; Damrauer, S.; et al. Identification of new susceptibility loci for type 2 diabetes and shared etiological pathways with coronary heart disease. Nat. Genet. 2017, 49, 1450–1457. [Google Scholar] [CrossRef]
- Lyssenko, V.; Bianchi, C.; Del Prato, S. Personalized Therapy by Phenotype and Genotype. Diabetes Care 2016, 39, S127–S136. [Google Scholar] [CrossRef]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef]
- Saxena, R.; Saleheen, D.; Been, L.F.; Garavito, M.L.; Braun, T.; Bjonnes, A.; Young, R.; Ho, W.K.; Rasheed, A.; Frossard, P.; et al. Genome-Wide Association Study Identifies a Novel Locus Contributing to Type 2 Diabetes Susceptibility in Sikhs of Punjabi Origin From India. Diabetes 2013, 62, 1746–1755. [Google Scholar] [CrossRef]
- Cook, J.P.; Morris, A.P. Multi-ethnic genome-wide association study identifies novel locus for type 2 diabetes susceptibility. Eur. J. Hum. Genet. 2016, 24, 1175–1180. [Google Scholar] [CrossRef]
- Langenberg, C.; Lotta, L. Genomic insights into the causes of type 2 diabetes. Lancet 2018, 391, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.M. Physiologic Interpretation of GWAS Signals for Type 2 Diabetes. Methods Mol Biol. 2018, 1706, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B. The Genetic Epidemiology of Type 2 Diabetes: Opportunities for Health Translation. Curr. Diabetes Rep. 2019, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Segerstolpe, Å.; Palasantza, A.; Eliasson, P.; Andersson, E.-M.; Andréasson, A.-C.; Sun, X.; Picelli, S.; Sabirsh, A.; Clausen, M.; Bjursell, M.K.; et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016, 24, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Kalai, F.Z.; Boulaaba, M.; Ferdousi, F.; Isoda, H. Effects of Isorhamnetin on Diabetes and Its Associated Complications: A Review of In Vitro and In Vivo Studies and a Post Hoc Transcriptome Analysis of Involved Molecular Pathways. Int. J. Mol. Sci. 2022, 23, 704. [Google Scholar] [CrossRef] [PubMed]
- Juttada, U.; Kumpatla, S.; Parveen, R.; Viswanathan, V. TCF7L2 polymorphism a prominent marker among subjects with Type-2-Diabetes with a positive family history of diabetes. Int. J. Biol. Macromol. 2020, 159, 402–405. [Google Scholar] [CrossRef]
- Mustafa, S.; Younus, D. Association of TCF7L2 rs7903146 Polymorphism with the Risk of Type 2 Diabetes Mellitus (T2DM) Among Kurdish Population in Erbil Province, Iraq. Indian J. Clin. Biochem. 2020, 36, 312–318. [Google Scholar] [CrossRef]
- Aboelkhair, N.T.; Kasem, H.E.; Abdelmoaty, A.A.; El-Edel, R.H. TCF7L2 gene polymorphism as a risk for type 2 diabetes mellitus and diabetic microvascular complications. Mol. Biol. Rep. 2021, 48, 5283–5290. [Google Scholar] [CrossRef]
- Elhourch, S.; Arrouchi, H.; Mekkaoui, N.; Allou, Y.; Ghrifi, F.; Allam, L.; Elhafidi, N.; Belyamani, L.; Ibrahimi, A.; Elomri, N.; et al. Significant Association of Polymorphisms in the TCF7L2 Gene with a Higher Risk of Type 2 Diabetes in a Moroccan Population. J. Pers. Med. 2021, 11, 461. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Das, M.; Lodh, I.; Goswami, R. Role of Metabolic Risk Factors, Family History, and Genetic Polymorphisms (PPARγ and TCF7L2) on Type 2 Diabetes Mellitus Risk in an Asian Indian Population. Public Health Genom. 2021, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bride, L.; Naslavsky, M.; Yamamoto, G.L.; Scliar, M.; Pimassoni, L.H.; Aguiar, P.S.; de Paula, F.; Wang, J.; Duarte, Y.; Passos-Bueno, M.R.; et al. TCF7L2 rs7903146 polymorphism association with diabetes and obesity in an elderly cohort from Brazil. Peerj 2021, 9, e11349. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Kaur, V.; Chamarthi, B.; Littleton, K.R.; Chen, L.; Manning, A.K.; Merino, J.; Thomas, M.K.; Hudson, M.; Goldfine, A.; et al. TCF7L2 Genetic Variation Augments Incretin Resistance and Influences Response to a Sulfonylurea and Metformin: The Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans (SUGAR-MGH). Diabetes Care 2018, 41, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, A.; Tricò, D.; Pierpont, B.; Shabanova, V.; Samuels, S.; Man, C.D.; Galuppo, B.; Santoro, N.; Caprio, S. A Reduced Incretin Effect Mediated by the rs7903146 Variant in the TCF7L2 Gene Is an Early Marker of β-Cell Dysfunction in Obese Youth. Diabetes Care 2020, 43, 2553–2563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tu, M.-S.; Xavier, G.D.S.; Leclerc, I.; Rutter, G.A. Transcription factor-7–like 2 (TCF7L2) gene acts downstream of the Lkb1/Stk11 kinase to control mTOR signaling, β cell growth, and insulin secretion. J. Biol. Chem. 2018, 293, 14178–14189, Erratum in J. Biol. Chem. 2018, 293, 18420. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, S.; Kohan, L.; Ghavami, S.; Azarpira, N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2020, 1868, 118926. [Google Scholar] [CrossRef]
- Nie, X.; Wei, X.; Ma, H.; Fan, L.; Chen, W.-D. The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J. Cell. Mol. Med. 2021, 25, 6479–6495. [Google Scholar] [CrossRef]
- Huang, T.; Wang, L.; Bai, M.; Zheng, J.; Yuan, D.; He, Y.; Wang, Y.; Jin, T.; Cui, W. Influence of IGF2BP2, HMG20A, and HNF1B genetic polymorphisms on the susceptibility to Type 2 diabetes mellitus in Chinese Han population. Biosci. Rep. 2020, 40, BSR20193955. [Google Scholar] [CrossRef]
- Azarova, I.E.; Klyosova, E.Y.; Lazarenko, V.A.; Konoplya, A.I.; Polonikov, A.V. rs11927381 Polymorphism and Type 2 Diabetes Mellitus: Contribution of Smoking to the Realization of Susceptibility to the Disease. Bull. Exp. Biol. Med. 2020, 168, 313–316. [Google Scholar] [CrossRef]
- Dai, N.; Zhao, L.; Wrighting, D.; Krämer, D.; Majithia, A.; Wang, Y.; Cracan, V.; Borges-Rivera, D.; Mootha, V.K.; Nahrendorf, M.; et al. IGF2BP2/IMP2-Deficient Mice Resist Obesity through Enhanced Translation of Ucp1 mRNA and Other mRNAs Encoding Mitochondrial Proteins. Cell Metab. 2015, 21, 609–621. [Google Scholar] [CrossRef]
- Kokkinopoulou, I.; Maratou, E.; Mitrou, P.; Boutati, E.; Sideris, D.C.; Fragoulis, E.G.; Christodoulou, M.-I. Decreased expression of microRNAs targeting type-2 diabetes susceptibility genes in peripheral blood of patients and predisposed individuals. Endocrine 2019, 66, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C.; Borai, A. Insulin-like growth factor-II: Its role in metabolic and endocrine disease. Clin. Endocrinol. 2014, 80, 773–781. [Google Scholar] [CrossRef]
- Mercader, J.M.; Liao, R.G.; Bell, A.D.; Dymek, Z.; Estrada, K.; Tukiainen, T.; Huerta-Chagoya, A.; Moreno-Macías, H.; Jablonski, K.A.; Hanson, R.L.; et al. A Loss-of-Function Splice Acceptor Variant in IGF2 Is Protective for Type 2 Diabetes. Diabetes 2017, 66, 2903–2914. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; Zhang, L.; Zhu, W.; Shen, H.; Yan, Y.-J.; Deng, H.-W. GWA-based pleiotropic analysis identified potential SNPs and genes related to type 2 diabetes and obesity. J. Hum. Genet. 2020, 66, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-Y.; Suzuki, T.; Watanabe, S.; Kimura, S.; Kaitsuka, T.; Fujimura, A.; Matsui, H.; Atta, M.G.; Michiue, H.; Fontecave, M.; et al. Deficit of tRNALys modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Investig. 2011, 121, 3598–3608. [Google Scholar] [CrossRef]
- Palmer, C.J.; Bruckner, R.J.; Paulo, J.A.; Kazak, L.; Long, J.Z.; Mina, A.I.; Deng, Z.; LeClair, K.B.; Hall, J.A.; Hong, S.; et al. Cdkal1, a type 2 diabetes susceptibility gene, regulates mitochondrial function in adipose tissue. Mol. Metab. 2017, 6, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- May, M.J.; Kopp, E.B. NF-κB AND REL PROTEINS: Evolutionarily Conserved Mediators of Immune Responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Arragain, S.; Handelman, S.K.; Forouhar, F.; Wei, F.-Y.; Tomizawa, K.; Hunt, J.F.; Douki, T.; Fontecave, M.; Mulliez, E.; Atta, M. Identification of Eukaryotic and Prokaryotic Methylthiotransferase for Biosynthesis of 2-Methylthio-N6-threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010, 285, 28425–28433. [Google Scholar] [CrossRef]
- Groenewoud, M.J.; Dekker, J.M.; Fritsche, A.; Reiling, E.; Nijpels, G.; Heine, R.J.; Maassen, J.A.; Machicao, F.; Schäfer, S.A.; Häring, H.U.; et al. Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia 2008, 51, 1659–1663. [Google Scholar] [CrossRef]
- Stančáková, A.; Pihlajamäki, J.; Kuusisto, J.; Stefan, N.; Fritsche, A.; Häring, H.; Andreozzi, F.; Succurro, E.; Sesti, G.; Boesgaard, T.W.; et al. Single-Nucleotide Polymorphism rs7754840 of CDKAL1 Is Associated with Impaired Insulin Secretion in Nondiabetic Offspring of Type 2 Diabetic Subjects and in a Large Sample of Men with Normal Glucose Tolerance. J. Clin. Endocrinol. Metab. 2008, 93, 1924–1930. [Google Scholar] [CrossRef]
- Li, J.; Niu, X.; Li, J.; Wang, Q. Association of PPARG Gene Polymorphisms Pro12Ala with Type 2 Diabetes Mellitus: A Meta-analysis. Curr. Diabetes Rev. 2019, 15, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Rathmann, W.; Strassburger, K.; Finner, H.; Grallert, H.; Huth, C.; Meisinger, C.; Gieger, C.; Martin, S.; Giani, G.; et al. Variants of the PPARG, IGF2BP2, CDKAL1, HHEX, and TCF7L2 Genes Confer Risk of Type 2 Diabetes Independently of BMI in the German KORA Studies. Horm. Metab. Res. 2008, 40, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Bhatti, A.; John, P.; Kiani, A.K. Data interpretation: Deciphering the biological function of Type 2 diabetes associated risk loci. Acta Diabetol. 2015, 52, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Erfani, T.; Sarhangi, N.; Afshari, M.; Abbasi, D.; Meybodi, H.R.A.; Hasanzad, M. KCNQ1 common genetic variant and type 2 diabetes mellitus risk. J. Diabetes Metab. Disord. 2019, 19, 47–51. [Google Scholar] [CrossRef]
- Unoki, H.; Takahashi, A.; Kawaguchi, T.; Hara, K.; Horikoshi, M.; Andersen, G.; Ng, D.P.K.; Holmkvist, J.; Borch-Johnsen, K.; Jørgensen, T.; et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat. Genet. 2008, 40, 1098–1102. [Google Scholar] [CrossRef]
- Cirillo, E.; Kutmon, M.; Hernandez, M.G.; Hooimeijer, T.; Adriaens, M.E.; Eijssen, L.M.T.; Parnell, L.D.; Coort, S.L.; Evelo, C.T. From SNPs to pathways: Biological interpretation of type 2 diabetes (T2DM) genome wide association study (GWAS) results. PLoS ONE 2018, 13, e0193515. [Google Scholar] [CrossRef]
- Rouf, A.S.M.R.; Amin, A.; Islam, K.; Haque, F.; Ahmed, K.R.; Rahman, A.; Islam, Z.; Kim, B. Statistical Bioinformatics to Uncover the Underlying Biological Mechanisms That Linked Smoking with Type 2 Diabetes Patients Using Transcritpomic and GWAS Analysis. Molecules 2022, 27, 4390. [Google Scholar] [CrossRef]
- Smushkin, G.; Vella, A. Genetics of type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 471–477. [Google Scholar] [CrossRef]
- Le Gal, K.; Schmidt, E.E.; Sayin, V.I. Cellular Redox Homeostasis. Antioxidants 2021, 10, 1377. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Lubrano, S.B.V. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Garcã a-Gimã©Nez, J.L.; Pallardã³, F.V. Maintenance of glutathione levels and its importance in epigenetic regulation. Front. Pharmacol. 2014, 5, 88. [Google Scholar] [CrossRef]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors 2018, 45, 152–168. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, L.; Zuo, S.; Zhang, Y.; Wan, D.; Long, C.; Huang, P.; Wu, X.; Wu, C.; Liu, G.; et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 488–498. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, A.; Chattopadhyay, B.; Bachhawat, A.K. Defining the cytosolic pathway of glutathione degradation in Arabidopsis thaliana: Role of the ChaC/GCG family of γ-glutamyl cyclotransferases as glutathione-degrading enzymes and AtLAP1 as the Cys-Gly peptidase. Biochem. J. 2015, 468, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Janowiak, B.E.; Hayward, M.A.; Peterson, F.C.; Volkman, B.F.; Griffith, O.W. γ-Glutamylcysteine Synthetase−Glutathione Synthetase: Domain Structure and Identification of Residues Important in Substrate and Glutathione Binding. Biochemistry 2006, 45, 10461–10473. [Google Scholar] [CrossRef]
- Oakley, A.; Yamada, T.; Liu, D.; Coggan, M.; Clark, A.G.; Board, P.G. The The identification and structural characterization of C7orf24 as gamma-glutamyl cyclotransferase. An essential enzyme in the gamma-glutamyl cycle. J. Biol. Chem. 2008, 283, 22031–22042, Erratum in J. Biol. Chem. 2008, 283, 32152. [Google Scholar] [CrossRef]
- Shenoy, N.; Stenson, M.; Lawson, J.; Abeykoon, J.; Patnaik, M.; Wu, X.; Witzig, T. Drugs with anti-oxidant properties can interfere with cell viability measurements by assays that rely on the reducing property of viable cells. Lab. Investig. 2017, 97, 494–497. [Google Scholar] [CrossRef]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Reactive oxygen species: Sources, consequences and targeted therapy in type 2 diabetes. J. Drug Target. 2016, 25, 93–101. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Mattevi, A. Structure and mechanisms of ROS generation by NADPH oxidases. Curr. Opin. Struct. Biol. 2019, 59, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Manea, A. NADPH oxidase-derived reactive oxygen species: Involvement in vascular physiology and pathology. Cell Tissue Res. 2010, 342, 325–339. [Google Scholar] [CrossRef]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2019, 2019, 1–17. [Google Scholar] [CrossRef]
- Teodoro, J.; Nunes, S.R.R.P.; Rolo, A.; Reis, F.; Palmeira, C.M. Therapeutic Options Targeting Oxidative Stress, Mitochondrial Dysfunction and Inflammation to Hinder the Progression of Vascular Complications of Diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef]
- Vignais, P.V. The superoxide-generating NADPH oxidase: Structural aspects and activation mechanism. Cell. Mol. Life Sci. CMLS 2002, 59, 1428–1459. [Google Scholar] [CrossRef]
- Sahoo, S.; Meijles, D.; Pagano, P.J. NADPH oxidases: Key modulators in aging and age-related cardiovascular diseases? Clin. Sci. 2016, 130, 317–335. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Rocha, M.; Falcon, R.; De Pablo, C.; Alvarez, A.; Jover, A.; Hernandez-Mijares, A.; Victor, V.M. Is Myeloperoxidase a Key Component in the ROS-Induced Vascular Damage Related to Nephropathy in Type 2 Diabetes? Antioxidants Redox Signal. 2013, 19, 1452–1458. [Google Scholar] [CrossRef]
- Banerjee, M.; Vats, P. Reactive metabolites and antioxidant gene polymorphisms in type 2 diabetes mellitus. Indian J. Hum. Genet. 2014, 20, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 56. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.G.; Crispim, D.; Canani, L.H.; Ferrugem, P.T.; Gross, J.L.; Roisenberg, I. Relationship of endothelial nitric oxide synthase (eNOS) gene polymorphisms with diabetic retinopathy in Caucasians with type 2 diabetes. Ophthalmic Genet. 2011, 33, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Porojan, M.D.; Catana, A.; Popp, R.; Dumitrascu, D.L.; Bala, C. The role of NOS2A -954G/C and vascular endothelial growth factor +936C/T polymorphisms in type 2 diabetes mellitus and diabetic nonproliferative retinopathy risk management. Ther. Clin. Risk Manag. 2015, 11, 1743–1748. [Google Scholar] [CrossRef]
- Priščáková, P.; Minárik, G.; Repiská, V. Candidate gene studies of diabetic retinopathy in human. Mol. Biol. Rep. 2016, 43, 1327–1345. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.-M.; Yang, L.-Q.; Zhong, C.-G.; Zhuang, Z.-X. Association of NOS2 and NOS3 gene polymorphisms with susceptibility to type 2 diabetes mellitus and diabetic nephropathy in the Chinese Han population. IUBMB Life 2016, 68, 516–525. [Google Scholar] [CrossRef]
- Ganjifrockwala, F.; Joseph, J.; George, G. Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 21–25. [Google Scholar] [CrossRef]
- Heinonen, S.; Lautala, S.; Koivuniemi, A.; Bunker, A. Insights into the behavior of unsaturated diacylglycerols in mixed lipid bilayers in relation to protein kinase C activation—A molecular dynamics simulation study. Biochim. Biophys. Acta (BBA) Biomembr. 2022, 1864, 183961. [Google Scholar] [CrossRef]
- Vats, P.; Sagar, N.; Singh, T.P.; Banerjee, M. Association of Superoxide dismutases (SOD1 and SOD2) and Glutathione peroxidase 1 (GPx1) gene polymorphisms with Type 2 diabetes mellitus. Free Radic. Res. 2014, 49, 17–24. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr Med. 2014, 13, 8–12. [Google Scholar]
- Diotallevi, M.; Checconi, P.; Palamara, A.T.; Celestino, I.; Coppo, L.; Holmgren, A.; Abbas, K.; Peyrot, F.; Mengozzi, M.; Ghezzi, P. Glutathione Fine-Tunes the Innate Immune Response toward Antiviral Pathways in a Macrophage Cell Line Independently of Its Antioxidant Properties. Front. Immunol. 2017, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 5028181. [Google Scholar] [CrossRef]
- McBean, G.J.; Aslan, M.; Griffiths, H.R.; Torrão, R.C. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015, 5, 186–194. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J. Polyol pathway and redox balance in diabetes. Pharmacol. Res. 2022, 182, 106326. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2018, 234, 8152–8161. [Google Scholar] [CrossRef]
- Traverso, N.; Furfaro, A.L.; Nitti, M.; Marengo, B.; Domenicotti, C.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A. Impaired synthesis contributes to diabetes-induced decrease in liver glutathione. Int. J. Mol. Med. 2012, 29, 899–905. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; An, H.; Ni, K.; Chen, B.; Li, H.; Li, Y.; Sheng, G.; Zhou, C.; Xie, M.; Chen, S.; et al. Glutathione prevents chronic oscillating glucose intake-induced β-cell dedifferentiation and failure. Cell Death Dis. 2019, 10, 321. [Google Scholar] [CrossRef]
- Sekhar, R.V. GlyNAC Supplementation Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Aging Hallmarks, Metabolic Defects, Muscle Strength, Cognitive Decline, and Body Composition: Implications for Healthy Aging. J. Nutr. 2021, 151, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, G.; Bravi, M.; Laurenti, O.; Cassone-Faldetta, M.; Armiento, A.; Ferri, C.; Balsano, F. Influence of reduced glutathione infusion on glucose metabolism in patients with non—Insulin-dependent diabetes mellitus. Metabolism 1998, 47, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Moon, M.K.; Kim, S.W.; Shin, H.D.; Hwang, Y.H.; Ahn, C.; Lee, H.K. Glutathion S-Transferase M1 Gene Polymorphism is Associated with Type 2 Diabetic Nephropathy. Korean Diabetes J. 2005, 29, 315–321. [Google Scholar]
- Murakami, K.; Takahito, K.; Ohtsuka, Y.; Fujiwara, Y.; Shimada, M.; Kawakami, Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism 1989, 38, 753–758. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirokawa, J.; Tagami, S.; Kawakami, Y.; Urata, Y.; Kondo, T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: Regulation of glutathione synthesis and efflux. Diabetologia 1995, 38, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P.; Reed, R.L.; Jones, D.P. Glutathione in Human Plasma: Decline in Association with Aging, Age-Related Macular Degeneration, and Diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cui, Q.; Yang, B.; Hou, Y.; Wang, H.; Xu, Y.; Wang, D.; Zhang, Q.; Pi, J. The impairment of glucose-stimulated insulin secretion in pancreatic β-cells caused by prolonged glucotoxicity and lipotoxicity is associated with elevated adaptive antioxidant response. Food Chem. Toxicol. 2017, 100, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Presti, M.E.; Wells, L.D. Glutathione Synthesis in the Exocrine Pancreas. Pancreas 1997, 14, 342–349. [Google Scholar] [CrossRef]
- Wallig, M.A. Xenobiotic Metabolism, Oxidant Stress and Chronic Pancreatitis. Digestion 1998, 59, 13–24. [Google Scholar] [CrossRef]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Githens, S. Glutathione metabolism in the pancreas compared with that in the liver, kidney, and small intestine. Int. J. Pancreatol. 1991, 8, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M. Glutathionists in the battlefield of gamma-glutamyl cycle. Arch. Biochem. Biophys. 2016, 595, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Noordam, R.; Smit, R.A.; Postmus, I.; Trompet, S.; Van Heemst, D. Assessment of causality between serum gamma-glutamyltransferase and type 2 diabetes mellitus using publicly available data: A Mendelian randomization study. Leuk. Res. 2016, 45, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Nano, J.; Muka, T.; Ligthart, S.; Hofman, A.; Murad, S.D.; LA Janssen, H.; Franco, O.H.; Dehghan, A. Gamma-glutamyltransferase levels, prediabetes and type 2 diabetes: A Mendelian randomization study. Leuk. Res. 2017, 46, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.M.; Hysenaj, G.; Wood, A.R.; Weedon, M.N.; Harries, L.W. Targeted Allelic Expression Profiling in Human Islets Identifies cis-Regulatory Effects for Multiple Variants Identified by Type 2 Diabetes Genome-Wide Association Studies. Diabetes 2014, 64, 1484–1491. [Google Scholar] [CrossRef]
- Ding, L.; Fan, L.; Xu, X.; Fu, J.; Xue, Y. Identification of core genes and pathways in type 2 diabetes mellitus by bioinformatics analysis. Mol. Med. Rep. 2019, 20, 2597–2608. [Google Scholar] [CrossRef]
- Azarova, I.; Klyosova, E.; Polonikov, A. The Link between Type 2 Diabetes Mellitus and the Polymorphisms of Glutathione-Metabolizing Genes Suggests a New Hypothesis Explaining Disease Initiation and Progression. Life 2021, 11, 886. [Google Scholar] [CrossRef]
- Arolas, J.L.; Aviles, F.X.; Chang, J.-Y.; Ventura, S. Folding of small disulfide-rich proteins: Clarifying the puzzle. Trends Biochem. Sci. 2006, 31, 292–301. [Google Scholar] [CrossRef]
- Tsunoda, S.; Avezov, E.; Zyryanova, A.; Konno, T.; Mendes-Silva, L.; Melo, E.P.; Harding, H.P.; Ron, D. Intact protein folding in the glutathione-depleted endoplasmic reticulum implicates alternative protein thiol reductants. Elife 2014, 3, e03421. [Google Scholar] [CrossRef]
- Costes, S.; Langen, R.; Gurlo, T.; Matveyenko, A.V.; Butler, P.C. β-Cell Failure in Type 2 Diabetes: A Case of Asking Too Much of Too Few? Diabetes 2013, 62, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cui, J.; He, Q.; Chen, Z.; Arvan, P.; Liu, M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes☆. Mol. Asp. Med. 2015, 42, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Weiss, M.A.; Arunagiri, A.; Yong, J.; Rege, N.; Sun, J.; Haataja, L.; Kaufman, R.J.; Arvan, P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes, Obes. Metab. 2018, 20, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Arunagiri, A.; Haataja, L.; Pottekat, A.; Pamenan, F.; Kim, S.; Zeltser, L.M.; Paton, A.W.; Paton, J.C.; Tsai, B.; Itkin-Ansari, P.; et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. Elife 2019, 8, e44532. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, D.; Kaufman, R.J. The Unfolded Protein Response: A Pathway That Links Insulin Demand with β-Cell Failure and Diabetes. Endocr. Rev. 2008, 29, 317–333, Erratum in Endocr. Rev. 2008, 29, 631. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Edwards, G., III; Salvadores, N.; Shahnawaz, M.; Diaz-Espinoza, R.; Soto, C. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 2017, 22, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.; Bushueva, O.; Konoplya, A.; Polonikov, A. Glutathione S-transferase genes and the risk of type 2 diabetes mellitus: Role of sexual dimorphism, gene-gene and gene-smoking interactions in disease susceptibility. J. Diabetes 2018, 10, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-T.; Wang, C.-J.; Tang, H.-Q.; Zhang, Q.; Wang, Y. Evaluation of glutathione S-transferase genetic variants affecting type 2 diabetes susceptibility: A meta-analysis. Gene 2013, 530, 301–308. [Google Scholar] [CrossRef]
- Mastana, S.S.; Kaur, A.; Hale, R.; Lindley, M.R. Influence of glutathione S-transferase polymorphisms (GSTT1, GSTM1, GSTP1) on type-2 diabetes mellitus (T2D) risk in an endogamous population from north India. Mol. Biol. Rep. 2013, 40, 7103–7110. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Bid, H.; Konwar, R.; Saxena, M.; Chaudhari, P.; Agrawal, C. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in north Indian population. J. Postgrad. Med. 2010, 56, 176–181. [Google Scholar] [CrossRef]
- Gönül, N.; Kadioglu, E.; Kocabaş, N.A.; Özkaya, M.; Karakaya, A.E.; Karahalil, B. The role of GSTM1, GSTT1, GSTP1, and OGG1 polymorphisms in type 2 diabetes mellitus risk: A case–control study in a Turkish population. Gene 2012, 505, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Moasser, E.; Kazemi-Nezhad, S.R.; Saadat, M.; Azarpira, N. Study of the association between glutathione S-transferase (GSTM1, GSTT1, GSTP1) polymorphisms with type II diabetes mellitus in southern of Iran. Mol. Biol. Rep. 2012, 39, 10187–10192. [Google Scholar] [CrossRef]
- Stoian, A.; Bănescu, C.; Bălaşa, R.I.; Moţăţăianu, A.; Stoian, M.; Moldovan, V.G.; Voidăzan, S.; Dobreanu, M. Influence of GSTM1, GSTT1, and GSTP1 Polymorphisms on Type 2 Diabetes Mellitus and Diabetic Sensorimotor Peripheral Neuropathy Risk. Dis. Markers 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.; Klyosova, E.; Lazarenko, V.; Konoplya, A.; Polonikov, A. Genetic variants in glutamate cysteine ligase confer protection against type 2 diabetes. Mol. Biol. Rep. 2020, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.E.; Klyosova, E.Y.; Polonikov, A.V. Polymorphic variants of glutathione reductase–new genetic markers of predisposition to type 2 diabetes mellitus. Ter. arkhiv 2021, 93, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.E.; Klyosova, E.Y.; Churilin, M.I.; Samgina, T.A.; Konoplya, A.I.; Polonikov, A.V. Genetic and biochemical investigation of the gamma-glutamylcyclotransferase role in predisposition to type 2 diabetes mellitus. Ecol. Genet. 2020, 18, 215–228. [Google Scholar] [CrossRef]
- Ramprasath, T.; Murugan, P.S.; Kalaiarasan, E.; Gomathi, P.; Rathinavel, A.; Selvam, G.S. Genetic association of Glutathione peroxidase-1 (GPx-1) and NAD(P)H:Quinone Oxidoreductase 1(NQO1) variants and their association of CAD in patients with type-2 diabetes. Mol. Cell. Biochem. 2011, 361, 143–150. [Google Scholar] [CrossRef]
- Azarova, I.E.; Klyosova, E.; Samgina, T.A.; Bushueva, O.; Yu Azarova, V.A.; Konoplya, A.I.; Polonikov, A.V. Polymorphic variant in gpx2 gene (rs4902346) and predisposition to type 2 diabetes mellitus. Med. Genet. 2020, 19, 17–27. (In Russian) [Google Scholar] [CrossRef]
- Ghattas, M.H.; Abo-Elmatty, D.M. Association of Polymorphic Markers of the Catalase and Superoxide Dismutase Genes with Type 2 Diabetes Mellitus. DNA Cell Biol. 2012, 31, 1598–1603. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Jin, A. Genetic polymorphisms in extracellular superoxide dismutase Leu53Leu, Arg213Gly, and Ala40Thr and susceptibility to type 2 diabetes mellitus. Genet. Mol. Res. 2016, 15, gmr15048418. [Google Scholar] [CrossRef]
- Nakamura, S.-I.; Kugiyama, K.; Sugiyama, S.; Miyamoto, S.; Koide, S.-I.; Fukushima, H.; Honda, O.; Yoshimura, M.; Ogawa, H. Polymorphism in the 5′-Flanking Region of Human Glutamate-Cysteine Ligase Modifier Subunit Gene Is Associated With Myocardial Infarction. Circulation 2002, 105, 2968–2973. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Liu, W.; Zhang, X.; Gao, X.; Yu, F.; Guo, W.; Meng, Y.; Gao, P.; Zhou, J.; Yuan, M.; et al. Oxidative Stress-Related Gene Polymorphisms Are Associated With Hepatitis B Virus-Induced Liver Disease in the Northern Chinese Han Population. Front. Genet. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rosa, H.; Schneider, R.; Bernstein, B.E.; Karabetsou, N.; Morillon, A.; Weise, C.; Schreiber, S.L.; Mellor, J.; Kouzarides, T. Methylation of Histone H3 K4 Mediates Association of the Isw1p ATPase with Chromatin. Mol. Cell 2003, 12, 1325–1332, Erratum in Mol. Cell. 2018, 70, 983. [Google Scholar] [CrossRef] [PubMed]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef] [PubMed]
- Tjeertes, J.V.; Miller, K.M.; Jackson, S.P. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009, 28, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Basu, S.; Kong, X.; Pankow, J.S.; Aleksic, N.; Tan, A.; Cushman, M.; Boerwinkle, E.; Folsom, A.R. Genome-wide association study identifies novel loci for plasma levels of protein C: The ARIC study. Blood 2010, 116, 5032–5036. [Google Scholar] [CrossRef]
- Long, Y.; Jia, D.; Wei, L.; Yang, Y.; Tian, H.; Chen, T. Liver-Specific Overexpression of Gamma-Glutamyltransferase Ameliorates Insulin Sensitivity of Male C57BL/6 Mice. J. Diabetes Res. 2017, 2017, 2654520. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Shankar, A.; Li, J.; Pollard, C.; Ducatman, A. Serum gamma-glutamyl transferase level and diabetes mellitus among US adults. Eur. J. Epidemiology 2009, 24, 369–373. [Google Scholar] [CrossRef]
- Zhao, W.; Tong, J.; Liu, J.; Liu, J.; Li, J.; Cao, Y. The Dose-Response Relationship between Gamma-Glutamyl Transferase and Risk of Diabetes Mellitus Using Publicly Available Data: A Longitudinal Study in Japan. Int. J. Endocrinol. 2020, 2020, 5356498. [Google Scholar] [CrossRef]
- Lee, Y.S.; Cho, Y.; Burgess, S.; Smith, G.D.; Relton, C.L.; Shin, S.-Y.; Shin, M.-J. Serum gamma-glutamyl transferase and risk of type 2 diabetes in the general Korean population: A Mendelian randomization study. Hum. Mol. Genet. 2016, 25, 3877–3886. [Google Scholar] [CrossRef]
- Jinnouchi, H.; Morita, K.; Tanaka, T.; Kajiwara, A.; Kawata, Y.; Oniki, K.; Saruwatari, J.; Nakagawa, K.; Otake, K.; Ogata, Y.; et al. Interactive effects of a common γ-glutamyltransferase 1 variant and low high-density lipoprotein-cholesterol on diabetic macro- and micro-angiopathy. Cardiovasc. Diabetol. 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Diergaarde, B.; Brand, R.; Lamb, J.; Cheong, S.Y.; Stello, K.; Barmada, M.M.; Feingold, E.; Whitcomb, D.C. Pooling-Based Genome-Wide Association Study Implicates Gamma-Glutamyltransferase 1 (GGT1) Gene in Pancreatic Carcinogenesis. Pancreatology 2010, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Brand, H.; Diergaarde, B.; O’Connell, M.R.; Whitcomb, D.C.; Brand, R.E. Variation in the γ-Glutamyltransferase 1 Gene and Risk of Chronic Pancreatitis. Pancreas 2013, 42, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Waterworth, D.; Perry, J.R.; Lim, N.; Song, K.; Chambers, J.C.; Zhang, W.; Vollenweider, P.; Stirnadel, H.; Johnson, T.; et al. Population-Based Genome-wide Association Studies Reveal Six Loci Influencing Plasma Levels of Liver Enzymes. Am. J. Hum. Genet. 2008, 83, 520–528. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Park, K.H.; Kim, C.-W.; Hur, Y.-I. The Relationship between Serum Gamma-glutamyltransferase Level and Overweight in Korean Urban Children. Korean J. Fam. Med. 2011, 32, 182–188. [Google Scholar] [CrossRef]
- Wickham, S.; West, M.B.; Cook, P.F.; Hanigan, M.H. Gamma-glutamyl compounds: Substrate specificity of gamma-glutamyl transpeptidase enzymes. Anal. Biochem. 2011, 414, 208–214. [Google Scholar] [CrossRef]
- Shin, D.M.S.; Yon, D.K. Apparently healthy adults with high serum gamma-glutamyl transferase levels are at increased risk of asthma development in the near future: A Korean nationwide cohort study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3958–3966. [Google Scholar] [CrossRef]
- Astle, W.J.; Elding, H.; Jiang, T.; Allen, D.; Ruklisa, D.; Mann, A.L.; Mead, D.; Bouman, H.; Riveros-Mckay, F.; Kostadima, M.A.; et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell 2016, 167, 1415–1429. [Google Scholar] [CrossRef]
- Heisterkamp, N.; Groffen, J.; Warburton, D.; Sneddon, T.P. The human gamma-glutamyltransferase gene family. Hum. Genet. 2008, 123, 321–332. [Google Scholar] [CrossRef]
- Whitfield, J.B. Gamma Glutamyl Transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Masuda, Y.; Maeda, S.; Watanabe, A.; Sano, Y.; Aiuchi, T.; Nakajo, S.; Itabe, H.; Nakaya, K. A novel 21-kDa cytochrome c-releasing factor is generated upon treatment of human leukemia U937 cells with geranylgeraniol. Biochem. Biophys. Res. Commun. 2006, 346, 454–460. [Google Scholar] [CrossRef]
- Bocharova, J.A.; Azarova, J.E.; Klyosova EYu Drozdova, E.L.; Solodilova, M.A.; Polonikov, A.V. Gene of gamma-glutamylcyclotransferase, a key enzyme of glutathione catabolism, and predisposition to ischemic stroke: Association analysis and functional annotation of gene polymorphisms. Med. Genet. 2020, 19, 32–39. (In Russian) [Google Scholar] [CrossRef]
- Singh, R.R.; Mohammad, J.; Orr, M.; Reindl, K.M. Glutathione S-Transferase pi-1 Knockdown Reduces Pancreatic Ductal Adenocarcinoma Growth by Activating Oxidative Stress Response Pathways. Cancers 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, Y.; Mori, M.; Tsukada, M.; Miyahara, S.; Sato-Fukushima, H.; Watanabe, E.; Murakami-Tonami, Y.; Inoue, H. Pi-Class Glutathione S-transferase (GSTP1)-Selective Fluorescent Probes for Multicolour Imaging with Various Cancer-Associated Enzymes. Chembiochem. 2022, 23, e202200443. [Google Scholar] [CrossRef]
- Bogaards, J.J.P.; Venekamp, J.C.; van Bladeren, P.J. Stereoselective Conjugation of Prostaglandin A2 and Prostaglandin J2 with Glutathione, Catalyzed by the Human Glutathione S-Transferases A1-1, A2-2, M1a-1a, and P1-1. Chem. Res. Toxicol. 1997, 10, 310–317. [Google Scholar] [CrossRef]
- Al-Eitan, L.N.; Rababa’H, D.M.; Alkhatib, R.Q.; Khasawneh, R.H.; Aljarrah, O.A. GSTM1 and GSTP1 Genetic Polymorphisms and Their Associations With Acute Lymphoblastic Leukemia Susceptibility in a Jordanian Population. J. Pediatr. Hematol. 2016, 38, e223–e229. [Google Scholar] [CrossRef]
- Hohaus, S.; Di Ruscio, A.; Di Febo, A.; Massini, G.; D’Alo’, F.; Guidi, F.; Mansueto, G.; Voso, M.T.; Leone, G. Glutathione S-transferase P1 Genotype and Prognosis in Hodgkin’s Lymphoma. Clin. Cancer Res. 2005, 11, 2175–2179. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Z.; Cui, D.; Liu, H.; Hao, X. Glutathione S-transferase P1 Ile105Val polymorphism and breast cancer risk: A meta-analysis involving 34,658 subjects. Breast Cancer Res. Treat. 2010, 125, 253–259. [Google Scholar] [CrossRef]
- Li, X.-M.; Yu, X.-W.; Yuan, Y.; Pu, M.-Z.; Zhang, H.-X.; Wang, K.-J.; Han, X.-D. Glutathione S-transferase P1, gene-gene interaction, and lung cancer susceptibility in the Chinese population: An updated meta-analysis and review. J. Cancer Res. Ther. 2015, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.D.O.; Gomes, B.C.; Rodrigues, A.S.; Rueff, J. Genetic Susceptibility in Acute Pancreatitis: Genotyping of GSTM1, GSTT1, GSTP1, CASP7, CASP8, CASP9, CASP10, LTA, TNFRSF1B, and TP53 Gene Variants. Pancreas 2017, 46, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Lin, L.; Song, G.; Deng, T. GSTM1 Null Genotype and GSTP1 Ile105Val Polymorphism Are Associated with Alzheimer’s Disease: A Meta-Analysis. Mol. Neurobiol. 2015, 53, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Minelli, C.; Granell, R.; Newson, R.; Rose-Zerilli, M.; Torrent, M.; Ring, S.M.; Holloway, J.; Shaheen, S.; Henderson, J. Glutathione-S-transferase genes and asthma phenotypes: A Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Leuk. Res. 2009, 39, 539–562. [Google Scholar] [CrossRef]
- MacIntyre, E.A.; Brauer, M.; Melén, E.; Bauer, C.P.; Bauer, M.; Berdel, D.; Bergström, A.; Brunekreef, B.; Chan-Yeung, M.; Klümper, C.; et al. GSTP1 and TNF Gene Variants and Associations between Air Pollution and Incident Childhood Asthma: The Traffic, Asthma and Genetics (TAG) Study. Environ. Health Perspect. 2014, 122, 418–424. [Google Scholar] [CrossRef]
- Huang, S.-X.; Wu, F.-X.; Luo, M.; Ma, L.; Gao, K.-F.; Li, J.; Wu, W.-J.; Huang, S.; Yang, Q.; Liu, K.; et al. The Glutathione S-Transferase P1 341C>T Polymorphism and Cancer Risk: A Meta-Analysis of 28 Case-Control Studies. PLoS ONE 2013, 8, e56722. [Google Scholar] [CrossRef]
- Kelada, S.N.; Stapleton, P.L.; Farin, F.M.; Bammler, T.K.; Eaton, D.L.; Smith-Weller, T.; Franklin, G.M.; Swanson, P.D.; Longstreth, W.; Checkoway, H. Glutathione S-transferase M1, T1, and P1 Polymorphisms and Parkinson’s Disease. Neurosci. Lett. 2002, 337, 5–8. [Google Scholar] [CrossRef]
- Awasthi, Y.C.; Sharma, R.; Cheng, J.; Yang, Y.; Sharma, A.; Singhal, S.S.; Awasthi, S. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol. Asp. Med. 2003, 24, 219–230. [Google Scholar] [CrossRef]
- Mastrocola, R.; Restivo, F.; Vercellinatto, I.; Danni, O.; Brignardello, E.; Aragno, M.; Boccuzzi, G. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J. Endocrinol. 2005, 187, 37–44. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tran, P.O.T.; Harmon, J.; Robertson, R.P. A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proc. Natl. Acad. Sci. USA 2002, 99, 12363–12368. [Google Scholar] [CrossRef]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Zhou, J.-C.; Wu, Y.-Y.; Ren, F.-Z.; Lei, X.G. Role of glutathione peroxidase 1 in glucose and lipid metabolism-related diseases. Free Radic. Biol. Med. 2018, 127, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Mohammedi, K.; Patente, T.A.; Bellili-Muñoz, N.; Driss, F.; Le Nagard, H.; Fumeron, F.; Roussel, R.; Hadjadj, S.; Corrêa-Giannella, M.L.; Marre, M.; et al. Glutathione peroxidase-1 gene (GPX1) variants, oxidative stress and risk of kidney complications in people with type 1 diabetes. Metabolism 2015, 65, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, L.; Hu, Z.; Song, Z.; Wang, Y.; Chen, Z. Evaluation of the oxidative stress–related genes ALOX5, ALOX5AP, GPX1, GPX3 and MPO for contribution to the risk of type 2 diabetes mellitus in the Han Chinese population. Diabetes Vasc. Dis. Res. 2018, 15, 336–339. [Google Scholar] [CrossRef]
- Lewis, P.; Stefanovic, N.; Pete, J.; Calkin, A.; Giunti, S.; Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Allen, T.J.; Kola, I.; Cooper, M.E.; et al. Lack of the Antioxidant Enzyme Glutathione Peroxidase-1 Accelerates Atherosclerosis in Diabetic Apolipoprotein E–Deficient Mice. Circulation 2007, 115, 2178–2187. [Google Scholar] [CrossRef]
- Wu, C.; Orozco, C.; Boyer, J.; Leglise, M.; Goodale, J.; Batalov, S.; Hodge, C.L.; Haase, J.; Janes, J.; Huss, J.W., 3rd; et al. BioGPS: An extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009, 10, R130. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Banting Lecture. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Zhou, B.; Cox, A.D.; Campbell, S.L. Rho GTPases, oxidation, and cell redox control. Small GTPases 2014, 5, e28579. [Google Scholar] [CrossRef]
- Osmenda, G.; Matusik, P.T.; Sliwa, T.; Czesnikiewicz-Guzik, M.; Skupien, J.; Malecki, M.T.; Siedlinski, M. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase p22phox subunit polymorphisms, systemic oxidative stress, endothelial dysfunction, and atherosclerosis in type 2 diabetes mellitus. Pol. Arch. Intern. Med. 2021, 131, 447–454. [Google Scholar] [CrossRef]
- Taylor, J.P.; Tse, H.M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021, 48, 102159. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Barman, S.; Fulton, D. Enzymatic regulation and functional relevance of NOX5. Curr. Pharm. Des. 2015, 21, 5999–6008. [Google Scholar] [CrossRef]
- Azarova, I.E.; Klyosova EYu Samgina, T.A.; Sakali SYu Kolomoets, I.I.; Azarova, V.A.; Konoplya, A.I.; Polonikov, A.V. Role of cyba gene polymorphisms in pathogenesis of type 2 diabetes mellitus. Med. Genet. 2019, 18, 37–48. (In Russian) [Google Scholar] [CrossRef]
- Sun, Q.; Yin, Y.; Zhu, Z.; Yan, Z. Association of the C242T polymorphism in the NAD(P)H oxidase P22 phox gene with type 2 diabetes mellitus risk: A meta-analysis. Curr. Med. Res. Opin. 2013, 30, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.E.; Klyosova, E.Y.; Kolomoets, I.I.; Azarova, V.A.; Ivakin, V.E.; Konoplya, A.I.; Polonikov, A.V. Polymorphisms of the Gene Encoding Cytochrome b-245 Beta Chain of NADPH Oxidase: Relationship with Redox Homeostasis Markers and Risk of Type 2 Diabetes Mellitus. Russ. J. Genet. 2020, 56, 856–862. [Google Scholar] [CrossRef]
- Azarova, I.E.; Klyosova, E.Y.; Kolomoets, I.I.; Polonikov, A.V. Polymorphic Variants of the Neutrophil Cytosolic Factor 2 Gene: Associations with Susceptibility to Type 2 Diabetes Mellitus and Cardiovascular Autonomic Neuropathy. Russ. J. Genet. 2022, 58, 593–602. [Google Scholar] [CrossRef]
- Moreno, M.U.; José, G.S.; Orbe, J.; Páramo, J.; Beloqui, O.; Díez, J.; Zalba, G. Preliminary characterisation of the promoter of the human p22phox gene: Identification of a new polymorphism associated with hypertension. FEBS Lett. 2003, 542, 27–31, Erratum in FEBS Lett. 2010, 584, 4709. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Hoffmann, M.; Kaya, E.; Tzvetkov, M.; Brockmoller, J. Genetic polymorphisms of NAD(P)H oxidase: Variation in subunit expression and enzyme activity. Pharm. J. 2007, 8, 297–304. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.-C.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48, e12951. [Google Scholar] [CrossRef]
- Meijles, D.N.; Fan, L.M.; Ghazaly, M.M.; Howlin, B.; Krönke, M.; Brooks, G.; Li, J.-M. p22 phox C242T Single-Nucleotide Polymorphism Inhibits Inflammatory Oxidative Damage to Endothelial Cells and Vessels. Circulation 2016, 133, 2391–2403. [Google Scholar] [CrossRef]
- Fang, S.; Wang, L.; Jia, C. Association of p22phox gene C242T polymorphism with coronary artery disease: A meta-analysis. Thromb. Res. 2010, 125, e197–e201. [Google Scholar] [CrossRef]
- Snahnicanova, Z.; Mendelova, A.; Grendar, M.; Holubekova, V.; Kostkova, M.; Pozorciakova, K.; Jancinová, M.; Kasubova, I.; Vojtkova, J.; Durdik, P.; et al. Association of Polymorphisms inCYBA, SOD1, andCATGenes with Type 1 Diabetes and Diabetic Peripheral Neuropathy in Children and Adolescents. Genet. Test. Mol. Biomark. 2018, 22, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Petrovič, D. Association of the −262C/T polymorphism in the catalase gene promoter and the C242T polymorphism of the NADPH oxidase P22phox gene with essential arterial hypertension in patients with diabetes mellitus type 2. Clin. Exp. Hypertens. 2013, 36, 36–39. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kiyohara, C.; Suetsugu-Ogata, S.; Hamada, N.; Nakanishi, Y. Biological interaction of cigarette smoking on the association between genetic polymorphisms involved in inflammation and the risk of lung cancer: A case-control study in Japan. Oncol. Lett. 2017, 13, 3873–3881. [Google Scholar] [CrossRef] [PubMed]
- Holla, L.I.; Kaňková, K.; Znojil, V. Haplotype Analysis of the NADPH Oxidase p22phox Gene in Patients with Bronchial Asthma. Int. Arch. Allergy Immunol. 2008, 148, 73–80. [Google Scholar] [CrossRef] [PubMed]
- José, G.S.; Moreno, M.U.; Oliván, S.; Beloqui, O.; Fortuño, A.; Díez, J.; Zalba, G. Functional Effect of the p22 phox −930 A/G Polymorphism on p22 phox Expression and NADPH Oxidase Activity in Hypertension. Hypertension 2004, 44, 163–169. [Google Scholar] [CrossRef]
- Moreno, M.U.; José, G.S.; Fortuño, A.; Beloqui, O.; Díez, J.; Zalba, G. The C242T CYBA polymorphism of NADPH oxidase is associated with essential hypertension. J. Hypertens. 2006, 24, 1299–1306. [Google Scholar] [CrossRef]
- Niemiec, P.; Nowak, T.; Iwanicki, T.; Krauze, J.; Górczyńska-Kosiorz, S.; Grzeszczak, W.; Ochalska-Tyka, A.; Żak, I. The −930A>G polymorphism of the CYBA gene is associated with premature coronary artery disease. A case–control study and gene–risk factors interactions. Mol. Biol. Rep. 2014, 41, 3287–3294. [Google Scholar] [CrossRef]
- Patente, T.A.; Mohammedi, K.; Bellili-Muñoz, N.; Driss, F.; Sanchez, M.; Fumeron, F.; Roussel, R.; Hadjadj, S.; Corrêa-Giannella, M.L.; Marre, M.; et al. Allelic variations in the CYBA gene of NADPH oxidase and risk of kidney complications in patients with type 1 diabetes. Free Radic. Biol. Med. 2015, 86, 16–24. [Google Scholar] [CrossRef]
- Frazão, J.B.; Thain, A.; Zhu, Z.; Luengo, M.; Condino-Neto, A.; Newburger, P.E. Regulation of CYBB Gene Expression in Human Phagocytes by a Distant Upstream NF-κB Binding Site. J. Cell. Biochem. 2015, 116, 2008–2017. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Sandford, A.J.; Wu, J.; Wang, Y.; Wu, S.; Ji, G.; Chen, G.; Feng, Y.; Tao, C.; et al. Association of CYBB polymorphisms with tuberculosis susceptibility in the Chinese Han population. Infect. Genet. Evol. 2015, 33, 169–175. [Google Scholar] [CrossRef]
- Acosta-Herrera, M.; Kerick, M.; González-Serna, D.; Wijmenga, C.; Franke, A.; Gregersen, P.K.; Padyukov, L.; Worthington, J.; Vyse, T.J.; Alarcón-Riquelme, M.E.; et al. Genome-wide meta-analysis reveals shared new loci in systemic seropositive rheumatic diseases. Ann. Rheum. Dis. 2018, 78, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Achury, J.; Zorro, M.M.; Ricaño-Ponce, I.; Zhernakova, D.V.; Diogo, D.; Raychaudhuri, S.; Franke, L.; Trynka, G.; Wijmenga, C.; Zhernakova, A. Functional implications of disease-specific variants in loci jointly associated with coeliac disease and rheumatoid arthritis. Hum. Mol. Genet. 2015, 25, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chen, Y.; Wu, Q.; Li, P.; Shao, Y.; Zhang, J.; Zhong, Q.; Peng, X.; Yang, H.; Hu, X.; et al. The association between single-nucleotide polymorphisms of NCF2 and systemic lupus erythematosus in Chinese mainland population. Clin. Rheumatol. 2010, 30, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277, Erratum in FEBS J. 2008, 275, 3984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-P.; Li, R.; Huang, Q.; Pan, H.-F.; Ye, D.-Q.; Li, X.-M. Association of NCF2, NCF4, and CYBA Gene Polymorphisms with Rheumatoid Arthritis in a Chinese Population. J. Immunol. Res. 2020, 2020, 8528976. [Google Scholar] [CrossRef]
- Roberts, R.L.; Hollis-Moffatt, J.; Gearry, R.; Kennedy, M.; Barclay, M.; Merriman, T. Confirmation of association of IRGM and NCF4 with ileal Crohn’s disease in a population-based cohort. Genes Immun. 2008, 9, 561–565. [Google Scholar] [CrossRef]
- Ryan, B.M.; Zanetti, K.A.; Robles, A.I.; Schetter, A.J.; Goodman, J.; Hayes, R.B.; Huang, W.-Y.; Gunter, M.J.; Yeager, M.; Burdette, L.; et al. Germline variation in NCF4, an innate immunity gene, is associated with an increased risk of colorectal cancer. Int. J. Cancer 2013, 134, 1399–1407. [Google Scholar] [CrossRef]
- Xing, Y.; Lin, Q.; Tong, Y.; Zhou, W.; Huang, J.; Wang, Y.; Huang, G.; Li, Y.; Xiang, Z.; Zhou, Z.; et al. Abnormal Neutrophil Transcriptional Signature May Predict Newly Diagnosed Latent Autoimmune Diabetes in Adults of South China. Front. Endocrinol. 2020, 11, 581902. [Google Scholar] [CrossRef]
- Azarova, I.E. NCF4 gene polymorphism, level of glutathione and glycated hemoglobin in type 2 diabetics with coronary artery disease. Med. Genet. 2021, 20, 37–47. (In Russian) [Google Scholar] [CrossRef]
- Vendrov, A.E.; Sumida, A.; Canugovi, C.; Lozhkin, A.; Hayami, T.; Madamanchi, N.R.; Runge, M.S. NOXA1-dependent NADPH oxidase regulates redox signaling and phenotype of vascular smooth muscle cell during atherogenesis. Redox Biol. 2019, 21, 101063. [Google Scholar] [CrossRef]
- Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H.; Ueno, N.; Takeya, R.; Miyano, K.; Kikuchi, H.; Sumimoto, H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: Its regulation by oxidase organizers and activators. J. Biol. Chem. 2005, 280, 23328–23339. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.J.; El Jamali, A.; Epperson, T.K.; Gamez, M.J.; Pearson, R.W.; Clark, R.A. NOX1 NADPH oxidase regulation by the NOXA1 SH3 domain. Free Radic. Biol. Med. 2007, 43, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, M.; Schickling, B.M.; Lopes, L.R.; Miller, F.J. Nox1 in cardiovascular diseases: Regulation and pathophysiology. Clin. Sci. 2015, 130, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Ritsick, D.; Cheng, G.; Lambeth, J.D. Point Mutations in the Proline-rich Region of p22 Are Dominant Inhibitors of Nox1- and Nox2-dependent Reactive Oxygen Generation. J. Biol. Chem. 2005, 280, 31859–31869. [Google Scholar] [CrossRef]
- Schroeder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 Is a Protective Reactive Oxygen Species Generating Vascular NADPH Oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef]
- Meng, W.; Shah, K.P.; Pollack, S.; Toppila, I.; Hebert, H.L.; McCarthy, M.I.; Groop, L.; Ahlqvist, E.; Lyssenko, V.; Agardh, E.; et al. A genome-wide association study suggests new evidence for an association of the NADPH Oxidase 4 (NOX4) gene with severe diabetic retinopathy in type 2 diabetes. Acta Ophthalmol. 2018, 96, e811–e819. [Google Scholar] [CrossRef]
- Roberts, K.E.; Kawut, S.M.; Krowka, M.J.; Brown, R.S.; Trotter, J.F.; Shah, V.; Peter, I.; Tighiouart, H.; Mitra, N.; Handorf, E.; et al. Genetic Risk Factors for Hepatopulmonary Syndrome in Patients With Advanced Liver Disease. Gastroenterology 2010, 139, 130–139. [Google Scholar] [CrossRef]
- Touyz, R.M.; Anagnostopoulou, A.; Rios, F.; Montezano, A.C.; Camargo, L.L. NOX5: Molecular biology and pathophysiology. Exp. Physiol. 2019, 104, 605–616. [Google Scholar] [CrossRef]
- Holterman, C.E.; Thibodeau, J.-F.; Towaij, C.; Gutsol, A.; Montezano, A.C.; Parks, R.J.; Cooper, M.E.; Touyz, R.M.; Kennedy, C.R. Nephropathy and Elevated BP in Mice with Podocyte-Specific NADPH Oxidase 5 Expression. J. Am. Soc. Nephrol. 2014, 25, 784–797. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Okabe, J.; Gray, S.P.; Hettige, T.; Chow, B.S.; Thallas-Bonke, V.; De Vos, L.; Holterman, C.E.; Coughlan, M.T.; et al. NADPH Oxidase Nox5 Accelerates Renal Injury in Diabetic Nephropathy. Diabetes 2017, 66, 2691–2703. [Google Scholar] [CrossRef]
- Shin, J.-G.; Seo, J.Y.; Seo, J.-M.; Kim, D.-Y.; Oh, J.-T.; Park, K.-W.; Kim, H.-Y.; Kim, J.-H.; Shin, H.D. Association analysis of NOX5 polymorphisms with Hirschsprung disease. J. Pediatr. Surg. 2019, 54, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Nagase, M.; Ayuzawa, N.; Kawarazaki, W.; Ishizawa, K.; Ueda, K.; Yoshida, S.; Fujita, T. Oxidative Stress Causes Mineralocorticoid Receptor Activation in Rat Cardiomyocytes. Hypertension 2012, 59, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Erickson, J.W.; Cerione, R.A. New Insights into How the Rho Guanine Nucleotide Dissociation Inhibitor Regulates the Interaction of Cdc42 with Membranes. J. Biol. Chem. 2009, 284, 23860–23871. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Jensen, T.E.; Kleinert, M.; Højlund, K.; Kiens, B.; Wojtaszewski, J.; Prats, C.; Schjerling, P.; Richter, E.A. Rac1 Signaling Is Required for Insulin-Stimulated Glucose Uptake and Is Dysregulated in Insulin-Resistant Murine and Human Skeletal Muscle. Diabetes 2013, 62, 1865–1875. [Google Scholar] [CrossRef]
- Kalwat, M.; Thurmond, D.C. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp. Mol. Med. 2013, 45, e37. [Google Scholar] [CrossRef]
- Subasinghe, W.; Syed, I.; Kowluru, A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic β-cells: Evidence for regulation by Rac1. Am. J. Physiol. Integr. Comp. Physiol. 2011, 300, R12–R20. [Google Scholar] [CrossRef]
- Newsholme, P.; Morgan, D.; Rebelato, E.; Oliveira-Emilio, H.C.; Procopio, J.; Curi, R.; Carpinelli, A. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009, 52, 2489–2498. [Google Scholar] [CrossRef]
- Syed, I.; Kyathanahalli, C.N.; Jayaram, B.; Govind, S.; Rhodes, C.J.; Kowluru, R.A.; Kowluru, A. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011, 60, 2843–2852. [Google Scholar] [CrossRef]
- Kowluru, A.; Kowluru, R.A. RACking up ceramide-induced islet β-cell dysfunction. Biochem. Pharmacol. 2018, 154, 161–169. [Google Scholar] [CrossRef]
- Marei, H.; Malliri, A. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases 2016, 8, 139–163. [Google Scholar] [CrossRef]
- Aguilar, B.J.; Zhu, Y.; Lu, Q. Rho GTPases as therapeutic targets in Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Azarova, I.; Klyosova, E.; Polonikov, A. Association between RAC1 gene variation, redox homeostasis and type 2 diabetes mellitus. Eur. J. Clin. Investig. 2022, 52, e13792. [Google Scholar] [CrossRef] [PubMed]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef]
- Pick, E. Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: Outsourcing a key task. Small GTPases 2014, 5, e27952. [Google Scholar] [CrossRef] [PubMed]
- Beckers, C.M.L.; van Hinsbergh, V.W.M.; Amerongen, G.P.V.N. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb. Haemost. 2010, 103, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Singh, A.K.; Dubey, M.; Kumar, S.; Saluja, R.; Keshari, R.S.; Verma, A.; Chandra, T.; Kumar, A.; Bajpai, V.K.; et al. Interaction of Inducible Nitric Oxide Synthase with Rac2 Regulates Reactive Oxygen and Nitrogen Species Generation in the Human Neutrophil Phagosomes: Implication in Microbial Killing. Antioxidants Redox Signal. 2014, 20, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Kinney, N.; Kang, L.; Bains, H.; Lawson, E.; Husain, M.; Husain, K.; Sandhu, I.; Shin, Y.; Carter, J.K.; Anandakrishnan, R.; et al. Ethnically biased microsatellites contribute to differential gene expression and glutathione metabolism in Africans and Europeans. PLoS ONE 2021, 16, e0249148. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A.; Bocharova, I.; Azarova, I.; Klyosova, E.; Bykanova, M.; Bushueva, O.; Polonikova, A.; Churnosov, M.; Solodilova, M. The Impact of Genetic Polymorphisms in Glutamate-Cysteine Ligase, a Key Enzyme of Glutathione Biosynthesis, on Ischemic Stroke Risk and Brain Infarct Size. Life 2022, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clin. Biochem. 2015, 48, 1200–1208. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine Increases Insulin Sensitivity and Glutathione Biosynthesis and Protects against Oxidative Stress in a Model of Sucrose-Induced Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef]
- Kalamkar, S.; Acharya, J.; Madathil, A.K.; Gajjar, V.; Divate, U.; Karandikar-Iyer, S.; Goel, P.; Ghaskadbi, S. Randomized Clinical Trial of How Long-Term Glutathione Supplementation Offers Protection from Oxidative Damage and Improves HbA1c in Elderly Type 2 Diabetic Patients. Antioxidants 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).