Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes
Abstract
1. Introduction
2. Results
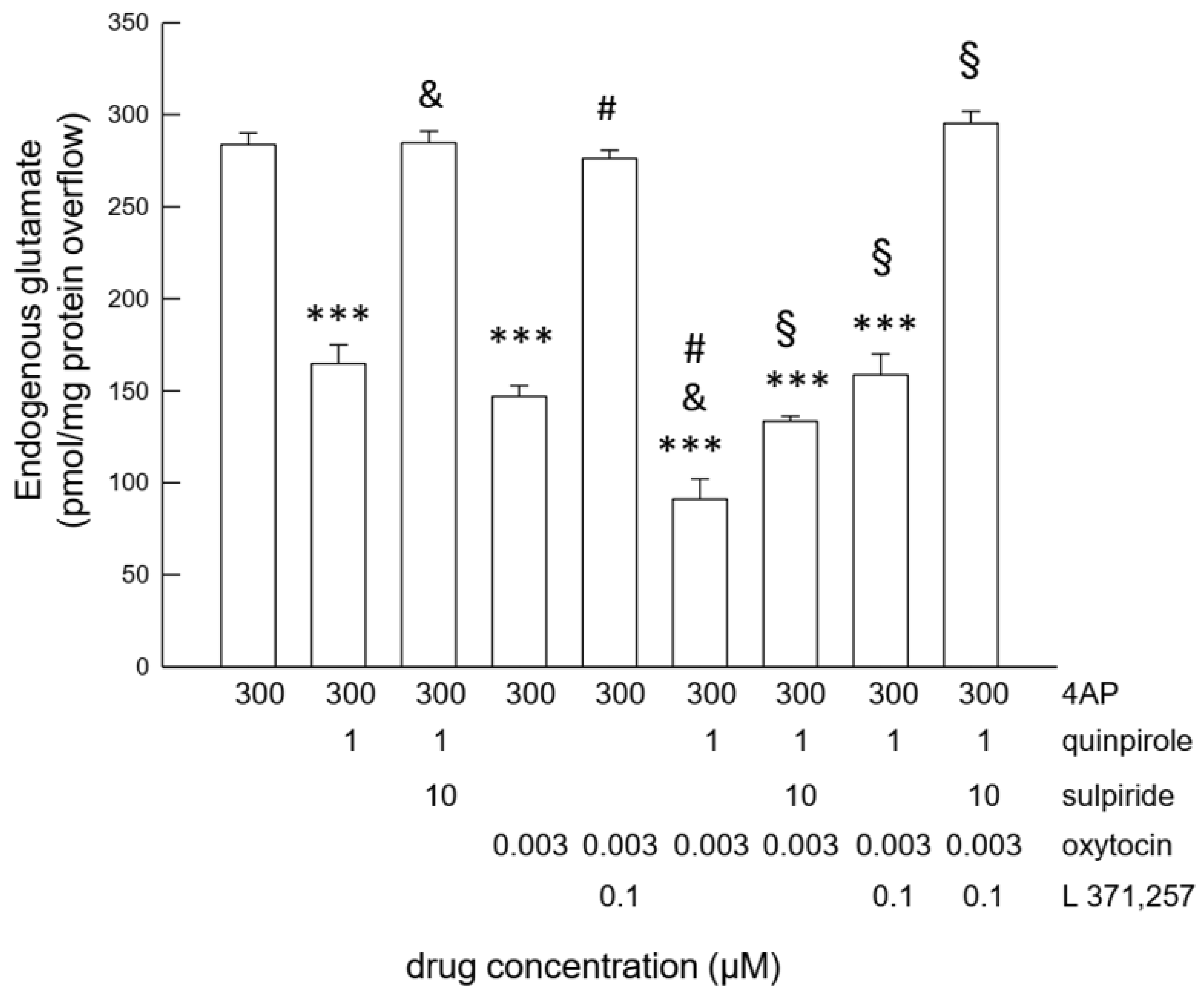
2.1. Activation of D2 or OT Receptor Inhibits the 4-AP-Evoked Glutamate Release
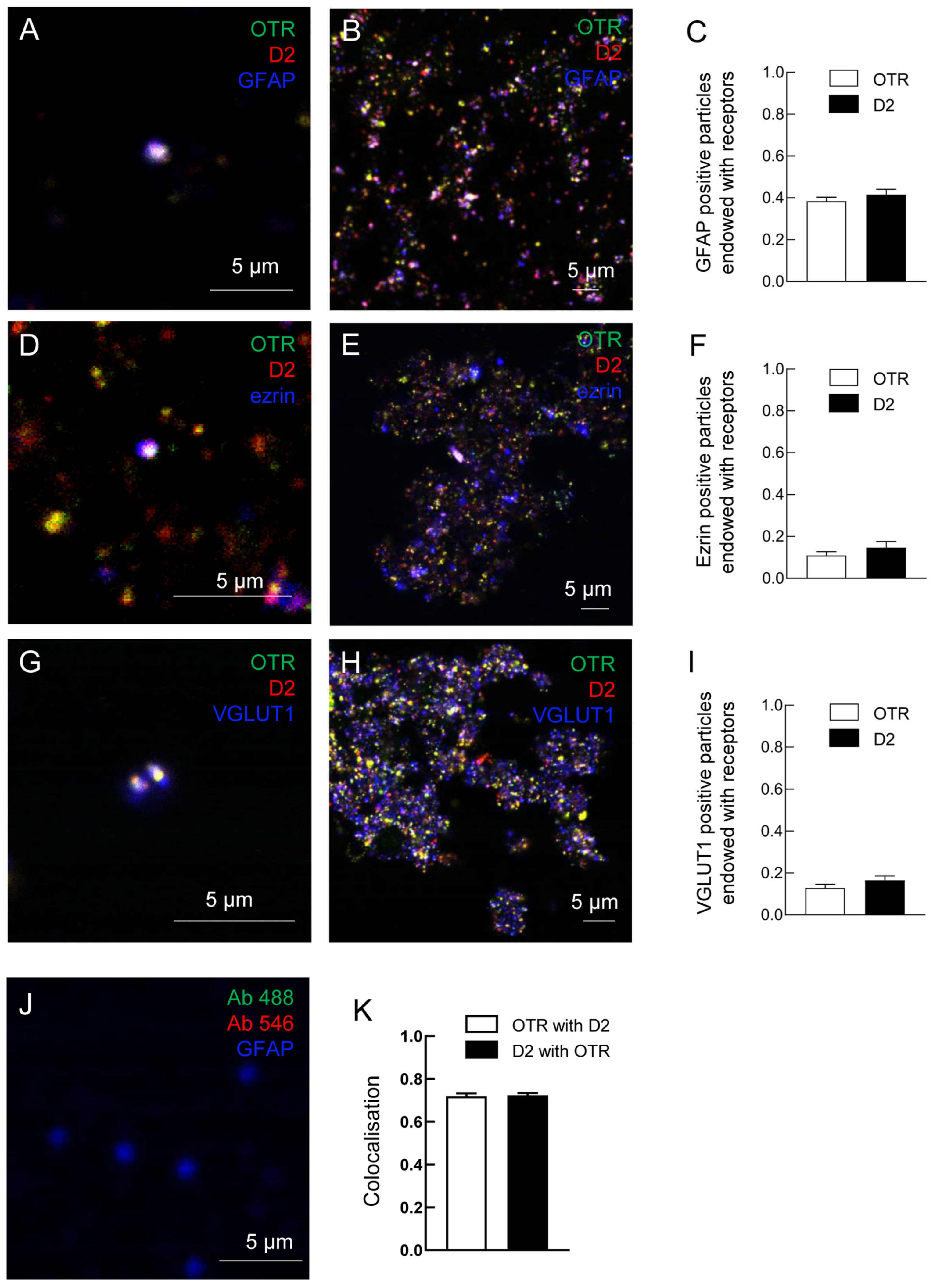
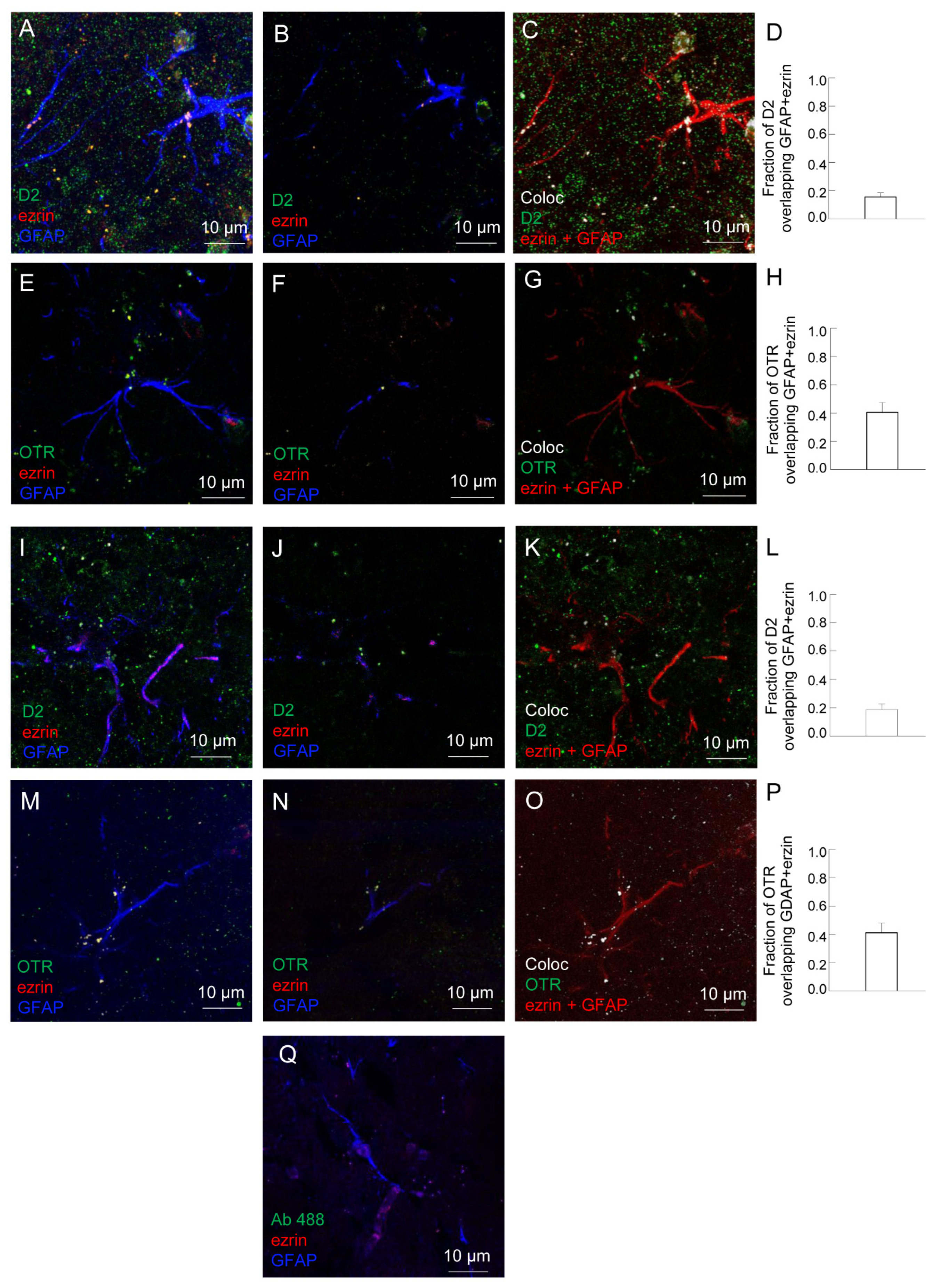
2.2. Both D2 and OT Receptors Are Co-Localized on Striatal Astrocytic Processes
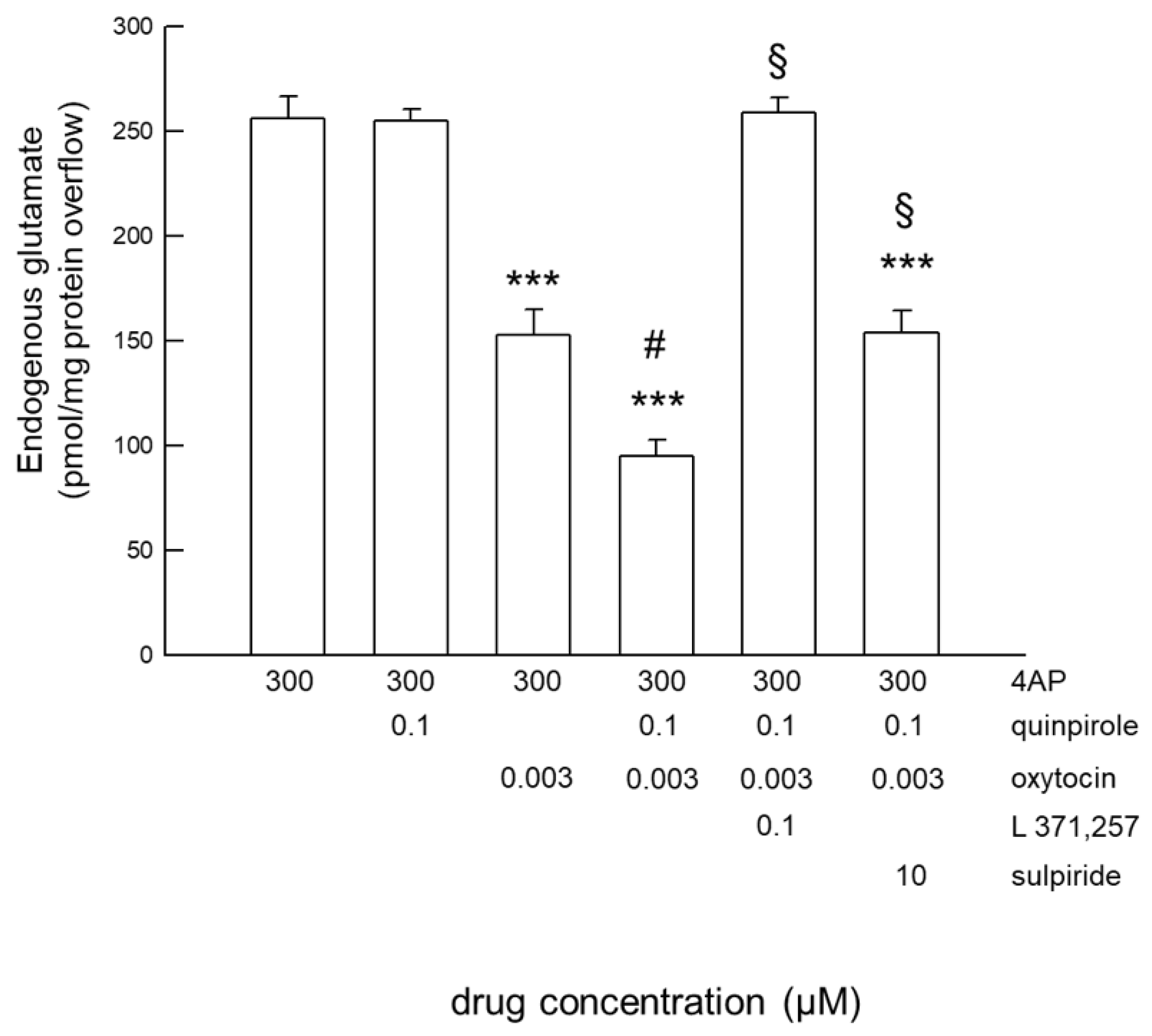
2.3. Functional Interaction between OT and D2 Receptors Expressed on Striatal Astrocytic Process
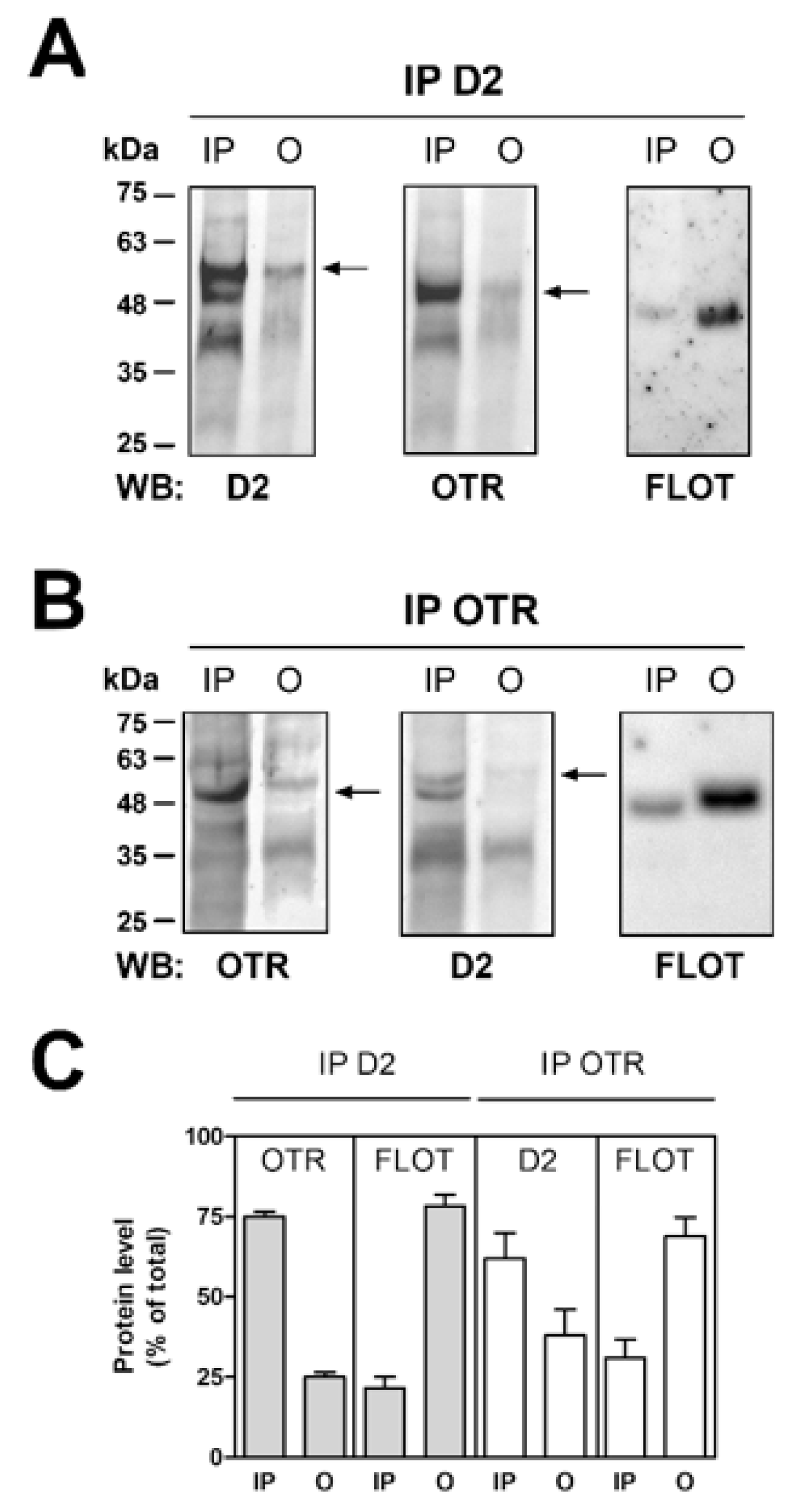
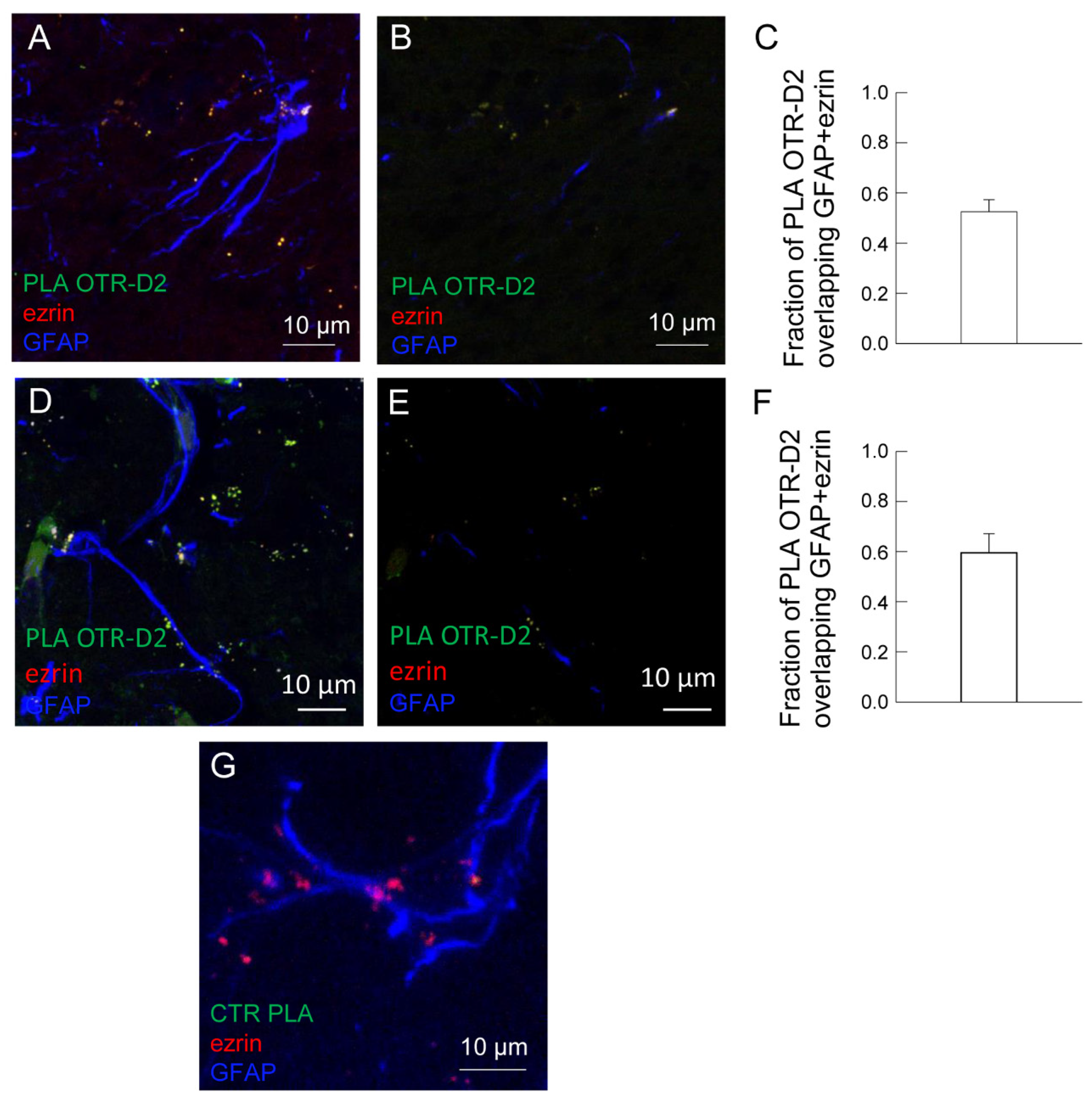
2.4. OT and D2 Receptors Expressed on Striatal Astrocytic Processes Physically Interact
2.5. OT and D2 Receptors Expressed on Striatal Astrocytes Can Form Heteromers
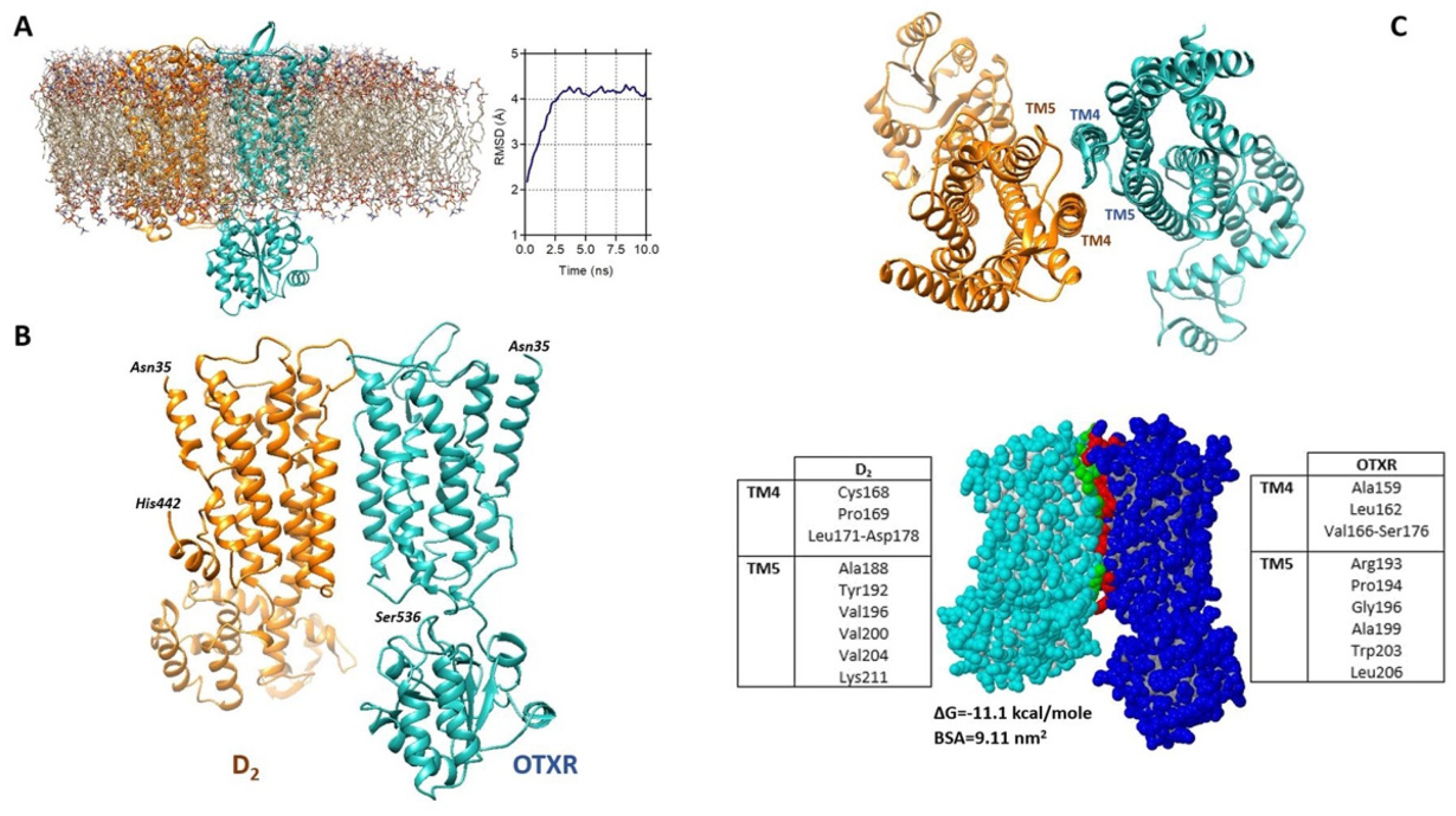
2.6. Estimated Model of the D2-OTR Heterodimer
3. Discussion
3.1. D2 and OT Receptors Are Expressed on the Same Striatal Astrocyte Processes
3.2. OT and D2 Receptors on Striatal Astrocyte Processes Functionally Interact
3.3. Astrocytic OTR and D2 Receptors Can Form Receptor Heteromers
3.4. Potential Relevance of Striatal Astrocytic D2-OTR Heteromers
4. Materials and Methods
4.1. Animals
4.2. Preparation of Purified Astrocytic Processes
4.3. Endogenous Glutamate Release
4.4. Immunofluorescent Labelling in Gliosomes
4.5. Immunoprecipitation and Immunoblot
4.6. Striatal Slices Preparation
4.7. Proximity Ligation Assay (PLA) and Immunofluorescent Confocal Microscopy on Slices
4.8. Confocal Microscopy on Gliosomes and Slices
4.9. Co-Localization Analysis
4.10. Receptor Structures
4.11. Modeling of the D2-OTR Heteroreceptor Complex
4.12. Calculations and Statistical Analysis
4.13. Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jourdain, P.; Bergersen, L.H.; Bhaukaurally, K.; Bezzi, P.; Santello, M.; Domercq, M.; Matute, C.; Tonello, F.; Gundersen, V.; Volterra, A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007, 10, 331–339. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The tripartite glutamatergic synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef]
- Eulenburg, V.; Gomeza, J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010, 63, 103–112. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Kékesi, O.; Morley, J.W.; Buskila, Y. Astrocytic modulation of neuronal excitability through K(+) spatial buffering. Neurosci. Biobehav. Rev. 2017, 77, 87–97. [Google Scholar] [CrossRef]
- Savtchouk, I.; Volterra, A. Gliotransmission: Beyond black-and-white. J. Neurosci. 2018, 38, 14–25. [Google Scholar] [CrossRef]
- Dvorzhak, A.; Melnick, I.; Grantyn, R. Astrocytes and presynaptic plasticity in the striatum: Evidence and unanswered questions. Brain Res. Bull. 2018, 136, 17–25. [Google Scholar] [CrossRef]
- Cavaccini, A.; Durkee, C.; Kofuji, P.; Tonini, R.; Araque, A. Astrocyte signaling gates long-term depression at corticostriatal synapses of the direct pathway. J. Neurosci. 2020, 40, 5757–5768. [Google Scholar] [CrossRef]
- Martín, R.; Bajo-Grañeras, R.; Moratalla, R.; Perea, G.; Araque, A. circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef]
- Corkrum, M.; Araque, A. Astrocyte-neuron signaling in the mesolimbic dopamine system: The hidden stars of dopamine signaling. Neuropsychopharmacology 2021, 46, 1864–1872. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Sardinha, V.M.; Guerra-Gomes, S.; Araque, A.; Sousa, N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015, 38, 535–549. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Villalba, R.M.; Smith, Y. Neuroglial plasticity at striatal glutamatergic synapses in Parkinson’s disease. Front. Syst. Neurosci. 2011, 5, 68. [Google Scholar] [CrossRef]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Sonninen, T.-M.; Hämäläinen, R.H.; Koskuvi, M.; Oksanen, M.; Shakirzyanova, A.; Wojciechowski, S.; Puttonen, K.; Naumenko, N.; Goldsteins, G.; Laham-Karam, N.; et al. Metabolic alterations in Parkinson’s disease astrocytes. Sci. Rep. 2020, 10, 14474. [Google Scholar] [CrossRef]
- Miyazaki, I.; Asanuma, M. Neuron-astrocyte interactions in Parkinson’s disease. Cells 2020, 9. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is oxytocin “nature’s medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-Oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Geng, Y.; Zhao, W.; Zhou, F.; Wang, J.; Markett, S.; Biswal, B.B.; Ma, Y.; Kendrick, K.M.; et al. Oxytocin differentially modulates specific dorsal and ventral striatal functional connections with frontal and cerebellar regions. Neuroimage 2019, 184, 781–789. [Google Scholar] [CrossRef]
- Loth, E.; Poline, J.-B.; Thyreau, B.; Jia, T.; Tao, C.; Lourdusamy, A.; Stacey, D.; Cattrell, A.; Desrivières, S.; Ruggeri, B.; et al. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol. Psychiatry 2014, 76, 367–376. [Google Scholar] [CrossRef]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, e92–e123. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; King, M.V.; Williams, S.; Edwards, A.; Ballard, T.M.; Steward, L.J.; Alberati, D.; Fone, K.C.F. Oxytocin attenuates phencyclidine hyperactivity and increases social interaction and nucleus accumben dopamine release in rats. Neuropsychopharmacology 2019, 44, 295–305. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Cuesta-Marti, C.; Lopez-Salas, A.; Chruścicka-Smaga, B.; Crespo-Ramírez, M.; Tesoro-Cruz, E.; Palacios-Lagunas, D.A.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The oxytocin receptor represents a key hub in the GPCR heteroreceptor network: Potential relevance for brain and behavior. Front. Mol. Neurosci. 2022, 15. [Google Scholar] [CrossRef]
- Wahis, J.; Baudon, A.; Althammer, F.; Kerspern, D.; Goyon, S.; Hagiwara, D.; Lefevre, A.; Barteczko, L.; Boury-Jamot, B.; Bellanger, B.; et al. Astrocytes mediate the effect of oxytocin in the central amygdala on neuronal activity and affective states in rodents. Nat. Neurosci. 2021, 24, 529–541. [Google Scholar] [CrossRef]
- Baudon, A.; Clauss Creusot, E.; Althammer, F.; Schaaf, C.P.; Charlet, A. Emerging role of astrocytes in oxytocin-mediated control of neural circuits and brain functions. Prog. Neurobiol. 2022, 217, 102328. [Google Scholar] [CrossRef] [PubMed]
- Bakos, J.; Srancikova, A.; Havranek, T.; Bacova, Z. Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast. 2018, 2018. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Pelassa, S.; Capraro, M.; Passalacqua, M.; Bozzo, M.; Gatta, E.; Anderlini, D.; et al. Heterodimer of A2A and oxytocin receptors regulating glutamate release in adult striatal astrocytes. Int. J. Mol. Sci. 2022, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- de la Mora, M.P.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the Rat. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 2075–2085. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.; Maura, G.; et al. A2A-D2 receptor–receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2017, 140, 268–279. [Google Scholar] [CrossRef]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef]
- Derouiche, A.; Geiger, K.D. Perspectives for ezrin and radixin in astrocytes: Kinases, functions and pathology. Int. J. Mol. Sci. 2019, 20, 3776. [Google Scholar] [CrossRef]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Pröls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef]
- Reichenbach, A.; Derouiche, A.; Kirchhoff, F. Morphology and dynamics of perisynaptic glia. Brain Res. Rev. 2010, 63, 11–25. [Google Scholar] [CrossRef]
- Ghézali, G.; Dallérac, G.; Rouach, N. Perisynaptic astroglial processes: Dynamic processors of neuronal information. Brain Struct. Funct. 2016, 221, 2427–2442. [Google Scholar] [CrossRef]
- Cervetto, C.; Frattaroli, D.; Venturini, A.; Passalacqua, M.; Nobile, M.; Alloisio, S.; Tacchetti, C.; Maura, G.; Agnati, L.; Marcoli, M. Calcium-permeable AMPA receptors trigger vesicular glutamate release from bergmann gliosomes. Neuropharmacology 2015, 99. [Google Scholar] [CrossRef]
- Ormel, L.; Stensrud, M.J.; Bergersen, L.H.; Gundersen, V. VGLUT1 is localized in astrocytic processes in several brain regions. Glia 2012, 60, 229–238. [Google Scholar] [CrossRef]
- Khammy, M.M.; Kim, S.; Bentzen, B.H.; Lee, S.; Choi, I.; Aalkjaer, C.; Jepps, T.A. 4-Aminopyridine: A pan voltage-gated potassium channel inhibitor that enhances K(v) 7.4 currents and inhibits noradrenaline-mediated contraction of rat mesenteric small arteries. Br. J. Pharmacol. 2018, 175, 501–516. [Google Scholar] [CrossRef]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K.; Reichenbach, A.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 55, 274–281. [Google Scholar] [CrossRef]
- Olsen, M.L.; Khakh, B.S.; Skatchkov, S.N.; Zhou, M.; Lee, C.J.; Rouach, N. New insights on astrocyte ion channels: Critical for homeostasis and neuron-glia signaling. J. Neurosci. 2015, 35, 13827–13835. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2017, 98, 239–389. [Google Scholar] [CrossRef]
- Wu, K.-C.; Kuo, C.-S.; Chao, C.-C.; Huang, C.-C.; Tu, Y.-K.; Chan, P.; Leung, Y.-M. Role of voltage-gated K(+) channels in regulating Ca(2+) entry in rat cortical astrocytes. J. Physiol. Sci. 2015, 65, 171–177. [Google Scholar] [CrossRef]
- Bekar, L.K.; Loewen, M.E.; Cao, K.; Sun, X.; Leis, J.; Wang, R.; Forsyth, G.W.; Walz, W. Complex expression and localization of inactivating Kv channels in cultured hippocampal astrocytes. J. Neurophysiol. 2005, 93, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- McNeill, J.; Rudyk, C.; Hildebrand, M.E.; Salmaso, N. Ion channels and electrophysiological properties of astrocytes: Implications for emergent stimulation technologies. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, L.K.; Zhang, Y. Kv3 channels: Enablers of rapid firing, neurotransmitter release, and neuronal endurance. Physiol. Rev. 2017, 97, 1431–1468. [Google Scholar] [CrossRef] [PubMed]
- Love, T.M. Oxytocin, Motivation and the role of dopamine. Pharmacol. Biochem. Behav. 2014, 119, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Walum, H.; Young, L.J. The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 2018, 19, 643–654. [Google Scholar] [CrossRef]
- Zhang, K.; Li, G.; Wang, L.; Cao, C.; Fang, R.; Luo, S.; Liu, P.; Zhang, X.Y. An epistasis between dopaminergic and oxytocinergic systems confers risk of post-traumatic stress disorder in a traumatized Chinese cohort. Sci. Rep. 2019, 9, 4–11. [Google Scholar] [CrossRef]
- Young, L.J.; Wang, Z. The neurobiology of pair bonding. Nat. Neurosci. 2004, 7, 1048–1054. [Google Scholar] [CrossRef]
- Skuse, D.H.; Gallagher, L. Dopaminergic-neuropeptide interactions in the social BRAIN. Trends Cogn. Sci. 2009, 13, 27–35. [Google Scholar] [CrossRef]
- Zanos, P.; Georgiou, P.; Weber, C.; Robinson, F.; Kouimtsidis, C.; Niforooshan, R.; Bailey, A. oxytocin and opioid addiction revisited: Old drug, new applications. Br. J. Pharmacol. 2018, 175, 2809–2824. [Google Scholar] [CrossRef]
- Leong, K.-C.; Cox, S.; King, C.; Becker, H.; Reichel, C.M. Oxytocin and rodent models of addiction. Int. Rev. Neurobiol. 2018, 140, 201–247. [Google Scholar] [CrossRef]
- Shigetomi, E.; Koizumi, S. The role of astrocytes in behaviors related to emotion and motivation. Neurosci. Res. 2022, 187, 21–39. [Google Scholar] [CrossRef]
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 heteromers on striatal astrocytes: Biochemical and biophysical evidence. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Agnati, L.F.; Tarakanov, A.O.; Ferré, S.; Fuxe, K.; Guidolin, D. Receptor-receptor interactions, receptor mosaics, and basic principles of molecular network organization. J. Mol. Neurosci. 2005, 26, 193–208. [Google Scholar] [CrossRef]
- Fuxe, K.; Canals, M.; Torvinen, M.; Marcellino, D.; Terasmaa, A.; Genedani, S.; Leo, G.; Guidolin, D.; Diaz-Cabiale, Z.; Rivera, A.; et al. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine. J. Neural Transm. 2007, 114, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Villalba, R.M.; Mathai, A.; Smith, Y. Morphological changes of glutamatergic synapses in animal models of Parkinson’s disease. Front. Neuroanat. 2015, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Chassain, C.; Melon, C.; Salin, P.; Vitale, F.; Couraud, S.; Durif, F.; Kerkerian-Le Goff, L.; Gubellini, P. Metabolic, synaptic and behavioral impact of 5-week chronic deep brain stimulation in hemiparkinsonian rats. J. Neurochem. 2016, 136, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Nickols, H.H.; Conn, P.J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol. Dis. 2014, 61, 55–71. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Poulain, D.A. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience 1993, 57, 501–535. [Google Scholar] [CrossRef]
- Hatton, G.I. Function-related plasticity in hypothalamus. Annu. Rev. Neurosci. 1997, 20, 375–397. [Google Scholar] [CrossRef]
- Meinung, C.-P. Oxytocin Receptor-Mediated Signaling in Astrocytes. Ph.D Thesis, Universität Regensburg, Regensburg, Germany, 2020. [Google Scholar]
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Hatton, G.I. Interaction of extracellular signal-regulated protein kinase 1/2 with actin cytoskeleton in supraoptic oxytocin neurons and astrocytes: Role in burst firing. J. Neurosci. 2007, 27, 13822–13834. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Hatton, G.I. Astrocytic plasticity and patterned oxytocin neuronal activity: Dynamic interactions. J. Neurosci. 2009, 29, 1743–1754. [Google Scholar] [CrossRef]
- Oliet, S.H.; Piet, R.; Poulain, D.A. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 2001, 292, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Hirrlinger, J.; Hülsmann, S.; Kirchhoff, F. Astroglial processes show spontaneous motility at active synaptic terminals In Situ. Eur. J. Neurosci. 2004, 20, 2235–2239. [Google Scholar] [CrossRef]
- Piet, R.; Vargová, L.; Syková, E.; Poulain, D.A.; Oliet, S.H.R. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc. Natl. Acad. Sci. USA 2004, 101, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Fuxe, K.; Zoli, M.; Ozini, I.; Toffano, G.; Ferraguti, F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. evidence for the existence of two main types of communication in the Central nervous. Acta Physiol. Scand. 1986, 128, 201–207. [Google Scholar] [CrossRef]
- Agnati, L.F.; Leo, G.; Zanardi, A.; Genedani, S.; Rivera, A.; Fuxe, K.; Guidolin, D. Volume transmission and wiring transmission from cellular to molecular networks: History and perspectives. Acta Physiol. 2006, 187, 329–344. [Google Scholar] [CrossRef]
- Fuxe, K.; Agnati, L.F.; Marcoli, M.; Borroto-Escuela, D.O. Volume Transmission in central dopamine and noradrenaline neurons and its astroglial targets. Neurochem. Res. 2015, 40, 2600–2614. [Google Scholar] [CrossRef] [PubMed]
- Marcoli, M.; Agnati, L.F.; Benedetti, F.; Genedani, S.; Guidolin, D.; Ferraro, L.; Maura, G.; Fuxe, K. On the role of the extracellular space on the holistic behavior of the brain. Rev. Neurosci. 2015, 26, 489–506. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Grinevich, V.; Neumann, I.D. Brain oxytocin: How puzzle stones from animal studies translate into psychiatry. Mol. Psychiatry 2021, 26, 265–279. [Google Scholar] [CrossRef]
- Bjelke, B.; Strömberg, I.; O’Connor, W.T.; Andbjer, B.; Agnati, L.F.; Fuxe, K. Evidence for volume transmission in the dopamine denervated neostriatum of the rat after a unilateral nigral 6-OHDA microinjection. studies with systemic D-amphetamine treatment. Brain Res. 1994, 662, 11–24. [Google Scholar] [CrossRef]
- Vizi, E.S.; Fekete, A.; Karoly, R.; Mike, A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 2010, 160, 785–809. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Perez De La Mora, M.; Manger, P.; Narváez, M.; Beggiato, S.; Crespo-Ramírez, M.; Navarro, G.; Wydra, K.; Díaz-Cabiale, Z.; Rivera, A.; et al. Brain dopamine transmission in health and parkinson’s disease: Modulation of synaptic transmission and plasticity through volume transmission and dopamine heteroreceptors. Front. Synaptic Neurosci. 2018, 10, 20. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.-I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.-S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Kam, T.-I.; Hinkle, J.T.; Dawson, T.M.; Dawson, V.L. Microglia and astrocyte dysfunction in Parkinson’s disease. Neurobiol. Dis. 2020, 144, 105028. [Google Scholar] [CrossRef]
- Que, R.; Zheng, J.; Chang, Z.; Zhang, W.; Li, H.; Xie, Z.; Huang, Z.; Wang, H.-T.; Xu, J.; Jin, D.; et al. Dl-3-n-butylphthalide rescues dopaminergic neurons in Parkinson’s disease models by inhibiting the NLRP3 inflammasome and ameliorating mitochondrial impairment. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.-K.; Chao, Y.-X.; West, A.; Chan, L.-L.; Poewe, W.; Jankovic, J. Parkinson disease and the immune system-Associations, mechanisms and therapeutics. Nat. Rev. Neurol. 2020, 16, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.; Morales, I.; Rodriguez-Sabate, C.; Sole-Sabater, M.; Rodriguez, M. Astrocytes, a promising opportunity to control the progress of Parkinson’s disease. Biomedicines 2021, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, T.; Liang, M.; Xie, J.; Song, N. Astrocyte dysfunction in Parkinson’s disease: From the perspectives of transmitted α-synuclein and genetic modulation. Transl. Neurodegener. 2021, 10, 39. [Google Scholar] [CrossRef]
- Sofroniew, M. V Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef]
- Parmar, M. Targets for astrocyte-based treatments of Parkinson’s disease (PD). Proc. Natl. Acad. Sci. USA 2022, 119, e2208876119. [Google Scholar] [CrossRef]
- Yang, Y.; Song, J.-J.; Choi, Y.R.; Kim, S.-H.; Seok, M.-J.; Wulansari, N.; Darsono, W.H.W.; Kwon, O.-C.; Chang, M.-Y.; Park, S.M.; et al. Therapeutic functions of astrocytes to treat α-synuclein pathology in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2110746119. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.; Tang, M.; Zhang, X.; Zhou, Z.; Yin, Y.; Zhou, Q.; Huang, Y.; Liu, Y.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via AB-crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Tang, M.; Fu, N.; Shao, W.; Zhang, S.; Yin, Y.; Zeng, R.; Wang, X.; Hu, G.; et al. Upregulation of alphab-crystallin expression in the substantia nigra of patients with Parkinson’s disease. Neurobiol. Aging 2015, 36, 1686–1691. [Google Scholar] [CrossRef]
- Kim, J.-H.; Rahman, M.H.; Lee, W.H.; Suk, K. Chemogenetic stimulation of the G(i) pathway in astrocytes suppresses neuroinflammation. Pharmacol. Res. Perspect. 2021, 9, e00822. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wu, J.; Manaenko, A.; Yang, P.; Tang, J.; Fu, W.; Zhang, J.H. Activation of dopamine D2 receptor suppresses neuroinflammation through AB-crystalline by inhibition of NF-ΚB nuclear translocation in experimental ICH mice model. Stroke 2015, 46, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.I.; Jo, M.G.; Park, T.J.; Ullah, R.; Ahmad, S.; Rehman, S.U.; Kim, M.O. Quinpirole-mediated regulation of dopamine D2 receptors inhibits glial cell-induced neuroinflammation in cortex and striatum after brain injury. Biomedicines 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Zhang, X.; Li, Y.-M. Antineuroinflammatory therapy: Potential treatment for autism spectrum disorder by inhibiting glial activation and restoring synaptic function. CNS Spectr. 2020, 25, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Campanelli, F.; Natale, G.; Marino, G.; Ghiglieri, V.; Calabresi, P. Striatal glutamatergic hyperactivity in Parkinson’s disease. Neurobiol. Dis. 2022, 168, 105697. [Google Scholar] [CrossRef]
- Purba, J.S.; Hofman, M.A.; Swaab, D.F. Decreased number of oxytocin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus in Parkinson’s disease. Neurology 1994, 44, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Angioni, L.; Cocco, C.; Ferri, G.-L.; Argiolas, A.; Melis, M.R.; Sanna, F. Involvement of nigral OXYTOCIN in locomotor activity: A behavioral, immunohistochemical and lesion study in male rats. Horm. Behav. 2016, 83, 23–38. [Google Scholar] [CrossRef]
- Erbaş, O.; Oltulu, F.; Taşkiran, D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides 2012, 38, 312–317. [Google Scholar] [CrossRef]
- Erbas, O.; Oltulu, F.; Taskiran, D. Suppression of exaggerated neuronal oscillations by oxytocin in a rat model of Parkinson’s disease. Gen. Physiol. Biophys. 2013, 32, 517–525. [Google Scholar] [CrossRef]
- Almansoub, H.A.M.M.; Tang, H.; Wu, Y.; Wang, D.-Q.; Mahaman, Y.A.R.; Salissou, M.T.M.; Lu, Y.; Hu, F.; Zhou, L.-T.; Almansob, Y.A.M.; et al. Oxytocin alleviates MPTP-induced neurotoxicity in mice by targeting MicroRNA-26a/death-associated protein kinase 1 pathway. J. Alzheimers Dis. 2020, 74, 883–901. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet. Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef]
- Erbaş, O.; Altuntaş, İ. Oxytocin and neuroprotective effects. In Oxytocin and Health; Wu, W., Kostoglou-Athanassiou, I., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. Ch. 4. ISBN 978-1-83969-138-6. [Google Scholar]
- Gamal-Eltrabily, M.; Manzano-García, A. Role of central oxytocin and dopamine systems in nociception and their POSSIBLE interactions: Suggested hypotheses. Rev. Neurosci. 2018, 29, 377–386. [Google Scholar] [CrossRef]
- Yeomans, D.C.; Hanson, L.R.; Carson, D.S.; Tunstall, B.J.; Lee, M.R.; Tzabazis, A.Z.; Jacobs, D.; Frey, W.H. Nasal oxytocin for the treatment of psychiatric disorders and pain: Achieving meaningful brain concentrations. Transl. Psychiatry 2021, 11, 388. [Google Scholar] [CrossRef]
- Ghazy, A.A.; Soliman, O.A.; Elbahnasi, A.I.; Alawy, A.Y.; Mansour, A.M.; Gowayed, M.A. Role of oxytocin in different neuropsychiatric, neurodegenerative, and neurodevelopmental disorders. Rev. Physiol. Biochem. Pharmacol. 2022, 186, 95–134. [Google Scholar] [CrossRef]
- Putnam, P.T.; Chang, S.W.C. Oxytocin does not stand alone. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210047. [Google Scholar] [CrossRef]
- Leng, G.; Ludwig, M. Intranasal oxytocin: Myths and delusions. Biol. Psychiatry 2016, 79, 243–250. [Google Scholar] [CrossRef]
- Carbone, F.; Ellmerer, P.; Spielberger, S.; Peball, M.; Sidoroff, V.; Ritter, M.; Seppi, K.; Poewe, W.; A. D. Effect of oxytocin on response inhibition in Parkinson’s disease: A pilot study [Abstract]. Mov Disord. 2021, 36, 65. [Google Scholar]
- Cervetto, C.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Pelassa, S.; Giuliani, S.; Baldini, F.; Maura, G.; Mariottini, P.; et al. Reactive astrocytosis in a mouse model of chronic polyamine catabolism activation. Biomolecules 2021, 11, 1247. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iga, K.; Shibata, T.; Shudo, M.; Kataoka, K. Glial plasmalemmal vesicles: A subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia 1993, 9, 48–56. [Google Scholar] [CrossRef]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-dependent vulnerability to excitotoxicity in spermine oxidase-overexpressing mouse. NeuroMolecular Med. 2016, 18, 50–68. [Google Scholar] [CrossRef]
- Cervetto, C.; Maura, G.; Marcoli, M. Inhibition of presynaptic release-facilitatory kainate autoreceptors by extracellular cyclic GMP. J. Pharmacol. Exp. Ther. 2010, 332, 210–219. [Google Scholar] [CrossRef]
- Di Cesare Mannelli, L.; Micheli, L.; Cervetto, C.; Toti, A.; Lucarini, E.; Parisio, C.; Marcoli, M.; Ghelardini, C. Neuronal alarmin IL-1α evokes astrocyte-mediated protective signals: Effectiveness in chemotherapy-induced neuropathic pain. Neurobiol. Dis. 2022, 168, 105716. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Mazzotta, M.C.; Frattaroli, D.; Alloisio, S.; Nobile, M.; Maura, G.; Marcoli, M. Calmidazolium selectively inhibits exocytotic glutamate release evoked by P2X7 receptor activation. Neurochem. Int. 2012, 60, 768–772. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, F.; Cervetto, C.; Mazzotta, M.C.; Bianchini, P.; Ronzitti, E.; Leprince, J.; Diaspro, A.; Maura, G.; Vallarino, M.; Vaudry, H.; et al. Urotensin II receptor and acetylcholine release from mouse cervical spinal cord nerve terminals. Neuroscience 2010, 170, 67–77. [Google Scholar] [CrossRef]
- Trifilieff, P.; Rives, M.-L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Slättman, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions Ex Vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef]
- Waltenspühl, Y.; Schöppe, J.; Ehrenmann, J.; Kummer, L.; Plückthun, A. crystal structure of the human oxytocin receptor. Sci. Adv. 2020, 6, eabb5419. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [CrossRef]
- Guidolin, D.; Tortorella, C.; Anderlini, D.; Marcoli, M.; Maura, G.; Agnati, F.L. Heteromerization as a mechanism modulating the AFFINITY of the ACE2 receptor to the receptor binding domain of SARS-CoV-2 spike protein. Curr. Proteom. 2021, 18, 695–704. [Google Scholar]
- Park, T.; Won, J.; Baek, M.; Seok, C. Galaxyheteromer: Protein heterodimer structure prediction by template-based and Ab Initio docking. Nucleic Acids Res. 2021, 49, W237–W241. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.E.M.; Guvench, O.; MacKerell, A.D.J. Current status of protein force fields for molecular dynamics simulations. Methods Mol. Biol. 2015, 1215, 47–71. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 27–28, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. https://doi.org/10.3390/ijms24054677
Amato S, Averna M, Guidolin D, Ceccoli C, Gatta E, Candiani S, Pedrazzi M, Capraro M, Maura G, Agnati LF, et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. International Journal of Molecular Sciences. 2023; 24(5):4677. https://doi.org/10.3390/ijms24054677
Chicago/Turabian StyleAmato, Sarah, Monica Averna, Diego Guidolin, Cristina Ceccoli, Elena Gatta, Simona Candiani, Marco Pedrazzi, Michela Capraro, Guido Maura, Luigi F. Agnati, and et al. 2023. "Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes" International Journal of Molecular Sciences 24, no. 5: 4677. https://doi.org/10.3390/ijms24054677
APA StyleAmato, S., Averna, M., Guidolin, D., Ceccoli, C., Gatta, E., Candiani, S., Pedrazzi, M., Capraro, M., Maura, G., Agnati, L. F., Cervetto, C., & Marcoli, M. (2023). Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. International Journal of Molecular Sciences, 24(5), 4677. https://doi.org/10.3390/ijms24054677










