Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats
Abstract
1. Introduction
2. Results
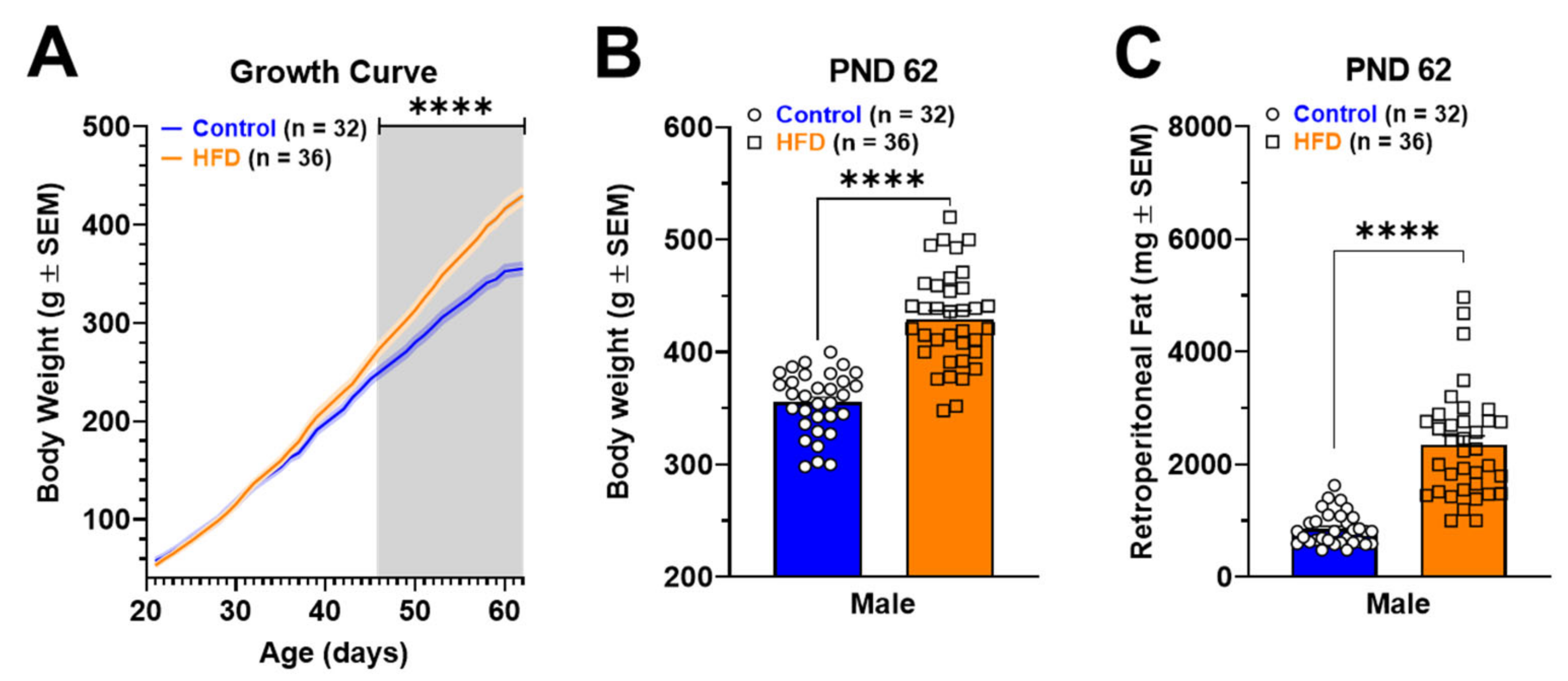
2.1. Murinometric Parameters in Rats Exposed to HFD
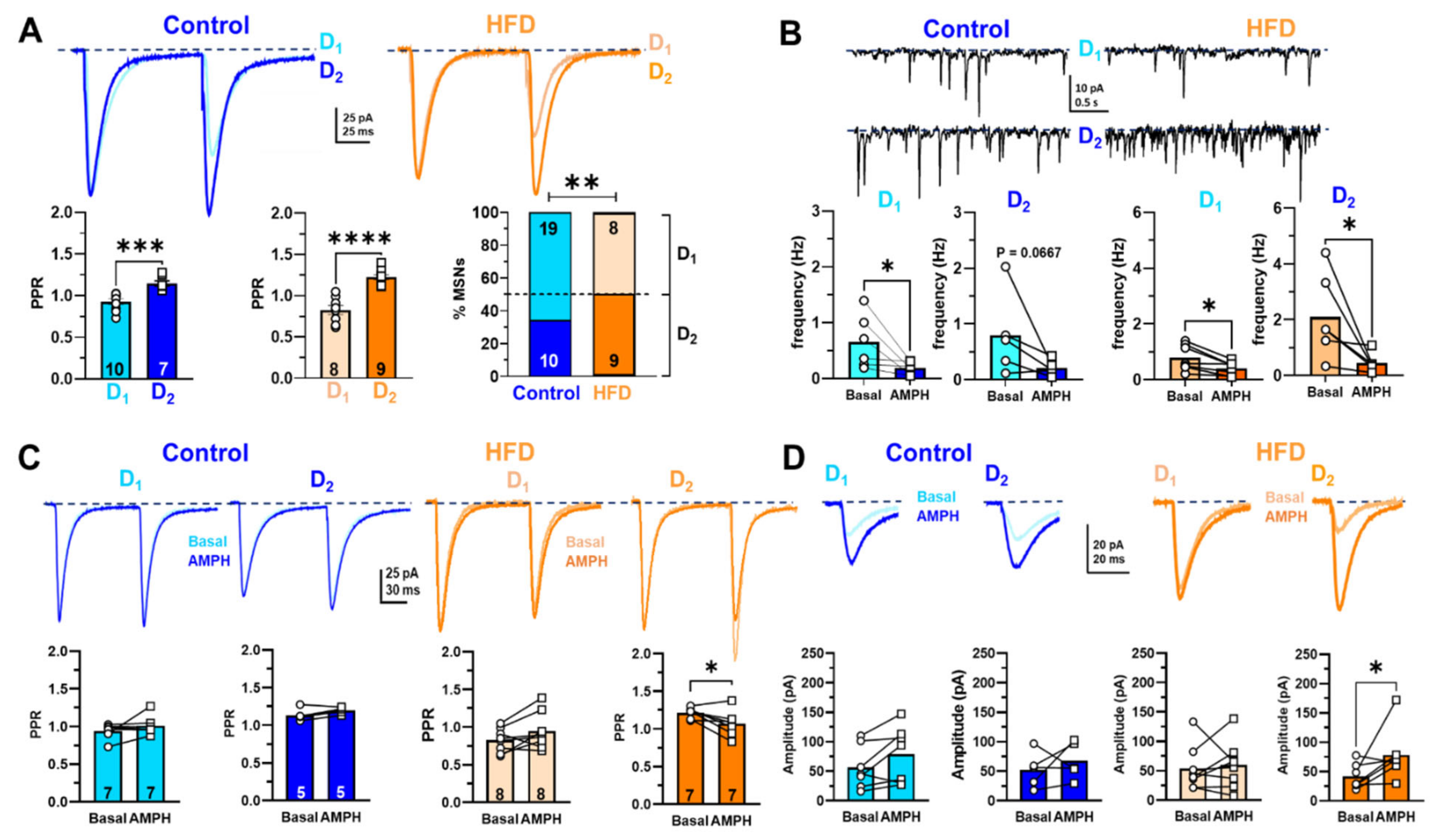
2.2. Electrophysiological Recordings in NAcc of Rats Exposed to HFD
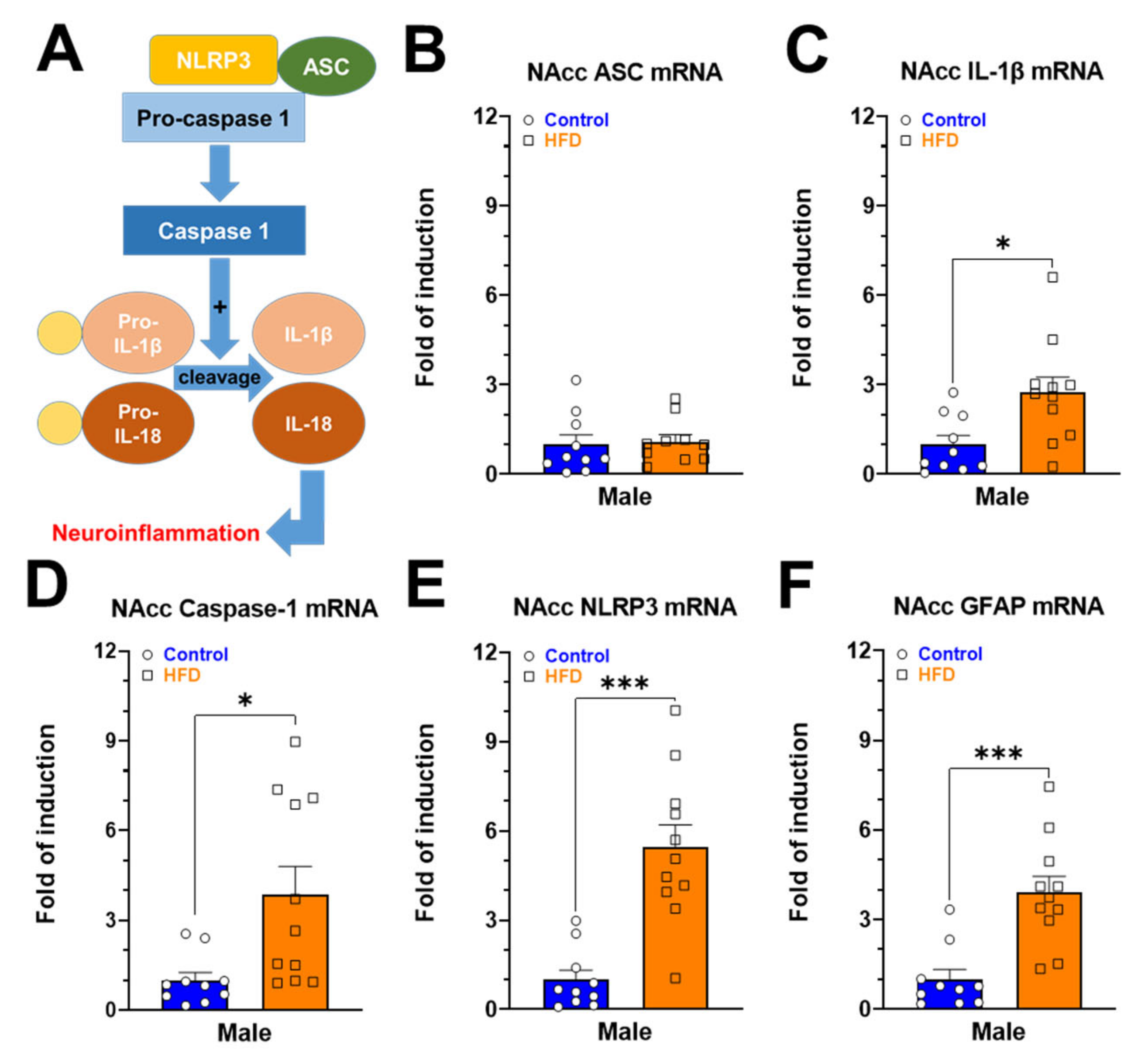
2.3. Gene Expression of Neuroinflammatory Markers in NAcc of Rats Exposed to HFD
2.4. Monoamine Contents in Brain Nuclei of the Mesolimbic and Nigrostriatal Pathways of Rats Exposed to HFD
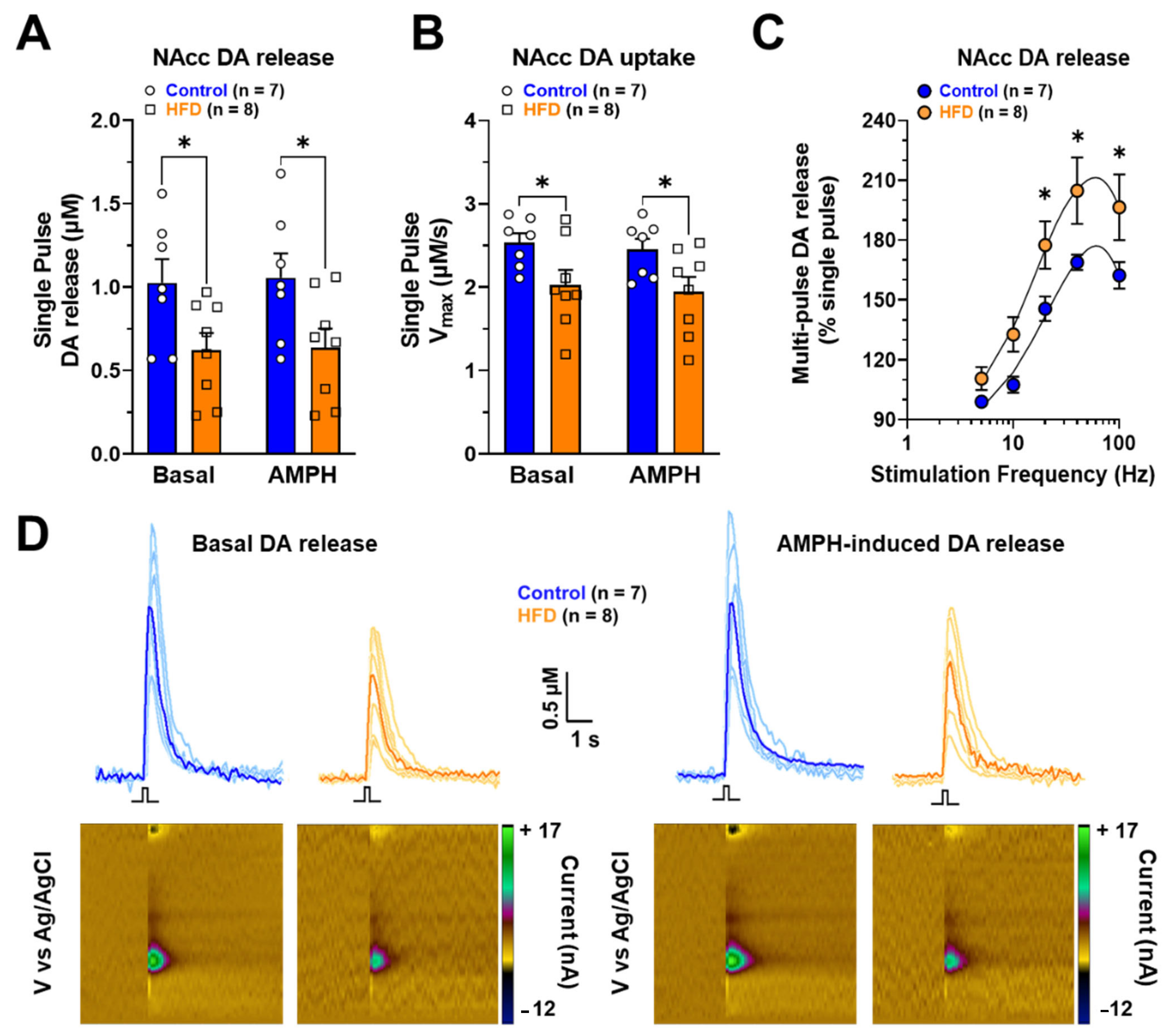
2.5. Ex-Vivo DA Release in NAcc of Rats Exposed to HFD
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Experimental Procedure
4.4. Electrophysiological Studies: Slice Preparation, Recording, and Analysis
4.5. RT-qPCR
4.6. Neurochemical Studies
4.6.1. Monoamine Content Quantification Using HPLC-ED
4.6.2. Ex-Vivo Fast Scan Cyclic Voltammetry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 December 2022).
- O’Donnell, P.; Grace, A.A. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 1993, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.N.; Hocking, S.L.; Markovic, T.P. Pharmacotherapy for the treatment of obesity. Mol. Cell Endocrinol. 2015, 418 Pt 2, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cason, A.M.; Smith, R.J.; Tahsili-Fahadan, P.; Moorman, D.E.; Sartor, G.C.; Aston-Jones, G. Role of orexin/hypocretin in reward-seeking and addiction: Implications for obesity. Physiol. Behav. 2010, 100, 419–428. [Google Scholar] [CrossRef]
- Sweeney, P.; Yang, Y. Neural Circuit Mechanisms Underlying Emotional Regulation of Homeostatic Feeding. Trends Endocrinol. Metab. 2017, 28, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Hernandez, L.; Hoebel, B.G. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res. Bull. 1990, 25, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Volkow, N.D. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry 2005, 162, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Gysling, K.; Wang, R.Y. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983, 277, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.W.; North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Pontieri, F.E.; Di Chiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 1997, 276, 2048–2050. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Gatley, S.J.; Logan, J.; Wang, G.J.; Ding, Y.S.; Dewey, S. PET evaluation of the dopamine system of the human brain. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1996, 37, 1242–1256. [Google Scholar]
- Cepeda, C.; Andre, V.M.; Yamazaki, I.; Wu, N.; Kleiman-Weiner, M.; Levine, M.S. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur. J. Neurosci. 2008, 27, 671–682. [Google Scholar] [CrossRef]
- Mariano, I.R.; Yamada, L.A.; Soares Rabassi, R.; Rissi Sabino, V.L.; Bataglini, C.; Azevedo, S.; Garcia, R.F.; Pedrosa, M.M.D. Differential Responses of Liver and Hypothalamus to the Nutritional Condition During Lactation and Adult Life. Front. Physiol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Lu, X.Y.; Ghasemzadeh, M.B.; Kalivas, P.W. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience 1998, 82, 767–780. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Brown, R.M.; Heinsbroek, J.A.; Lobo, M.K.; Schwartz, D.J.; Kalivas, P.W. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 2015, 18, 1230–1232. [Google Scholar] [CrossRef]
- Godfrey, N.; Borgland, S.L. Diversity in the lateral hypothalamic input to the ventral tegmental area. Neuropharmacology 2019, 154, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Espana, R.A. Hypocretin/orexin regulation of dopamine signaling: Implications for reward and reinforcement mechanisms. Front. Behav. Neurosci. 2012, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.H.; Tahsili-Fahadan, P.; Wise, R.A.; Lupica, C.R.; Aston-Jones, G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science 2011, 333, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Vega-Quiroga, I.; Yarur, H.E.; Gysling, K. Lateral septum stimulation disinhibits dopaminergic neurons in the antero-ventral region of the ventral tegmental area: Role of GABA-A alpha 1 receptors. Neuropharmacology 2018, 128, 76–85. [Google Scholar] [CrossRef]
- Yoshida, K.; McCormack, S.; Espana, R.A.; Crocker, A.; Scammell, T.E. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006, 494, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Zeltser, L.M. Feeding circuit development and early-life influences on future feeding behaviour. Nat. Rev. Neurosci. 2018, 19, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Dixon, J.B. Food for Thought: Reward Mechanisms and Hedonic Overeating in Obesity. Curr. Obes. Rep. 2017, 6, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef]
- Frank, M.G.; Adhikary, S.; Sobesky, J.L.; Weber, M.D.; Watkins, L.R.; Maier, S.F. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav. Immun. 2016, 51, 99–108. [Google Scholar] [CrossRef]
- Gutierrez-Martos, M.; Girard, B.; Mendonca-Netto, S.; Perroy, J.; Valjent, E.; Maldonado, R.; Martin, M. Cafeteria diet induces neuroplastic modifications in the nucleus accumbens mediated by microglia activation. Addict. Biol. 2018, 23, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Decarie-Spain, L.; Sharma, S.; Hryhorczuk, C.; Issa-Garcia, V.; Barker, P.A.; Arbour, N.; Alquier, T.; Fulton, S. Nucleus accumbens inflammation mediates anxiodepressive behavior and compulsive sucrose seeking elicited by saturated dietary fat. Mol. Metab. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Pennartz, C.M.; Groenewegen, H.J.; Lopes da Silva, F.H. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 1994, 42, 719–761. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 2011, 69, 628–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dever, D.; Lowe, J.; Storey, G.P.; Bhansali, A.; Eck, E.K.; Nitulescu, I.; Weimer, J.; Bamford, N.S. Regulation of prefrontal excitatory neurotransmission by dopamine in the nucleus accumbens core. J. Physiol. 2012, 590, 3743–3769. [Google Scholar] [CrossRef]
- Planert, H.; Berger, T.K.; Silberberg, G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS ONE 2013, 8, e57054. [Google Scholar] [CrossRef]
- Krentzel, A.A.; Willett, J.A.; Johnson, A.G.; Meitzen, J. Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell. J. Comp. Neurol. 2021, 529, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Gertler, T.S.; Chan, C.S.; Surmeier, D.J. Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. 2008, 28, 10814–10824. [Google Scholar] [CrossRef]
- Cao, J.; Dorris, D.M.; Meitzen, J. Electrophysiological properties of medium spiny neurons in the nucleus accumbens core of prepubertal male and female Drd1a-tdTomato line 6 BAC transgenic mice. J. Neurophysiol. 2018, 120, 1712–1727. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Russell, J.; Monsalves-Alvarez, M.; Cruz, G.; Valladares-Ide, D.; Basualto-Alarcon, C.; Barrientos, G.; Estrada, M.; Llanos, P. NLRP3 Inflammasome: Potential Role in Obesity Related Low-Grade Inflammation and Insulin Resistance in Skeletal Muscle. Int. J. Mol. Sci. 2021, 22, 3254. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997, 17, 851–861. [Google Scholar] [CrossRef]
- Hajnal, A.; Smith, G.P.; Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R31–R37. [Google Scholar] [CrossRef]
- Bassareo, V.; Di Chiara, G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur. J. Neurosci. 1999, 11, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. In Brain Imaging in Behavioral Neuroscience; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2012; Volume 11, pp. 1–24. [Google Scholar]
- Fernandez-Aranda, F.; Karwautz, A.; Treasure, J. Food addiction: A transdiagnostic construct of increasing interest. Eur. Eat Disord. Rev. 2018, 26, 536–540. [Google Scholar] [CrossRef]
- Dang, L.C.; Samanez-Larkin, G.R.; Castrellon, J.J.; Perkins, S.F.; Cowan, R.L.; Zald, D.H. Associations between dopamine D2 receptor availability and BMI depend on age. NeuroImage 2016, 138, 176–183. [Google Scholar] [CrossRef]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lalchandani, R.R.; van der Goes, M.S.; Partridge, J.G.; Vicini, S. Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J. Neurosci. 2013, 33, 14075–14086. [Google Scholar] [CrossRef] [PubMed]
- Bamford, N.S.; Wightman, R.M.; Sulzer, D. Dopamine’s Effects on Corticostriatal Synapses during Reward-Based Behaviors. Neuron 2018, 97, 494–510. [Google Scholar] [CrossRef]
- Pardo-Garcia, T.R.; Garcia-Keller, C.; Penaloza, T.; Richie, C.T.; Pickel, J.; Hope, B.T.; Harvey, B.K.; Kalivas, P.W.; Heinsbroek, J.A. Ventral Pallidum Is the Primary Target for Accumbens D1 Projections Driving Cocaine Seeking. J. Neurosci. 2019, 39, 2041–2051. [Google Scholar] [CrossRef]
- Marcos-Gomez, B.; Bustos, M.; Prieto, J.; Martinez, J.A.; Moreno-Aliaga, M.J. Obesity, inflammation and insulin resistance: Role of gp 130 receptor ligands. Sist. Sanit. Navar. 2008, 31, 113–123. [Google Scholar]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef]
- Karczewski, J.; Zielinska, A.; Staszewski, R.; Eder, P.; Dobrowolska, A.; Souto, E.B. Obesity and the Brain. Int. J. Mol. Sci. 2022, 23, 6145. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.G.; Dos Santos Quaresma, M.V.L.; Nakamoto, F.P.; Magalhaes, A.C.O.; Lucin, G.A.; Thomatieli-Santos, R.V. Does Modern Lifestyle Favor Neuroimmunometabolic Changes? A Path to Obesity. Front. Nutr. 2021, 8, 705545. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, S.; Hancock, D.P.; Petocz, P.; Ceriello, A.; Brand-Miller, J. High-glycemic index carbohydrate increases nuclear factor-kappaB activation in mononuclear cells of young, lean healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1188–1193. [Google Scholar]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Rheinheimer, J.; de Souza, B.M.; Cardoso, N.S.; Bauer, A.C.; Crispim, D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Henriksbo, B.D.; Anhe, F.F.; Schertzer, J.D. The NLRP3 inflammasome regulates adipose tissue metabolism. Biochem. J. 2020, 477, 1089–1107. [Google Scholar] [CrossRef]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Merali, Z.; Anisman, H. Variations of nucleus accumbens dopamine and serotonin following systemic interleukin-1, interleukin-2 or interleukin-6 treatment. Neuroscience 1999, 88, 823–836. [Google Scholar] [CrossRef]
- Emmons, H.A.; Wallace, C.W.; Fordahl, S.C. Interleukin-6 and tumor necrosis factor-alpha attenuate dopamine release in mice fed a high-fat diet, but not medium or low-fat diets. Nutr. Neurosci. 2022, 1–11. [Google Scholar] [CrossRef]
- South, T.; Huang, X.F. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem. Res. 2008, 33, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Woods, C.; Zhen, J.; Antonio, T.; Carr, K.D.; Reith, M.E. Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain. J. Neurochem. 2017, 140, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.C.; Stouffer, M.A.; Mancini, M.; Nicholson, C.; Carr, K.D.; Rice, M.E. Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. Eur. J. Neurosci. 2019, 49, 794–804. [Google Scholar] [CrossRef]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.R.; Fordahl, S.C. Bingeing on High-Fat Food Enhances Evoked Dopamine Release and Reduces Dopamine Uptake in the Nucleus Accumbens. Obesity 2021, 29, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.W.; Beatty, N.S.; Hutcherson, S.A.; Emmons, H.A.; Loudermilt, M.C.; Fordahl, S.C. Replacing a Palatable High-Fat Diet with a Low-Fat Alternative Heightens kappa-Opioid Receptor Control over Nucleus Accumbens Dopamine. Nutrients 2021, 13, 2341. [Google Scholar] [CrossRef]
- Elgueta-Reyes, M.; Martinez-Pinto, J.; Renard, G.M.; Sotomayor-Zarate, R. Neonatal programming with sex hormones: Effect on expression of dopamine D1 receptor and neurotransmitters release in nucleus accumbens in adult male and female rats. Eur. J. Pharmacol. 2021, 902, 174118. [Google Scholar] [CrossRef] [PubMed]
- Elgueta-Reyes, M.; Velasquez, V.B.; Espinosa, P.; Riquelme, R.; Dib, T.; Sanguinetti, N.K.; Escobar, A.P.; Martinez-Pinto, J.; Renard, G.M.; Sotomayor-Zarate, R. Effects of Early Life Exposure to Sex Hormones on Neurochemical and Behavioral Responses to Psychostimulants in Adulthood: Implications in Drug Addiction. Int. J. Mol. Sci. 2022, 23, 6575. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yorgason, J.T.; Espana, R.A.; Jones, S.R. Demon voltammetry and analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 2011, 202, 158–164. [Google Scholar] [CrossRef] [PubMed]


| Gene | GenBank Access (N°) | Forward Primer | Reverse Primer |
|---|---|---|---|
| ASC | NM_172322.1 | 5′-TGCTGCAGATGGACCCCATA-3′ | 5′-CACAGCTCCAGACTCTTCCATA-3′ |
| IL-1β | NM_031512.2 | 5′-AGCTTCAGGAAGGCAGTGTC-3′ | 5′-TCAGACAGCACGAGGCATTT-3′ |
| Caspase-1 | NM_012762.3 | 5′-CACGAGACCTGTGCGATCAT-3′ | 5′-CTTGAGGGAACCACTCGGTC-3′ |
| NLRP3 | NM_001191642.1 | 5′-CTCTGCATGCCGTATCTGGT-3′ | 5′-GTCCTGAGCCATGGAAGCAA-3′ |
| GFAP | NM_017009.2 | 5′-CAACCTCCAGATCCGAGAAACC-3′ | 5′-GCATCTCCACCGTCTTTACCA-3′ |
| 18s | NR_046237.2 | 5′-TCAAGAACGAAAGTCGGAGG-3′ | 5′-GGACATCTAAGGGCATCACA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Briceño, W.; Velásquez, V.B.; Silva-Olivares, F.; Ceballo, K.; Céspedes, R.; Jorquera, G.; Cruz, G.; Martínez-Pinto, J.; Bonansco, C.; Sotomayor-Zárate, R. Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats. Int. J. Mol. Sci. 2023, 24, 4703. https://doi.org/10.3390/ijms24054703
Plaza-Briceño W, Velásquez VB, Silva-Olivares F, Ceballo K, Céspedes R, Jorquera G, Cruz G, Martínez-Pinto J, Bonansco C, Sotomayor-Zárate R. Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats. International Journal of Molecular Sciences. 2023; 24(5):4703. https://doi.org/10.3390/ijms24054703
Chicago/Turabian StylePlaza-Briceño, Wladimir, Victoria B. Velásquez, Francisco Silva-Olivares, Karina Ceballo, Ricardo Céspedes, Gonzalo Jorquera, Gonzalo Cruz, Jonathan Martínez-Pinto, Christian Bonansco, and Ramón Sotomayor-Zárate. 2023. "Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats" International Journal of Molecular Sciences 24, no. 5: 4703. https://doi.org/10.3390/ijms24054703
APA StylePlaza-Briceño, W., Velásquez, V. B., Silva-Olivares, F., Ceballo, K., Céspedes, R., Jorquera, G., Cruz, G., Martínez-Pinto, J., Bonansco, C., & Sotomayor-Zárate, R. (2023). Chronic Exposure to High Fat Diet Affects the Synaptic Transmission That Regulates the Dopamine Release in the Nucleus Accumbens of Adolescent Male Rats. International Journal of Molecular Sciences, 24(5), 4703. https://doi.org/10.3390/ijms24054703









