Vitamin D as a Shield against Aging
Abstract
1. Introduction
2. Aging Is a Cellular and Molecular Matter
2.1. The Cellular Route to Aging
2.2. Immunosenescence: The First Step toward Aging
3. Vitamin D and Geroprotection
3.1. Vitamin D against Infections
3.2. Vitamin D against Inflammaging, Cellular Senescence, and Mitochondrial Dysfunction
4. Vitamin D-Dependent NF-kB Regulation: A Putative Anti-Aging Strategy
Vitamin D Regulates Heart and Skeletal Muscle Functioning
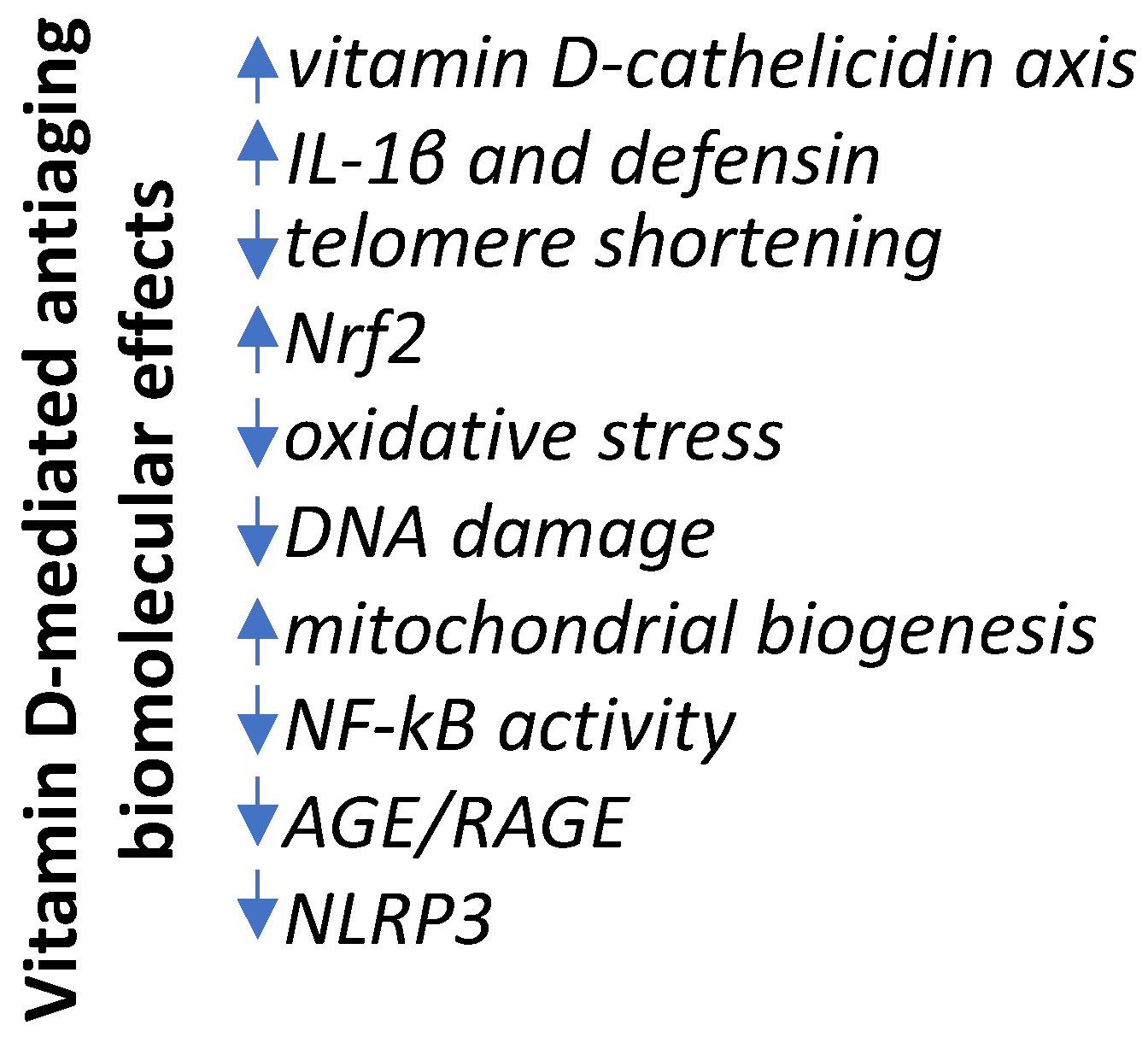
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirkwood, T.B.L. Why and how are we living longer?: Why and how are we living longer? Exp. Physiol. 2017, 102, 1067–1074. [Google Scholar] [CrossRef]
- Marsman, D.; Belsky, D.W.; Gregori, D.; Johnson, M.A.; Low Dog, T.; Meydani, S.; Pigat, S.; Sadana, R.; Shao, A.; Griffiths, J.C. Healthy ageing: The natural consequences of good nutrition—A conference report. Eur. J. Nutr. 2018, 57, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef]
- Johnson, A.A.; Shokhirev, M.N.; Wyss-Coray, T.; Lehallier, B. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res. Rev. 2020, 60, 101070. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Uthaiah, C.A.; Beeraka, N.M.; Rajalakshmi, R.; Ramya, C.M.; Madhunapantula, S.V. Role of Neural Stem Cells and Vitamin D Receptor (VDR)–Mediated Cellular Signaling in the Mitigation of Neurological Diseases. Mol. Neurobiol. 2022, 59, 4065–4105. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, A.; Choudhury, B.K.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S.; Karishma. Vitamin D and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 001–005. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Jie, Y.; Sun, Y.; Wang, X.; Fan, Y. Predictive value of 25-hydroxyvitamin D level in patients with coronary artery disease: A meta-analysis. Front. Nutr. 2022, 9, 984487. [Google Scholar] [CrossRef]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Ganmaa, D.; Enkhmaa, D.; Nasantogtokh, E.; Sukhbaatar, S.; Tumur-Ochir, K.; Manson, J. Vitamin D, respiratory infections, and chronic disease: Review of meta-analyses and randomized clinical trials. J. Intern. Med. 2022, 291, 141–164. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.-M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Livadas, S.; Goulis, D.G.; Bretz, S.; Ceausu, I.; Durmusoglu, F.; Erkkola, R.; Fistonic, I.; Gambacciani, M.; Geukes, M.; et al. EMAS position statement: Vitamin D and menopausal health. Maturitas 2023, 169, 2–9. [Google Scholar] [CrossRef]
- Bergman, P. Low vitamin D is a marker for poor health and increased risk for disease: But causality is still unclear in most cases. J. Intern. Med. 2023, 293, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, S.J. Articulating the Case for the Longevity Dividend. Cold Spring Harb. Perspect. Med. 2016, 6, a025940. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.-A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef]
- Whiting, C.C.; Siebert, J.; Newman, A.M.; Du, H.; Alizadeh, A.A.; Goronzy, J.; Weyand, C.M.; Krishnan, E.; Fathman, C.G.; Maecker, H.T. Large-Scale and Comprehensive Immune Profiling and Functional Analysis of Normal Human Aging. PLoS ONE 2015, 10, e0133627. [Google Scholar] [CrossRef]
- Duggal, N.A.; Niemiro, G.; Harridge, S.D.R.; Simpson, R.J.; Lord, J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat. Rev. Immunol. 2019, 19, 563–572. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2006, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Qi, Q.; Olshen, R.A.; Weyand, C.M. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.; Cheng, Y.; Glanville, J.; Zhang, D.; Lee, J.-Y.; Olshen, R.A.; Weyand, C.M.; Boyd, S.D.; Goronzy, J.J. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 13139–13144. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fang, F.; Cavanagh, M.M.; Qi, Q.; Weyand, C.M. Naive T Cell Maintenance and Function in Human Aging. J. Immunol. 2015, 194, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive Cell Growth Causes Cytoplasm Dilution And Contributes to Senescence. Cell 2019, 176, 1083–1097.E18. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Faget, D.V.; Ren, Q.; Stewart, S.A. Unmasking senescence: Context-dependent effects of SASP in cancer. Nat. Rev. Cancer 2019, 19, 439–453. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Wiley, C.D.; Flynn, J.M.; Morrissey, C.; Lebofsky, R.; Shuga, J.; Dong, X.; Unger, M.A.; Vijg, J.; Melov, S.; Campisi, J. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell 2017, 16, 1043–1050. [Google Scholar] [CrossRef]
- Pae, M.; Meydani, S.N.; Wu, D. The role of nutrition in enhancing immunity in aging. Aging Dis. 2012, 3, 91–129. [Google Scholar]
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Fisher, P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006, 236, 13–23. [Google Scholar] [CrossRef]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Shao, L.; Weyand, C.M. Immune Aging and Rheumatoid Arthritis. Rheum. Dis. Clin. North Am. 2010, 36, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Li, G.; Yang, Z.; Weyand, C.M. The Janus Head of T Cell Aging—Autoimmunity and Immunodeficiency. Front. Immunol. 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Yang, Z.; Goronzy, J.J. T-cell aging in rheumatoid arthritis. Curr. Opin. Rheumatol. 2014, 26, 93–100. [Google Scholar] [CrossRef]
- Smith, K.A. Interleukin-2: Inception, Impact, and Implications. Science 1988, 240, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.; Rink, L.; Uciechowski, P. The Th17/Treg balance is disturbed during aging. Exp. Gerontol. 2013, 48, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wei, C.; Chen, Y.; Wu, Y.; Shou, X.; Chen, W.; Lu, D.; Sun, H.; Li, W.; Yu, B.; et al. IL-33 induces thymic involution-associated naive T cell aging and impairs host control of severe infection. Nat. Commun. 2022, 13, 6881. [Google Scholar] [CrossRef] [PubMed]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef]
- Pahlavani, M.A. T cell signaling effect of age. Front. Biosci. 1998, 3, 1120–1133. [Google Scholar] [CrossRef]
- Kirk, C.J.; Miller, R.A. Analysis of Raf-1 Activation in Response to TCR Activation and Costimulation in Murine T-Lymphocytes: Effect of Age. Cell. Immunol. 1998, 190, 33–42. [Google Scholar] [CrossRef]
- Pahlavani, M.A.; Harris, M.D.; Richardson, A. Activation of p21ras/MAPK Signal Transduction Molecules Decreases with Age in Mitogen-Stimulated T Cells from Rats. Cell. Immunol. 1998, 185, 39–48. [Google Scholar] [CrossRef]
- Pahlavani, M.A.; Harris, M.D.; Richardson, A. The Age-Related Decline in the Induction of IL-2 Transcription Is Correlated to Changes in the Transcription Factor NFAT. Cell. Immunol. 1995, 165, 84–91. [Google Scholar] [CrossRef]
- Whisler, R.L.; Beiqing, L.; Chen, M. Age-Related Decreases in IL-2 Production by Human T Cells Are Associated with Impaired Activation of Nuclear Transcriptional Factors AP-1 and NF-AT. Cell. Immunol. 1996, 169, 185–195. [Google Scholar] [CrossRef]
- Whisler, R.L.; Chen, M.; Beiqing, L.; Carle, K.W. Impaired Induction of c-fos/c-jun Genes and of Transcriptional Regulatory Proteins Binding Distinct c-fos/c-jun Promoter Elements in Activated Human T Cells during Aging. Cell. Immunol. 1997, 175, 41–50. [Google Scholar] [CrossRef]
- Trebilcock, G.U.; Ponnappan, U. Evidence for Lowered Induction of Nuclear Factor Kappa B in Activated Human T Lymphocytes during Aging. Gerontology 1996, 42, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Trebilcock, G.U.; Ponnappan, U. Nuclear factor-κB induction in CD45RO+ and CD45RA+ T cell subsets during aging. Mech. Ageing Dev. 1998, 102, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Claycombe, K.J.; Wu, D.; Nikolova-Karakashian, M.; Palmer, H.; Beharka, A.; Paulson, K.E.; Meydani, S.N. Ceramide Mediates Age-associated Increase in Macrophage Cyclooxygenase-2 Expression. J. Biol. Chem. 2002, 277, 30784–30791. [Google Scholar] [CrossRef] [PubMed]
- Hayek, M.G.; Mura, C.; Wu, D.; Beharka, A.A.; Han, S.N.; Paulson, K.E.; Hwang, D.; Meydani, S.N. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J. Immunol. 1997, 159, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Marko, M.; Claycombe, K.; Paulson, K.E.; Meydani, S.N. Ceramide-induced and Age-associated Increase in Macrophage COX-2 Expression Is Mediated through Up-regulation of NF-κB Activity. J. Biol. Chem. 2003, 278, 10983–10992. [Google Scholar] [CrossRef]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef]
- Panda, A.; Arjona, A.; Sapey, E.; Bai, F.; Fikrig, E.; Montgomery, R.R.; Lord, J.M.; Shaw, A.C. Human innate immunosenescence: Causes and consequences for immunity in old age. Trends Immunol. 2009, 30, 325–333. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M. NK and NKT cell functions in immunosenescence. Aging Cell 2004, 3, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; De la Fuente, M. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016, 158, 27–37. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef]
- Pawelec, G. Does the human immune system ever really become “senescent”? F1000Research 2017, 6, 1323. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ortega, E.F.; Meydani, S.N.; Adkins, Y.; Stephensen, C.B.; Thompson, B.; Zwickey, H. Nutrition, Immunosenescence, and Infectious Disease: An Overview of the Scientific Evidence on Micronutrients and on Modulation of the Gut Microbiota. Adv. Nutr. 2022, 13, S1–S26. [Google Scholar] [CrossRef]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun. Rev. 2011, 11, 84–87. [Google Scholar] [CrossRef]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Holick, M.F.; Matsuoka, L.Y.; Wortsman, J. Age, vitamin D, and solar ultraviolet. Lancet 1989, 334, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.-H.W.; Baylink, D.J. Vitamin D Therapy of Osteoporosis: Plain Vitamin D Therapy Versus Active Vitamin D Analog (D-Hormone) Therapy. Calcif. Tissue Int. 1999, 65, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, L. Vitamin D and the elderly. Clin. Endocrinol. 2005, 62, 265–281. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D Deficiency and Secondary Hyperparathyroidism in the Elderly: Consequences for Bone Loss and Fractures and Therapeutic Implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lovegrove, J.A.; Givens, D.I. 25(OH)D3-enriched or fortified foods are more efficient at tackling inadequate vitamin D status than vitamin D3. Proc. Nutr. Soc. 2018, 77, 282–291. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Hewison, M. An update on vitamin D and human immunity: An update on vitamin D. Clin. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator: Vitamin D3 as immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef]
- Cantorna, M.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Medrano, M.; Carrillo-Cruz, E.; Montero, I.; Perez-Simon, J. Vitamin D: Effect on Haematopoiesis and Immune System and Clinical Applications. IJMS 2018, 19, 2663. [Google Scholar] [CrossRef]
- Prietl, B.; Treiber, G.; Pieber, T.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Fritsche, J.; Mondal, K.; Ehrnsperger, A.; Andreesen, R.; Kreutz, M. Regulation of 25-hydroxyvitamin D3-1α-hydroxylase and production of 1α,25-dihydroxyvitamin D3 by human dendritic cells. Blood 2003, 102, 3314–3316. [Google Scholar] [CrossRef]
- Gottfried, E.; Rehli, M.; Hahn, J.; Holler, E.; Andreesen, R.; Kreutz, M. Monocyte-derived cells express CYP27A1 and convert vitamin D3 into its active metabolite. Biochem. Biophys. Res. Commun. 2006, 349, 209–213. [Google Scholar] [CrossRef]
- Korf, H.; Decallonne, B.; Mathieu, C. Vitamin D for infections. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 431–436. [Google Scholar] [CrossRef]
- Shin, D.-M.; Jo, E.-K. Antimicrobial Peptides in Innate Immunity against Mycobacteria. Immune Netw. 2011, 11, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bellmann-Weiler, R.; Weiss, G. Pitfalls in the Diagnosis and Therapy of Infections in Elderly Patients—A Mini-Review. Gerontology 2009, 55, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Mily, A.; Sultana, T.; Haq, A.; Ahmed, S.; Mostafa Kamal, S.M.; van Schadewijk, A.; Hiemstra, P.S.; Gudmundsson, G.H.; Agerberth, B.; et al. Immune responses in the treatment of drug-sensitive pulmonary tuberculosis with phenylbutyrate and vitamin D3 as host directed therapy. BMC Infect. Dis. 2018, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J.; Garrett, J.; Camargo, C.A.; Scragg, R.; Vandal, A.; Sisk, R.; Milne, D.; Tai, R.; Jeon, G.; Cursons, R.; et al. Vitamin D3 supplementation in adults with bronchiectasis: A pilot study. Chronic Respir. Dis. 2018, 15, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Akhtar, E.; Sarker, P.; Rahim, Z.; Gudmundsson, G.H.; Agerberth, B.; Raqib, R. Oral intake of phenylbutyrate with or without vitamin D3upregulates the cathelicidin LL-37 in human macrophages: A dose finding study for treatment of tuberculosis. BMC Pulm. Med. 2013, 13, 23. [Google Scholar] [CrossRef]
- Fabri, M.; Stenger, S.; Shin, D.-M.; Yuk, J.-M.; Liu, P.T.; Realegeno, S.; Lee, H.-M.; Krutzik, S.R.; Schenk, M.; Sieling, P.A.; et al. Vitamin D Is Required for IFN-γ–Mediated Antimicrobial Activity of Human Macrophages. Sci. Transl. Med. 2011, 3, 104ra102. [Google Scholar] [CrossRef]
- Chung, C.; Silwal, P.; Kim, I.; Modlin, R.L.; Jo, E.-K. Vitamin D-Cathelicidin Axis: At the Crossroads between Protective Immunity and Pathological Inflammation during Infection. Immune Netw. 2020, 20, e12. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Toll-Like Receptor 8 Ligands Activate a Vitamin D Mediated Autophagic Response that Inhibits Human Immunodeficiency Virus Type 1. PLoS Pathog. 2012, 8, e1003017. [Google Scholar] [CrossRef]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Klug-Micu, G.M.; Stenger, S.; Sommer, A.; Liu, P.T.; Krutzik, S.R.; Modlin, R.L.; Fabri, M. CD40 ligand and interferon-γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 2013, 139, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Rekha, R.S.; Rao Muvva, S.J.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Shin, D.-M.; Lee, H.-M.; Yang, C.-S.; Jin, H.S.; Kim, K.-K.; Lee, Z.-W.; Lee, S.-H.; Kim, J.-M.; Jo, E.-K. Vitamin D3 Induces Autophagy in Human Monocytes/Macrophages via Cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef]
- Verway, M.; Bouttier, M.; Wang, T.-T.; Carrier, M.; Calderon, M.; An, B.-S.; Devemy, E.; McIntosh, F.; Divangahi, M.; Behr, M.A.; et al. Vitamin D Induces Interleukin-1β Expression: Paracrine Macrophage Epithelial Signaling Controls M. tuberculosis Infection. PLoS Pathog. 2013, 9, e1003407. [Google Scholar] [CrossRef]
- Dimitrov, V.; White, J.H. Species-specific regulation of innate immunity by vitamin D signaling. J. Steroid Biochem. Mol. Biol. 2016, 164, 246–253. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Role of Vitamin D in the Hygiene Hypothesis: The Interplay between Vitamin D, Vitamin D Receptors, Gut Microbiota, and Immune Response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-S.; Shin, D.-M.; Kim, K.-H.; Lee, Z.-W.; Lee, C.-H.; Park, S.G.; Bae, Y.S.; Jo, E.-K. NADPH Oxidase 2 Interaction with TLR2 Is Required for Efficient Innate Immune Responses to Mycobacteria via Cathelicidin Expression. J. Immunol. 2009, 182, 3696–3705. [Google Scholar] [CrossRef]
- Yan, Y.; Finkel, T. Autophagy as a regulator of cardiovascular redox homeostasis. Free. Radic. Biol. Med. 2017, 109, 108–113. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.Y.; Pena, O.M.; Hancock, R.E.W. Therapeutic Potential of Host Defense Peptides in Antibiotic-resistant Infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Hilbring, C.; Augustin, M.; Kirsten, N.; Mohr, N. Epidemiology of rosacea in a population-based study of 161,269 German employees. Int. J. Dermatol. 2022, 61, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Ha, J.M.; Cho, E.B.; Jin, J.K.; Park, E.J.; Park, H.R.; Kang, H.J.; Ko, S.H.; Kim, K.H.; Kim, K.J. A Study on Vitamin D and Cathelicidin Status in Patients with Rosacea: Serum Level and Tissue Expression. Ann. Dermatol. 2018, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Rigby, W.F.; Stacy, T.; Fanger, M.W. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J. Clin. Investig. 1984, 74, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune Modulatory Treatment of Trinitrobenzene Sulfonic Acid Colitis with Calcitriol Is Associated with a Change of a T Helper (Th) 1/Th17 to a Th2 and Regulatory T Cell Profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1α,25-Dihydroxyvitamin D3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; O’Garra, A. 1α,25-Dihydroxyvitamin D3 Has a Direct Effect on Naive CD4+ T Cells to Enhance the Development of Th2 Cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef]
- Gorman, S.; Kuritzky, L.A.; Judge, M.A.; Dixon, K.M.; McGlade, J.P.; Mason, R.S.; Finlay-Jones, J.J.; Hart, P.H. Topically Applied 1,25-Dihydroxyvitamin D3 Enhances the Suppressive Activity of CD4+CD25+ Cells in the Draining Lymph Nodes. J. Immunol. 2007, 179, 6273–6283. [Google Scholar] [CrossRef]
- Hamzaoui, A.; Berraïes, A.; Hamdi, B.; Kaabachi, W.; Ammar, J.; Hamzaoui, K. Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology 2014, 219, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Berti, E.; Colonna, M.; Adorini, L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood 2005, 106, 3490–3497. [Google Scholar] [CrossRef]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 1137–1142. [Google Scholar] [CrossRef]
- Fletcher, J.; Bishop, E.L.; Harrison, S.R.; Swift, A.; Cooper, S.C.; Dimeloe, S.K.; Raza, K.; Hewison, M. Autoimmune disease and interconnections with vitamin D. Endocr. Connect. 2022, 11, e210554. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Ginaldi, L.; De Martinis, M. The IL-33/ST2 axis and vitamin D as a possible emerging therapeutic target in osteoarthritis. Rheumatology 2021, 60, e300. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Pioggia, G.; Gangemi, S. IL-33/Vitamin D Crosstalk in Psoriasis-Associated Osteoporosis. Front. Immunol. 2020, 11, 604055. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Guillain-Barré syndrome, the IL-33/ST2 axis, and vitamin D. Eur. J. Neurol. 2022, 29, e20–e21. [Google Scholar] [CrossRef]
- Facciotti, F.; Gagliani, N.; Häringer, B.; Alfen, J.S.; Penatti, A.; Maglie, S.; Paroni, M.; Iseppon, A.; Moro, M.; Crosti, M.C.; et al. IL-10–producing forkhead box protein 3–negative regulatory T cells inhibit B-cell responses and are involved in systemic lupus erythematosus. J. Allergy Clin. Immunol. 2016, 137, 318–321.E5. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef]
- van der Wielen, R.P.J.; de Groot, L.C.P.G.M.; van Staveren, W.A.; Löwik, M.R.H.; van den Berg, H.; Haller, J.; Moreiras, O. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef]
- Oliveri, B.; Plantalech, L.; Bagur, A.; Wittich, A.C.; Rovai, G.; Pusiol, E.; Giovanelli, J.L.; Ponce, G.; Nieva, A.; Chaperón, A.; et al. High prevalence of vitamin D insufficiency in healthy elderly people living at home in Argentina. Eur. J. Clin. Nutr. 2004, 58, 337–342. [Google Scholar] [CrossRef]
- Portela, M.L.P.M.; Mónico, A.; Barahona, A.; Dupraz, H.; Sol Gonzales-Chaves, M.M.; Zeni, S.N. Comparative 25-OH-vitamin D level in institutionalized women older than 65 years from two cities in Spain and Argentina having a similar solar radiation index. Nutrition 2010, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.M.; Abo El-Khair, S.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Satilmis, S.; Celik, O.; Biyik, I.; Ozturk, D.; Celik, K.; Akın, F.; Ayca, B.; Yalcin, B.; Dagdelen, S. Association between serum vitamin D levels and subclinical coronary atherosclerosis and plaque burden/composition in young adult population. Bosn. J. Basic Med Sci. 2015, 15, 67–72. [Google Scholar] [CrossRef]
- Oh, J.; Weng, S.; Felton, S.K.; Bhandare, S.; Riek, A.; Butler, B.; Proctor, B.M.; Petty, M.; Chen, Z.; Schechtman, K.B.; et al. 1,25(OH)2 Vitamin D Inhibits Foam Cell Formation and Suppresses Macrophage Cholesterol Uptake in Patients With Type 2 Diabetes Mellitus. Circulation 2009, 120, 687–698. [Google Scholar] [CrossRef]
- Katsuumi, G.; Shimizu, I.; Yoshida, Y.; Minamino, T. Vascular Senescence in Cardiovascular and Metabolic Diseases. Front. Cardiovasc. Med. 2018, 5, 18. [Google Scholar] [CrossRef]
- Chen, L.; Yang, R.; Qiao, W.; Zhang, W.; Chen, J.; Mao, L.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D exerts an antiaging role by activation of Nrf2-antioxidant signaling and inactivation of p16/p53-senescence signaling. Aging Cell 2019, 18, e12951. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.-Q.; Yang, Z.; Fan, D.; Tang, Q.-Z. BMI1 in the heart: Novel functions beyond tumorigenesis. eBioMedicine 2021, 63, 103193. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D deficiency accelerates ageing and age-related diseases: A novel hypothesis: Vitamin D deficiency and ageing. J. Physiol. 2017, 595, 6825–6836. [Google Scholar] [CrossRef] [PubMed]
- Scaini, G.; Rezin, G.T.; Carvalho, A.F.; Streck, E.L.; Berk, M.; Quevedo, J. Mitochondrial dysfunction in bipolar disorder: Evidence, pathophysiology and translational implications. Neurosci. Biobehav. Rev. 2016, 68, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci. 2020, 10, 660. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease: Mechanisms of cardiovascular ageing. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Fernandez-Medarde, A.; Santos, E. Ras in Cancer and Developmental Diseases. Genes Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D Inhibits COX-2 Expression and Inflammatory Response by Targeting Thioesterase Superfamily Member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef]
- Songkiatisak, P.; Rahman, S.M.T.; Aqdas, M.; Sung, M.-H. NF-κB, a culprit of both inflamm-ageing and declining immunity? Immun. Ageing 2022, 19, 20. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; O’Loghlen, A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020, 30, 628–639. [Google Scholar] [CrossRef]
- Jiang, X.; Takahashi, N.; Matsui, N.; Tetsuka, T.; Okamoto, T. The NF-κB Activation in Lymphotoxin β Receptor Signaling Depends on the Phosphorylation of p65 at Serine 536. J. Biol. Chem. 2003, 278, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Purkayastha, S.; Tang, Y.; Zhang, H.; Yin, Y.; Li, B.; Liu, G.; Cai, D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013, 497, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Thoma, A.; Lightfoot, A.P. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. In Muscle Atrophy; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1088, pp. 267–279. ISBN 9789811314346. [Google Scholar]
- Mousa, A.; Naderpoor, N.; Johnson, J.; Sourris, K.; de Courten, M.P.J.; Wilson, K.; Scragg, R.; Plebanski, M.; de Courten, B. Effect of vitamin D supplementation on inflammation and nuclear factor kappa-B activity in overweight/obese adults: A randomized placebo-controlled trial. Sci. Rep. 2017, 7, 15154. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κB and the immune response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [PubMed]
- García-García, V.A.; Alameda, J.P.; Page, A.; Casanova, M.L. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-κB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. NF-κB Signaling in the Aging Process. J. Clin. Immunol. 2009, 29, 397–405. [Google Scholar] [CrossRef]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef]
- Petroulakis, E.; Mamane, Y.; Le Bacquer, O.; Shahbazian, D.; Sonenberg, N. mTOR signaling: Implications for cancer and anticancer therapy. Br. J. Cancer 2006, 94, 195–199. [Google Scholar] [CrossRef]
- Antinozzi, C.; Marampon, F.; Corinaldesi, C.; Vicini, E.; Sgrò, P.; Vannelli, G.B.; Lenzi, A.; Crescioli, C.; Di Luigi, L. Testosterone insulin-like effects: An in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells. J. Endocrinol. Investig. 2017, 40, 1133–1143. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Ju, Z.; Djojosubroto, M.W.; Schienke, A.; Lechel, A.; Schaetzlein, S.; Jiang, H.; Stepczynska, A.; Wang, C.; Buer, J.; et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 2007, 39, 99–105. [Google Scholar] [CrossRef]
- Lee, W.-P.; Hou, M.-C.; Lan, K.-H.; Li, C.-P.; Chao, Y.; Lin, H.-C.; Lee, S.-D. Helicobacter pylori-induced chronic inflammation causes telomere shortening of gastric mucosa by promoting PARP-1-mediated non-homologous end joining of DNA. Arch. Biochem. Biophys. 2016, 606, 90–98. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y.; Li, W.; Cheng, Y.; Li, X.; Huang, C.; Meng, X.; Wu, B.; Liu, X.; Zhang, L.; et al. Telomerase reverse transcriptase acts in a feedback loop with NF-κB pathway to regulate macrophage polarization in alcoholic liver disease. Sci. Rep. 2016, 6, 18685. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.L.A.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.L.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 Links Histone H3 Lysine 9 Deacetylation to NF-κB-Dependent Gene Expression and Organismal Life Span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef]
- Cai, D.; Frantz, J.D.; Tawa, N.E.; Melendez, P.A.; Oh, B.-C.; Lidov, H.G.W.; Hasselgren, P.-O.; Frontera, W.R.; Lee, J.; Glass, D.J.; et al. IKKβ/NF-κB Activation Causes Severe Muscle Wasting in Mice. Cell 2004, 119, 285–298. [Google Scholar] [CrossRef]
- Liu, Y.; Drozdov, I.; Shroff, R.; Beltran, L.E.; Shanahan, C.M. Prelamin A Accelerates Vascular Calcification Via Activation of the DNA Damage Response and Senescence-Associated Secretory Phenotype in Vascular Smooth Muscle Cells. Circ. Res. 2013, 112, e99–e109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, K.; Robinson, A.R.; Clauson, C.L.; Blair, H.C.; Robbins, P.D.; Niedernhofer, L.J.; Ouyang, H. DNA damage drives accelerated bone aging via an NF-κB-dependent mechanism. J. Bone Miner. Res. 2013, 28, 1214–1228. [Google Scholar] [CrossRef]
- Ghosh, A.; Roy, A.; Liu, X.; Kordower, J.H.; Mufson, E.J.; Hartley, D.M.; Ghosh, S.; Mosley, R.L.; Gendelman, H.E.; Pahan, K. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 18754–18759. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.J.; Choi, D.-Y.; Park, M.H.; Hong, J.T. NF-κB as a Key Mediator of Brain Inflammation in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor κB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Geldmeyer-Hilt, K.; Heine, G.; Hartmann, B.; Baumgrass, R.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem. Biophys. Res. Commun. 2011, 407, 699–702. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sottili, M.; Antinozzi, C.; Vannelli, G.B.; Romanelli, F.; Riccieri, V.; Valesini, G.; Lenzi, A.; Crescioli, C. The Vitamin D Receptor Agonist BXL-01-0029 as a Potential New Pharmacological Tool for the Treatment of Inflammatory Myopathies. PLoS ONE 2013, 8, e77745. [Google Scholar] [CrossRef]
- Sottili, M.; Cosmi, L.; Borgogni, E.; Sarchielli, E.; Maggi, L.; Francalanci, M.; Vannelli, G.B.; Ronconi, E.; Adorini, L.; Annunziato, F.; et al. Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp. Cell Res. 2009, 315, 264–273. [Google Scholar] [CrossRef]
- Borgogni, E.; Sarchielli, E.; Sottili, M.; Santarlasci, V.; Cosmi, L.; Gelmini, S.; Lombardi, A.; Cantini, G.; Perigli, G.; Luconi, M.; et al. Elocalcitol Inhibits Inflammatory Responses in Human Thyroid Cells and T Cells. Endocrinology 2008, 149, 3626–3634. [Google Scholar] [CrossRef]
- Zhang, C.; Tong, T.; Miao, D.; Wang, L. Vitamin D inhibits TNF-α induced apoptosis of human nucleus pulposus cells through regulation of NF-kB signaling pathway. J. Orthop. Surg. Res. 2021, 16, 411. [Google Scholar] [CrossRef]
- Anversa, P.; Leri, A.; Kajstura, J. Cardiac Regeneration. J. Am. Coll. Cardiol. 2006, 47, 1769–1776. [Google Scholar] [CrossRef]
- Aslan, E.; Komurcu, N.; Beji, N.K.; Yalcin, O. Bladder Training and Kegel Exercises for Women with Urinary Complaints Living in a Rest Home. Gerontology 2008, 54, 224–231. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in autophagy signaling for health and diseases: Insights on potential mechanisms and future perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Li, W.; Che, X.; Chen, X.; Zhou, M.; Luo, X.; Liu, T. Study of calcitriol anti-aging effects on human natural killer cells in vitro. Bioengineered 2021, 12, 6844–6854. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Arefi, S.; Sadri, M.; Delbandi, A.-A. Effect of active vitamin D on proliferation, cell cycle and apoptosis in endometriotic stromal cells. Reprod. Biomed. Online 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, D.; Yongyut, P.; Li, G.; Esteban, M.Á.; Jintasataporn, O.; Deng, J.; Zhang, W.; Ai, Q.; Mai, K.; et al. Vitamin D3 deficiency induced intestinal inflammatory response of turbot through nuclear factor-κB/inflammasome pathway, accompanied by the mutually exclusive apoptosis and autophagy. Front. Immunol. 2022, 13, 986593. [Google Scholar] [CrossRef]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-Z.; Chen, Z.-C.; Wang, S.-S.; Liu, W.-B.; Zhao, C.-L.; Zhuang, X.-D. NLRP3 inflammasome activation contributes to the pathogenesis of cardiocytes aging. Aging 2021, 13, 20534–20551. [Google Scholar] [CrossRef]
- Filardi, T.; Ghinassi, B.; Di Baldassarre, A.; Tanzilli, G.; Morano, S.; Lenzi, A.; Basili, S.; Crescioli, C. Cardiomyopathy Associated with Diabetes: The Central Role of the Cardiomyocyte. IJMS 2019, 20, 3299. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Palm, N.W.; Zhu, S.; Flavell, R.A. Inflammasomes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016287. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Lopez-Castejon, G.; Blandizzi, C.; Fornai, M. Canonical and Non-Canonical Activation of NLRP3 Inflammasome at the Crossroad between Immune Tolerance and Intestinal Inflammation. Front. Immunol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Sebastian-Valverde, M.; Pasinetti, G.M. The NLRP3 Inflammasome as a Critical Actor in the Inflammaging Process. Cells 2020, 9, 1552. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, X.; Wu, J.; Xiao, M.; Zhang, J.; Wang, B.; Fang, L.; Zhang, H.; Wang, X.; Yang, S.; et al. Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination. Front. Immunol. 2019, 10, 2783. [Google Scholar] [CrossRef]
- Cao, R.; Ma, Y.; Li, S.; Shen, D.; Yang, S.; Wang, X.; Cao, Y.; Wang, Z.; Wei, Y.; Li, S.; et al. 1,25(OH)2D3 alleviates DSS-induced ulcerative colitis via inhibiting NLRP3 inflammasome activation. J. Leukoc. Biol. 2020, 108, 283–295. [Google Scholar] [CrossRef]
- Tate, M.D.; Mansell, A. An update on the NLRP3 inflammasome and influenza: The road to redemption or perdition? Curr. Opin. Immunol. 2018, 54, 80–85. [Google Scholar] [CrossRef]
- Crescioli, C. Vitamin D, exercise, and immune health in athletes: A narrative review. Front. Immunol. 2022, 13, 954994. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Leeuwenburgh, C.; Coelho-Junior, H.J.; Bernabei, R.; Landi, F.; Marzetti, E. Targeting mitochondrial quality control for treating sarcopenia: Lessons from physical exercise. Expert Opin. Ther. Targets 2019, 23, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef] [PubMed]
- Zembron-Lacny, A.; Dziubek, W.; Wolny-Rokicka, E.; Dabrowska, G.; Wozniewski, M. The Relation of Inflammaging With Skeletal Muscle Properties in Elderly Men. Am. J. Men's Health 2019, 13, 155798831984193. [Google Scholar] [CrossRef]
- Brown, E.C.; DiSilvestro, R.A.; Babaknia, A.; Devor, S.T. Soy versus whey protein bars: Effects on exercise training impact on lean body mass and antioxidant status. Nutr. J. 2004, 3, 22. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The Chronic Effects of Whey Proteins on Blood Pressure, Vascular Function, and Inflammatory Markers in Overweight Individuals. Obesity 2010, 18, 1354–1359. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women. Br. J. Nutr. 2011, 105, 1512–1519. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Rössler, K.; Thorp, R.M.; Graham, D.F.; Timmons, B.W.; Stannard, S.R.; Tarnopolsky, M.A. Effect of dietary protein content during recovery from high-intensity cycling on subsequent performance and markers of stress, inflammation, and muscle damage in well-trained men. Appl. Physiol. Nutr. Metab. 2008, 33, 39–51. [Google Scholar] [CrossRef]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired proteostasis during skeletal muscle aging. Free. Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef]
- Guillet, C.; Zangarelli, A.; Gachon, P.; Morio, B.; Giraudet, C.; Rousset, P.; Boirie, Y. Whole Body Protein Breakdown Is Less Inhibited by Insulin, But Still Responsive to Amino Acid, in Nondiabetic Elderly Subjects. J. Clin. Endocrinol. Metab. 2004, 89, 6017–6024. [Google Scholar] [CrossRef]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef]
- Wilkes, E.A.; Selby, A.L.; Atherton, P.J.; Patel, R.; Rankin, D.; Smith, K.; Rennie, M.J. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 2009, 90, 1343–1350. [Google Scholar] [CrossRef]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Verstuyf, A. Vitamin D, Mitochondria, and Muscle. J. Clin. Endocrinol. Metab. 2013, 98, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Ceglia, L.; Harris, S.S. Vitamin D and Its Role in Skeletal Muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef]
- Lin, A.M.Y. Antioxidative Effect of Vitamin D3 on Zinc-Induced Oxidative Stress in CNS. Ann. N. Y. Acad. Sci. 2005, 1053, 319–329. [Google Scholar] [CrossRef]
- Nakai, K.; Fujii, H.; Kono, K.; Goto, S.; Kitazawa, R.; Kitazawa, S.; Hirata, M.; Shinohara, M.; Fukagawa, M.; Nishi, S. Vitamin D Activates the Nrf2-Keap1 Antioxidant Pathway and Ameliorates Nephropathy in Diabetic Rats. Am. J. Hypertens. 2014, 27, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tokunaga, K.; Komaba, H.; Itoh, K.; Matsushita, K.; Watanabe, H.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Fukagawa, M. Vitamin D Receptor Activator Reduces Oxidative Stress in Hemodialysis Patients With Secondary Hyperparathyroidism: VDRA and Oxidative Stress. Ther. Apher. Dial. 2011, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Srikuea, R.; Hirunsai, M. Effects of intramuscular administration of 1α,25(OH)2D3 during skeletal muscle regeneration on regenerative capacity, muscular fibrosis, and angiogenesis. J. Appl. Physiol. 2016, 120, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Lundell, L.S.; Perry, B.D.; Patil, K.V.; Chibalin, A.V.; Levinger, I.; McQuade, L.R.; Stepto, N.K. Effect of N-acetylcysteine infusion on exercise-induced modulation of insulin sensitivity and signaling pathways in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E388–E397. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Blank, S.; Scanlon, K.S.; Sinks, T.H.; Lett, S.; Falk, H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. Am. J. Public Health 1995, 85, 656–659. [Google Scholar] [CrossRef]
- Scanlon, K.S.; Blank, S.; Sinks, T.; Lett, S.; Mueller, P.; Freedman, D.S.; Serdula, M.; Falk, H. Subclinical health effects in a population exposed to excess vitamin D in milk. Am. J. Public Health 1995, 85, 1418–1422. [Google Scholar] [CrossRef]
- Holick, M.F.; Shao, Q.; Liu, W.W.; Chen, T.C. The vitamin D content of fortified milk and infant formula. N. Engl. J. Med. 1992, 326, 1178–1181. [Google Scholar] [CrossRef]
- Larsen, E.R.; Mosekilde, L.; Foldspang, A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: A pragmatic population-based 3-year intervention study. J. Bone Miner. Res. 2004, 19, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; de Godoi Rezende Costa Molino, C.; Rival, S.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Kanis, J.A.; Manson, J.E.; Dawson-Hughes, B.; Orav, E.J.; et al. DO-HEALTH: Vitamin D3-Omega-3—Home exercise—Healthy aging and longevity trial—Design of a multinational clinical trial on healthy aging among European seniors. Contemp. Clin. Trials 2021, 100, 106124. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. https://doi.org/10.3390/ijms24054546
Fantini C, Corinaldesi C, Lenzi A, Migliaccio S, Crescioli C. Vitamin D as a Shield against Aging. International Journal of Molecular Sciences. 2023; 24(5):4546. https://doi.org/10.3390/ijms24054546
Chicago/Turabian StyleFantini, Cristina, Clarissa Corinaldesi, Andrea Lenzi, Silvia Migliaccio, and Clara Crescioli. 2023. "Vitamin D as a Shield against Aging" International Journal of Molecular Sciences 24, no. 5: 4546. https://doi.org/10.3390/ijms24054546
APA StyleFantini, C., Corinaldesi, C., Lenzi, A., Migliaccio, S., & Crescioli, C. (2023). Vitamin D as a Shield against Aging. International Journal of Molecular Sciences, 24(5), 4546. https://doi.org/10.3390/ijms24054546





