Inhibition of Macrophage-Specific CHIT1 as an Approach to Treat Airway Remodeling in Severe Asthma
Abstract
1. Introduction
2. Results
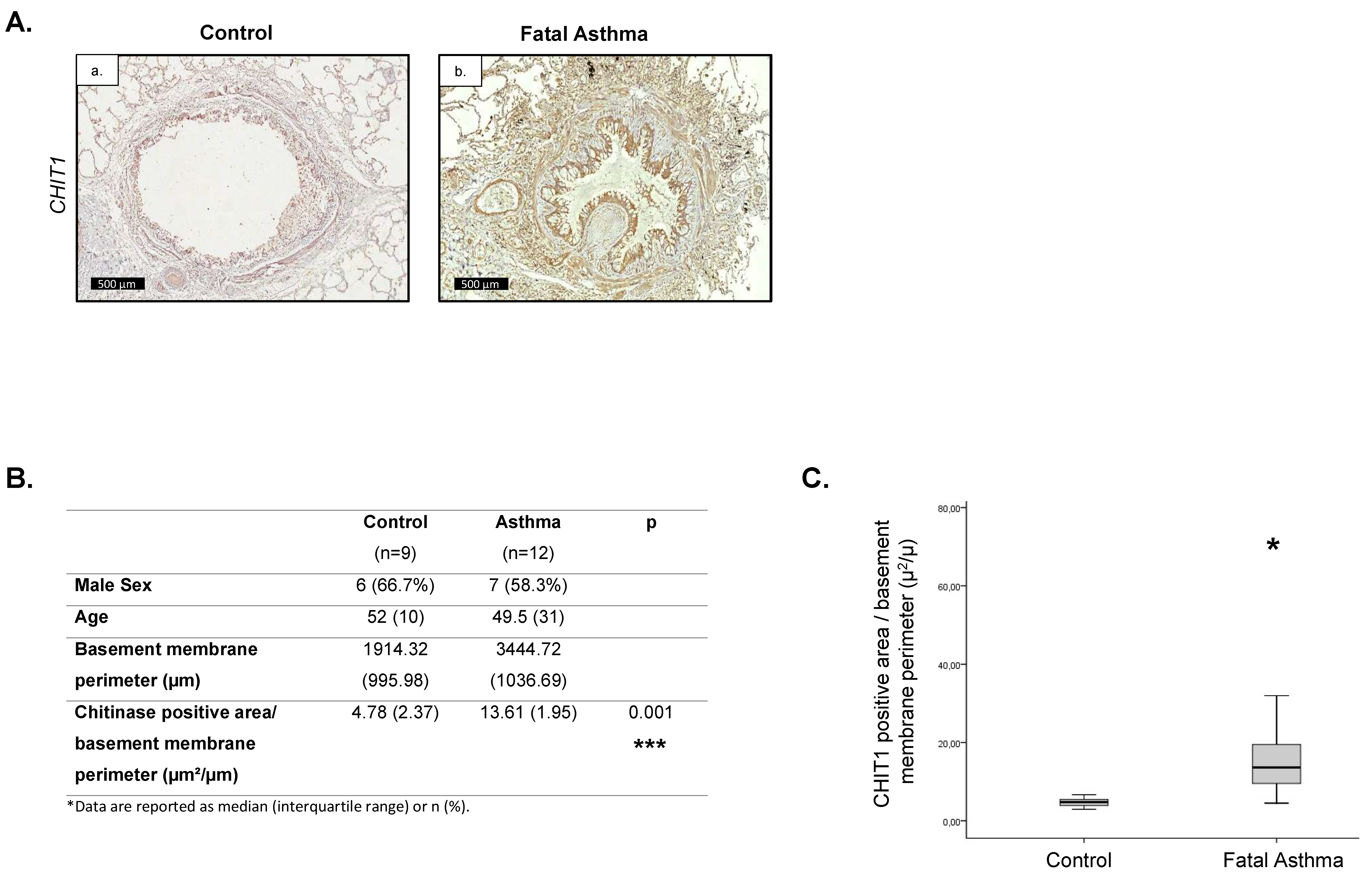
2.1. Increased CHIT1 Expression in Fatal Asthma
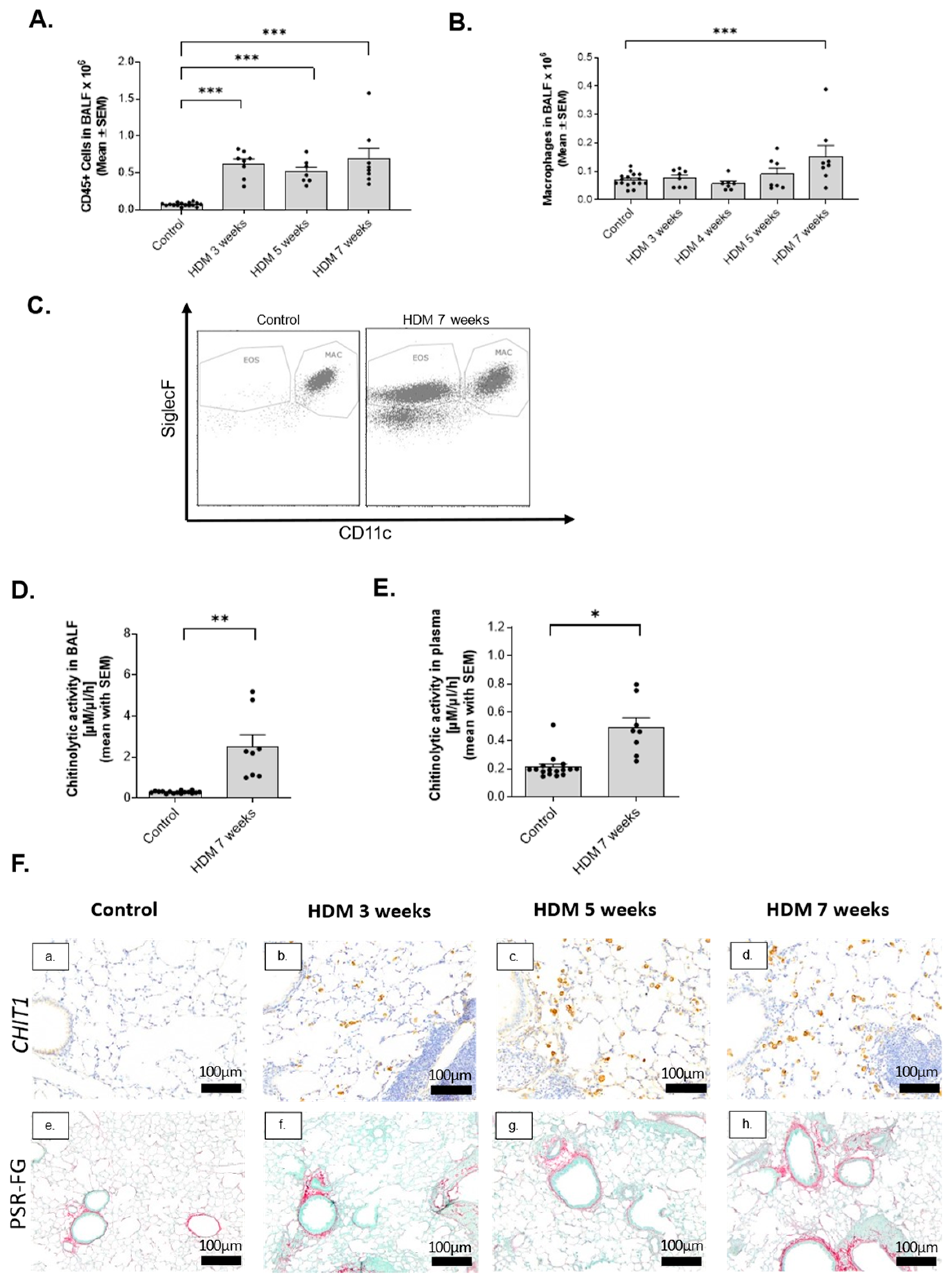
2.2. Activation of Macrophage-Specific CHIT1 in Chronic House Dust Mite (HDM) Asthma Model
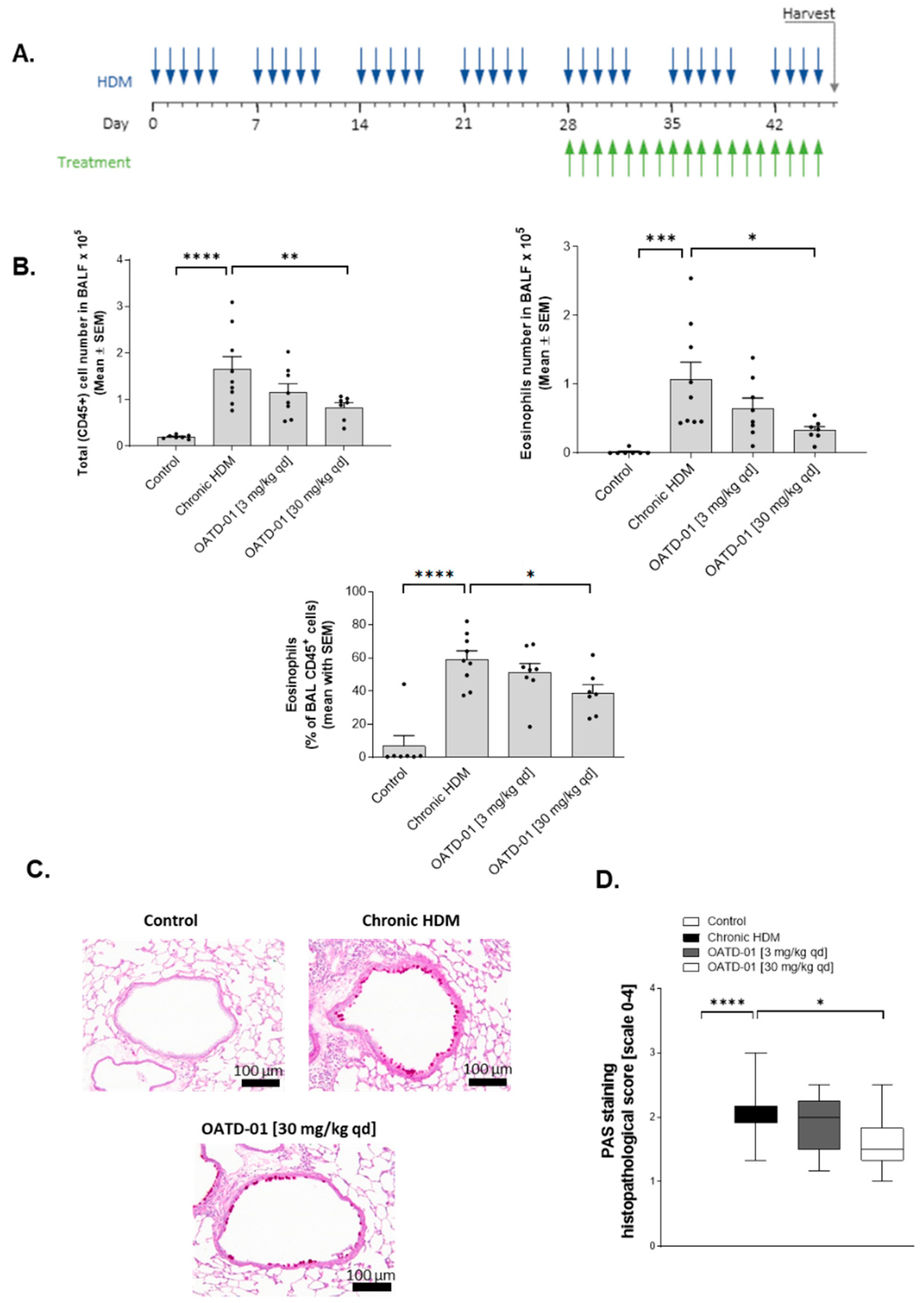
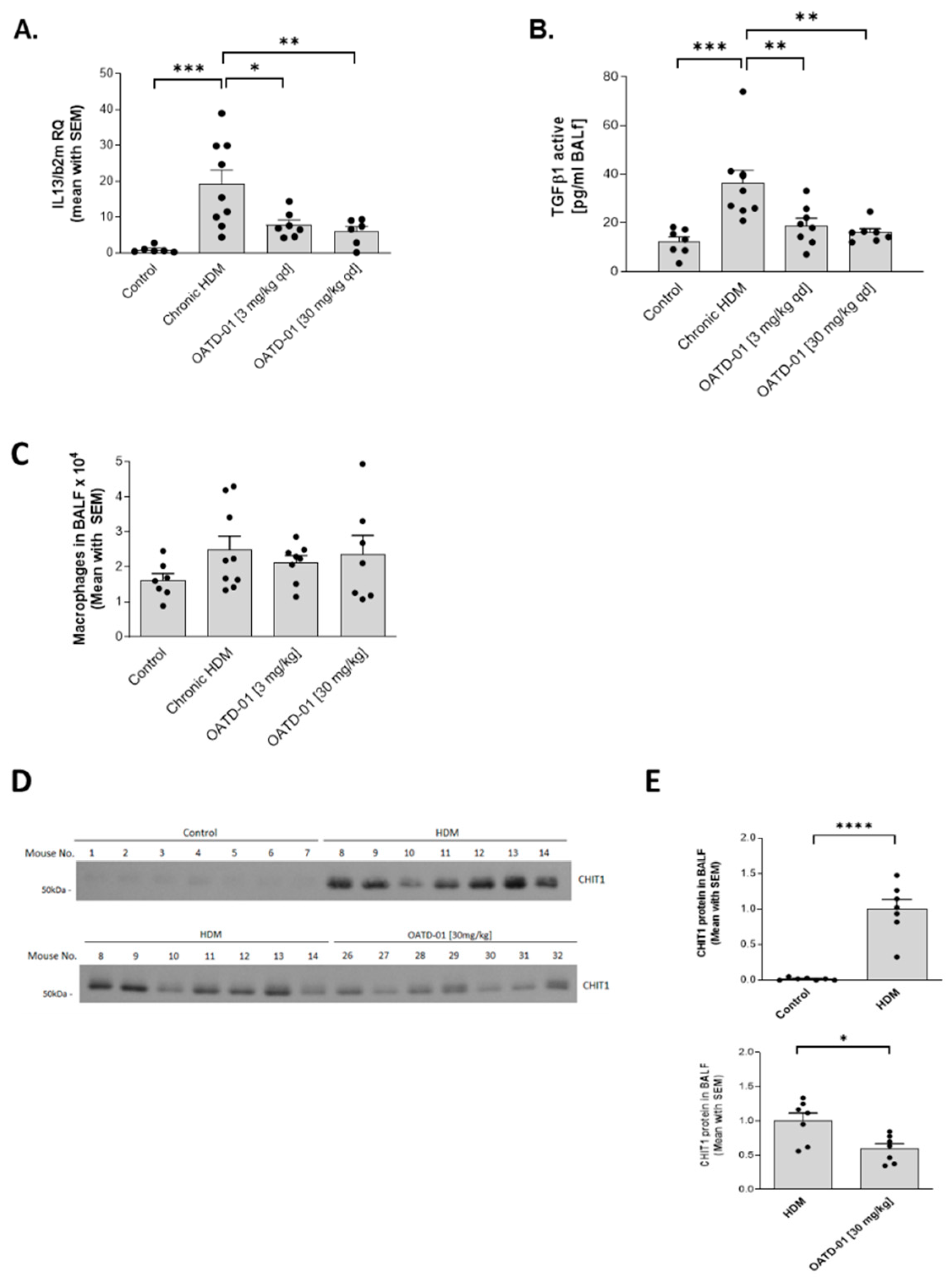
2.3. Chitinase Inhibition with OATD-01 Dose-Dependently Ameliorates Pulmonary Inflammation and Goblet Cell Metaplasia in Chronic Asthma Model
2.4. OATD-01 Changes Phenotype, but Not Number of Profibrotic Lung Macrophages
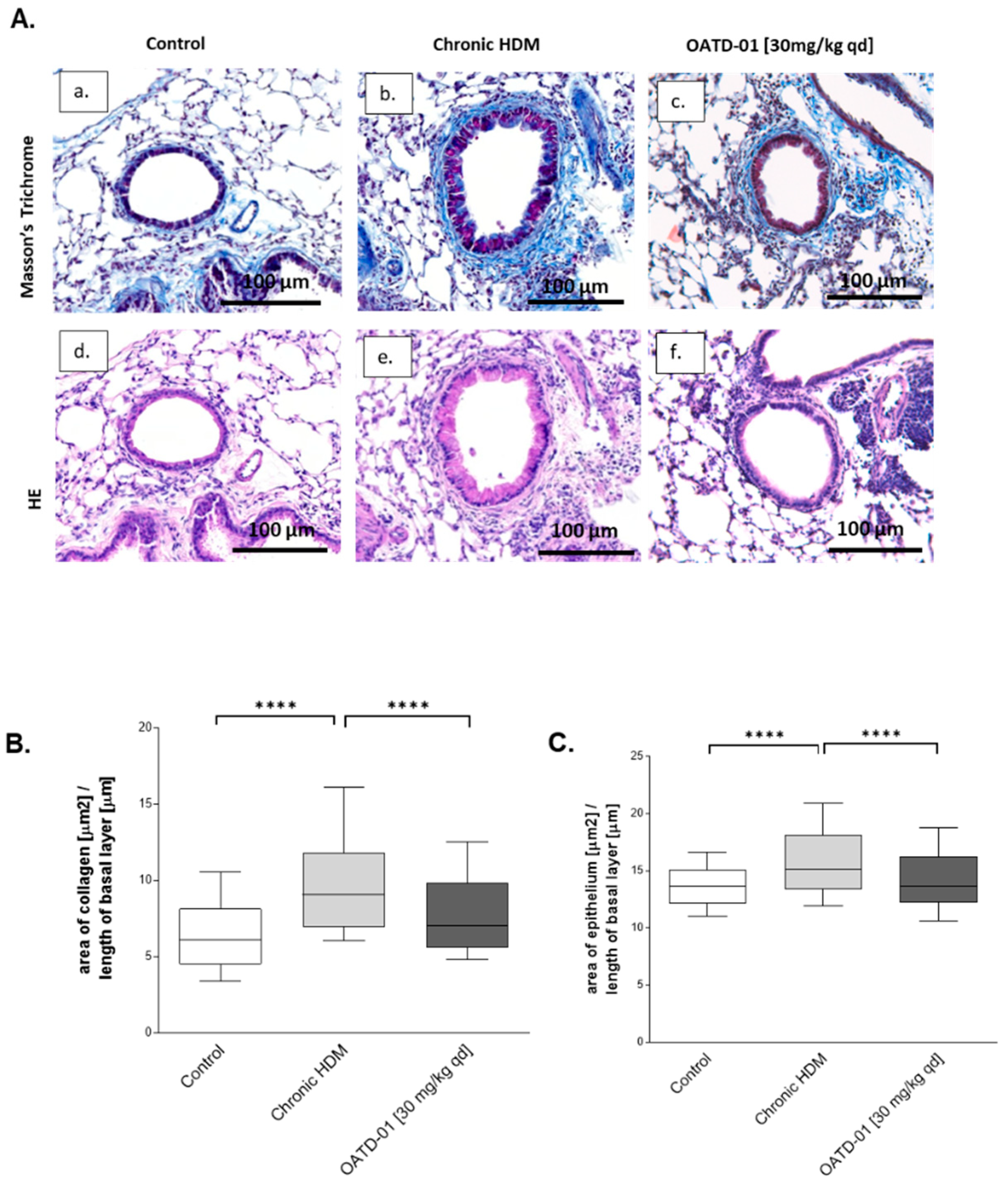
2.5. Chitinase Inhibition Prevents Development of Airway Remodeling after Chronic HDM Administration
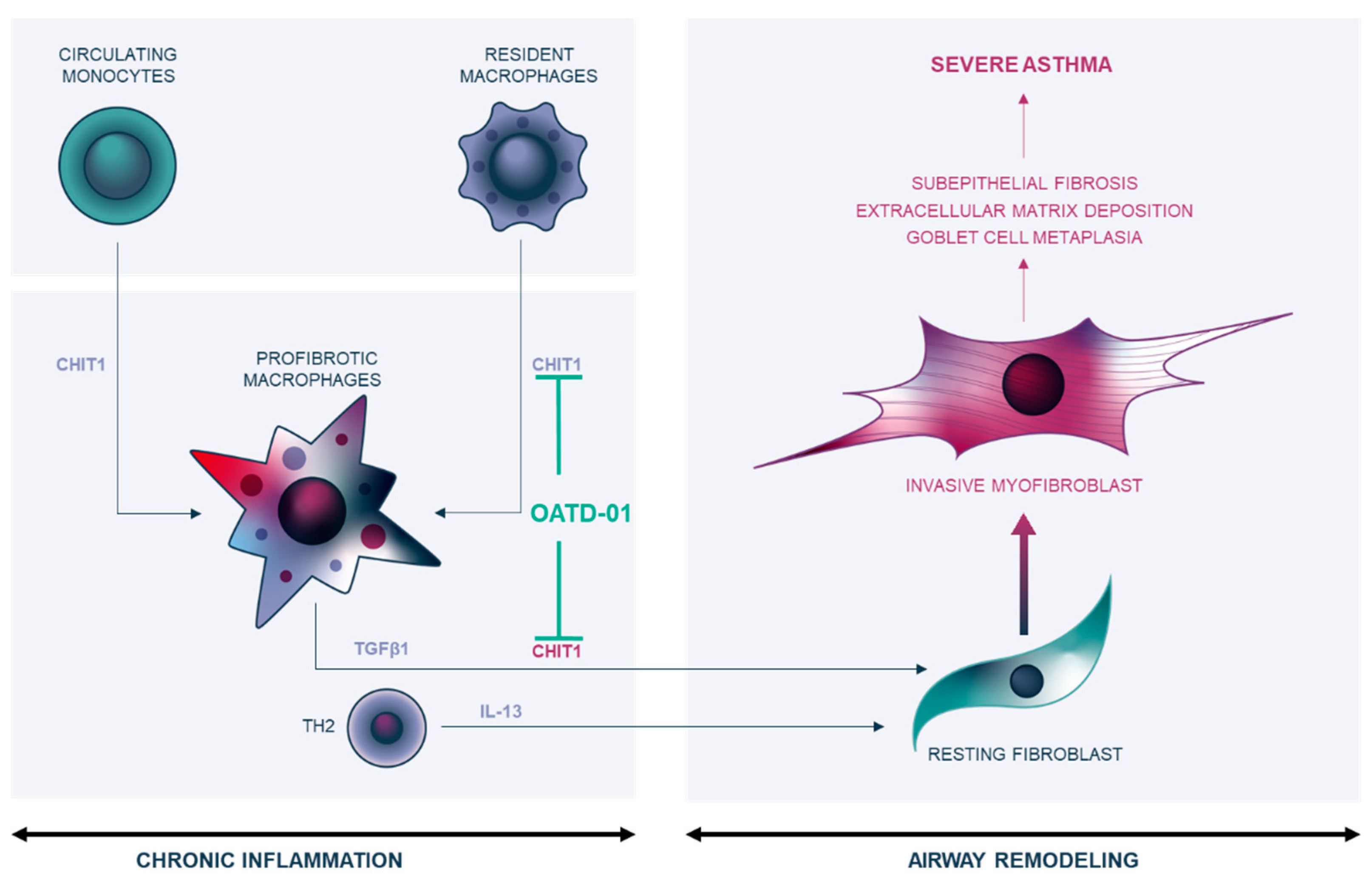
3. Discussion
4. Materials and Methods
4.1. Human Sample Collection and Analysis
4.2. Animal Studies
4.3. HDM-Induced Chronic Airway Inflammation and Remodeling Mouse Model
4.4. BAL Collection
4.5. Plasma Collection
4.6. Chitinolytic Activity in Murine BALF and Plasma
4.7. Lung Collection
4.8. Flow Cytometry Analysis
4.9. Real-Time PCR
4.10. ELISA
4.11. Western Blot Analysis
4.12. Histology
4.13. Immunohistochemistry
4.14. Semi-Quantitative Scoring of Goblet Cell Metaplasia in HDM-Induced Mouse Model of Asthma
4.15. Quantitative Analysis of Bronchiole Collagen Wall Thickness and Bronchiole Epithelium Thickness in HDM-Induced Mouse Model of Asthma
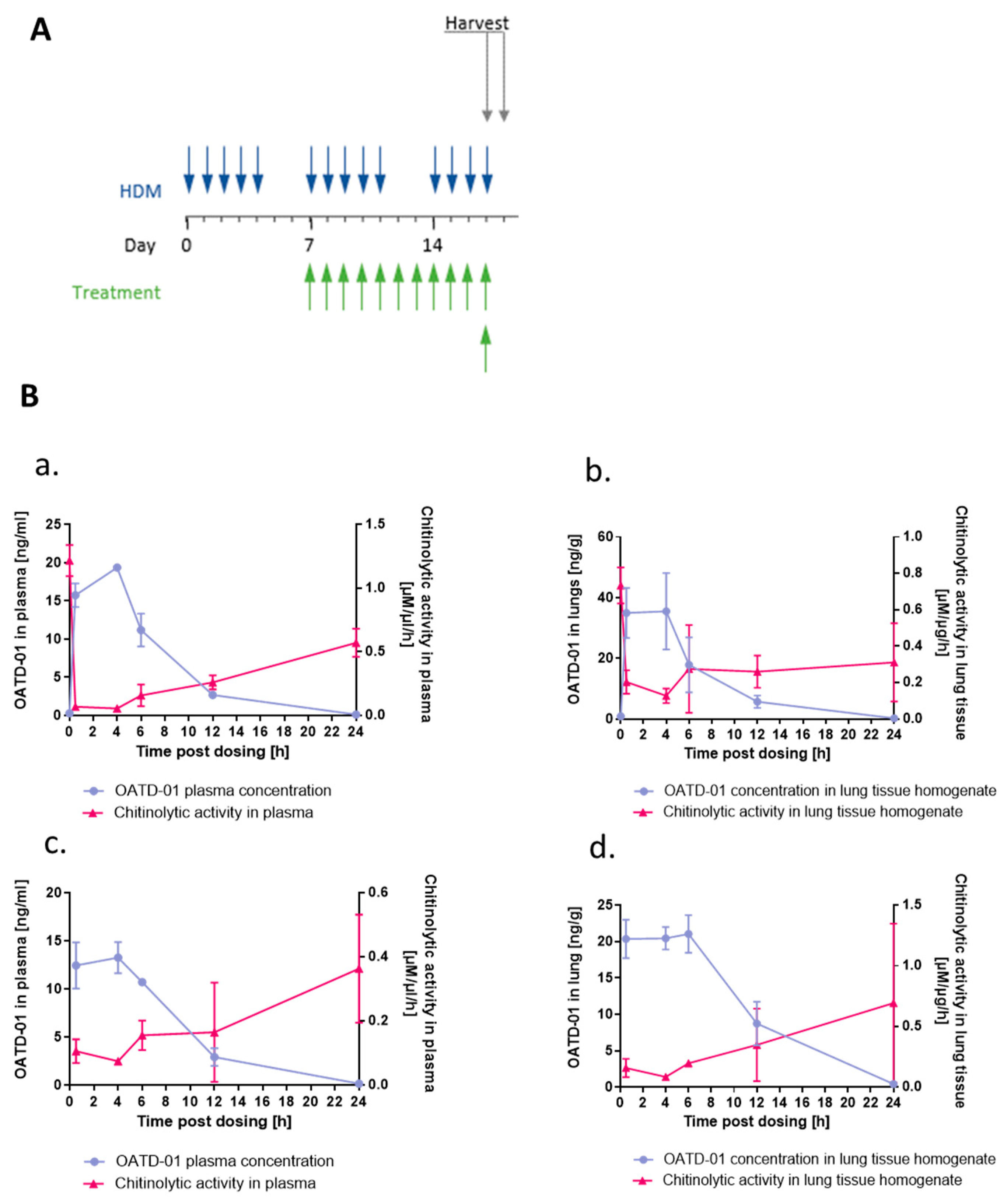
4.16. OATD-01 Pharmacokinetics/Pharmacodynamics (PK/PD) Studies in Mice
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trejo Bittar, H.E.; Yousem, S.A.; Wenzel, S.E. Pathobiology of Severe Asthma. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 511–545. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Das, J.; Wenzel, S.E. Determining Asthma Endotypes and Outcomes: Complementing Existing Clinical Practice with Modern Machine Learning. Cell Rep. Med. 2022, 3, 100857. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Curtiss, M.L.; Blain, T.J.; Liu, R.-M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.A.; Zhu, Z.; Chupp, G.; Homer, R.J. Airway Remodeling in Asthma. J. Clin. Investig. 1999, 104, 1001–1006. [Google Scholar] [CrossRef]
- Saglani, S.; Lloyd, C.M. Novel Concepts in Airway Inflammation and Remodelling in Asthma. Eur. Respir. J. 2015, 46, 1796–1804. [Google Scholar] [CrossRef]
- Jeffery, P.; Holgate, S.; Wenzel, S. Methods for the Assessment of Endobronchial Biopsies in Clinical Research. Am. J. Respir. Crit. Care Med. 2003, 168, S1–S17. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Prim. 2015, 1, 15025. [Google Scholar] [CrossRef]
- Thepen, T.; Kraal, G.; Holt, P.G. The Role of Alveolar Macrophages in Regulation of Lung Inflammation. Ann. N. Y. Acad. Sci. 1994, 725, 200–206. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar Macrophages: Plasticity in a Tissue-Specific Context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Kanneganti, M.; Kamba, A.; Mizoguchi, E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J. Epithel. Biol. Pharmacol. 2012, 5, 1–9. [Google Scholar] [CrossRef]
- van Eijk, M.; van Roomen, C.P.A.A.; Renkema, G.H.; Bussink, A.P.; Andrews, L.; Blommaart, E.F.C.; Sugar, A.; Verhoeven, A.J.; Boot, R.G.; Aerts, J.M.F.G. Characterization of Human Phagocyte-Derived Chitotriosidase, a Component of Innate Immunity. Int. Immunol. 2005, 17, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.; McCurdy, S.; Alcala, M.; Irei, J.; Garo, J.; Regan, W.; Lee, B.-H.; Kitamoto, S.; Boisvert, W.A. Expression of Chitotriosidase in Macrophages Modulates Atherosclerotic Plaque Formation in Hyperlipidemic Mice. Front. Physiol. 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Verde, F.; Fang, L.; Feneberg, E.; Oeckl, P.; Roeber, S.; Anderl-Straub, S.; Danek, A.; Diehl-Schmid, J.; Fassbender, K.; et al. Chitotriosidase (CHIT1) Is Increased in Microglia and Macrophages in Spinal Cord of Amyotrophic Lateral Sclerosis and Cerebrospinal Fluid Levels Correlate with Disease Severity and Progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Tibullo, D.; Cambria, D.; Distefano, G.; Saccone, S.; Di Raimondo, F.; Malaguarnera, L. Chitotriosidase Expression during Monocyte-Derived Dendritic Cells Differentiation and Maturation. Inflammation 2015, 38, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Sharma, L.; Dela Cruz, C.S. Chitotriosidase: A Marker and Modulator of Lung Disease. Eur. Respir. Rev. 2020, 29, 190143. [Google Scholar] [CrossRef]
- Logue, E.C.; Neff, C.P.; Mack, D.G.; Martin, A.K.; Fiorillo, S.; Lavelle, J.; Vandivier, R.W.; Campbell, T.B.; Palmer, B.E.; Fontenot, A.P. Upregulation of Chitinase 1 in Alveolar Macrophages of HIV-Infected Smokers. J. Immunol. 2019, 202, 1363–1372. [Google Scholar] [CrossRef]
- Elias, J.A.; Homer, R.J.; Hamid, Q.; Lee, C.G. Chitinases and Chitinase-like Proteins in TH2 Inflammation and Asthma. J. Allergy Clin. Immunol. 2005, 116, 497–500. [Google Scholar] [CrossRef]
- Byers, D.E.; Wu, K.; Dang-Vu, G.; Jin, X.; Agapov, E.; Zhang, X.; Battaile, J.T.; Schechtman, K.; Yusen, R.; Pierce, R.A.; et al. Triggering Receptor Expressed on Myeloid Cells-2 Expression Tracks with M2-Like Macrophage Activity and Disease Severity in COPD. Chest 2018, 153, 77–86. [Google Scholar] [CrossRef]
- Cho, S.J.; Weiden, M.D.; Lee, C.G. Chitotriosidase in the Pathogenesis of Inflammation, Interstitial Lung Diseases and COPD. Allergy. Asthma Immunol. Res. 2015, 7, 14–21. [Google Scholar] [CrossRef]
- Holt, P.G.; Oliver, J.; Bilyk, N.; McMenamin, C.; McMenamin, P.G.; Kraal, G.; Thepen, T. Downregulation of the Antigen Presenting Cell Function(s) of Pulmonary Dendritic Cells in Vivo by Resident Alveolar Macrophages. J. Exp. Med. 1993, 177, 397–407. [Google Scholar] [CrossRef]
- Zasłona, Z.; Przybranowski, S.; Wilke, C.; van Rooijen, N.; Teitz-Tennenbaum, S.; Osterholzer, J.J.; Wilkinson, J.E.; Moore, B.B.; Peters-Golden, M. Resident Alveolar Macrophages Suppress, Whereas Recruited Monocytes Promote, Allergic Lung Inflammation in Murine Models of Asthma. J. Immunol. 2014, 193, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.; Malaguarnera, G.; Gregorio, C.; D’Amico, F.; Mazzarino, M.C.; Malaguarnera, L. Modulation of Chitotriosidase During Macrophage Differentiation. Cell Biochem. Biophys. 2013, 66, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Huss, E.; Prell, T.; Steinbach, R.; Guerra, J.; Srivastava, A.; Witte, O.W.; Grosskreutz, J. Monocyte-Derived Macrophages Contribute to Chitinase Dysregulation in Amyotrophic Lateral Sclerosis: A Pilot Study. Front. Neurol. 2021, 12, 629332. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.B.; et al. Recruited Alveolar Macrophages, in Response to Airway Epithelial–Derived Monocyte Chemoattractant Protein 1/CCL2, Regulate Airway Inflammation and Remodeling in Allergic Asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef]
- Kitamoto, S.; Egashira, K.; Ichiki, T.; Han, X.; McCurdy, S.; Sakuda, S.; Sunagawa, K.; Boisvert, W.A. Chitinase Inhibition Promotes Atherosclerosis in Hyperlipidemic Mice. Am. J. Pathol. 2013, 183, 313–325. [Google Scholar] [CrossRef]
- Draijer, C.; Florez-Sampedro, L.; Reker-Smit, C.; Post, E.; van Dijk, F.; Melgert, B.N. Explaining the Polarized Macrophage Pool during Murine Allergic Lung Inflammation. Front. Immunol. 2022, 13, 7681. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.K.; Schloesser, D.; Fundel-Clemens, K.; Lerner, C.; Gabler, S.; Baskaran, P.; Wohnhaas, C.T.; Dichtl, S.; Huber, H.J.; Ask, K.; et al. Anti-Fibrotic Drug Nintedanib Inhibits CSF1R to Promote IL-4-Associated Tissue Repair Macrophages. Am. J. Respir. Cell Mol. Biol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dymek, B.; Sklepkiewicz, P.; Mlacki, M.; Güner, N.C.; Nejman-Gryz, P.; Drzewicka, K.; Przysucha, N.; Rymaszewska, A.; Paplinska-Goryca, M.; Zagozdzon, A.; et al. Pharmacological Inhibition of Chitotriosidase (CHIT1) as a Novel Therapeutic Approach for Sarcoidosis. J. Inflamm. Res. 2022, 15, 5621–5634. [Google Scholar] [CrossRef]
- Koralewski, R.; Dymek, B.; Mazur, M.; Sklepkiewicz, P.; Olejniczak, S.; Czestkowski, W.; Matyszewski, K.; Andryianau, G.; Niedziejko, P.; Kowalski, M.; et al. Discovery of OATD-01, a First-in-Class Chitinase Inhibitor as Potential New Therapeutics for Idiopathic Pulmonary Fibrosis. J. Med. Chem. 2020, 63, 15527–15540. [Google Scholar] [CrossRef]
- Agapov, E.; Battaile, J.T.; Tidwell, R.; Hachem, R.; Patterson, G.A.; Pierce, R.A.; Atkinson, J.J.; Holtzman, M.J. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am. J. Respir. Cell Mol. Biol. 2009, 41, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lee, C.-M.; Ma, B.; Kamle, S.; Elias, J.A.; Zhou, Y.; Lee, C.G. Targeting Chitinase 1 and Chitinase 3-Like 1 as Novel Therapeutic Strategy of Pulmonary Fibrosis. Front. Pharmacol. 2022, 13, 826471. [Google Scholar] [CrossRef] [PubMed]
- Sklepkiewicz, P.; Dymek, B.A.; Mlacki, M.; Koralewski, R.; Mazur, M.; Nejman-Gryz, P.; Korur, S.; Zagozdzon, A.; Rymaszewska, A.; von der Thüsen, J.H.; et al. Inhibition of CHIT1 as a Novel Therapeutic Approach in Idiopathic Pulmonary Fibrosis. Eur. J. Pharmacol. 2022, 919, 174792. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Tamaoki, J.; Takeyama, K.; Nakata, J.; Nagai, A. Interleukin-13 Induces Goblet Cell Differentiation in Primary Cell Culture from Guinea Pig Tracheal Epithelium. Am. J. Respir. Cell Mol. Biol. 2002, 27, 536–541. [Google Scholar] [CrossRef]
- Atherton, H.C.; Jones, G.; Danahay, H. IL-13-Induced Changes in the Goblet Cell Density of Human Bronchial Epithelial Cell Cultures: MAP Kinase and Phosphatidylinositol 3-Kinase Regulation. Am. J. Physiol. Cell. Mol. Physiol. 2003, 285, L730–L739. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Park, J.W.; Cho, W.-K.; Zhou, Y.; Han, B.; Yoon, P.O.; Chae, J.; Elias, J.A.; Lee, C.G. Modifiers of TGF-Β1 Effector Function as Novel Therapeutic Targets of Pulmonary Fibrosis. Korean J. Intern. Med. 2014, 29, 281. [Google Scholar] [CrossRef]
- James, A.J.; Reinius, L.E.; Verhoek, M.; Gomes, A.; Kupczyk, M.; Hammar, U.; Ono, J.; Ohta, S.; Izuhara, K.; Bel, E.; et al. The Chitinase Proteins YKL-40 and Chitotriosidase Are Increased in Both Asthma and COPD. Am. J. Respir. Crit. Care Med. 2015, 193, 1–68. [Google Scholar] [CrossRef]
- Harlander, M.; Lestan, D.; Turel, M. Chitotriosidase Activity in Plasma and COPD Exacerbations. Lung 2020, 198, 299–306. [Google Scholar] [CrossRef]
- Przysucha, N.; Górska, K.; Maskey-Warzęchowska, M.; Proboszcz, M.; Nejman-Gryz, P.; Paplińska-Goryca, M.; Dymek, B.; Zagozdzon, A.; Krenke, R. The Role of Chitinases in Chronic Airway Inflammation Associated with Tobacco Smoke Exposure. Cells 2022, 11, 3765. [Google Scholar] [CrossRef]
- Bargagli, E.; Margollicci, M.; Luddi, A.; Nikiforakis, N.; Grazia Perari, M.; Grosso, S.; Perrone, A.; Rottoli, P. Chitotriosidase Activity in Patients with Interstitial Lung Diseases. Respir. Med. 2007, 101, 2176–2181. [Google Scholar] [CrossRef]
- Bennett, D.; Cameli, P.; Lanzarone, N.; Carobene, L.; Bianchi, N.; Fui, A.; Rizzi, L.; Bergantini, L.; Cillis, G.; Miriana, D.A.; et al. Chitotriosidase: A Biomarker of Activity and Severity in Patients with Sarcoidosis. Respir. Res. 2020, 21, 12. [Google Scholar] [CrossRef]
- Seibold, M.A.; Donnelly, S.; Solon, M.; Innes, A.; Woodruff, P.G.; Boot, R.G.; Burchard, E.G.; Fahy, J.V. Chitotriosidase Is the Primary Active Chitinase in the Human Lung and Is Modulated by Genotype and Smoking Habit. J. Allergy Clin. Immunol. 2008, 122, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; He, C.-H.; Park, J.W.; Lee, J.H.; Kamle, S.; Ma, B.; Akosman, B.; Cotez, R.; Chen, E.; Zhou, Y.; et al. Chitinase 1 Regulates Pulmonary Fibrosis by Modulating TGF-β/SMAD7 Pathway via TGFBRAP1 and FOXO3. Life Sci. Alliance 2019, 2, e201900350. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Herzog, E.L.; Ahangari, F.; Zhou, Y.; Gulati, M.; Lee, C.-M.; Peng, X.; Feghali-Bostwick, C.; Jimenez, S.A.; Varga, J.; et al. Chitinase 1 Is a Biomarker for and Therapeutic Target in Scleroderma-Associated Interstitial Lung Disease That Augments TGF-1 Signaling. J. Immunol. 2012, 189, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Reyfman, P.A.; Walter, J.M.; Joshi, N.; Anekalla, K.R.; McQuattie-Pimentel, A.C.; Chiu, S.; Fernandez, R.; Akbarpour, M.; Chen, C.-I.; Ren, Z.; et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1517–1536. [Google Scholar] [CrossRef] [PubMed]
- Letuve, S.; Kozhich, A.; Humbles, A.; Brewah, Y.; Dombret, M.C.; Grandsaigne, M.; Adle, H.; Kolbeck, R.; Aubier, M.; Coyle, A.J.; et al. Lung Chitinolytic Activity and Chitotriosidase Are Elevated in Chronic Obstructive Pulmonary Disease and Contribute to Lung Inflammation. Am. J. Pathol. 2010, 176, 638–649. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Garcia, D.; Porter, P.; Huang, X.; Quinlan, P.J.; Blanc, P.D.; Corry, D.B.; Locksley, R.M. Fungal Chitin from Asthma-Associated Home Environments Induces Eosinophilic Lung Infiltration. J. Immunol. 2011, 187, 2261–2267. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic Mammalian Chitinase in Asthmatic Th2 Inflammation and IL-13 Pathway Activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef]
- Mazur, M.; Olczak, J.; Olejniczak, S.; Koralewski, R.; Czestkowski, W.; Jedrzejczak, A.; Golab, J.; Dzwonek, K.; Dymek, B.; Sklepkiewicz, P.L.; et al. Targeting Acidic Mammalian Chitinase Is Effective in Animal Model of Asthma. J. Med. Chem. 2018, 61, 695–710. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, C.-M.; Lee, J.H.; Kim, M.-O.; Park, J.W.; Kamle, S.; Akosman, B.; Herzog, E.L.; Peng, X.Y.; Elias, J.A.; et al. Kasugamycin Is a Novel Chitinase 1 Inhibitor with Strong Antifibrotic Effects on Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2022, 67, 309–319. [Google Scholar] [CrossRef]
- Hong, J.Y.; Kim, M.; Sol, I.S.; Kim, K.W.; Lee, C.-M.; Elias, J.A.; Sohn, M.H.; Lee, C.G. Chitotriosidase Inhibits Allergic Asthmatic Airways via Regulation of TGF-β Expression and Foxp3 + Treg Cells. Allergy 2018, 73, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Makinde, T.; Murphy, R.F.; Agrawal, D.K. The Regulatory Role of TGF- β in Airway Remodeling in Asthma. Immunol. Cell Biol. 2007, 85, 348–356. [Google Scholar] [CrossRef]
- Tomkowicz, A.; Kraus-Filarska, M.; Bar, J.; Rabczyński, J.; Jeleń, M.; Piesiak, P.; Fal, A.; Panaszek, B. Bronchial Hyper-Responsiveness, Subepithelial Fibrosis, and Transforming Growth Factor-Β1 Expression in Patients with Long-Standing and Recently Diagnosed Asthma. Arch. Immunol. Ther. Exp. 2008, 56, 401–408. [Google Scholar] [CrossRef]
- Kim, K.K.; Sheppard, D.; Chapman, H.A. TGF-Β1 Signaling and Tissue Fibrosis. Cold Spring Harb. Perspect. Biol. 2018, 10, a022293. [Google Scholar] [CrossRef]
- Kenyon, N.J. TGF-1 Causes Airway Fibrosis and Increased Collagen I and III MRNA in Mice. Thorax 2003, 58, 772–777. [Google Scholar] [CrossRef]
- Voynow, J.A.; Fischer, B.M.; Malarkey, D.E.; Burch, L.H.; Wong, T.; Longphre, M.; Ho, S.B.; Foster, W.M. Neutrophil Elastase Induces Mucus Cell Metaplasia in Mouse Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1293–L1302. [Google Scholar] [CrossRef]
| Antigen | Conjugate | Dilution | Cat. | No. |
|---|---|---|---|---|
| Gr1 | Alexa 488 | 1:800 | Biolegend, San Diego, CA, USA | 108417 |
| Siglec F | PE | 1:400 | BD Biosciences, San Jose, CA, USA | 552126 |
| CD11c | APC | 1:400 | eBiosciences San Diego, CA, USA | 17-0114-82 |
| CD45.2 | PerCp-Cy5 | 1:100 | BD Biosciences, San Jose, CA, USA | 552950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sklepkiewicz, P.; Dymek, B.; Mlacki, M.; Zagozdzon, A.; Salamon, M.; Siwińska, A.M.; Mazurkiewicz, M.P.; de Souza Xavier Costa, N.; Mazur, M.; Mauad, T.; et al. Inhibition of Macrophage-Specific CHIT1 as an Approach to Treat Airway Remodeling in Severe Asthma. Int. J. Mol. Sci. 2023, 24, 4719. https://doi.org/10.3390/ijms24054719
Sklepkiewicz P, Dymek B, Mlacki M, Zagozdzon A, Salamon M, Siwińska AM, Mazurkiewicz MP, de Souza Xavier Costa N, Mazur M, Mauad T, et al. Inhibition of Macrophage-Specific CHIT1 as an Approach to Treat Airway Remodeling in Severe Asthma. International Journal of Molecular Sciences. 2023; 24(5):4719. https://doi.org/10.3390/ijms24054719
Chicago/Turabian StyleSklepkiewicz, Piotr, Barbara Dymek, Michal Mlacki, Agnieszka Zagozdzon, Magdalena Salamon, Anna Maria Siwińska, Marcin Piotr Mazurkiewicz, Natalia de Souza Xavier Costa, Marzena Mazur, Thais Mauad, and et al. 2023. "Inhibition of Macrophage-Specific CHIT1 as an Approach to Treat Airway Remodeling in Severe Asthma" International Journal of Molecular Sciences 24, no. 5: 4719. https://doi.org/10.3390/ijms24054719
APA StyleSklepkiewicz, P., Dymek, B., Mlacki, M., Zagozdzon, A., Salamon, M., Siwińska, A. M., Mazurkiewicz, M. P., de Souza Xavier Costa, N., Mazur, M., Mauad, T., Gołębiowski, A., Dzwonek, K., Gołąb, J., & Zasłona, Z. (2023). Inhibition of Macrophage-Specific CHIT1 as an Approach to Treat Airway Remodeling in Severe Asthma. International Journal of Molecular Sciences, 24(5), 4719. https://doi.org/10.3390/ijms24054719






