Assessment of sST2 Behaviors to Evaluate Severity/Clinical Impact of Acute Pulmonary Embolism
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galiè, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.B.; Ferreira, D.; Janssens, U.; et al. Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism. Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef] [PubMed]
- Gkana, A.; Papadopoulou, A.; Mermiri, M.; Beltsios, E.; Chatzis, D.; Malli, F.; Adamou, A.; Gourgoulianis, K.; Mavrovounis, G.; Pantazopoulos, I. Contemporary Biomarkers in Pulmonary Embolism Diagnosis: Moving beyond D-Dimers. J. Pers. Med. 2022, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Lax, A.; Perez-Martinez, M.T.; Asensio-Lopez, M.D.C.; Sanchez-Mas, J. Clinical Relevance of SST2 in Cardiac Diseases. Clin. Chem. Lab. Med. 2016, 54, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Concistrè, A.; Suppa, M.; Galardo, G.; Rosa, A.; Bertazzoni, G.; Pugliese, F.; Letizia, C.; Petramala, L. Prognostic Role of SST2 in Acute Heart Failure and COVID-19 Infection—A Narrative Review on Pathophysiology and Clinical Prospective. Int. J. Mol. Sci. 2022, 23, 8230. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; MacDonald, T.; Hermoso, M.A. The IL-33/ST2 axis: Role in health and disease. Cytokine Growth Factor Rev. 2015, 26, 615–623. [Google Scholar] [CrossRef]
- Jimenez, D.; Lobo, J.L.; Fernandez-Golfin, C.; Portillo, A.K.; Nieto, R.; Lankeit, M.; Konstantinides, S.; Prandoni, P.; Muriel, A.; Yusen, R.D. Effectiveness of Prognosticating Pulmonary Embolism Using the ESC Algorithm and the Bova Score. Thromb. Haemost. 2016, 115, 827–834. [Google Scholar] [CrossRef]
- Weinberg, E.O. ST2 Protein in Heart Disease: From Discovery to Mechanisms and Prognostic Value. Biomark. Med. 2009, 3, 495–511. [Google Scholar] [CrossRef]
- Maisel, A.S.; Di Somma, S. Dowe Need Another Heart Failure Biomarker: Focus on Soluble Suppression of Tumorigenicity 2 (SST2). Eur. Heart J. 2017, 38, 2325–2332. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, X.; Xu, H.; Wu, Y.; Jia, X.; Fang, Y.; Lu, Y.; Xu, Y.; Zhang, J.; Su, Y. Serum Soluble ST2 Is a Valuable Prognostic Biomarker in Patients with Acute Heart Failure. Front. Cardiovasc. Med. 2022, 9, 123. [Google Scholar] [CrossRef]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. IL-33/ST2 Axis in Organ Fibrosis. Front. Immunol. 2018, 9, 2432. [Google Scholar] [CrossRef]
- Tajima, S.; Oshikawa, K.; Tominaga, S.I.; Sugiyama, Y. The Increase in Serum Soluble ST2 Protein Upon Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Chest 2003, 124, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; De Los Santos, F.G.; Wu, Z.; Liu, T.; Phan, S.H. An ST2-Dependent Role of Bone Marrow-Derived Group 2 Innate Lymphoid Cells in Pulmonary Fibrosis. J. Pathol. 2018, 245, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of SST2 in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, H.; Maisel, A.S. Soluble ST2 Testing: A Promising Biomarker in the Management of Heart Failure. Arq. Bras. Cardiol. 2016, 106, 145–152. [Google Scholar] [CrossRef]
- Mildner, M.; Storka, A.; Lichtenauer, M.; Mlitz, V.; Ghannadan, M.; Hoetzenecker, K.; Nickl, S.; Dome, B.; Tschachler, E.; Ankersmit, H.J. Primary Sources and Immunological Prerequisites for SST2 Secretion in Humans. Cardiovasc. Res. 2010, 87, 769–777. [Google Scholar] [CrossRef]
- Harjola, V.P.; Mebazaa, A.; Čelutkiene, J.; Bettex, D.; Bueno, H.; Chioncel, O.; Crespo-Leiro, M.G.; Falk, V.; Filippatos, G.; Gibbs, S.; et al. Contemporary Management of Acute Right Ventricular Failure: A Statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 226–241. [Google Scholar] [CrossRef]
- Smulders, Y.M. Pathophysiology and Treatment of Haemodynamic Instability in Acute Pulmonary Embolism: The Pivotal Role of Pulmonary Vasoconstriction. Cardiovasc. Res. 2000, 48, 23–33. [Google Scholar] [CrossRef]
- Begieneman, M.P.V.; Van De Goot, F.R.W.; Van Der Bilt, I.A.C.; Vonk Noordegraaf, A.; Spreeuwenberg, M.D.; Paulus, W.J.; Van Hinsbergh, V.W.M.; Visser, F.C.; Niessen, H.W.M. Pulmonary Embolism Causes Endomyocarditis in the Human Heart. Heart 2008, 94, 450–456. [Google Scholar] [CrossRef]
- Vuilleumier, N.; Simona, A.; Méan, M.; Limacher, A.; Lescuye, P.; Gerstel, E.; Bounameaux, H.; Aujesky, D.; Righini, M. Comparison of Cardiac and Non-Cardiac Biomarkers for Risk Stratification in Elderly Patients with Non-Massive Pulmonary Embolism. PLoS ONE 2016, 11, e0155973. [Google Scholar] [CrossRef]
- Mauritz, G.J.; Marcus, J.T.; Westerhof, N.; Postmus, P.E.; Vonk-Noordegraaf, A. Prolonged Right Ventricular Post-Systolic Isovolumic Period in Pulmonary Arterial Hypertension Is Not a Reflection of Diastolic Dysfunction. Heart 2011, 97, 473–478. [Google Scholar] [CrossRef]
- Lankeit, M.; Jiménez, D.; Kostrubiec, M.; Dellas, C.; Hasenfuss, G.; Pruszczyk, P.; Konstantinides, S. Predictive Value of the High-Sensitivity Troponin T Assay and the Simplified Pulmonary Embolism Severity Index in Hemodynamically Stable Patients with Acute Pulmonary Embolism: A Prospective Validation Study. Circulation 2011, 124, 2716–2724. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, K.S.; Clark, A.R.; Tawhai, M.H. Blood Flow Redistribution and Ventilation perfusion Mismatch during Embolic Pulmonary Arterial Occlusion. Pulm. Circ. 2011, 1, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Alladina, J.W.; Levy, S.D.; Hibbert, K.A.; Januzzi, J.L.; Harris, R.S.; Matthay, M.A.; Thompson, B.T.; Bajwa, E.K. Plasma Concentrations of Soluble Suppression of Tumorigenicity-2 and Interleukin-6 Are Predictive of Successful Liberation from Mechanical Ventilation in Patients with the Acute Respiratory Distress Syndrome. Crit. Care Med. 2016, 44, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.; Geibel, A.; Kasper, W.; Olschewski, M.; Blümel, L.; Just, H. Patent Foramen Ovale Is an Important Predictor of Adverse Outcome in Patients with Major Pulmonary Embolism. Circulation 1998, 97, 1946–1951. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Qiao, L.; Tan, Z.; Jin, J.; Yang, J.; Zhang, L.; Fang, B.M.; Xu, X. The Predictive Value of PaO2/FIO2 and Additional Parameters for in-Hospital Mortality in Patients with Acute Pulmonary Embolism: An 8-Year Prospective Observational Single-Center Cohort Study. BMC Pulm. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
- Vanni, S.; Jiménez, D.; Nazerian, P.; Morello, F.; Parisi, M.; Daghini, E.; Pratesi, M.; López, R.; Bedate, P.; Lobo, J.L.; et al. Short-Term Clinical Outcome of Normotensive Patients with Acute PE and High Plasma Lactate. Thorax 2015, 70, 333–338. [Google Scholar] [CrossRef]
- Hobohm, L.; Hellenkamp, K.; Hasenfuß, G.; Münzel, T.; Konstantinides, S.; Lankeit, M. Comparison of Risk Assessment Strategies for Not-High-Risk Pulmonary Embolism. Eur. Respir. J. 2016, 47, 1170–1178. [Google Scholar] [CrossRef]
- Fernández, C.; Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Fernández-Golfín, C.; Yusen, R.D.; Jiménez, D. Validation of a Model for Identification of Patients at Intermediate to High Risk for Complications Associated with Acute Symptomatic Pulmonary Embolism. Chest 2015, 148, 211–218. [Google Scholar] [CrossRef]
- Bova, C.; Sanchez, O.; Prandoni, P.; Lankeit, M.; Konstantinides, S.; Vanni, S.; Jiménez, D. Identification of Intermediate-Risk Patients with Acute Symptomatic Pulmonary Embolism. Eur. Respir. J. 2014, 44, 694–703. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Bueno, H.; Galié, N.; Gibbs, J.S.R.; Ageno, W.; Agewall, S.; Almeida, A.G.; Andreotti, F.; Barbato, E.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Acute Pulmonary Embolism Developed in Collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Donzé, J.; Le Gal, G.; Fine, M.J.; Roy, P.M.; Sanchez, O.; Verschuren, F.; Cornuz, J.; Meyer, G.; Perrier, A.; Righini, M.; et al. Prospective Validation of the Pulmonary Embolism Severity Index: A Clinical Prognostic Model for Pulmonary Embolism. Thromb. Haemost. 2008, 100, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.; Mallett, S.; Daoud-Elias, M.; Poggi, J.N.; Clarke, M. Prognostic Models in Acute Pulmonary Embolism: A Systematic Review and Meta-Analysis. BMJ Open 2016, 6, e010324. [Google Scholar] [CrossRef] [PubMed]
- Kohn, C.G.; Mearns, E.S.; Parker, M.W.; Hernandez, A.V.; Coleman, C.I. Prognostic Accuracy of Clinical Prediction Rules for Early Post-Pulmonary Embolism All-Cause Mortality: A Bivariate Meta-Analysis. Chest 2015, 147, 1043–1062. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Roy, P.M.; Meyer, G.; Verschuren, F.; Aujesky, D.; Le Gal, G. The Simplified Pulmonary Embolism Severity Index (PESI): Validation of a Clinical Prognostic Model for Pulmonary Embolism. J. Thromb. Haemost. 2011, 9, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Sam, A.; Sánchez, D.; Gómez, V.; Wagner, C.; Kopecna, D.; Zamarro, C.; Moores, L.; Aujesky, D.; Yusen, R.; Jiménez Castro, D. The Shock Index and the Simplified PESI for Identification of Low-Risk Patients with Acute Pulmonary Embolism. Eur. Respir. J. 2011, 37, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Vicaut, E.; Danays, T.; Agnelli, G.; Becattini, C.; Beyer-Westendorf, J.; Bluhmki, E.; Bouvaist, H.; Brenner, B.; Couturaud, F.; et al. Fibrinolysis for Patients with Intermediate-Risk Pulmonary Embolism. N. Engl. J. Med. 2014, 370, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Mahmoudpour, S.H.; Planquette, B.; Sanchez, O.; Konstantinides, S.V.; Meyer, G. Prognostic Value of Right Ventricular Dysfunction or Elevated Cardiac Biomarkers in Patients with Low-Risk Pulmonary Embolism: A Systematic Review and Meta-Analysis. Eur. Heart J. 2019, 40, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kazerooni, E.A.; Cascade, P.N. Pulmonary Embolism: Optimization of Small Pulmonary Artery Visualization at Multi-Detector Row CT. Radiology 2003, 227, 455–460. [Google Scholar] [CrossRef]
- Carrier, M.; Righini, M.; Wells, P.S.; Perrier, A.; Anderson, D.R.; Rodger, M.A.; Pleasance, S.; Le Gal, G. Subsegmental Pulmonary Embolism Diagnosed by Computed Tomography: Incidence and Clinical Implications. A Systematic Review and Meta-Analysis of the Management Outcome Studies. J. Thromb. Haemost 2010, 8, 1716–1722. [Google Scholar] [CrossRef]
- Stein, P.D.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V.; Popovich, J.; Quinn, D.A.; Sos, T.A.; et al. Multidetector Computed Tomography for Acute Pulmonary Embolism. N. Engl. J. Med. 2006, 354, 2317–2327. [Google Scholar] [CrossRef]
- Andrade, I.; Mehdipoor, G.; Le Mao, R.; García-Sánchez, A.; Pintado, B.; Pérez, A.; Rodríguez, C.; Velasco, D.; Bikdeli, B.; Jiménez, D. Prognostic Significance of Computed Tomography-Assessed Right Ventricular Enlargement in Low-Risk Patients with Pulmonary Embolism: Systematic Review and Meta-Analysis. Thromb. Res. 2021, 197, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Peacock, W.F.; Maisel, A.S.; Chae, C.U.; Jesse, R.L.; Baggish, A.L.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; Van Kimmenade, R.R.J.; et al. Measurement of the Interleukin Family Member ST2 in Patients with Acute Dyspnea. Results From the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) Study. J. Am. Coll. Cardiol 2007, 50, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, E.K.; Mebazaa, A.; Januzzi, J.L. ST2 in Pulmonary Disease. Am. J. Cardiol. 2015, 115, 44B–47B. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, B.; Severinsen, M.Z.; Von Wowern, F.; Miguel, C.S.; Goetze, J.P.; Melander, O. St2 Predicts Mortality in Patients with Acute Hypercapnic Respiratory Failure Treated with Noninvasive Positive Pressure Ventilation. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, B.; Zelnick, L.; Anderson, A.H.; Feldman, H.; Go, A.; He, J.; Kansal, M.; Mohanty, M.J.; Mehta, R.; Shlipak, M.G.; et al. Cardiac Biomarkers and Risk of Atherosclerotic Cardiovascular Disease in Patients with Chronic Kidney Disease. Kidney360 2022, 3, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Dimitropoulos, S.; Mystakidi, V.C.; Oikonomou, E.; Siasos, G.; Tsigkou, V.; Athanasiou, D.; Gouliopoulos, N.; Bletsa, E.; Kalampogias, A.; Charalambous, G.; et al. Association of Soluble Suppression of Tumorigenesis-2 (ST2) with Endothelial Function in Patients with Ischemic Heart Failure. Int. J. Mol. Sci. 2020, 21, 9385. [Google Scholar] [CrossRef]
- Konstantinides, S.; Meyer, G.; Lang, I.; Verschuren, F.; Meneveau, N.; Charbonnier, B.; Bouvaist, H.; Geibel, A.; Beyer-Westendorf, J.; Dellas, C.; et al. Single-Bolus Tenecteplase plus Heparin Compared with Heparin Alone for Normotensive Patients with Acute Pulmonary Embolism Who Have Evidence of Right Ventricular Dysfunction and Myocardial Injury: Rationale and Design of the Pulmonary Embolism Thrombolysi. Am. Heart J. 2012, 163, 33–38.e1. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of Amer. J. Card. Fail. 2017, 23, 628–651. [Google Scholar] [CrossRef]
- Sarlo, F.; De Luca, C.; Moretti, G.; Urbani, A.; Baroni, S. Analytical Performance Evaluation of the New SST2 Turbidimetric Assay Implemented in Laboratory Automation Systems. Clin. Chem. Lab. Med. 2022, 60, E54–E56. [Google Scholar] [CrossRef]
- Armbruster, D.A.; Pry, T. Limit of Blank, Limit of Detection and Limit of Quantitation. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S49–S52. [Google Scholar]
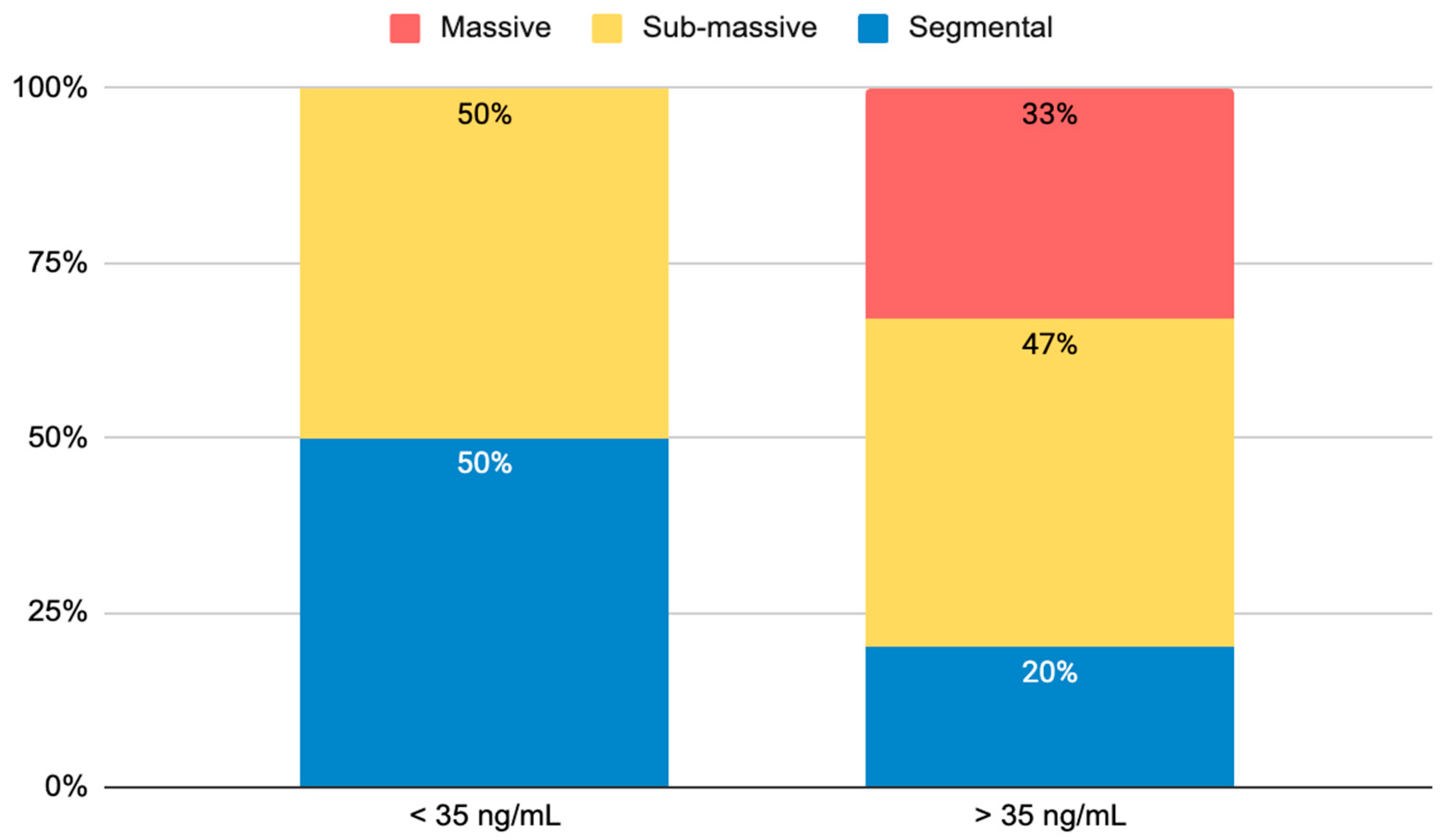
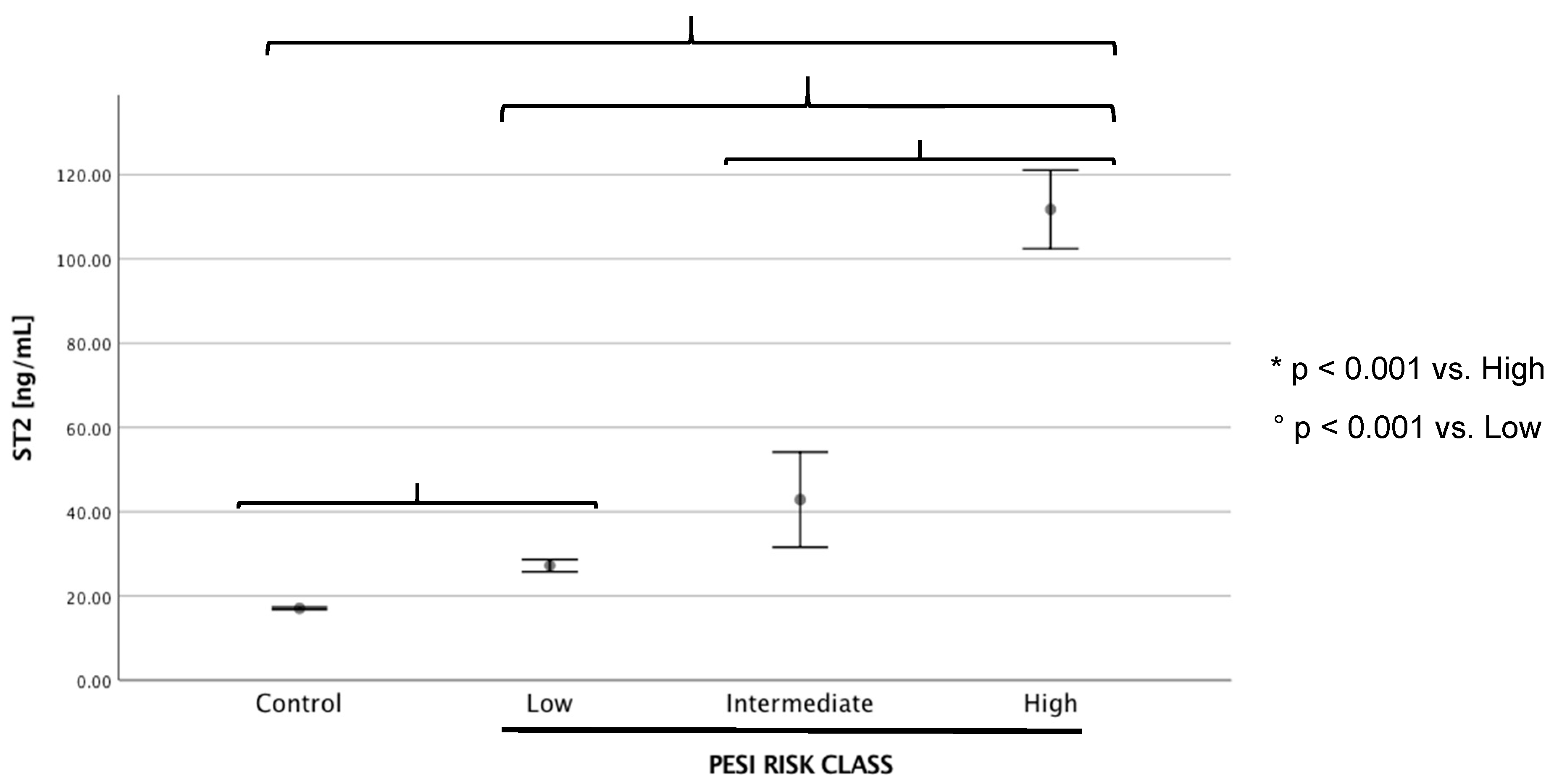
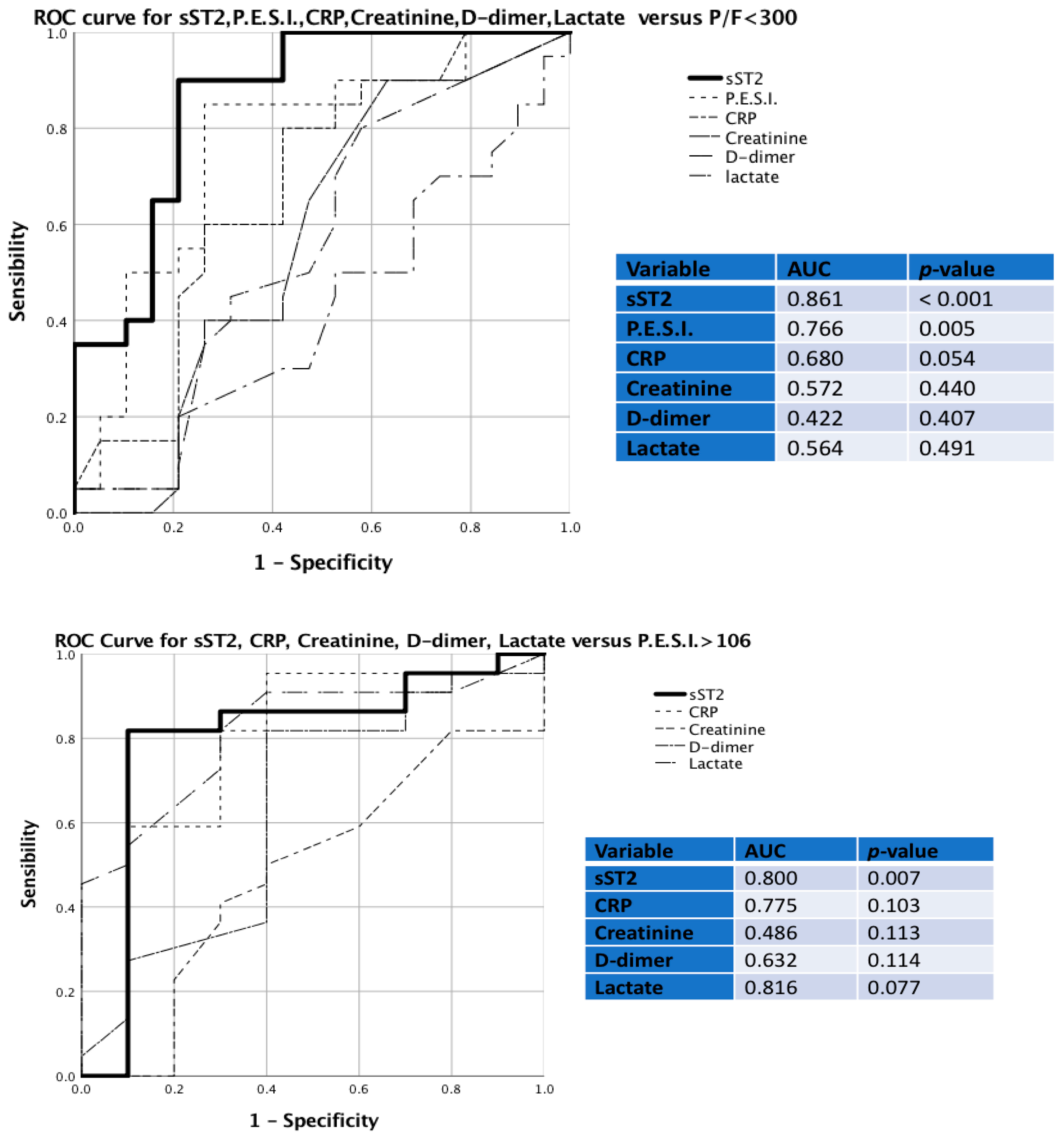
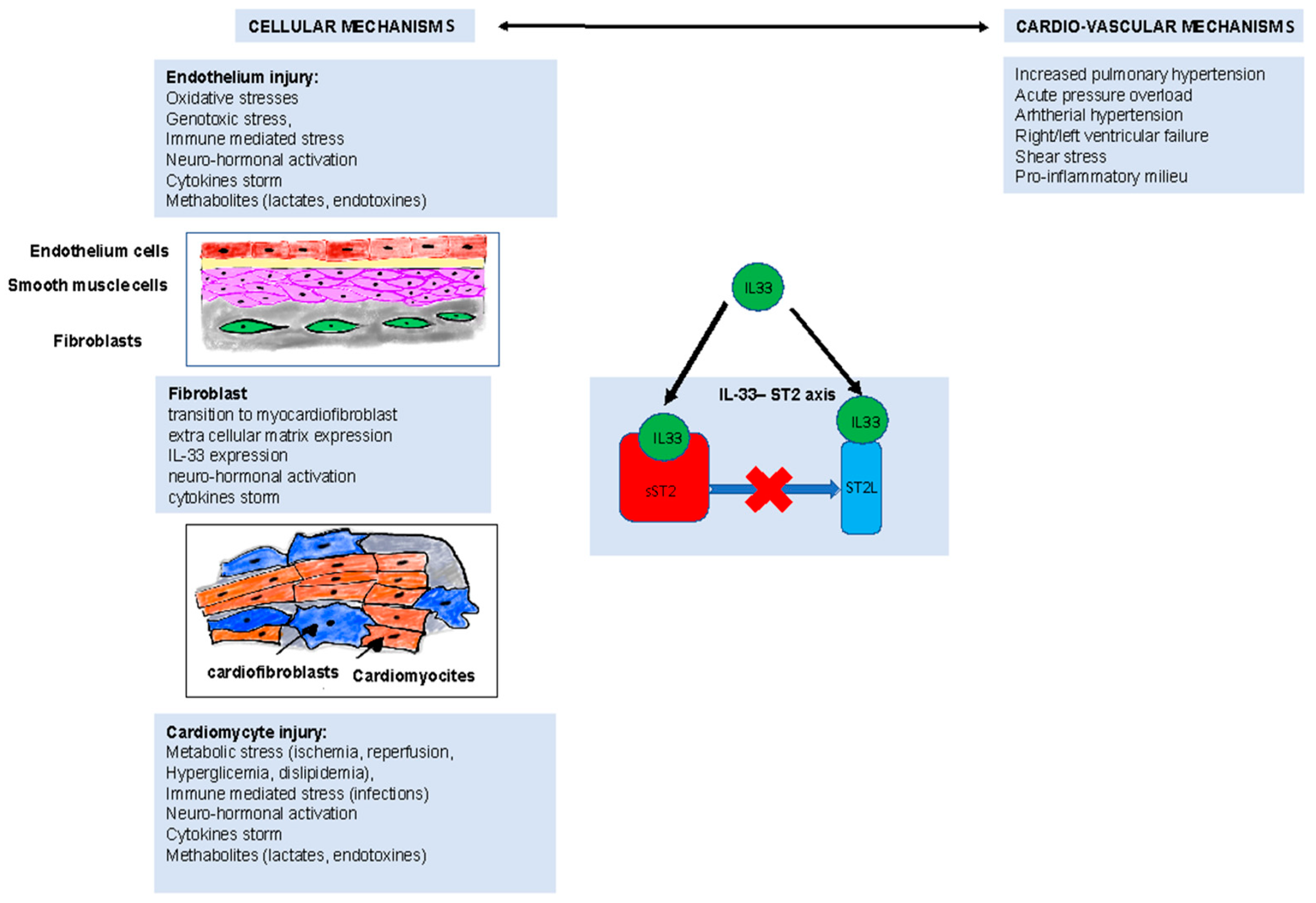
| Controls (n = 38) | PE (n = 72) | p-Value | PE < 35 ng/mL (n = 34) | PE > 35 ng/mL (n = 38) | p-Value | |
|---|---|---|---|---|---|---|
| Anthropometric parameters | ||||||
| Age (years) | 58 ± 6.4 | 68 ± 2.8 | 0.71 | 67 ± 4.7 | 70 ± 24.1 | 0.64 |
| Males (%) | 45 | 51.4 | 0.9 | 50 | 52.6 | 0.88 |
| Hypertension (%) | 30 | 43 | 0.57 | 41.2 | 44.7 | 0.83 |
| Diabetes (%) | 3 | 6.9 | <0.001 | 8.8 | 5.2 | 0.12 |
| Heart failure/cardiomyopathy (%) | 3 | 1.08 | 0.567 | 0.0 | 2.6 | 0.07 |
| Obesity (%) | 17 | 41.6 | 0.35 | 47 | 36.8 | 0.43 |
| Oncologic (%) | 0 | 27.6 | <0.001 | 17.6 | 34.72 | 0.18 |
| SBP (mmHg) | 127 ± 11.8 | 134 ± 11.8 | 0.08 | 134 ± 3.9 | 132 ± 9.8 | 0.89 |
| DBP (mmHg) | 62 ± 8.8 | 76 ± 15.49 | 0.54 | 79 ± 4.6 | 75 ± 4.3 | 0.51 |
| HR (bpm) | 77 ± 12 | 102 ± 25.12 | 0.03 | 100 ± 8.5 | 104 ± 6.0 | 0.74 |
| RR (bpm) | 17 ± 6 | 19.12 ± 5.62 | 0.76 | 18.3± 5.8 | 20.1 ± 4.2 | 0.44 |
| Laboratory parameters | ||||||
| D-dimer (n.r. 0–550 ng/mL) | 345 ± 110 | 3681 ± 236 | <0.001 | 3164 ± 472 | 4030 ± 196 | 0.08 |
| Creatinine (n.r. 0.1-1.2 mg/dL) | 0.8 ± 0.2 | 1.06 ± 0.16 | 0.075 | 1.23 ± 0.34 | 0.94 ± 0.08 | 0.36 |
| TNT-hs (n.r. < 0.0014 μg/L) | 0.008 ± 0.002 | 0.041 ± 0.0081 | 0.04 | 0.040 ± 0.010 | 0.045 ± 0.012 | 0.78 |
| Fibrinogen (n.r. 200–400 mg/dL) | 155 ± 43 | 467.5 ± 18.8 | <0.001 | 433.9 ± 33.9 | 491 ± 22.5 | 0.15 |
| C-reactive protein (n.r. < 0.5 mg/dL) | 1.5 ± 0.92 | 9.07 ± 5.64 | 0.065 | 4.81 ± 1.52 | 12.65 ± 2.59 | 0.02 |
| PT-INR (n.r. 0.8–1.2) | 0.95 ± 0.3 | 1.10 ± 0.033 | 0.035 | 1.05 ± 0.04 | 1.16 ± 0.05 | 0.12 |
| aPTT (n.r. 0.8–1.2) | 0.64 ± 0.28 | 0.92 ± 0.031 | 0.2 | 0.91 ± 0.042 | 0.92 ± 0.051 | 0.88 |
| sST2 (ng/mL) | 17.1 ± 0.4 | 87.74 ± 17.1 | <0.001 | 24 ± 1.71 | 138 ± 23.8 | <0.001 |
| PESI score | 35 ± 18.3 | 117.5 ± 10.6 | <0.05 | 103.7 ±15.1 | 138.7 ± 14.9 | 0.05 |
| Blood gas parameters | ||||||
| pH | 7.40 ± 0.23 | 7.47 ± 0.14 | 0.8 | 7.44 ± 0.12 | 7.48 ± 0.21 | 0.038 |
| pO2 (mmHg) | 91.7 ± 5.1 | 89.7 ± 3.9 | 0.2 | 88.1 ± 5.14 | 91.6 ± 6.08 | 0.011 |
| pCO2 (mmHg) | 39.2 ± 2.3 | 35.55 ± 1.42 | 0.025 | 36.6 ± 5.7 | 33.9 ± 2.08 | 0.37 |
| FiO2 (%) | 21 | 34.86 ± 2.32 | 0.02 | 29.5 ± 2.80 | 40.53 ± 3.27 | 0.04 |
| SpO2 (%) | 96.7 ± 1.3 | 95.2 ± 1.01 | 0.15 | 96.4 ± 2.3 | 93.4 ± 2.5 | 0.28 |
| P/F ratio | 410 ± 30.5 | 273.2 ± 101.7 | 0.02 | 301.43 ± 39.48 | 228.67 ± 30.19 | 0.07 |
| Lactate (mmol/L) | 0.8 ± 0.02 | 1.76 ± 0.37 | <0.001 | 1.025 ± 0.05 | 2.43 ± 0.69 | 0.05 |
| Echocardiographic parameters | ||||||
| LVIDD (mm) | 48.3 ± 5.2 | 49.9 ± 4.7 | 0.231 | 50.4 ± 4.9 | 49.2 ± 4.4 | 0.65 |
| IVSd (mm) | 9.41 ± 0.42 | 10.36 ± 1.12 | 0.032 | 9.78 ± 0.99 | 10.8 ± 1.33 | 0.43 |
| LVPWd (mm) | 9.11 ± 0.92 | 9.81 ± 1.25 | 0.348 | 9.62 ± 1.23 | 10.1 ± 1.25 | 0.22 |
| LAVI (mL/m2) | 24.13 ± 3.12 | 27.8 ± 6.38 | 0.094 | 26.7 ± 5.88 | 28.7 ± 6.63 | 0.31 |
| LVM (g) | 179.2 ± 26.2 | 189.27 ± 37.1 | 0.128 | 186.7 ± 29.89 | 195.2 ± 39.82 | 0.04 |
| LVMi (g/m2) | 90.2 ± 12.3 | 99.32 ± 17.56 | 0.099 | 94.75 ± 15.67 | 102.28 ± 19.7 | 0.035 |
| LVEF (%) | 58.3 ± 5.2 | 54.27 ± 7.86 | 0.134 | 55.14 ± 9.32 | 52.27 ± 6.34 | 0.12 |
| LVH (%) | 5 | 28 | 0.02 | 42 | 58 | 0.11 |
| RVd (mm) | 32.2 ± 5.5 | 39.54 ± 4.71 | 0.012 | 37.54 ± 4.71 | 40.72 ± 3.12 | 0.06 |
| TAPSE (mm) | 22.21± 3.11 | 18.72 ± 3.13 | 0.002 | 19.88 ± 4.12 | 17.12 ± 2.92 | 0.05 |
| RVFW S’ (cm/sec) | 12.6 ± 1.23 | 11.37 ± 2.88 | 0.04 | 11.88 ± 3.79 | 10.97 ± 2.2 | 0.12 |
| RVFAC (%) | 42.3 ± 5.12 | 36.2 ± 8.5 | 0.02 | 39.5 ± 9.9 | 34.7 ± 7.8 | 0.021 |
| RVSP (mmHg) | 20.12 ± 8.34 | 34.36 ± 7.1 | 0.003 | 33.6 ± 3.5 | 34.94 ± 7.9 | 0.38 |
| Pearson Correlation Coefficient p-Value | Age | FBG | PT-INR | aPTT | d-DIM | TNT-hs | Creat | CRP | PESI | sST2 | FiO2 | pO2 | pH | pCO2 | Lactate | P/F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.160 | 0.070 | 0.068 | 0.108 | 0.006 | 0.221 | 0.452 | 0.444 | 0.160 | −0.045 | −0.134 | −0.034 | 0.084 | 0.032 | 0.23 | |
| 0.415 | 0.725 | 0.732 | 0.616 | 0.980 | 0.268 | 0.018 | 0.050 | 0.425 | 0.817 | 0.554 | 0.888 | 0.725 | 0.898 | 0.92 | ||
| FBG | −0.002 | 0.239 | −0.263 | −0.104 | −0.080 | 0.508 | 0.086 | 0.163 | −0.001 | 0.096 | −0.124 | -.124 | 0.026 | −0.095 | ||
| 0.993 | 0.221 | 0.215 | 0.653 | 0.692 | 0.007 | 0.720 | 0.425 | 0.994 | 0.671 | 0.602 | 0.602 | 0.916 | 0.70 | |||
| PT-INR | 0.595 | 0.235 | 0.042 | 0.175 | 0.316 | 0.206 | 0.427 | 0.380 | −0.079 | −0.321 | −0.092 | 0.643 | −0.423 | |||
| 0.001 | 0.268 | 0.856 | 0.382 | 0.108 | 0.384 | 0.029 | 0.046 | 0.725 | 0.168 | 0.700 | 0.003 | 0.05 | ||||
| aPTT | 0.013 | −0.292 | 0.078 | 0.313 | 0.090 | 0.349 | 0.149 | −0.193 | −0.406 | 0.216 | 0.315 | −0.479 | ||||
| 0.953 | 0.200 | 0.699 | 0.111 | 0.706 | 0.081 | 0.448 | 0.389 | 0.076 | 0.360 | 0.190 | 0.07 | |||||
| d-DIM | 0.295 | −0.025 | −0.202 | 0.338 | 0.116 | 0.171 | 0.031 | 0.189 | −0.047 | 0.329 | 0.011 | |||||
| 0.207 | 0.908 | 0.356 | 0.170 | 0.598 | 0.424 | 0.899 | 0.467 | 0.859 | 0.214 | 0.9 | ||||||
| TNT-hs | 0.114 | −0.203 | 0.009 | −0.126 | 0.144 | 0.716 | 0.050 | −0.570 | 0.152 | 0.303 | ||||||
| 0.623 | 0.376 | 0.971 | 0.595 | 0.534 | 0.236 | 0.850 | 0.017 | 0.574 | 0.25 | |||||||
| Creatinine | 0.070 | −0.124 | −0.075 | 0.083 | −0.324 | 0.001 | 0.255 | −0.061 | −0.032 | |||||||
| 0.734 | 0.603 | 0.717 | 0.680 | 0.152 | 0.996 | 0.292 | 0.810 | 0.90 | ||||||||
| CRP | 0.063 | 0.304 | 0.277 | 0.003 | −0.155 | 0.002 | 0.199 | −0.295 | ||||||||
| 0.793 | 0.139 | 0.163 | 0.990 | 0.514 | 0.992 | 0.414 | 0.23 | |||||||||
| PESI | 0.434 | 0.520 | 0.009 | 0.107 | 0.133 | 0.150 | −0.28 | |||||||||
| 0.036 | 0.019 | 0.974 | 0.693 | 0.623 | 0.594 | 0.33 | ||||||||||
| sST2 | 0.554 | −0.171 | 0.104 | −0.127 | 0.441 | −0.596 | ||||||||||
| 0.003 | 0.471 | 0.682 | 0.615 | 0.076 | 0.015 | |||||||||||
| FiO2 | −0.132 | −0.030 | −0.092 | 0.367 | −0.906 | |||||||||||
| 0.901 | 0.699 | 0.123 | 0.001 | |||||||||||||
| pO2 | −0.141 | −0.227 | −0.199 | 0.39 | ||||||||||||
| 0.553 | 0.336 | 0.413 | 0.1 | |||||||||||||
| pH | 0.076 | 0.152 | 0.134 | |||||||||||||
| 0.749 | 0.535 | 0.59 | ||||||||||||||
| pCO2 | −0.467 | 0.09 | ||||||||||||||
| 0.044 | 0.72 | |||||||||||||||
| Lactate | −0.35 | |||||||||||||||
| 016 | ||||||||||||||||
| P/F |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petramala, L.; Concistrè, A.; Sarlo, F.; Baroni, S.; Suppa, M.; Servello, A.; Circosta, F.; Galardo, G.; Gandini, O.; Marino, L.; et al. Assessment of sST2 Behaviors to Evaluate Severity/Clinical Impact of Acute Pulmonary Embolism. Int. J. Mol. Sci. 2023, 24, 4591. https://doi.org/10.3390/ijms24054591
Petramala L, Concistrè A, Sarlo F, Baroni S, Suppa M, Servello A, Circosta F, Galardo G, Gandini O, Marino L, et al. Assessment of sST2 Behaviors to Evaluate Severity/Clinical Impact of Acute Pulmonary Embolism. International Journal of Molecular Sciences. 2023; 24(5):4591. https://doi.org/10.3390/ijms24054591
Chicago/Turabian StylePetramala, Luigi, Antonio Concistrè, Francesca Sarlo, Silvia Baroni, Marianna Suppa, Adriana Servello, Francesco Circosta, Gioacchino Galardo, Orietta Gandini, Luca Marino, and et al. 2023. "Assessment of sST2 Behaviors to Evaluate Severity/Clinical Impact of Acute Pulmonary Embolism" International Journal of Molecular Sciences 24, no. 5: 4591. https://doi.org/10.3390/ijms24054591
APA StylePetramala, L., Concistrè, A., Sarlo, F., Baroni, S., Suppa, M., Servello, A., Circosta, F., Galardo, G., Gandini, O., Marino, L., Cavallaro, G., Iannucci, G., & Letizia, C. (2023). Assessment of sST2 Behaviors to Evaluate Severity/Clinical Impact of Acute Pulmonary Embolism. International Journal of Molecular Sciences, 24(5), 4591. https://doi.org/10.3390/ijms24054591






