Focus on the Use of Resveratrol in Bladder Cancer
Abstract
1. Introduction
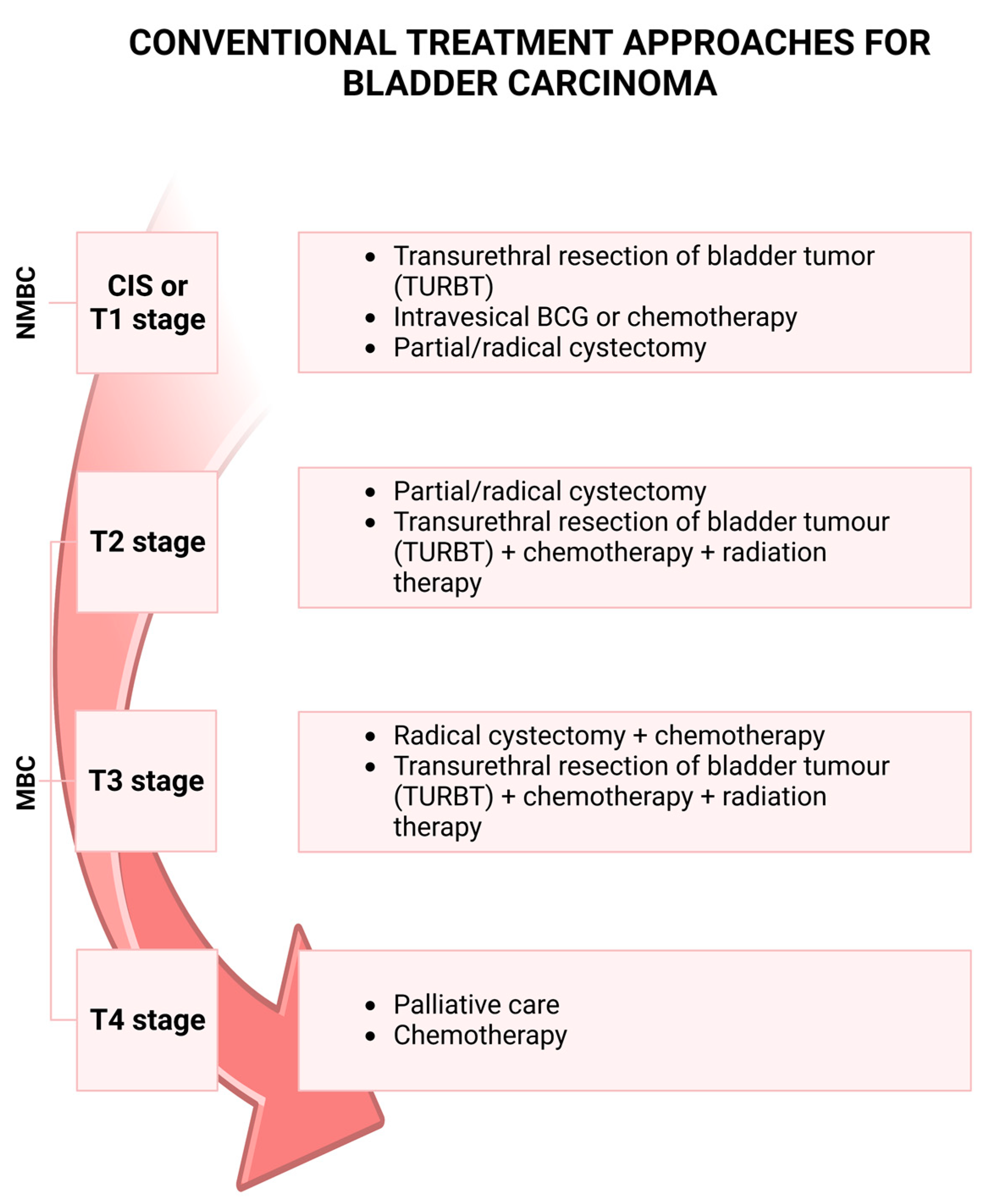
2. Bladder Cancer
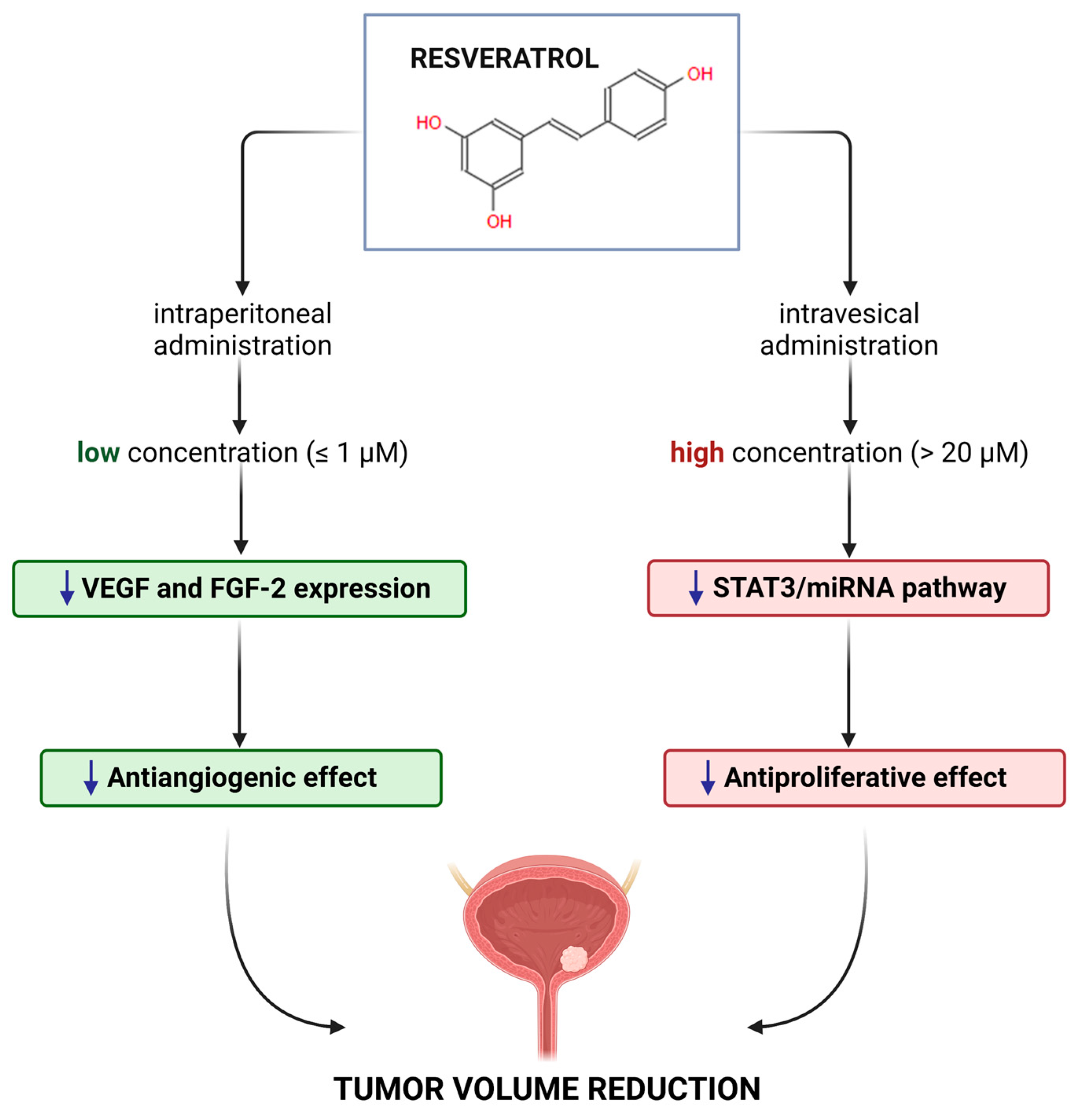
3. Resveratrol and Bladder Cancer
4. Other Mechanisms of Action of Resveratrol
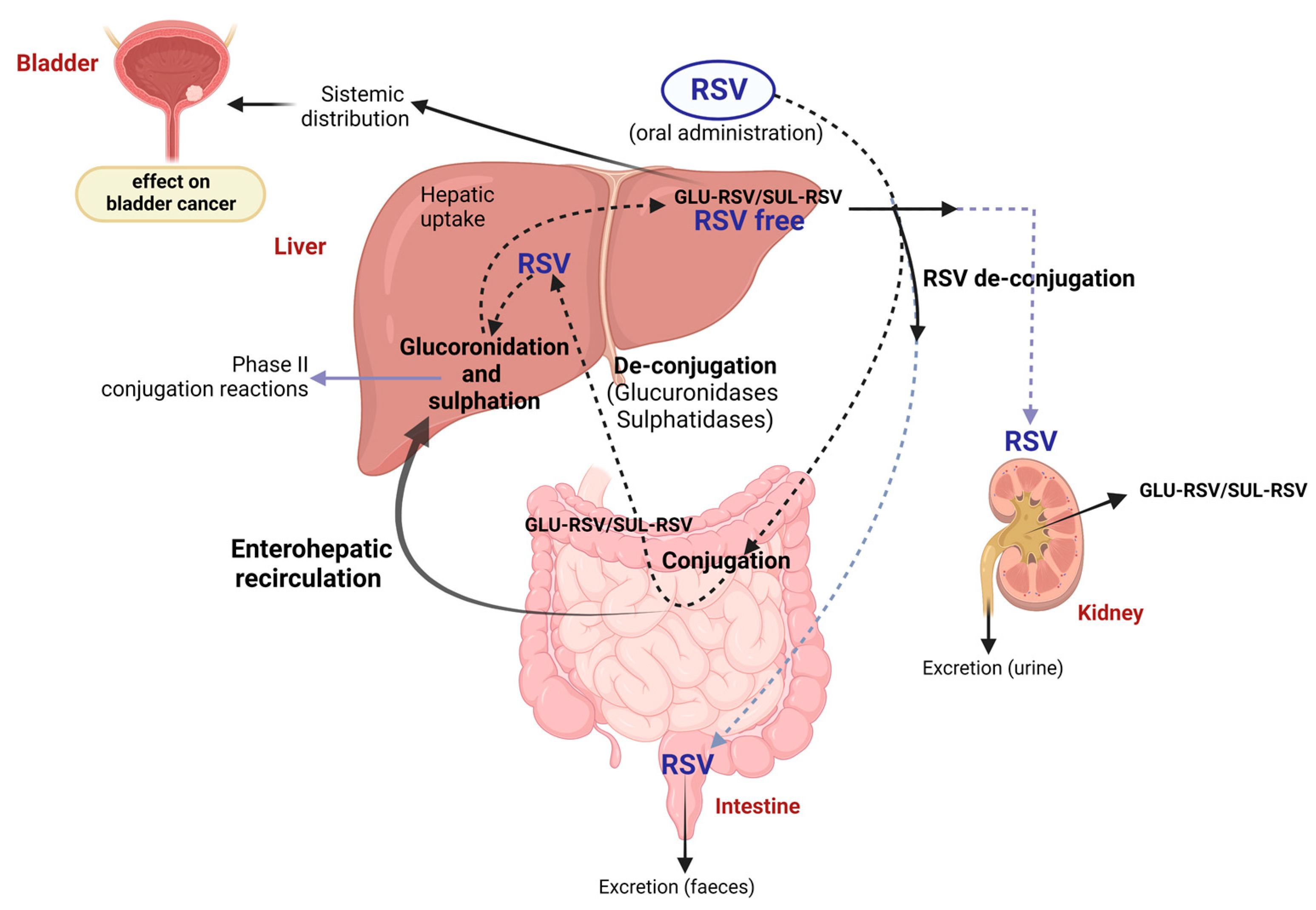
5. Pharmacokinetics of Resveratrol
6. Combination of Resveratrol with Other Compounds
7. Final Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Marques, F.Z.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009, 41, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, X.; Zhao, X.; Zhang, Y.; Wang, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Insights into the Anti-inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022, 2022, 7138756. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Nafady, M.H.; Islam, M.R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, M.H.; Akhtar, M.F.; Perveen, A.; Md Ashraf, G.; et al. Resveratrol and neuroprotection: An insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef] [PubMed]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Almeida, T.C.; Silva, G.N.D. Resveratrol effects in bladder cancer: A mini review. Genet. Mol. Biol. 2021, 44, e20200371. [Google Scholar] [CrossRef] [PubMed]
- IARC. Cancer Today. Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. 2021. 2022. Available online: https://gco.iarc.fr/today/online-analysis-table (accessed on 4 January 2023).
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Larré, S.; Roupret, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houéde, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015, 466, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; UICC International Union against Cancer; Wiley-Blackwell and UICC: New York, NY, USA, 2017. [Google Scholar]
- Andersson, M.; Berger, M.; Zieger, K.; Malmström, P.U.; Bläckberg, M. The diagnostic challenge of suspicious or positive malignant urine cytology findings when cystoscopy findings are normal: An outpatient blue-light flexible cystoscopy may solve the problem. Scand. J. Urol. 2021, 55, 263–267. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- MacLennan, G.T.; Kirkali, Z.; Cheng, L. Histologic grading of noninvasive papillary urothelial neoplasms. Eur. Urol. 2007, 51, 889–898. [Google Scholar] [CrossRef]
- Smaldone, M.C.; Jacobs, B.L.; Smaldone, A.M.; Hrebinko, R.L., Jr. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology 2008, 72, 613–616. [Google Scholar] [CrossRef]
- Prout, G.R., Jr. Bladder carcinoma and a TNM system of classification. J. Urol. 1977, 117, 583–590. [Google Scholar] [CrossRef]
- Lopez-Beltran, A. Bladder cancer: Clinical and pathological profile. Scand. J. Urol. Nephrol. 2008, 42, 95–109. [Google Scholar] [CrossRef]
- Brausi, M.; Collette, L.; Kurth, K.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Newling, D.; Bouffioux, C.; Sylvester, R.J.; EORTC Genito-Urinary Tract Cancer Collaborative Group. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur. Urol. 2002, 41, 523–531. [Google Scholar] [CrossRef]
- Sharma, S.; Ksheersagar, P.; Sharma, P. Diagnosis and treatment of bladder cancer. Am. Fam. Physician 2009, 80, 717–723. [Google Scholar]
- Sylvester, R.J.; Oosterlinck, W.; Holmang, S.; Sydes, M.R.; Birtle, A.; Gudjonsson, S.; De Nunzio, C.; Okamura, K.; Kaasinen, E.; Solsona, E.; et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur. Urol. 2016, 69, 231–244. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Oosterlinck, W.; van der Meijden, A.P. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J. Urol. 2004, 171, 2186–2435. [Google Scholar] [CrossRef]
- Abern, M.R.; Owusu, R.A.; Anderson, M.R.; Rampersaud, E.N.; Inman, B.A. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. J. Natl. Compr. Cancer Netw. 2013, 11, 477–484. [Google Scholar] [CrossRef]
- Perlis, N.; Zlotta, A.R.; Beyene, J.; Finelli, A.; Fleshner, N.E.; Kulkarni, G.S. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: An updated meta-analysis on 2548 patients and quality-of-evidence review. Eur. Urol. 2013, 64, 421–430. [Google Scholar] [CrossRef]
- Tolley, D.A.; Parmar, M.K.; Grigor, K.M.; Lallemand, G.; Benyon, L.L.; Fellows, J.; Freedman, L.S.; Grigor, K.M.; Hall, R.R.; Hargreave, T.B.; et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: A further report with 7 years of follow up. J. Urol. 1996, 155, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Malmström, P.; Sylvester, R.J.U.; Crawford, D.E.; Friedrich, M.; Krege, S.; Rintala, E.; Solsona, E.; Di Stasi, S.M.; Witjes, J.A. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 2009, 56, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Shelley, M.D.; Kynaston, H.; Court, J.; Wilt, T.J.; Coles, B.; Burgon, K.; Mason, M.D. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001, 88, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Han, R.F.; Pan, J.G. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 2006, 67, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Shelley, M.D.; Wilt, T.J.; Court, J.; Coles, B.; Kynaston, H.; Mason, M.D. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004, 93, 485–490. [Google Scholar] [CrossRef]
- Böhle, A.; Jocham, D.; Bock, P.R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 2003, 169, 90–95. [Google Scholar] [CrossRef]
- Raj, G.V.; Herr, H.; Serio, A.M.; Donat, S.M.; Bochner, B.H.; Vickers, A.J.; Dalbagni, G. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J. Urol. 2007, 177, 1283–1286. [Google Scholar] [CrossRef]
- Bai, Y.; Mao, Q.Q.; Qin, J.; Zheng, X.Y.; Wang, Y.B.; Yang, K.; Shen, H.F.; Xie, L.P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Choi, B.Y.; Kundu, J.K.; Shin, Y.K.; Na, H.K.; Surh, Y.J. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: Eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009, 69, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- El-Mowafy, A.M.; El-Mesery, M.E.; Salem, H.A.; Al-Gayyar, M.M.; Darweish, M.M. Prominent chemopreventive and chemoenhancing effects for resveratrol: Unraveling molecular targets and the role of C-reactive protein. Chemotherapy 2010, 56, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Kenealey, J.D.; Subramanian, L.; Van Ginkel, P.R.; Darjatmoko, S.; Lindstrom, M.J.; Somoza, V.; Ghosh, S.K.; Song, Z.; Hsung, R.P.; Kwon, G.S.; et al. Resveratrol metabolites do not elicit early pro-apoptotic mechanisms in neuroblastoma cells. J. Agric. Food. Chem. 2011, 59, 4979–4986. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H.; Newman, J.W.; Pedersen, T.L.; Keen, C.L.; Ducore, J.M. Resveratrol given intraperitoneally does not inhibit the growth of high-risk t(4;11) acute lymphoblastic leukemia cells in a NOD/SCID mouse model. Int. J. Oncol. 2012, 40, 1277–1284. [Google Scholar] [CrossRef]
- Tseng, S.H.; Lin, S.M.; Chen, J.C.; Su, Y.H.; Huang, H.Y.; Chen, C.K.; Lin, P.Y.; Chen, Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef]
- Stocco, B.; Toledo, K.; Salvador, M.; Paulo, M.; Koyama, N.; Torqueti Toloi, M.R. Dose-dependent effect of resveratrol on bladder cancer cells: Chemoprevention and oxidative stress. Maturitas 2012, 72, 72–78. [Google Scholar] [CrossRef]
- Lin, X.; Wu, G.; Huo, W.Q.; Zhang, Y.; Jin, F.S. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int. J. Urol. 2012, 19, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Li, H.; Yu, L.J.; Chen, X.Y.; Kong, Q.Y.; Song, X.; Shu, X.H.; Liu, J. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS ONE 2014, 9, e89806. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Q.; Xie, Q.; Zhang, M. Effect and mechanism of resveratrol on drug resistance in human bladder cancer cells. Mol. Med. Rep. 2017, 15, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, C.; Li, H.; Wu, M.; Ren, C.; Zhen, Y.; Ma, X.; Diao, Y.; Ma, X.; Deng, S.; et al. Differential sensitivities of bladder cancer cell lines to resveratol are unrelated to its metabolic profile. Oncotarget 2017, 8, 40289–40304. [Google Scholar] [CrossRef]
- Almeida, T.C.; Guerra, C.C.C.; De Assis, B.L.G.; de Oliveira Aguiar Soares, R.D.; Garcia, C.C.M.; Lima, A.A.; da Silva, G.N. Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ. Mol. Mutagen. 2019, 60, 740–751. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Li, C.; Wang, S.; Zhu, M.; Wang, J.; Yue, H.; Ma, X.; Zhen, Y.; Shu, X. Metabolic profile and structure-activity relationship of resveratrol and its analogs in human bladder cancer cells. Cancer Manag. Res. 2019, 11, 4631–4642. [Google Scholar] [CrossRef]
- Zhou, C.; Ding, J.; Wu, Y. Resveratrol induces apoptosis of bladder cancer cells via miR 21 regulation of the Akt/Bcl 2 signaling pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Lançon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- Bhaskara, V.K.; Mittal, B.; Mysorekar, V.V.; Amaresh, N.; Simal-Gandara, J. Resveratrol, cancer and cancer stem cells: A review on past to future. Curr. Res. Food Sci. 2020, 3, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.G.; Landry, J.; Sternglanz, R.; Denu, J.M. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl. Acad. Sci. USA 2000, 97, 14178–14182. [Google Scholar] [CrossRef]
- Brachmann, C.B.; Sherman, J.M.; Devine, S.E.; Cameron, E.E.; Pillus, L.; Boeke, J.D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995, 9, 2888–2902. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins, aging, and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 81–90. [Google Scholar] [CrossRef]
- Jackson, M.D.; Denu, J.M. Structural identification of 2′- and 3′-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta -NAD+-dependent histone/protein deacetylases. J. Biol. Chem. 2002, 277, 18535–18544. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E.; CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xu, S.; Maitland-Toolan, K.A.; Sato, K.; Jiang, B.; Ido, Y.; Lan, F.; Walsh, K.; Wierzbicki, M.; Verbeuren, T.J.; et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMPactivated protein kinase. J. Biol. Chem. 2008, 283, 20015–20026. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- Hong, H.J.; Kang, W.; Kim, D.G.; Lee, D.H.; Lee, Y.; Han, C.H. Effects of resveratrol on the insulin signaling pathway of obese mice. J. Vet. Sci. 2014, 15, 179–185. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Yu, C.; Geun Shin, Y.; Chow, A.; Li, Y.; Kosmeder, J.W.; Sup Lee, Y.; Hirschelman, W.H.; Pezzuto, J.M.; Mehta, R.G.; Van Breemen, R.B. Human, Rat, And Mouse Metabolism Of Resveratrol. Pharm. Res. 2002, 19, 1907–1914. [Google Scholar] [CrossRef]
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Mérillon, J.-M. Cellular Uptake And Efflux Of Trans -Piceid And Its Aglycone Trans -Resveratrol On The Apical Membrane Of Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2005, 53, 798–803. [Google Scholar] [CrossRef]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The Bioavailability And Distribution Of Trans-Resveratrol Are Constrained By Abc Transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef]
- Böhmdorfer, M.; Szakmary, A.; Schiestl, R.; Vaquero, J.; Riha, J.; Brenner, S.; Thalhammer, T.; Szekeres, T.; Jäger, W. Involvement of UDP-Glucuronosyltransferases and Sulfotransferases in the Excretion and Tissue Distribution of Resveratrol in Mice. Nutrients 2017, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Brill, S.S.; Furimsky, A.M.; Ho, M.N.; Furniss, M.J.; Li, Y.; Green, A.G.; Bradford, W.W.; Green, C.E.; Kapetanovic, I.M.; Iyer, L.V. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J. Pharm. Pharmacol. 2006, 58, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Miksits, M.; Maier-Salamon, A.; Aust, S.; Thalhammer, T.; Reznicek, G.; Kunert, O.; Haslinger, E.; Szekeres, T.; Jaeger, W. Sulfation of resveratrol in human liver: Evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica 2005, 35, 1101–1119. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In Vivo And In Vitro Metabolism Of Trans-Resveratrol By Human Gut Microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-Da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Singh, S.K.; Makadia, V.; Sharma, S.; Rashid, M.; Shahi, S.; Mishra, P.R.; Wahajuddin, M.; Gayen, J.R. Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017, 7, 395–407. [Google Scholar] [CrossRef]
- Peñalva, R.; Morales, J.; González-Navarro, C.J.; Larrañeta, E.; Quincooces, G.; Peñuelas, I.; Irache, J. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef]
- Vasconcelos, T.; Araújo, F.; Lopes, C.; Loureiro, A.; Das Neves, J.; Marques, S.; Sarmento, B. Multicomponent self-nano emulsifying delivery systems of resveratrol with enhanced pharmacokinetics profile. Eur. J. Pharm. Sci. 2019, 137, 105011. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Xie, Y.; Liu, G.; Lu, Y.; Wu, W.; Chen, L. Oat protein-shellac nanoparticles as a delivery vehicle for resveratrol to improve bioavailability in vitro and in vivo. Nanomedicine 2019, 14, 2853–2871. [Google Scholar] [CrossRef]
- Santos, A.C.; Veiga, F.; Sequeira, J.A.D.; Fortuna, A.; Falcão, A.; Souto, E.B.; Pattekari, P.; Ribeiro, C.F.; Ribeiro, A.J. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats—A pharmacokinetics study. Analyst 2019, 144, 2062–2079. [Google Scholar] [CrossRef] [PubMed]
- Katekar, R.; Thombre, G.; Riyazuddin, M.; Husain, A.; Rani, H.; Praveena, K.S.; Gayen, J.R. Pharmacokinetics and brain targeting of trans-resveratrol loaded mixed micelles in rats following intravenous administration. Pharm. Dev. Technol. 2019, 25, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhong, C.; Deng, Y.; Zhang, Q.; Zhang, X.; Zhao, X. Resveratrol loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles for tail vein injection II: Pharmacokinetics, tissue distribution and bioavailability. Drug Deliv. 2020, 27, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Spogli, R.; Bastianini, M.; Ragonese, F.; Iannitti, R.G.; Monarca, L.; Bastioli, F.; Nakashidze, I.; Brecchia, G.; Menchetti, L.; Codini, M.; et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients 2018, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Floridi, A.; Lazzarini, A.; Tantucci, A.; Russo, R.; Ragonese, F.; Monarca, L.; Caglioti, C.; Spogli, R.; Leonardi, L.; et al. Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Represents an Oral Formulation of Resveratrol With Better Gastric Absorption and Bioavailability Respect to Pure Resveratrol. Front. Nutr. 2020, 7, 570047. [Google Scholar] [CrossRef]
- Cho, C.J.; Yang, C.W.; Wu, C.L.; Ho, J.Y.; Yu, C.P.; Wu, S.T.; Yu, D.S. The modulation study of multiple drug resistance in bladder cancer by curcumin and resveratrol. Oncol. Lett. 2019, 18, 6869–6876. [Google Scholar] [CrossRef]
- Cho, C.J.; Yu, C.P.; Wu, C.L.; Ho, J.Y.; Yang, C.W.; Yu, D.S. Decreased drug resistance of bladder cancer using phytochemicals treatment. Kaohsiung J. Med. Sci. 2021, 37, 128–135. [Google Scholar] [CrossRef]
- Alayev, A.; Salamon, R.S.; Schwartz, N.S.; Berman, A.Y.; Wiener, S.L.; Holz, M.K. Combination of Rapamycin and Resveratrol for Treatment of Bladder Cancer. J. Cell Physiol. 2017, 232, 436–446. [Google Scholar] [CrossRef]
- Soares, L.B.M.; Lima, A.P.B.; Melo, A.S.; Almeida, T.C.; de Medeiros Teixeira, L.F.; da Silva, G.N. Additive effects of resveratrol and doxorubicin on bladder cancer cells. Anticancer Drugs 2022, 33, e389–e397. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sincropi, M.S.; Puoci, F.; Casaburi, I.; Saturmino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381–1408. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Yin, H.B.; Li, X.Y.; Zhu, G.M.; He, W.Y.; Gou, X. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020, 56, 151–164. [Google Scholar] [CrossRef]
- Ohno, R.; Uozaki, H.; Kikuchi, Y.; Kumagai, A.; Aso, T.; Watanabe, M.; Watabe, S.; Muto, S.; Yamaguchi, R. Both cancerous miR-21 and stromal miR-21 in urothelial carcinoma are related to tumour progression. Histopathology 2016, 69, 993–999. [Google Scholar] [CrossRef]
- Wu, Z.; Zhou, Z.; Zhang, W.; Yu, Y. MiR-21-5p inhibition attenuates Warburg effect and stemness maintenance in osteosarcoma cells via inactivation of Wnt/β-catenin signaling. Acta Biochim. Pol. 2021, 68, 725–732. [Google Scholar] [CrossRef]
| Combination | Cell Line | Effect | Reference |
|---|---|---|---|
| Resveratrol + Gemcitabine | T24-GCB |
| [94] |
| Resveratrol + Rapamycin | TSC1-null MEFs WTMEFs 639V HCV29 MGH-U1 |
| [95] |
| Resveratrol + Adriamycin | Pumc-91 |
| [45] |
| Resveratrol + Doxorubicin | 5637 T24 |
| [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zucchi, A.; Claps, F.; Pastore, A.L.; Perotti, A.; Biagini, A.; Sallicandro, L.; Gentile, R.; Caglioti, C.; Palazzetti, F.; Fioretti, B. Focus on the Use of Resveratrol in Bladder Cancer. Int. J. Mol. Sci. 2023, 24, 4562. https://doi.org/10.3390/ijms24054562
Zucchi A, Claps F, Pastore AL, Perotti A, Biagini A, Sallicandro L, Gentile R, Caglioti C, Palazzetti F, Fioretti B. Focus on the Use of Resveratrol in Bladder Cancer. International Journal of Molecular Sciences. 2023; 24(5):4562. https://doi.org/10.3390/ijms24054562
Chicago/Turabian StyleZucchi, Alessandro, Francesco Claps, Antonio Luigi Pastore, Alessandro Perotti, Andrea Biagini, Luana Sallicandro, Rosaria Gentile, Concetta Caglioti, Federico Palazzetti, and Bernard Fioretti. 2023. "Focus on the Use of Resveratrol in Bladder Cancer" International Journal of Molecular Sciences 24, no. 5: 4562. https://doi.org/10.3390/ijms24054562
APA StyleZucchi, A., Claps, F., Pastore, A. L., Perotti, A., Biagini, A., Sallicandro, L., Gentile, R., Caglioti, C., Palazzetti, F., & Fioretti, B. (2023). Focus on the Use of Resveratrol in Bladder Cancer. International Journal of Molecular Sciences, 24(5), 4562. https://doi.org/10.3390/ijms24054562









