Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome
Abstract
1. Introduction
2. Results
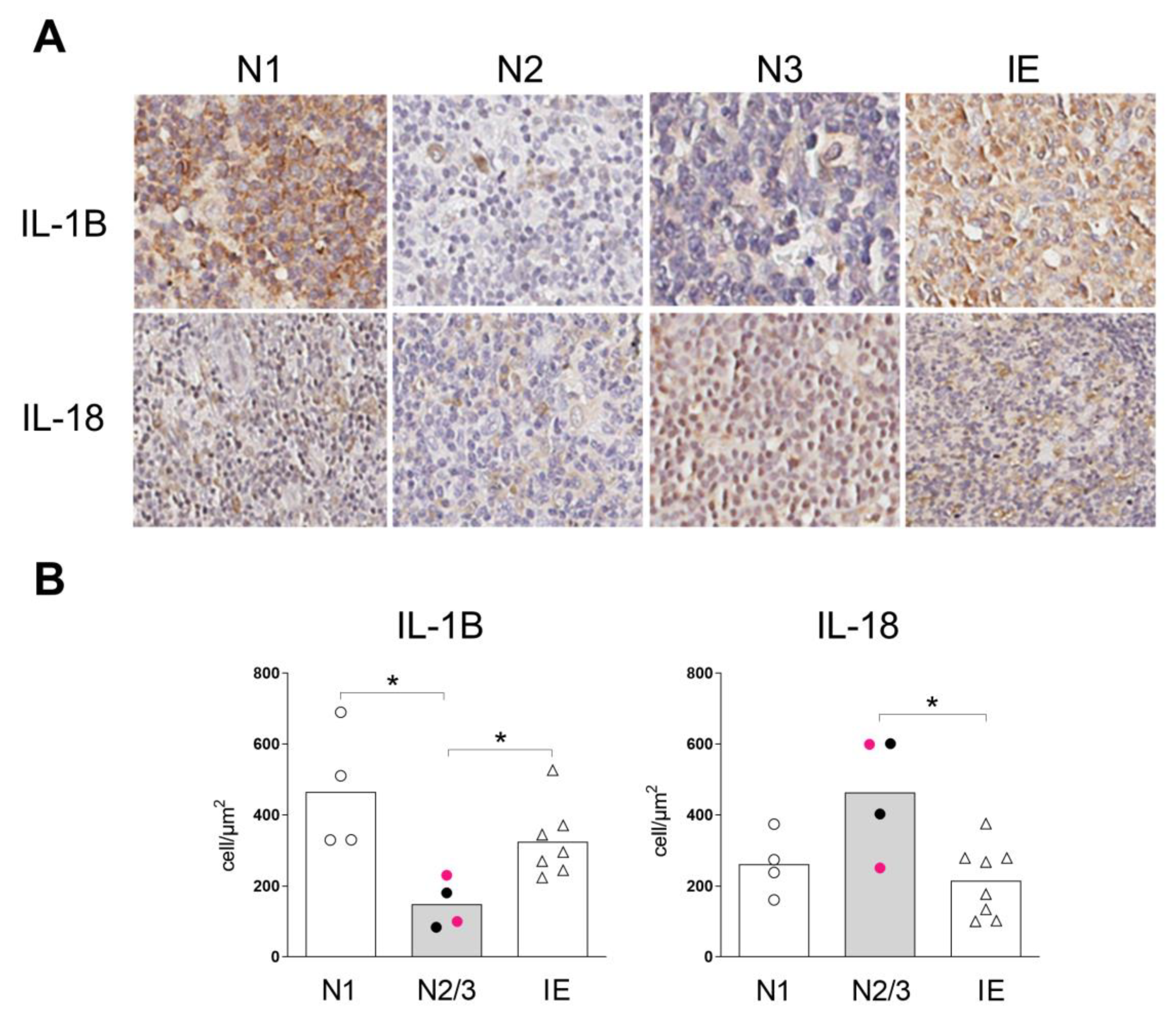
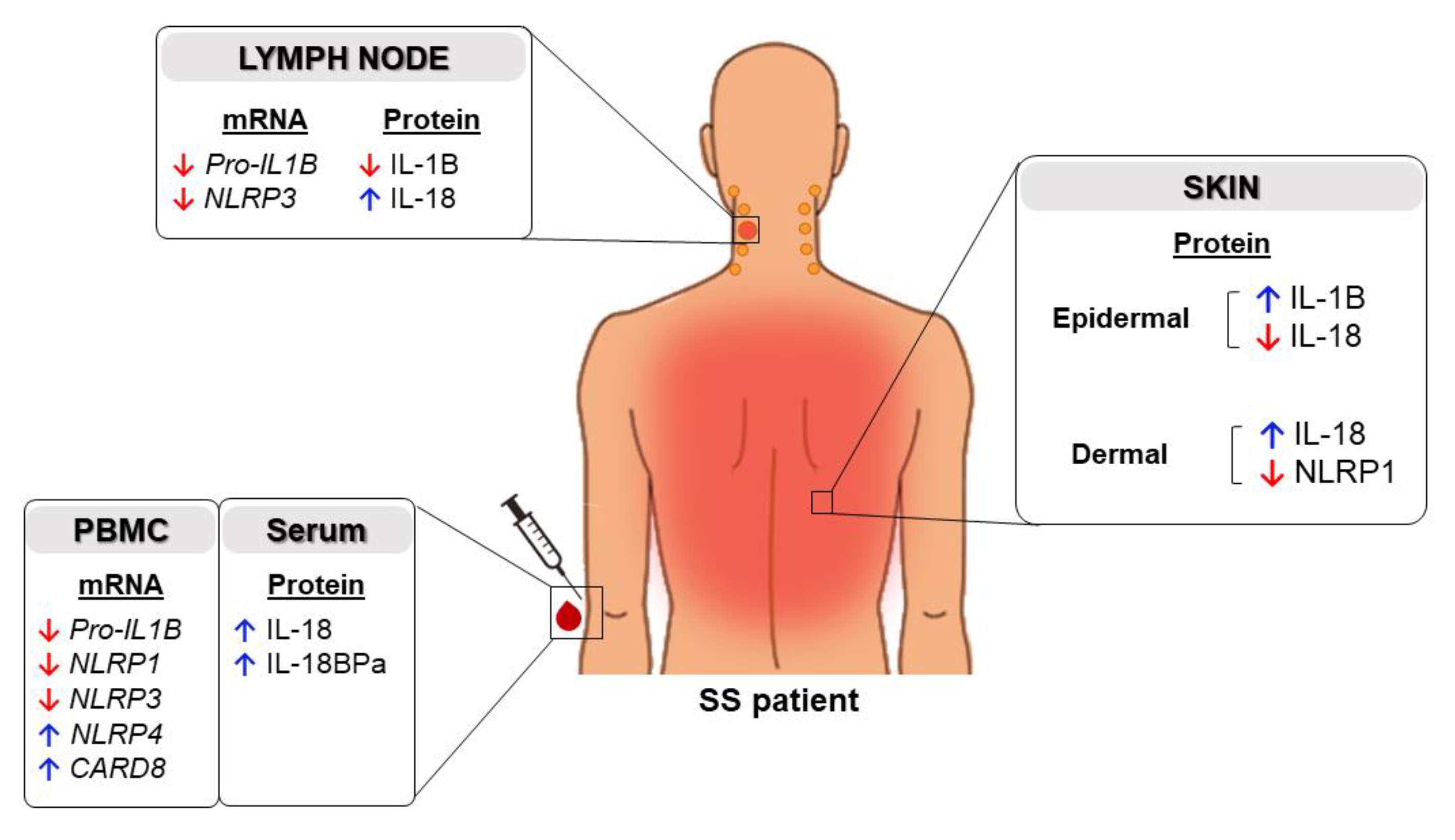
2.1. Expression of Inflammasome Components in the Skin of Patients with SS
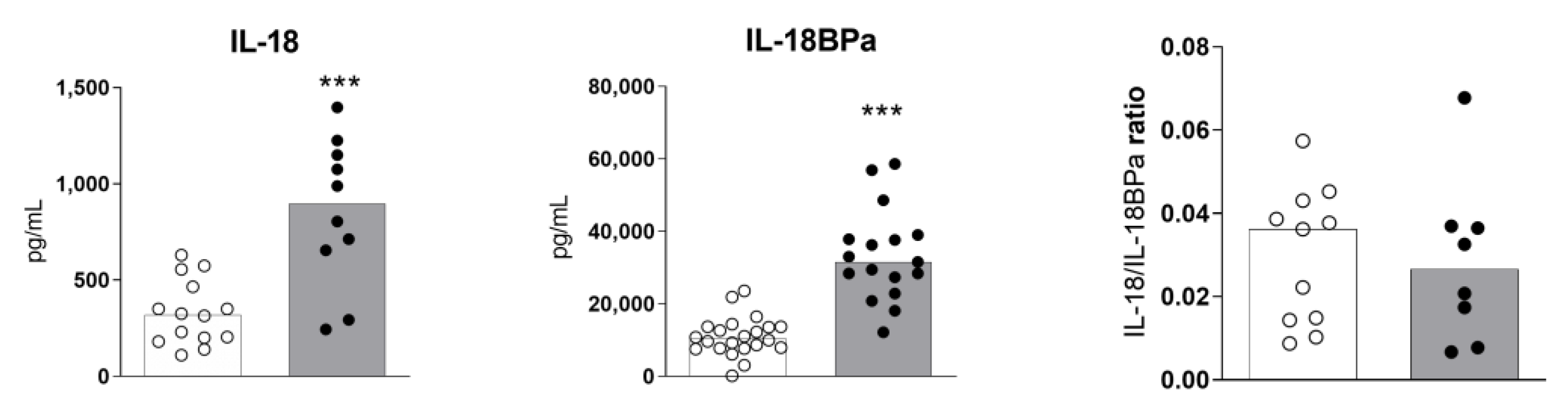
2.2. High Levels of Serum IL-18 and IL-18BPa in Sézary Syndrome
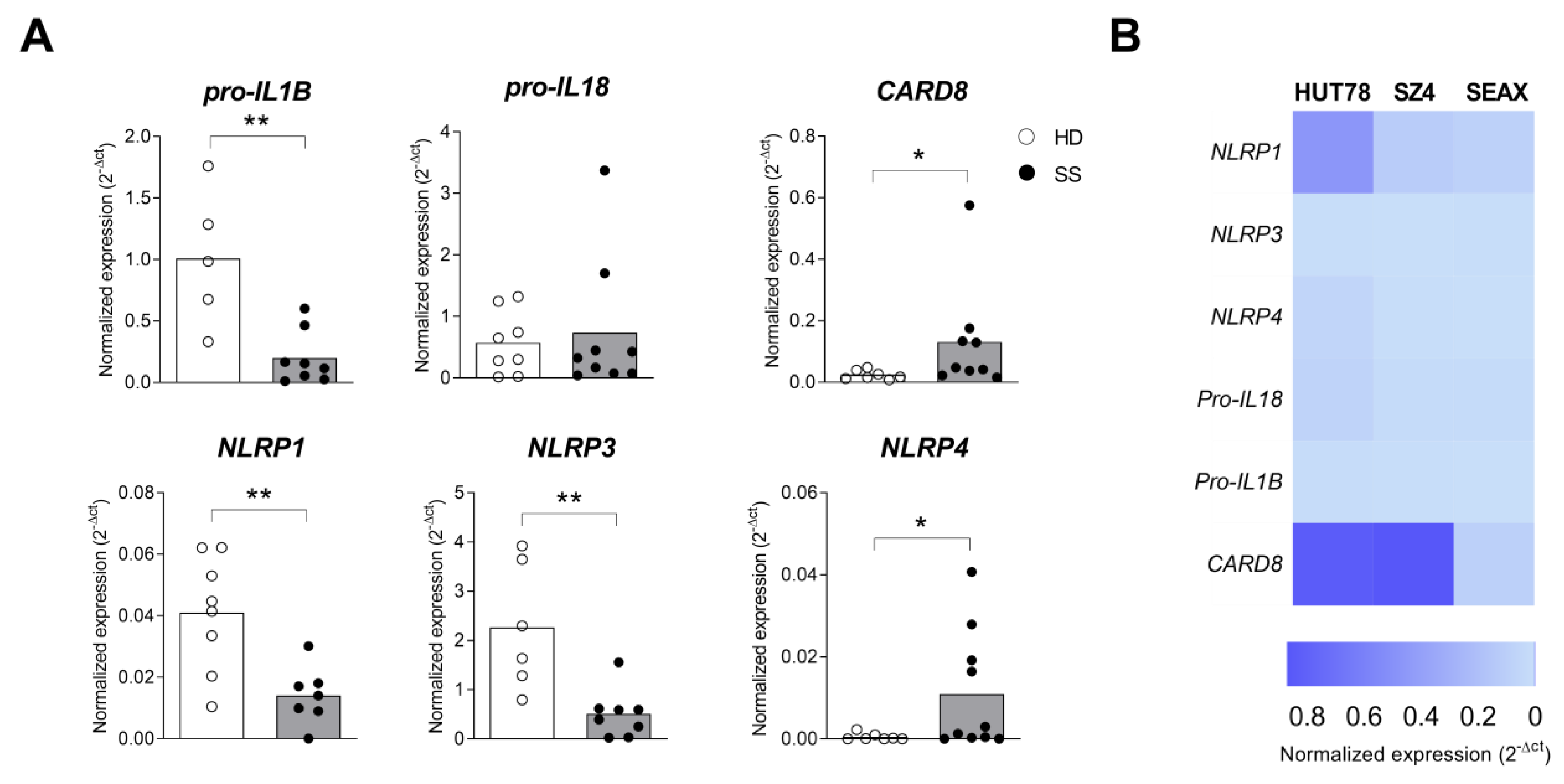
2.3. Inflammasome Profile in PBMC from Sézary Patients
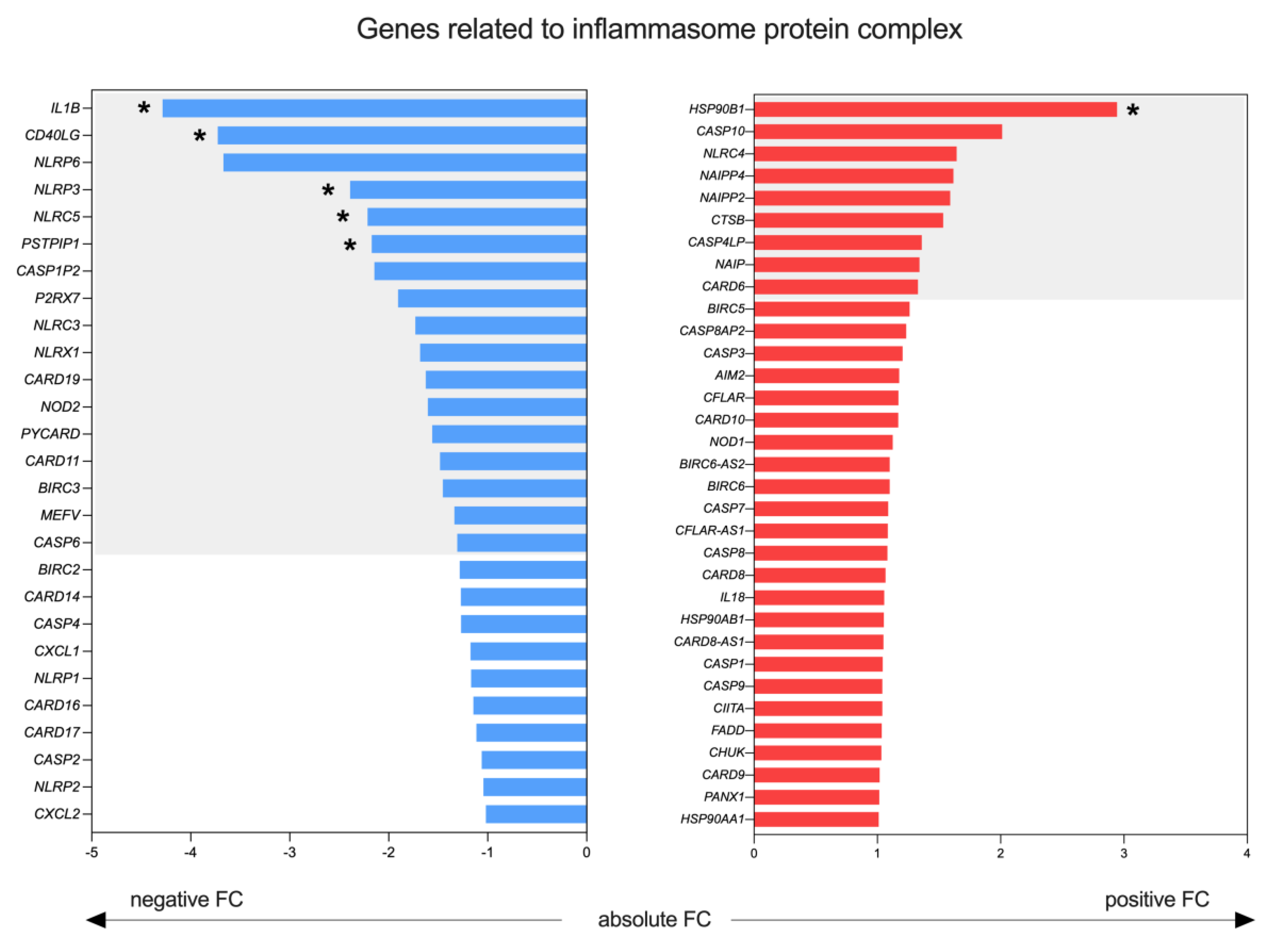
2.4. Inflammasome Profile in Lymph Nodes of Sézary Patients
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. PBMC and Cell Lines
4.3. RNA Extraction, Sequencing and Transcriptomic Analyses
4.4. Gene Expression by Real-Time Polymerase Chain Reaction
4.5. Immunohistochemistry
4.6. Determination of IL-18 and IL-18BPa
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agar, N.S.; Wedgeworth, E.; Cr ichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M.; et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: Validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, P.I.; Lorber, B.; Vonderheid, E.C. Infections Complicating Mycosis Fungoides and Sezary Síndrome. JAMA 1992, 267, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, M.P.; Manfrere, K.C.; Miyashiro, D.R.; Lima, J.F.; de, M.O.L.; Pereira, N.Z.; Cury-Martins, J.; Pereira, J.; Duarte, A.J.S.; Sato, M.N.; et al. Chronic activation profile of circulating CD8+ T cells in Sézary syndrome. Oncotarget 2018, 9, 3497–3506. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2014, 89, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Manfrere, K.C.G.; Torrealba, M.P.; Miyashiro, D.R.; Oliveira, L.M.S.; de Carvalho, G.C.; Lima, J.F.; Branco, A.; Pereira, N.Z.; Pereira, J.; Sanches, J.A.; et al. Toll-like receptor agonists partially restore the production of pro-inflammatory cytokines and type I interferon in Sézary syndrome. Oncotarget 2016, 7, 74592–74601. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, J.D.; Ortonne, N.; Giustiniani, J.; Schiavon, V.; Huet, D.; Bagot, M.; Bensussan, A. Circulating natural killer lymphocytes are potential cytotoxic effectors against autologous malignant cells in sezary syndrome patients. J. Invest. Dermatol. 2005, 125, 1273–1278. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.; Denlinger, N.; McLaughlin, E.; Chakravarti, N.; Hwang, S.; Chen, L.; Mao, H.C.; Kline, D.; Youssef, Y.; Kohnken, R.; et al. Highly cytotoxic natural killer cells are associated with poor prognosis in patients with cutaneous T-cell lymphoma. Blood Adv. 2018, 2, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, D.; Souza, B.C.E.; Torrealba, M.P.; Manfrere, K.C.G.; Sato, M.N.; Sanches, J.A. The Role of Tumor Microenvironment in the Pathogenesis of Sézary Syndrome. Int. J. Mol. Sci. 2022, 23, 936. [Google Scholar] [CrossRef] [PubMed]
- Najidh, S.; Tensen, C.P.; van der Sluijs-Gelling, A.J.; Teodosio, C.; Cats, D.; Mei, H.; Kuipers, T.B.; Out-Luijting, J.J.; Zoutman, W.H.; van Hall, T.; et al. Improved Sézary cell detection and novel insights into immunophenotypic and molecular heterogeneity in Sézary syndrome. Blood 2021, 138, 2539–2554. [Google Scholar] [CrossRef]
- Monteleone, M.; Stow, J.L.; Schroder, K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 2015, 74, 213–218. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Hacham, M.; Argov, S.; White, R.M.; Segal, S.; Apte, R.N. Different patterns of interleukin-1alpha and interleukin-1beta expression in organs of normal young and old mice. Eur. Cytokine Netw. 2002, 13, 55–65. [Google Scholar] [PubMed]
- Bazan, J.F.; Timans, J.C.; Kastelein, R.A. A newly defined interleukin-1? Nature 1996, 379, 591. [Google Scholar] [CrossRef]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. 2021, 8, 639103. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Vogel, P.; Body-Malapel, M.; Lamkanfi, M.; Kanneganti, T.D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 2010, 185, 4912–4920. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Yamanaka, K.; Clark, R.; Dowgiert, R.; Hurwitz, D.; Shibata, M.; Rich, B.E.; Hirahara, K.; Jones, D.A.; Eapen, S.; Mizutani, H.; et al. Expression of interleukin-18 and caspase-1 in cutaneous T-cell lymphoma. Clin. Cancer Res. 2006, 12, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Huggins, R.H.; Lertsburapa, T.; Bauer, K.; Rademaker, A.; Gerami, P.; Guitart, J. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J. Am. Acad. Dermatol. 2008, 59, 949–952. [Google Scholar] [CrossRef]
- Rothe, M.J.; Bernstein, M.L.; Grant-Kels, J.M. Life-threatening erythroderma: Diagnosing and treating the "red man". Clin. Dermatol. 2005, 23, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Bossaller, L.; Chiang, P.I.; Schmidt-Lauber, C.; Ganesan, S.; Kaiser, W.J.; Rathinam, V.A.; Mocarski, E.S.; Subramanian, D.; Green, D.R.; Silverman, N.; et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1B and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 2012, 189, 5508–5512. [Google Scholar] [CrossRef]
- Omoto, Y.; Tokime, K.; Yamanaka, K.; Habe, K.; Morioka, T.; Kurokawa, I.; Tsutsui, H.; Yamanishi, K.; Nakanishi, K.; Mizutani, H. Human mast cell chymase cleaves pro-IL-18 and generates a novel and biologically active IL-18 fragment. J. Immunol. 2006, 177, 8315–8319. [Google Scholar] [CrossRef]
- Akeda, T.; Yamanaka, K.; Tsuda, K.; Omoto, Y.; Gabazza, E.C.; Mizutani, H. CD8+ T cell granzyme B activates keratinocyte endogenous IL-18. Arch. Dermatol. Res. 2014, 306, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.L.; Mamaï, O.; Sborgi, L.; Boussofara, L.; Hopkins, R.; Robinson, K.; Szeverényi, I.; Takeichi, T.; Balaji, R.; Lau, A.; et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016, 167, 187–202.e117. [Google Scholar] [CrossRef]
- Hasegawa, T.; Nakashima, M.; Suzuki, Y. Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem. Biophys. Res. Commun. 2016, 477, 329–335. [Google Scholar] [CrossRef]
- Olsen, E.A.; Hodak, E.; Anderson, T.; Carter, J.B.; Henderson, M.; Cooper, K.; Lim, H.W. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J. Am. Acad. Dermatol. 2016, 74, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Sun, S.C. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor κB and Mitogen-Activated Protein Kinase Pathways. Front. Immunol. 2018, 9, 1849. [Google Scholar] [CrossRef] [PubMed]
- Caamaño, J.; Hunter, C.A. NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 2002, 15, 414–429. [Google Scholar] [CrossRef]
- Cariti, C.; Quaglino, P.; Lupia, T.; Novelli, M.; Marra, E.; Fava, P.; Caliendo, V.; Tonella, L.; De Rosa, F.G.; Fierro, M.T.; et al. Infections in Sézary syndrome: A retrospective cohort study of 113 patients. J. Am. Acad. Dermatol. 2022, 86, 943–946. [Google Scholar] [CrossRef]
- Gross, O.; Thomas, C.J.; Guarda, G.; Tschopp, J. The inflammasome: An integrated view. Immunol. Rev. 2011, 243, 136–151. [Google Scholar] [CrossRef]
- Linder, A.; Hornung, V. Live Cell Imaging of T Cell Pyroptosis. Methods Mol. Biol. 2022, 2523, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mazodier, K.; Marin, V.; Novick, D.; Farnarier, C.; Robitail, S.; Schleinitz, N.; Veit, V.; Paul, P.; Rubinstein, M.; Dinarello, C.A.; et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 2005, 106, 3483–3489. [Google Scholar] [CrossRef]
- Novick, D.; Schwartsburd, B.; Pinkus, R.; Suissa, D.; Belzer, I.; Sthoeger, Z.; Keane, W.F.; Chvatchko, Y.; Kim, S.H.; Fantuzzi, G.; et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 2001, 14, 334–342. [Google Scholar] [CrossRef]
- Sors, A.; Jean-Louis, F.; Pellet, C.; Laroche, L.; Dubertret, L.; Courtois, G.; Bachelez, H.; Michel, L. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood 2006, 107, 2354–2363. [Google Scholar] [CrossRef]
- Yang, X.P.; Ghoreschi, K.; Steward-Tharp, S.M.; Rodriguez-Canales, J.; Zhu, J.; Grainger, J.R.; Hirahara, K.; Sun, H.W.; Wei, L.; Vahedi, G.; et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 2011, 12, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Akilov, O.E.; Wu, M.X.; Ustyugova, I.V.; Falo, L.D., Jr.; Geskin, L.J. Resistance of Sezary cells to TNF-alpha-induced apoptosis is mediated in part by a loss of TNFR1 and a high level of the IER3 expression. Exp. Dermatol. 2012, 21, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.F.; Berkova, Z.; Mathur, R.; Zhu, H.; Braun, F.K.; Tao, R.H.; Sabichi, A.L.; Ao, X.; Maeng, H.; Samaniego, F. Nucleolin inhibits Fas ligand binding and suppresses Fas-mediated apoptosis in vivo via a surface nucleolin-Fas complex. Blood 2013, 121, 4729–4739. [Google Scholar] [CrossRef]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S.; et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Carney, D.N.; Bunn, P.A.; Russell, E.K.; Jaffe, E.S.; Schechter, G.P.; Guccion, J.G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood 1980, 55, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kaltoft, K.; Bisballe, S.; Rasmussen, H.F.; Thestrup-Pedersen, K.; Thomsen, K.; Sterry, W. A continuous T-cell line from a patient with Sezary syndrome. Arch. Dermatol. Res. 1987, 279, 293–298. [Google Scholar] [CrossRef]
- Abrams, J.T.; Lessin, S.; Ghosh, S.K.; Ju, W.; Vonderheid, E.C.; Nowell, P.; Murphy, G.; Elfenbein, B.; DeFreitas, E. A clonal CD4-positive T-cell line established from the blood of a patient with Sézary syndrome. J. Invest. Dermatol. 1991, 96, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 24 September 2022).
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Breuer, K.; Foroushani, A.K.; Laird, M.R.; Chen, C.; Sribnaia, A.; Lo, R.; Winsor, G.L.; Hancock, R.E.; Brinkman, F.S.; Lynn, D.J. InnateDB: Systems biology of innate immunity and beyond--recent updates and continuing curation. Nucleic Acids Res. 2013, 41, D1228–D1233. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manfrere, K.C.G.; Torrealba, M.P.; Ferreira, F.M.; de Sousa, E.S.A.; Miyashiro, D.; Teixeira, F.M.E.; Custódio, R.W.A.; Nakaya, H.I.; Ramos, Y.A.L.; Sotto, M.N.; et al. Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. Int. J. Mol. Sci. 2023, 24, 4674. https://doi.org/10.3390/ijms24054674
Manfrere KCG, Torrealba MP, Ferreira FM, de Sousa ESA, Miyashiro D, Teixeira FME, Custódio RWA, Nakaya HI, Ramos YAL, Sotto MN, et al. Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. International Journal of Molecular Sciences. 2023; 24(5):4674. https://doi.org/10.3390/ijms24054674
Chicago/Turabian StyleManfrere, Kelly Cristina Gomes, Marina Passos Torrealba, Frederico Moraes Ferreira, Emanuella Sarmento Alho de Sousa, Denis Miyashiro, Franciane Mouradian Emidio Teixeira, Ricardo Wesley Alberca Custódio, Helder I. Nakaya, Yasmin Alefe Leuzzi Ramos, Mirian Nacagami Sotto, and et al. 2023. "Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome" International Journal of Molecular Sciences 24, no. 5: 4674. https://doi.org/10.3390/ijms24054674
APA StyleManfrere, K. C. G., Torrealba, M. P., Ferreira, F. M., de Sousa, E. S. A., Miyashiro, D., Teixeira, F. M. E., Custódio, R. W. A., Nakaya, H. I., Ramos, Y. A. L., Sotto, M. N., Woetmann, A., Ødum, N., Duarte, A. J. d. S., Sanches, J. A., & Sato, M. N. (2023). Imbalanced IL-1B and IL-18 Expression in Sézary Syndrome. International Journal of Molecular Sciences, 24(5), 4674. https://doi.org/10.3390/ijms24054674








