Sterile Pancreas Inflammation during Preservation and after Transplantation
Abstract
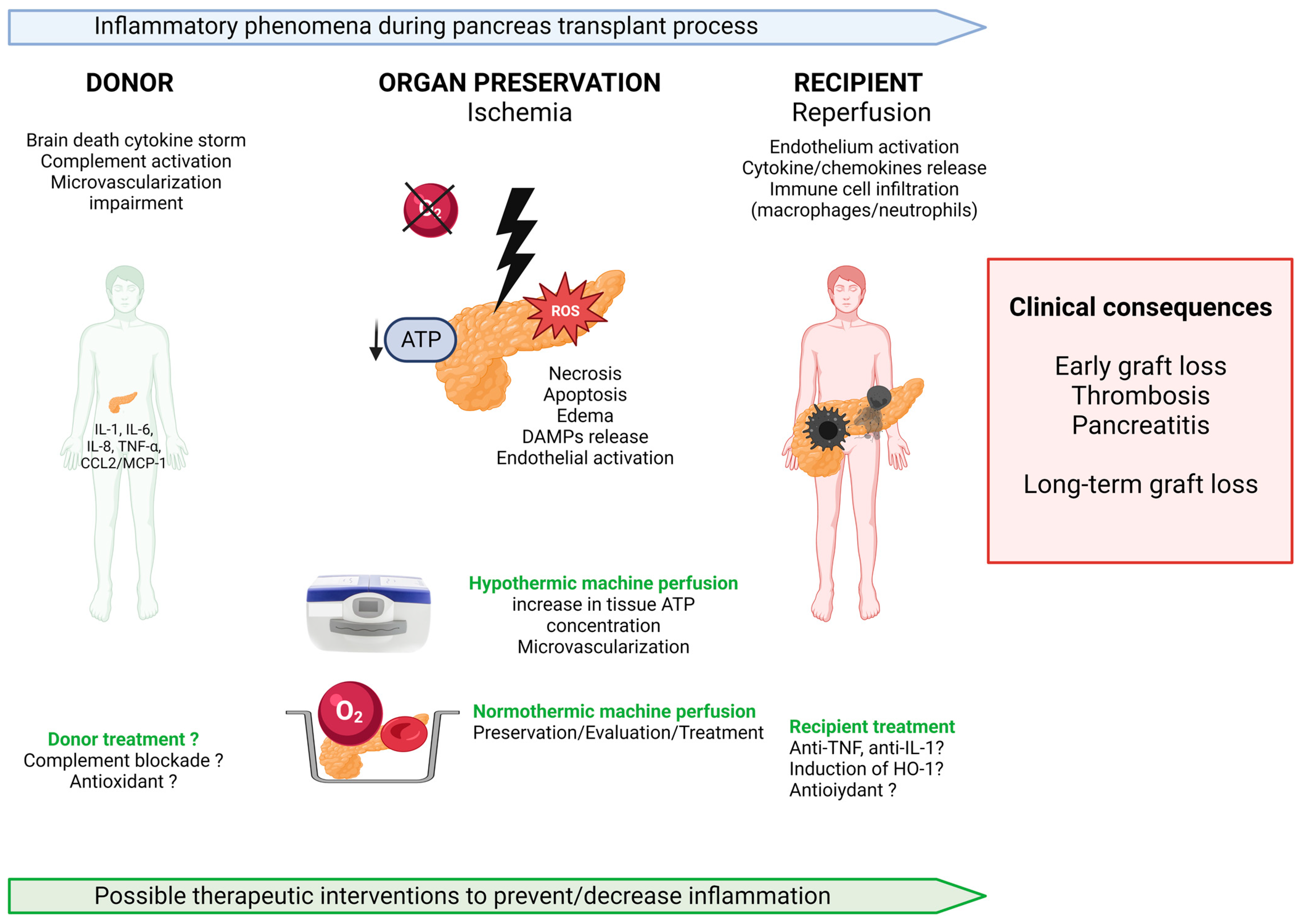
1. Introduction
2. Clinical Impact of Ischemia-Reperfusion after Pancreas Transplantation
3. Mechanism of Sterile Inflammation during Organ Procurement and Ischemia-Reperfusion
3.1. Sterile Inflammation Starts in the Donor: Role of Brain Death
3.2. Ischemia-Reperfusion and Innate Immunity
3.3. Activation of Adaptive Immunity
3.4. Specificities Related to Early Graft Thrombosis
3.5. Specificities Related to Donation after Cardiac Death
4. Therapeutic Options to Decrease Sterile Inflammation of the Pancreas
4.1. Donor Treatment
4.2. Anti-Inflammatory Therapies in Recipients
4.3. Refinement of Preservation Techniques
4.3.1. Static Cold Storage
4.3.2. Hypothermic Machine Perfusion
4.3.3. Normothermic Machine Perfusion
5. How to Improve Our Knowledge of the Mechanisms of IRI and Related Sterile Inflammation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BMI | body mass index |
| CIT | cold ischemia time |
| CCL2 | chemokine ligand 2 |
| CCR2 | C-C chemokine receptor type 2 |
| CD | cluster of differentiation |
| DAMP | Damage-associated molecular pattern |
| DBD | donation after brain death |
| DC | dendritic cell |
| DCD | donation after cardiac death |
| DNA | desoxyribonucleic acid |
| G-CSF | granulocyte colony-stimulating factor |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| HIF | hypoxia inducible factors |
| HO-1 | heme oxygenase 1 |
| HMP | hypothermic machine perfusion |
| H2S | hydrogen sulfide |
| ICAM1 | intercellular adhesion molecule 1 |
| IFN | γ interferon gamma |
| IL | interleukine |
| IR | Ischemia-reperfusion |
| IRI | ischemia-reperfusion injuries |
| MIP2 | macrophage inflammatory protein 2 |
| MCP-1 | monocyte chemoattractant protein 1 |
| NETs | neutrophil extracellular traps |
| NF-κB | nuclear factor kappa B |
| NMP | normothermic machine perfusion |
| NRP | normothermic regional perfusion |
| PAK | pancreas after kidney |
| PTA | pancreas transplant alone |
| RNA | ribonucleic acid |
| SIRPα | signal regulatory protein alpha |
| SPK | simultaneous pancreas and kidney transplantation |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor alpha |
References
- Smith, G.C.; Trauer, T.; Kerr, P.G.; Chadban, S.J. Prospective quality-of-life monitoring of simultaneous pancreas and kidney transplant recipients using the 36-item short form health survey. Am. J. Kidney Dis. 2010, 55, 698–707. [Google Scholar] [CrossRef]
- Mohan, P.; Safi, K.; Little, D.M.; Donohoe, J.; Conlon, P.; Walshe, J.J.; O’Kelly, P.; Thompson, C.J.; Hickey, D.P. Improved patient survival in recipients of simultaneous pancreas-kidney transplant compared with kidney transplant alone in patients with type 1 diabetes mellitus and end-stage renal disease. Br. J. Surg. 2003, 90, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.S.; Zhang, M.; Schaubel, D.E.; Shu, X.; Magee, J.C. A Reassessment of the Survival Advantage of Simultaneous Kidney-Pancreas Versus Kidney-Alone Transplantation. Transplantation 2015, 99, 1900–1906. [Google Scholar] [CrossRef]
- Boggi, U.; Baronti, W.; Amorese, G.; Pilotti, S.; Occhipinti, M.; Perrone, V.; Marselli, L.; Barsotti, M.; Campani, D.; Gianetti, E.; et al. Treating Type 1 Diabetes by Pancreas Transplant Alone: A Cohort Study on Actual Long-term (10 Years) Efficacy and Safety. Transplantation 2022, 106, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Boggi, U.; Vistoli, F.; Andres, A.; Arbogast, H.P.; Badet, L.; Baronti, W.; Bartlett, S.T.; Benedetti, E.; Branchereau, J.; Burke, G.W., 3rd; et al. First World Consensus Conference on pancreas transplantation: Part II—recommendations. Am. J. Transplant. 2021, 21, 17–59. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W.G. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2016, 13, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Debout, A.; Foucher, Y.; Trébern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2014, 87, 343–349. [Google Scholar] [CrossRef]
- Postalcioglu, M.; Kaze, A.D.; Byun, B.C.; Siedlecki, A.; Tullius, S.G.; Milford, E.L.; Paik, J.M.; Abdi, R. Association of Cold Ischemia Time With Acute Renal Transplant Rejection. Transplantation 2018, 102, 1188–1194. [Google Scholar] [CrossRef]
- Lozanovski, V.J.; Döhler, B.; Weiss, K.H.; Mehrabi, A.; Süsal, C. The Differential Influence of Cold Ischemia Time on Outcome After Liver Transplantation for Different Indications—Who Is at Risk? A Collaborative Transplant Study Report. Front. Immunol. 2020, 11, 892. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Gruessner, R.W.G. Long-term outcome after pancreas transplantation: A registry analysis. Curr. Opin. Organ Transplant. 2016, 21, 377. [Google Scholar] [CrossRef]
- Axelrod, D.A.; Sung, R.S.; Meyer, K.H.; Wolfe, R.A.; Kaufman, D.B. Systematic Evaluation of Pancreas Allograft Quality, Outcomes and Geographic Variation in Utilization. Am. J. Transplant. 2010, 10, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Lee, F.J.; Bradbury, L.; Collett, D.; Reddy, S.; Sinha, S.; Sharples, E.; Ploeg, R.J.; Friend, P.J.; Vaidya, A. Validation of the Pancreas Donor Risk Index for use in a UK population. Transpl. Int. 2015, 28, 1028–1033. [Google Scholar] [CrossRef]
- Finger, E.B.; Radosevich, D.M.; Dunn, T.B.; Chinnakotla, S.; Sutherland, D.E.R.; Matas, A.J.; Pruett, T.L.; Kandaswamy, R. A Composite Risk Model for Predicting Technical Failure in Pancreas Transplantation. Am. J. Transplant. 2013, 13, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; Ramcharan, T.; Kandaswamy, R.; Gruessner, R.W.G.; Gruessner, A.C.; Sutherland, D.E.R. Technical failures after pancreas transplants: Why grafts fail and the risk factors--a multivariate analysis. Transplantation 2004, 78, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, E.N.; Dunn, T.B.; Sutherland, D.E.R.; Kandaswamy, R.; Finger, E.B. Optimizing outcomes in pancreas transplantation: Impact of organ preservation time. Clin. Transplant. 2017, 31, e13035. [Google Scholar] [CrossRef]
- Kopp, W.H.; Verhagen, M.J.J.; Blok, J.J.; Huurman, V.A.L.; de Fijter, J.W.; de Koning, E.J.; Putter, H.; Baranski, A.G.; Schaapherder, A.F.M.; Braat, A.E.; et al. Thirty Years of Pancreas Transplantation at Leiden University Medical Center: Long-term Follow-up in a Large Eurotransplant Center. Transplantation 2015, 99, e145. [Google Scholar] [CrossRef]
- Öllinger, R.; Margreiter, C.; Bösmüller, C.; Weissenbacher, A.; Frank, F.; Schneeberger, S.; Mark, W.; Margreiter, R.; Pratschke, J. Evolution of Pancreas Transplantation: Long-Term Results and Perspectives From a High-Volume Center. Ann. Surg. 2012, 256, 780. [Google Scholar] [CrossRef]
- Dumbill, R.; Laurenson-Schafer, H.; Sharples, E.J.; Barnes, J.; Mittal, S.; Friend, P.J.; Clark, A. Pancreatic Islet Changes in Human Whole Organ Pancreas Explants: What Can Be Learned From Explanted Samples? Transplant Direct 2020, 6, e613. [Google Scholar] [CrossRef]
- Ogliari, A.C.; Caldara, R.; Socci, C.; Sordi, V.; Cagni, N.; Moretti, M.P.; Dell’acqua, A.; Mercalli, A.; Scavini, M.; Secchi, A.; et al. High levels of donor CCL2/MCP-1 predict graft-related complications and poor graft survival after kidney-pancreas transplantation. Am. J. Transplant. 2008, 8, 1303–1311. [Google Scholar] [CrossRef]
- Rech, T.H.; Crispim, D.; Rheinheimer, J.; Barkan, S.S.; Osvaldt, A.B.; Grezzana Filho, T.J.M.; Kruel, C.R.P.; Martini, J.; Gross, J.L.; Leitão, C.B. Brain death-induced inflammatory activity in human pancreatic tissue: A case-control study. Transplantation 2014, 97, 212–219. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Baird, B.J.; Sempowski, G.D.; Cardona, D.M.; Li, Z.; Weinhold, K.J.; Sudan, D.L.; Brennan, T.V. Upregulation of IL-1β, IL-6, and CCL-2 by a novel mouse model of pancreatic ischemia-reperfusion injury. Transplantation 2013, 95, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, R.; Eisold, S.; Linnebacher, M.; Bünger, C.; Nizze, H.; Wacke, R.; Benz, S.; Schareck, W.; Klar, E. Up-regulation of ICAM-1 during cold ischemia triggers early neutrophil infiltration in human pancreas allograft reperfusion. Transplant. Proc. 2009, 41, 3622–3627. [Google Scholar] [CrossRef] [PubMed]
- Floerchinger, B.; Oberhuber, R.; Tullius, S.G. Effects of brain death on organ quality and transplant outcome. Transplant. Rev. 2012, 26, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Morariu, A.M.; Schuurs, T.A.; Leuvenink, H.G.D.; van Oeveren, W.; Rakhorst, G.; Ploeg, R.J. Early events in kidney donation: Progression of endothelial activation, oxidative stress and tubular injury after brain death. Am. J. Transplant. 2008, 8, 933–941. [Google Scholar] [CrossRef]
- Obermaier, R.; von Dobschuetz, E.; Keck, T.; Hopp, H.-H.; Drognitz, O.; Schareck, W.; Hopt, U.T.; Benz, S. Brain death impairs pancreatic microcirculation. Am. J. Transplant. 2004, 4, 210–215. [Google Scholar] [CrossRef]
- Benz, S.; Bergt, S.; Obermaier, R.; Wiessner, R.; Pfeffer, F.; Schareck, W.; Hopt, U.T. Impairment of microcirculation in the early reperfusion period predicts the degree of graft pancreatitis in clinical pancreas transplantation. Transplantation 2001, 71, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Damman, J.; Daha, M.R.; van Son, W.J.; Leuvenink, H.G.; Ploeg, R.J.; Seelen, M.A. Crosstalk between Complement and Toll-like Receptor Activation in Relation to Donor Brain Death and Renal Ischemia-Reperfusion Injury. Am. J. Transplant. 2011, 11, 660–669. [Google Scholar] [CrossRef]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Castaneda, M.P.; Swiatecka-Urban, A.; Mitsnefes, M.M.; Feuerstein, D.; Kaskel, F.J.; Tellis, V.; Devarajan, P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 2003, 76, 50–54. [Google Scholar] [CrossRef]
- Boor, P.; Floege, J. Renal Allograft Fibrosis: Biology and Therapeutic Targets. Am. J. Transplant. 2015, 15, 863–886. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. Immune cells in experimental acute kidney injury. Nat. Rev. Nephrol. 2015, 11, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sharkey, D.; Cantley, L.G. Tubular GM-CSF Promotes Late MCP-1/CCR2-Mediated Fibrosis and Inflammation after Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2019, 30, 1825–1840. [Google Scholar] [CrossRef] [PubMed]
- Sung, F.L.; Zhu, T.Y.; Au-Yeung, K.K.W.; Siow, Y.L.; Karmin, O. Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-kappaB. Kidney Int. 2002, 62, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Bräsen, J.H.; Khalifa, A.; Schmitz, J.; Dai, W.; Einecke, G.; Schwarz, A.; Hallensleben, M.; Schmidt, B.M.W.; Kreipe, H.H.; Haller, H.; et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017, 92, 479–489. [Google Scholar] [CrossRef]
- Kageyama, S.; Hirao, H.; Nakamura, K.; Ke, B.; Zhang, M.; Ito, T.; Aziz, A.; Oncel, D.; Kaldas, F.M.; Busuttil, R.W.; et al. Recipient HO-1 inducibility is essential for posttransplant hepatic HO-1 expression and graft protection: From bench-to-bedside. Am. J. Transplant. 2019, 19, 356–367. [Google Scholar] [CrossRef]
- Lakkis, F.G.; Li, X.C. Innate allorecognition by monocytic cells and its role in graft rejection. Am. J. Transplant. 2018, 18, 289–292. [Google Scholar] [CrossRef]
- Dai, H.; Lan, P.; Zhao, D.; Abou-Daya, K.; Liu, W.; Chen, W.; Friday, A.J.; Williams, A.L.; Sun, T.; Chen, J.; et al. PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science 2020, 368, 1122–1127. [Google Scholar] [CrossRef]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; et al. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. JASN 2017, 28, 1753–1768. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Azzollini, N.; Todeschini, M.; Fiori, S.; Cavinato, R.A.; Cassis, P.; Solini, S.; Pezzuto, F.; Mister, M.; Thurman, J.M.; et al. Complement Alternative Pathway Deficiency in Recipients Protects Kidney Allograft From Ischemia/Reperfusion Injury and Alloreactive T Cell Response. Am. J. Transplant. 2017, 17, 2312–2325. [Google Scholar] [CrossRef]
- Lakey, J.R.T.; Kneteman, N.M.; Rajotte, R.V.; Wu, D.C.; Bigam, D.; Shapiro, A.M.J. Effect of core pancreas temperature during cadaveric procurement on human islet isolation and functional viability. Transplantation 2002, 73, 1106–1110. [Google Scholar] [CrossRef]
- Weegman, B.P.; Suszynski, T.M.; Scott, W.E.; Ferrer Fábrega, J.; Avgoustiniatos, E.S.; Anazawa, T.; O’Brien, T.D.; Rizzari, M.D.; Karatzas, T.; Jie, T.; et al. Temperature profiles of different cooling methods in porcine pancreas procurement. Xenotransplantation 2014, 21, 574–581. [Google Scholar] [CrossRef]
- Wassmer, C.-H.; Perrier, Q.; Combescure, C.; Pernin, N.; Parnaud, G.; Cottet-Dumoulin, D.; Brioudes, E.; Bellofatto, K.; Lebreton, F.; Berishvili, E.; et al. Impact of ischemia time on islet isolation success and posttransplantation outcomes: A retrospective study of 452 pancreas isolations. Am. J. Transplant. 2021, 21, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Kobayashi, E.; Rawson, J.; Takahashi, M.; Mullen, Y. Mechanisms of islet damage mediated by pancreas cold ischemia/rewarming. Cryobiology 2016, 73, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Vignali, D.; Piemonti, L. Monitoring Inflammation, Humoral and Cell-mediated Immunity in Pancreas and Islet Transplants. Curr. Diabetes Rev. 2015, 11, 135–143. [Google Scholar] [CrossRef]
- Ozaki, K.S.; Kimura, S.; Nalesnik, M.A.; Sico, R.M.; Zhang, M.; Ueki, S.; Ross, M.; Stolz, D.B.; Murase, N. The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngenic kidney transplantation. Kidney Int. 2012, 81, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Batal, I.; Mohan, S.; De Serres, S.A.; Vasilescu, E.-R.; Tsapepas, D.; Crew, R.J.; Patel, S.S.; Serban, G.; McCune, K.; Husain, S.A.; et al. Analysis of dendritic cells and ischemia-reperfusion changes in postimplantation renal allograft biopsies may serve as predictors of subsequent rejection episodes. Kidney Int. 2018, 93, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Uehara, M.; Solhjou, Z.; Banouni, N.; Kasinath, V.; Xiaqun, Y.; Dai, L.; Yilmam, O.; Yilmaz, M.; Ichimura, T.; Fiorina, P.; et al. Ischemia augments alloimmune injury through IL-6-driven CD4+ alloreactivity. Sci. Rep. 2018, 8, 2461. [Google Scholar] [CrossRef]
- Mikhalski, D.; Wissing, K.M.; Ghisdal, L.; Broeders, N.; Touly, M.; Hoang, A.-D.; Loi, P.; Mboti, F.; Donckier, V.; Vereerstraeten, P.; et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation 2008, 85, S3–S9. [Google Scholar] [CrossRef]
- Fuquay, R.; Renner, B.; Kulik, L.; McCullough, J.W.; Amura, C.; Strassheim, D.; Pelanda, R.; Torres, R.; Thurman, J.M. Renal Ischemia-Reperfusion Injury Amplifies the Humoral Immune Response. JASN 2013, 24, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Braza, M.S.; Conde, P.; Garcia, M.; Cortegano, I.; Brahmachary, M.; Pothula, V.; Fay, F.; Boros, P.; Werner, S.A.; Ginhoux, F.; et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am. J. Transplant. 2018, 18, 1247–1255. [Google Scholar] [CrossRef]
- Conde, P.; Rodriguez, M.; van der Touw, W.; Jimenez, A.; Burns, M.; Miller, J.; Brahmachary, M.; Chen, H.; Boros, P.; Rausell-Palamos, F.; et al. DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity 2015, 42, 1143–1158. [Google Scholar] [CrossRef]
- Vendrame, F.; Pileggi, A.; Laughlin, E.; Allende, G.; Martin-Pagola, A.; Molano, R.D.; Diamantopoulos, S.; Standifer, N.; Geubtner, K.; Falk, B.A.; et al. Recurrence of Type 1 Diabetes After Simultaneous Pancreas-Kidney Transplantation, Despite Immunosuppression, Is Associated With Autoantibodies and Pathogenic Autoreactive CD4 T-Cells. Diabetes 2010, 59, 947–957. [Google Scholar] [CrossRef]
- Muthusamy, A.S.R.; Giangrande, P.L.F.; Friend, P.J. Pancreas allograft thrombosis. Transplantation 2010, 90, 705–707. [Google Scholar] [CrossRef]
- Troppmann, C.; Gruessner, A.C.; Benedetti, E.; Papalois, B.E.; Dunn, D.L.; Najarian, J.S.; Sutherland, D.E.; Gruessner, R.W. Vascular graft thrombosis after pancreatic transplantation: Univariate and multivariate operative and nonoperative risk factor analysis. J. Am. Coll. Surg. 1996, 182, 285–316. [Google Scholar]
- Stratta, R.J.; Taylor, R.J.; Gill, I.S. Pancreas transplantation: A managed cure approach to diabetes. Curr. Probl. Surg. 1996, 33, 709–808. [Google Scholar] [CrossRef]
- Burke, G.W.; Ciancio, G.; Figueiro, J.; Buigas, R.; Olson, L.; Roth, D.; Kupin, W.; Miller, J. Hypercoagulable state associated with kidney-pancreas transplantation. Thromboelastogram-directed anti-coagulation and implications for future therapy. Clin. Transplant. 2004, 18, 423–428. [Google Scholar] [CrossRef]
- Kopp, W.H.; van Leeuwen, C.A.T.; Lam, H.D.; Huurman, V.A.L.; de Fijter, J.W.; Schaapherder, A.F.; Baranski, A.G.; Braat, A.E. Retrospective study on detection, treatment, and clinical outcome of graft thrombosis following pancreas transplantation. Transpl. Int. 2019, 32, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Harbell, J.W.; Morgan, T.; Feldstein, V.A.; Roll, G.R.; Posselt, A.; Kang, S.-M.; Feng, S.; Hirose, R.; Freise, C.E.; Stock, P. Splenic Vein Thrombosis Following Pancreas Transplantation: Identification of Factors That Support Conservative Management. Am. J. Transplant. 2017, 17, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Berney, T.; Boffa, C.; Augustine, T.; Badet, L.; de Koning, E.; Pratschke, J.; Socci, C.; Friend, P. Utilization of organs from donors after circulatory death for vascularized pancreas and islet of Langerhans transplantation: Recommendations from an expert group. Transpl. Int. 2016, 29, 798–806. [Google Scholar] [CrossRef]
- Callaghan, C.J.; Ibrahim, M.; Counter, C.; Casey, J.; Friend, P.J.; Watson, C.J.E.; Karydis, N. Outcomes after simultaneous pancreas-kidney transplantation from donation after circulatory death donors: A UK registry analysis. Am. J. Transplant. 2021, 21, 3673–3683. [Google Scholar] [CrossRef]
- Romano, A.; Alsabeah, K.; Wilczek, H.; Söderdahl, G.; Nordström, J.; Sandberg, J.; Ericzon, B.-G.; Nowak, G. Simultaneous Pancreas-Kidney Transplant From Donors After Brain Death vs Donors After Circulatory Death: A Single-Center Follow-up Study Over 3 Decades. Transplant. Proc. 2019, 51, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Siskind, E.; Akerman, M.; Maloney, C.; Huntoon, K.; Alex, A.; Siskind, T.; Bhaskeran, M.; Ali, N.; Basu, A.; Molmenti, E.; et al. Pancreas transplantation from donors after cardiac death: An update of the UNOS database. Pancreas 2014, 43, 544–547. [Google Scholar] [CrossRef]
- Anderson, P.T.; Aquil, S.; McLean, K.; McAlister, V.C.; Sener, A.; Luke, P.P. First Canadian experience with donation after cardiac death simultaneous pancreas and kidney transplants. Can J. Surg. 2017, 60, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W.; Lam, H.-D.; Schaapherder, A.F.M.; Huurman, V.; van der Boog, P.; de Koning, E.; de Fijter, J.W.; Baranski, A.; Braat, A. Pancreas transplantation with grafts from donors deceased after circulatory death (DCD): 5 years single center experience. Transplantation 2017, 102, 333–339. [Google Scholar] [CrossRef] [PubMed]
- van Loo, E.S.; Krikke, C.; Hofker, H.S.; Berger, S.P.; Leuvenink, H.G.D.; Pol, R.A. Outcome of pancreas transplantation from donation after circulatory death compared to donation after brain death. Pancreatology 2017, 17, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Leemkuil, M.; Lier, G.; Engelse, M.A.; Ploeg, R.J.; de Koning, E.J.P.; ‘t Hart, N.A.; Krikke, C.; Leuvenink, H.G.D. Hypothermic Oxygenated Machine Perfusion of the Human Donor Pancreas. Transplant. Direct 2018, 4, e388. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.A.; Roberts, J.L.; Fedotovs, A.; Paul, S.; Cottee, S.; Defries, G.; Watson, C.J.E.; Pettigrew, G.J. Outcomes for circulatory death and brainstem death pancreas transplantation with or without use of normothermic regional perfusion. Br. J. Surg. 2021, 108, 1406–1408. [Google Scholar] [CrossRef]
- Contreras, J.L.; Eckstein, C.; Smyth, C.A.; Sellers, M.T.; Vilatoba, M.; Bilbao, G.; Rahemtulla, F.G.; Young, C.J.; Thompson, J.A.; Chaudry, I.H.; et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 2003, 52, 2935–2942. [Google Scholar] [CrossRef]
- Danobeitia, J.S.; Zens, T.J.; Chlebeck, P.J.; Zitur, L.J.; Reyes, J.A.; Eerhart, M.J.; Coonen, J.; Capuano, S.; D’Alessandro, A.M.; Torrealba, J.R.; et al. Targeted donor complement blockade after brain death prevents delayed graft function in a nonhuman primate model of kidney transplantation. Am. J. Transplant. 2020, 20, 1513–1526. [Google Scholar] [CrossRef]
- Ambrosi, N.; Arrosagaray, V.; Guerrieri, D.; Uva, P.D.; Petroni, J.; Herrera, M.B.; Iovanna, J.L.; León, L.; Incardona, C.; Chuluyan, H.E.; et al. α-Lipoic Acid Protects Against Ischemia-Reperfusion Injury in Simultaneous Kidney-Pancreas Transplantation. Transplantation 2016, 100, 908–915. [Google Scholar] [CrossRef]
- Jaster, R. Molecular regulation of pancreatic stellate cell function. Mol. Cancer 2004, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Masuya, M.; Tawara, I.; Miyata, E.; Oda, K.; Nakamori, Y.; Suzuki, K.; Ohishi, K.; Katayama, N. Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells. PLoS ONE 2014, 9, e84889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Ito, T.; Gibo, J.; Kawabe, K.; Oono, T.; Kaku, T.; Arita, Y.; Zhao, Q.W.; Usui, M.; Egashira, K.; et al. Anti-monocyte chemoattractant protein 1 gene therapy attenuates experimental chronic pancreatitis induced by dibutyltin dichloride in rats. Gut 2005, 54, 1759–1767. [Google Scholar] [CrossRef]
- Hering, B.J.; Kandaswamy, R.; Ansite, J.D.; Eckman, P.M.; Nakano, M.; Sawada, T.; Matsumoto, I.; Ihm, S.-H.; Zhang, H.-J.; Parkey, J.; et al. Single-Donor, Marginal-Dose Islet Transplantation in Patients With Type 1 Diabetes. JAMA 2005, 293, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Kandaswamy, R.; Parkey, J.; Zhang, H.-J.; Liu, B.; Ihm, S.H.; Ansite, J.D.; Witson, J.; Bansal-Pakala, P.; Balamurugan, A.N.; et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am. J. Transplant. 2008, 8, 2463–2470. [Google Scholar] [CrossRef]
- Matsumoto, S.; Takita, M.; Chaussabel, D.; Noguchi, H.; Shimoda, M.; Sugimoto, K.; Itoh, T.; Chujo, D.; SoRelle, J.; Onaca, N.; et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant 2011, 20, 1641–1647. [Google Scholar] [CrossRef]
- Pascher, A.; Klupp, J. Biologics in the Treatment of Transplant Rejection and Ischemia/Reperfusion Injury. BioDrugs 2005, 19, 211–231. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Zu Vilsendorf, A.M.; Terbish, T.; Klempnauer, J.; Jörns, A. Induction of heme oxygenase-1 improves the survival of pancreas grafts by prevention of pancreatitis after transplantation. Transplantation 2007, 84, 1644–1655. [Google Scholar] [CrossRef]
- Barlow, A.D.; Hosgood, S.A.; Nicholson, M.L. Current State of Pancreas Preservation and Implications for DCD Pancreas Transplantation. Transplantation 2013, 95, 1419. [Google Scholar] [CrossRef]
- Nishime, K.; Miyagi-Shiohira, C.; Kuwae, K.; Tamaki, Y.; Yonaha, T.; Sakai-Yonaha, M.; Saitoh, I.; Watanabe, M.; Noguchi, H. Preservation of pancreas in the University of Wisconsin solution supplemented with AP39 reduces reactive oxygen species production and improves islet graft function. Am. J. Transplant. 2021, 21, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, F.; Sigrist, S.; Delpy, E.; Cherfan, J.; Peronet, C.; Zal, F.; Bouzakri, K.; Pinget, M.; Maillard, E. Beneficial effects of the novel marine oxygen carrier M101 during cold preservation of rat and human pancreas. J. Cell. Mol. Med. 2019, 23, 8025–8034. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Smith, K.E.; Purvis, W.G.; Min, C.G.; Weber, C.S.; Cooksey, A.M.; Hasilo, C.; Paraskevas, S.; Suszynski, T.M.; Weegman, B.P.; et al. Oxygen Perfusion (Persufflation) of Human Pancreata Enhances Insulin Secretion and Attenuates Islet Proinflammatory Signaling. Transplantation 2019, 103, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kuan, K.G.; Wee, M.N.; Chung, W.Y.; Kumar, R.; Mees, S.T.; Dennison, A.; Maddern, G.; Trochsler, M. Extracorporeal machine perfusion of the pancreas: Technical aspects and its clinical implications—a systematic review of experimental models. Transplant. Rev. 2016, 30, 31–47. [Google Scholar] [CrossRef]
- Zakkar, M.; Angelini, G.D.; Emanueli, C. Regulation of Vascular Endothelium Inflammatory Signalling by Shear Stress. Curr. Vasc. Pharmacol. 2016, 14, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pereyra, L.H.; Valgee, K.D.; Castellanos, J.; Chee, M. Hypothermic Pulsatile Perfusion: Its Use in the Preservation of Pancreases for 24 to 48 Hours Before Islet Cell Transplantation. Arch. Surg. 1980, 115, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.N.; Stavrou, E.X.; Fang, C.; Merkulova, A.; Alaiti, M.A.; Nakajima, K.; Morooka, T.; Merkulov, S.; LaRusch, G.A.; Simon, D.I.; et al. Prolylcarboxypeptidase promotes angiogenesis and vascular repair. Blood 2013, 122, 1522–1531. [Google Scholar] [CrossRef]
- Fukae, K.; Tominaga, R.; Tokunaga, S.; Kawachi, Y.; Imaizumi, T.; Yasui, H. The effects of pulsatile and nonpulsatile systemic perfusion on renal sympathetic nerve activity in anesthetized dogs. J. Thorac. Cardiovasc. Surg. 1996, 111, 478–484. [Google Scholar] [CrossRef]
- Moers, C.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; Squifflet, J.-P.; Rahmel, A.; Ploeg, R.J. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Samoylova, M.L.; Nash, A.; Kuchibhatla, M.; Barbas, A.S.; Brennan, T.V. Machine perfusion of donor kidneys may reduce graft rejection. Clin. Transplant. 2019, 33, e13716. [Google Scholar] [CrossRef]
- Reutzel-Selke, A.; Jurisch, A.; Denecke, C.; Pascher, A.; Martins, P.N.A.; Keßler, H.; Tamura, A.; Utku, N.; Pratschke, J.; Neuhaus, P.; et al. Donor age intensifies the early immune response after transplantation. Kidney Int. 2007, 71, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Kvietkauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Machine Perfusion of Extended Criteria Donor Organs: Immunological Aspects. Front. Immunol. 2020, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Branchereau, J.; Renaudin, K.; Kervella, D.; Bernadet, S.; Karam, G.; Blancho, G.; Cantarovich, D. Hypothermic pulsatile perfusion of human pancreas: Preliminary technical feasibility study based on histology. Cryobiology 2018, 85, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Prudhomme, T.; Kervella, D.; Ogbemudia, A.E.; Gauttier, V.; Le Bas-Bernardet, S.; Minault, D.; Hervouet, J.; Cantarovich, D.; Karam, G.; Renaudin, K.; et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: A controlled study. Transplant. Int. 2021, 34, 353–364. [Google Scholar] [CrossRef]
- Hosgood, S.A.; van Heurn, E.; Nicholson, M.L. Normothermic machine perfusion of the kidney: Better conditioning and repair? Transpl. Int. 2015, 28, 657–664. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Jassem, W.; Xystrakis, E.; Ghnewa, Y.G.; Yuksel, M.; Pop, O.; Martinez-Llordella, M.; Jabri, Y.; Huang, X.; Lozano, J.J.; Quaglia, A.; et al. Normothermic Machine Perfusion (NMP) Inhibits Proinflammatory Responses in the Liver and Promotes Regeneration. Hepatology 2019, 70, 682–695. [Google Scholar] [CrossRef]
- Barlow, A.D.; Hamed, M.O.; Mallon, D.H.; Brais, R.J.; Gribble, F.M.; Scott, M.A.; Howat, W.J.; Bradley, J.A.; Bolton, E.M.; Pettigrew, G.J.; et al. Use of Ex Vivo Normothermic Perfusion for Quality Assessment of Discarded Human Donor Pancreases. Am. J. Transplant. 2015, 15, 2475–2482. [Google Scholar] [CrossRef]
- Ahmed, N.; Liu, Q.; Matthew, W. Quintini Cristiano Normothermic Ex Vivo Perfusion of Discarded Human Pancreas. Artif. Organs 2018, 42, 334–335. [Google Scholar] [CrossRef]
- Kuan, K.G.; Wee, M.N.; Chung, W.Y.; Kumar, R.; Mees, S.T.; Dennison, A.; Maddern, G.; Trochsler, M. A Study of Normothermic Hemoperfusion of the Porcine Pancreas and Kidney. Artif. Organs 2017, 41, 490–495. [Google Scholar] [CrossRef]
- Ogbemudia, A.E.; Hakim, G.; Dengu, F.; El-Gilani, F.; Dumbill, R.; Mulvey, J.; Sayal, K.; Prudhomme, T.; Mesnard, B.; Rozenberg, K.; et al. Development of ex situ normothermic reperfusion as an innovative method to assess pancreases after preservation. Transpl. Int. 2021, 34, 1630–1642. [Google Scholar] [CrossRef] [PubMed]
- Mazilescu, L.I.; Parmentier, C.; Kalimuthu, S.N.; Ganesh, S.; Kawamura, M.; Goto, T.; Noguchi, Y.; Selzner, M.; Reichman, T.W. Normothermic ex situ pancreas perfusion for the preservation of porcine pancreas grafts. Am. J. Transplant. 2022, 22, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.; Barbas, A.; Goldaracena, N.; Fernandez, L.; Al-Adra, D.P. Immunological organ modification during Ex Vivo machine perfusion: The future of organ acceptance. Transplant. Rev. 2021, 35, 100586. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.P.; Ball, A.L.; Critchley, W.R.; Major, T.; Edge, R.J.; Amin, K.; Clancy, M.J.; Fildes, J.E. Ex Vivo Normothermic Perfusion Induces Donor-Derived Leukocyte Mobilization and Removal Prior to Renal Transplantation. Kidney Int. Rep. 2016, 1, 230–239. [Google Scholar] [CrossRef]
- Delpech, P.-O.; Thuillier, R.; SaintYves, T.; Danion, J.; Le Pape, S.; van Amersfoort, E.S.; Oortwijn, B.; Blancho, G.; Hauet, T. Inhibition of complement improves graft outcome in a pig model of kidney autotransplantation. J. Transl. Med. 2016, 14, 277. [Google Scholar] [CrossRef]
- Manook, M.; Kwun, J.; Burghuber, C.; Samy, K.; Mulvihill, M.; Yoon, J.; Xu, H.; MacDonald, A.L.; Freischlag, K.; Curfman, V.; et al. Thrombalexin: Use of a Cytotopic Anticoagulant to Reduce Thrombotic Microangiopathy in a Highly Sensitized Model of Kidney Transplantation. Am. J. Transplant. 2017, 17, 2055–2064. [Google Scholar] [CrossRef]
- Soussi, D.; Danion, J.; Baulier, E.; Favreau, F.; Sauvageon, Y.; Bossard, V.; Matillon, X.; Turpin, F.; Belgsir, E.M.; Thuillier, R.; et al. Vectisol Formulation Enhances Solubility of Resveratrol and Brings Its Benefits to Kidney Transplantation in a Preclinical Porcine Model. Int. J. Mol. Sci. 2019, 20, 2268. [Google Scholar] [CrossRef]
- Yuzefovych, Y.; Valdivia, E.; Rong, S.; Hack, F.; Rother, T.; Schmitz, J.; Bräsen, J.H.; Wedekind, D.; Moers, C.; Wenzel, N.; et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Front. Immunol. 2020, 11, 265. [Google Scholar] [CrossRef]
- Tietjen, G.T.; Hosgood, S.A.; DiRito, J.; Cui, J.; Deep, D.; Song, E.; Kraehling, J.R.; Piotrowski-Daspit, A.S.; Kirkiles-Smith, N.C.; Al-Lamki, R.; et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci. Transl. Med. 2017, 9, eaam6764. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Z.; Fei, L.; Sun, H.; Wang, R.; Chen, Y.; Chen, H.; Wang, J.; Tang, H.; Ge, W.; et al. Construction of a human cell landscape at single-cell level. Nature 2020, 581, 303–309. [Google Scholar] [CrossRef]
- Zimmerman, K.A.; Bentley, M.R.; Lever, J.M.; Li, Z.; Crossman, D.K.; Song, C.J.; Liu, S.; Crowley, M.R.; George, J.F.; Mrug, M.; et al. Single-Cell RNA Sequencing Identifies Candidate Renal Resident Macrophage Gene Expression Signatures across Species. J. Am. Soc. Nephrol. 2019, 30, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J.; He, S.; Liu, Y.; Chen, H.; He, S.; Yin, M.; Zou, D.; Chen, S.; Luo, T.; et al. Resolving the graft ischemia-reperfusion injury during liver transplantation at the single cell resolution. Cell Death Dis. 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Veres, A.; Wolock, S.L.; Faust, A.L.; Gaujoux, R.; Vetere, A.; Ryu, J.H.; Wagner, B.K.; Shen-Orr, S.S.; Klein, A.M.; et al. A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst. 2016, 3, 346–360.e4. [Google Scholar] [CrossRef] [PubMed]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; van Gurp, L.; Engelse, M.A.; Carlotti, F.; de Koning, E.J.; et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst. 2016, 3, 385–394.e3. [Google Scholar] [CrossRef]
| Reference | Number of Patients/Time Period/Country | Outcome Considered | Type of Donor | Incidence of Technical Failure and Thrombosis | CIT Time Considered | Results | Other Variables Associated with Outcome |
|---|---|---|---|---|---|---|---|
| Axelrod et al. [11] | 9401 patients 2000–2006 SRTR (USA) | 1-year graft survival (development of pancreas donor risk index (PDRI)) | DBD and DCD (1.4 %) | Preservation time > 12 h associated with outcome, integrated in PDRI | |||
| Öllinger et al. [17] | 509 patients 1979–2011 Austria | Long-term graft survival | DBD | Thrombosis cause of 6.5% of pancreas losses | Continuous variable | CIT > 14 h associated with worse 10-year graft survival (44% vs. 65%, p = 0.04) but not in multivariate analysis | Donor age Type of transplantation Time of transplantation Number of transplants |
| Finger et al. [10] | 1115 patients 1998–2011 USA | Technical failure (graft loss <90 days due to thrombosis, bleeding, pancreatitis or intra-abdominal infections). | DBD and DCD (2.9%) | Incidence of TF 10.2% Incidence of thrombosis 5.6% | Cut-off 20h | Multivariate model preservation time >20 h associated with TF (HR 2.17 [1.45;3.23], p < 0.001) | Donor BMI Donor Cr Donor age |
| Kopp et al. [16] | 349 patients 1984–2012 Netherlands | Pancreas allograft survival | DBD and DCD (2%) | Early graft failure (<3 months) due to technical failure 9.4%, including thrombosis (8.3 % of total graft failure) | Continuous variable | Death-censored pancreas allograft associated with CIT (p = 0.005) Pancreas graft survival multivariate model CIT HR 0.9 [0.81–0.99], p = 0.033 | Transplant type (SPK vs. PTA/PAK) Procurement center local/non-local Recipient cerebrovascular disease |
| Mittal et al. [12] | 1201 patients 2004–2011 UK | 1-year graft survival | DBD and DCD (10.8%) | Continuous variable | Cold ischemia time associated with outcome (p < 0.001) | PDRI | |
| Gruessner et al. [6] | 11 104 2005–2014 World (UNOS/IPTR) | Allograft survival Technical failure Graft thrombosis | DBD and DCD (3% of SPK) | Early TF (<3 months) 7.4% for 2005–2009 era and 5.4% for 2010-2014 (SPK) Thrombosis 5.5% for 2005-2009 era and 4.1% for 2010–2014 (SPK) | Cut-offs 12h and 24h | Preservation time associated with pancreas failure (SPK) (RR 12 h–24 h 1.18 [1.06;1.33], >=24 h 2.38 [1.60;3.53], vs. 0–11 h, p < 0.0001, multivariate analysis) Preservation time associated with early graft failure due to graft thrombosis (SPK) (RR 12h-24h 1.18 [0.94;1.49], >=24 h 3.14 [1.51;6.51], vs. 0–11h, p = 0.005 multivariate analysis) | Allograft failure (SPK) Era Recipient gender Recipient BMI PRA Donor age Donor Cause of death Immunosuppression Center volume Early graft failure due to thrombosis (SPK) Era Recipient BMI PRA Donor cause of death |
| Reference | Molecules Implicated | Cell Source | Animal/Human | Setting | Outcome/Associated Event |
|---|---|---|---|---|---|
| Ogliari et al. [19] | CCL-2 | Monocytes, macrophages, dendritic cells | Human (77 SPK recipients) | Donor brain death | High donor circulating CCL2 levels associated with pancreas loss HR 4.4 [1.14–16.7] p = 0.031 multivariate analysis High incidence of pancreas graft thrombosis |
| Rech et al. [20] | TNF-α, IL-6 | Various | Human (17 brain death and 20 control pancreas) | Donor brain death | Higher serum concentration of TNF and IL-6 in brain death patients. Increased TNF protein levels in pancreatic tissue in brain death patients. |
| Lunsford et al. [21] | Various cytokines and chemokines | Various | Mouse | Ischemia reperfusion (reversible vascular isolation of distal pancreas) | Upregulation of G-CSF, IFN-γ, TNF- α, IL-2, IL-1β, IL-6, CCL-2, CCL-5, CXCL-1, MIP2 protein levels in mice serum (30 min IRI). Upregulation CCL-2, IL-1β, IL-6, fos, hsp1a, hspd1, cd14 gene expression in pancreatic tissue (30 min IRI, marked inflammation) |
| Wiessner et al. [22] | ICAM-1, P-selectin | Endothelial cells | Human | Ischemia reperfusion (pancreas biopsies during cold ischemia, after reperfusion (allogeneic transplantation) and controls) | Increased expression of P-selectin after reperfusion Increased expression of ICAM-1 during cold ischemia Extensive infiltration of CD11b cells (neutrophils) in venules and capillaries after reperfusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kervella, D.; Mesnard, B.; Prudhomme, T.; Bruneau, S.; Masset, C.; Cantarovich, D.; Blancho, G.; Branchereau, J. Sterile Pancreas Inflammation during Preservation and after Transplantation. Int. J. Mol. Sci. 2023, 24, 4636. https://doi.org/10.3390/ijms24054636
Kervella D, Mesnard B, Prudhomme T, Bruneau S, Masset C, Cantarovich D, Blancho G, Branchereau J. Sterile Pancreas Inflammation during Preservation and after Transplantation. International Journal of Molecular Sciences. 2023; 24(5):4636. https://doi.org/10.3390/ijms24054636
Chicago/Turabian StyleKervella, Delphine, Benoît Mesnard, Thomas Prudhomme, Sarah Bruneau, Christophe Masset, Diego Cantarovich, Gilles Blancho, and Julien Branchereau. 2023. "Sterile Pancreas Inflammation during Preservation and after Transplantation" International Journal of Molecular Sciences 24, no. 5: 4636. https://doi.org/10.3390/ijms24054636
APA StyleKervella, D., Mesnard, B., Prudhomme, T., Bruneau, S., Masset, C., Cantarovich, D., Blancho, G., & Branchereau, J. (2023). Sterile Pancreas Inflammation during Preservation and after Transplantation. International Journal of Molecular Sciences, 24(5), 4636. https://doi.org/10.3390/ijms24054636










