Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials
Abstract
1. Introduction
2. Results
2.1. Identification of Relevant Citations
2.2. Types and Trends in Conditions Studied
2.3. Measures of Curcumin Clinical Trial Quality
2.4. Side Effects Reported in Curcumin Clinical Trial Citations
2.5. Clinical Trials for Metabolic Disorders
2.6. Clinical Trials for Musculoskeletal Disorders
2.7. Clinical Trials for Neuropsychiatric Disorders
2.8. Clinical Trials for Gastrointestinal Disorders (Excluding Cancer)
2.9. Clinical Trials for Cancer
2.10. Clinical Trials for Less Commonly Studied Disorders
3. Discussion
4. Methods
4.1. Design of Systematic Literature Search
4.2. Methods for Assessing Citation Inclusion
4.3. Data Extraction and Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Velayudhan, K.C.; Dikshit, N.; Nizar, M.A. Ethnobotany of turmeric (Curcuma longa L.). Indian J. Tradit. Knowl. 2012, 11, 607–614. [Google Scholar]
- Funk, J.L.; Schneider, C. Perspective on Improving the Relevance, Rigor, and Reproducibility of Botanical Clinical Trials: Lessons Learned From Turmeric Trials. Front. Nutr. 2021, 8, 782912. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.A.; Karde, M.; Mundle, N.; Jadhav, P.R.; Mehra, K. Phytochemical Evaluation and Curcumin Content Determination of Turmeric Rhizomes Collected From Bhandara District of Maharashtra (India). Med. Chem. 2014, 4, 588–591. [Google Scholar] [CrossRef]
- Skiba, M.B.; Luis, P.B.; Alfafara, C.; Billheimer, D.; Schneider, C.; Funk, J.L. Curcuminoid Content and Safety-Related Markers of Quality of Turmeric Dietary Supplements Sold in an Urban Retail Marketplace in the United States. Mol. Nutr. Food Res. 2018, 62, 1800143. [Google Scholar] [CrossRef] [PubMed]
- Bates, J. Oilseeds, spices, fruits and flavour in the Indus Civilisation. J. Archaeol. Sci. Rep. 2019, 24, 879–887. [Google Scholar] [CrossRef]
- Blaze, J. A Comparison of Current Regulatory Frameworks for Nutraceuticals in Australia, Canada, Japan, and the United States. Innov. Pharm. 2021, 12. [Google Scholar] [CrossRef]
- Smith, T.; Resetar, H.; Morton, C. US Sales of Herbal Supplements Increase by 9.7% in 2021. HerbalEgram 2022. Available online: https://www.herbalgram.org/resources/herbalegram/volumes/volume-19/issue-11-november/news-and-features-1/2021-herb-market-report/ (accessed on 20 February 2023).
- Linstrom, A.; Ooyen, C.; Lynch, M.E.; Blumenthal, M. Herb Supplement Sales Increase 5.5% in 2012: Herbal Supplement Sales Rise for the 9th Consecutive Year; Turmeric Sales Jump 40% in Natural Channel. HerbalGram 2013, 99, 60–65. [Google Scholar]
- Skiba, M.B.; Hopkins, L.L.; Hopkins, A.L.; Billheimer, D.; Funk, J.L. Nonvitamin, Nonmineral Dietary Supplement Use in Individuals with Rheumatoid Arthritis. J. Nutr. 2020, 150, 2451–2459. [Google Scholar] [CrossRef]
- Hauer, M.; Rossi, A.; Wertheim, B.; Kleppel, H.; Bea, J.; Funk, J.L. Dietary Supplement Use in Women Diagnosed with Breast Cancer. J. Nutr. 2023. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; Macdonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Sharma, S.; Xu, S.; Tewari, D.; Fang, J. Curcumin as a Natural Remedy for Atherosclerosis: A Pharmacological Review. Molecules 2021, 26, 4036. [Google Scholar] [CrossRef] [PubMed]
- Šudomová, M.; Hassan, S.T.S. Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways. Microorganisms 2021, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. Curcumin May (Not) Defy Science. ACS Med. Chem. Lett. 2017, 8, 467–470. [Google Scholar] [CrossRef]
- Kunihiro, A.G.; Luis, P.B.; Frye, J.B.; Chew, W.; Chow, H.H.; Schneider, C.; Funk, J.L. Bone-Specific Metabolism of Dietary Polyphenols in Resorptive Bone Diseases. Mol. Nutr. Food Res. 2020, 64, e2000072. [Google Scholar] [CrossRef]
- Kunihiro, A.G.; Luis, P.B.; Brickey, J.A.; Frye, J.B.; Chow, H.S.; Schneider, C.; Funk, J.L. Beta-Glucuronidase Catalyzes Deconjugation and Activation of Curcumin-Glucuronide in Bone. J. Nat. Prod. 2019, 82, 500–509. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.I.; Edwards, R.L.; Luis, P.B.; Presley, S.H.; Porter, N.A.; Schneider, C. Stability and anti-inflammatory activity of the reduction-resistant curcumin analog, 2,6-dimethyl-curcumin. Org. Biomol. Chem. 2018, 16, 3273–3281. [Google Scholar] [CrossRef]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling curcumin degradation: Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Luis, P.B.; Nakashima, F.; Kunihiro, A.G.; Presley, S.H.; Funk, J.L.; Schneider, C. Mechanistic Differences in the Inhibition of NF-κB by Turmeric and Its Curcuminoid Constituents. J. Agric. Food Chem. 2020, 68, 6154–6160. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Orally effective FDA-approved protein kinase targeted covalent inhibitors (TCIs). Pharmacol. Res. 2021, 165, 105422. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, R.; Patouret, R.; Winssinger, N. Covalent inhibitors: An opportunity for rational target selectivity. Curr. Opin. Chem. Biol. 2017, 39, 54–63. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Thota, R.N.; Dias, C.B.; Abbott, K.A.; Acharya, S.H.; Garg, M.L. Curcumin alleviates postprandial glycaemic response in healthy subjects: A cross-over, randomized controlled study. Sci. Rep. 2018, 8, 13679. [Google Scholar] [CrossRef]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.; Hui, E.; Ng, C.C.Y.; Chow, M.; Ho, P.C.; Lam, S.; et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef]
- Mela, D.J.; Cao, X.Z.; Dobriyal, R.; Fowler, M.I.; Lin, L.; Joshi, M.; Mulder, T.J.P.; Murray, P.G.; Peters, H.P.F.; Vermeer, M.A.; et al. The effect of 8 plant extracts and combinations on post-prandial blood glucose and insulin responses in healthy adults: A randomized controlled trial. Nutr. Metab. 2020, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Pungcharoenkul, K.; Thongnopnua, P. Effect of different curcuminoid supplement dosages on total in vivo antioxidant capacity and cholesterol levels of healthy human subjects. Phytother. Res. 2011, 25, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.; Kutian, R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992, 36, 273–275. [Google Scholar] [PubMed]
- Tang, M.; Larson-Meyer, D.E.; Liebman, M. Effect of cinnamon and turmeric on urinary oxalate excretion, plasma lipids, and plasma glucose in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Batista, Â.G.; Björck, I.; Östman, E. Turmeric-based beverage blunted acute high fat meal-induced oxidative stress by lowering serum malondialdehyde (MDA) levels: A randomized cross-over study. Planta Med. 2016, 82, P991. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals. A randomised controlled trial. Metabolism 2018, 82, 22–35. [Google Scholar] [CrossRef]
- Ferguson, J.J.; Wolska, A.; Remaley, A.T.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Bread enriched with phytosterols with or without curcumin modulates lipoprotein profiles in hypercholesterolaemic individuals. A randomised controlled trial. Food Funct. 2019, 10, 2515–2527. [Google Scholar] [CrossRef]
- Funamoto, M.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Imaizumi, A.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Komiyama, M.; Wada, H.; et al. Highly absorptive curcumin reduces serum atherosclerotic low-density lipoprotein levels in patients with mild COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2029–2034. [Google Scholar] [CrossRef]
- Kocher, A.; Bohnert, L.; Schiborr, C.; Frank, J. Highly bioavailable micellar curcuminoids accumulate in blood, are safe and do not reduce blood lipids and inflammation markers in moderately hyperlipidemic individuals. Mol. Nutr. Food Res. 2016, 60, 1555–1563. [Google Scholar] [CrossRef]
- Mirzabeigi, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The Effect of Curcumin on some of Traditional and Non-traditional Cardiovascular Risk Factors: A Pilot Randomized, Double-blind, Placebo-controlled Trial. Iran 2015, 14, 479–486. [Google Scholar]
- Venkatesan, H. Hypolipidaemic effect of alcoholic extracts of the plants Curcuma longa and Guatteria gaumeri. Int. J. Res. Pharm. Sci. 2019, 10, 1062–1068. [Google Scholar] [CrossRef]
- Amirkhani, Z.; Azarbayjani, M.A.; Peeri, M.; Matin Homaei, H. Effect of combining resistance training and curcumin supplementation on lipid profile in obese women. Iran. J. Obstet. Gynecol. Infertil. 2017, 20, 24–32. [Google Scholar] [CrossRef]
- Blanton, C.; Gordon, B. Effect of Morning vs. Evening Turmeric Consumption on Urine Oxidative Stress Biomarkers in Obese, Middle-Aged Adults: A Feasibility Study. Int. J. Environ. Res. Public Health 2020, 17, 4088. [Google Scholar] [CrossRef]
- Bupparenoo, P.; Pakchotanon, R.; Narongroeknawin, P.; Asavatanabodee, P.; Chaiamnuay, S. Effect of Curcumin on Serum Urate in Asymptomatic Hyperuricemia: A Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2020, 18, 248–260. [Google Scholar] [CrossRef]
- Campbell, M.S.; Ouyang, A.; Krishnakumar, I.M.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef]
- Campos-Cervantes, A.; Murillo-Ortiz, B.O.; Alvarado-Caudillo, Y.; Pérez-Vázquez, V.; RamírezEmiliano, J. Curcumin Decreases the Oxidative Damage Indexes and Increases the Adiponectin Levels in Serum of Obese Subjects. Free Radic. Biol. Med. 2011, 51, S95. [Google Scholar] [CrossRef]
- Dolati, S.; Namiranian, K.; Amerian, R.; Mansouri, S.; Arshadi, S.; Azarbayjani, M.A. The Effect of Curcumin Supplementation and Aerobic Training on Anthropometric Indices, Serum Lipid Profiles, C-Reactive Protein and Insulin Resistance in Overweight Women: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Obes. Metab. Syndr. 2020, 29, 47–57. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Mohammad, S.; Parizadeh, R.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef]
- Ismail, N.; El Dayem, S.; Hamed, M. Curcumin intake could lower serum macrophage migration inhibitory factor and monocyte chemoattractant protein-1 levels in obese subjects. Trends Med. Res. 2016, 11, 82–87. [Google Scholar] [CrossRef]
- Ismail, N.A.; Abd El Dayem, S.M.; Salama, E.; Ragab, S.; Abd El Baky, A.N.; Ezzat, W.M. Impact of curcumin intake on gluco-insulin homeostasis, leptin and adiponectin in obese subjects. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1891–1897. [Google Scholar]
- Ismail, N.A.; Ragab, S.; El-Baky AN, E.A.; Hamed, M.; Ibrahim, A.A. Effect of oral curcumin administration on insulin resistance, serum resistin and fetuin-A in obese children: Randomized placebo-controlled study. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 887–896. [Google Scholar]
- Kabaran, S.; Atakan, A. Effects of dietary intervention with or without turmeric on blood lipids and weight loss in dyslipidemic overweight/obese women. Clin. Nutr. 2018, 37, S218. [Google Scholar] [CrossRef]
- Kawasaki, K.F.; Muroyama, K.; Murosaki, S. Effect of a combination of hot water extract of curcuma longa and curcumin on serum liver enzymes, inflammatory markers, and emotional states in healthy participants with moderately high body mass index—A randomized, double-blind, placebo-controlled clinical trial. Jpn. Pharmacol. Ther. 2017, 45, 243–252. [Google Scholar]
- Latif, R.; Mumtaz, S.; Al Sheikh, M.H.; Chathoth, S.; Nasser Al Naimi, S. Effects of Turmeric on Cardiovascular Risk Factors, Mental Health, and Serum Homocysteine in Overweight, Obese Females. Altern. Ther. Health Med. 2020, 21, 21. [Google Scholar]
- Mohajer, A.; Ghayour-Mobarhan, M.; Parizadeh SM, R.; Tavallaie, S.; Rajabian, M.; Sahebkar, A. Effects of supplementation with curcuminoids on serum copper and zinc concentrations and superoxide dismutase enzyme activity in obese subjects. Trace Elem. Electrolytes 2014, 32, 16–21. [Google Scholar] [CrossRef]
- Mohamadi, A.; Sahebkar, A.H.; Iranshahi, M.; Akhlaghi, S.; Mobarhan, M.G. Curcumin effects on blood lipid profile in obese individuals. Clin. Biochem. 2011, 44, S239. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef]
- Moohebati, M.; Yazdandoust, S.; Sahebkar, A.; Mazidi, M.; Sharghi-Shahri, Z.; Ferns, G.; Ghayour-Mobarhan, M. Investigation of the effect of short-term supplementation with curcuminoids on circulating small dense low-density lipoprotein concentrations in obese dyslipidemic subjects: A randomized double-blind placebo-controlled cross-over trial. ARYA Atheroscler. 2014, 10, 280–286. [Google Scholar]
- Nieman, D.C.; Cialdella-Kam, L.; Knab, A.M.; Shanely, R.A. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: A metabolomics approach. Plant Foods Hum. Nutr. 2012, 67, 415–421. [Google Scholar] [CrossRef]
- Nuraiza, M.; Edwards, C.A.; Combet, E. Impact of a 3-weeks randomized double-blind cross-over study curuminoid supplementation on endotoxemia, inflammatory markers, and lipid profiles in healthy overweight and obese adults. Proc. Nutr. Soc. 2016, 75, E160. [Google Scholar] [CrossRef]
- Pashine, L.; Singh, J.V.; Vaish, A.K.; Ojha, S.K.; Mahdi, A.A. Effect of turmeric (Curcuma longa) on overweight hyperlipidemic subjects: Double blind study. Indian J. Community Health 2012, 24, 113–117. [Google Scholar]
- Sahebkar, A.; Ghayour-Mobarhan, M.; Ganjali, S.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R. Effects of curcuminoids supplementation on circulating concentrations of interleukins. Eur. J. Pharm. Sci. 2013, 50, E164. [Google Scholar]
- Sahebkar, A.; Mohammadi, A.; Atabati, A.; Rahiman, S.; Tavallaie, S.; Iranshahi, M.; Akhlaghi, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013, 27, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Uchio, R.; Muroyama, K.; Okuda-Hanafusa, C.; Kawasaki, K.; Yamamoto, Y.; Murosaki, S. Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 1822. [Google Scholar] [CrossRef]
- Amin, F.; Islam, N.; Anila, N.; Gilani, A.H. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome—A double blind randomized controlled trial—TAK-MetS trial. Complement. Ther. Med. 2015, 23, 165–174. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Ghazimoradi, M.; Saberi-Karimian, M.; Mohammadi, F.; Sahebkar, A.; Tavallaie, S.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Moohebati, M.; Esmaeili, H.; et al. The Effects of Curcumin and Curcumin-Phospholipid Complex on the Serum Pro-oxidant-Antioxidant Balance in Subjects with Metabolic Syndrome. Phytother. Res. 2017, 31, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Javandoost, A.; Afshari, A.; Saberi-Karimian, M.; Sahebkar, A.; Safarian, H.; Moammeri, M.; Dizaji, B.F.; Tavalaei, S.; Ferns, G.A.; Pasdar, A.; et al. The effects of curcumin and a modified curcumin formulation on serum Cholesteryl Ester Transfer Protein concentrations in patients with metabolic syndrome: A randomized, placebo-controlled clinical trial. Avicenna J. 2018, 8, 330–337. [Google Scholar]
- Mohammadi, A.; Sadeghnia, H.R.; Saberi-Karimian, M.; Safarian, H.; Ferns, G.A.; Ghayour-Mobarhan, M.; Sahebkar, A. Effects of Curcumin on Serum Vitamin E Concentrations in Individuals with Metabolic Syndrome. Phytother. Res. 2017, 31, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, F.; Ghazi-Moradi, M.; Ghayour-Mobarhan, M.; Esmaeili, H.; Moohebati, M.; Saberi-Karimian, M.; Safarian, H.; Tavallaie, S.; Ferns, G.A.; Sahebkar, A. The Effects of Curcumin on Serum Heat Shock Protein 27 Antibody Titers in Patients with Metabolic Syndrome. J. Diet. Suppl. 2018, 16, 592–601. [Google Scholar] [CrossRef]
- Osali, A. Aerobic exercise and nano-curcumin supplementation improve inflammation in elderly females with metabolic syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Soflaei, S.S.; Majeed, M.; Sahebkar, A. Effects of supplementation with curcumin on serum adipokine concentrations: A randomized controlled trial. Nutrition 2016, 32, 1116–1122. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Hosseini, M.S.; Abbasinazari, M.; Sahebkar, A. Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement. Ther. Med. 2014, 22, 851–857. [Google Scholar] [CrossRef]
- Safarian, H.; Parizadeh, S.M.R.; Saberi-Karimain, M.; Darroudi, S.; Javandoost, A.; Mohammadi, F.; Moammeri, M.; Ferns, G.A.; Ghayour-Mobarhan, M.; Mohebati, M. The Effect of Curcumin on Serum Copper and Zinc and Zn/Cu Ratio in Individuals with Metabolic Syndrome: A Double-Blind Clinical Trial. J. Diet. Suppl. 2018, 16, 625–634. [Google Scholar] [CrossRef]
- Sahebkar, A.; Panahi, Y.; Khalili, N. Beneficial effects of adjunctive therapy with bioavailability-enhanced curcumin in subjects with metabolic syndrome receiving low-dose atorvastatin: A randomized parallel-group trial. Eur. J. Pharm. Sci. 2013, 50, E234. [Google Scholar]
- Shirmohammadi, L.; Ghayour-Mobarhan, M.; Saberi-Karimian, M.; Iranshahi, M.; Tavallaie, S.; Emamian, M.; Sahebkar, A. Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome. Cardiovasc. Hematol Disord Drug Targets 2020, 20, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Su, Y.F.; Yang, H.W.; Lee, Y.H.; Chou, J.I.; Ueng, K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Abbood, M.S. Hypolipidaemic and anti-inflammatory effects of curcumin versus atorvastatin in type 2 diabetic patients. Int. J. Pharm. Sci. Rev. Res. 2018, 49, 1–7. [Google Scholar]
- Adab, Z.; Eghtesadi, S.; Vafa, M.R.; Heydari, I.; Shojaii, A.; Haqqani, H.; Arablou, T.; Eghtesadi, M. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother. Res. 2019, 33, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 12, 12. [Google Scholar] [CrossRef]
- Appendino, G.; Belcaro, G.; Cornelli, U.; Luzzi, R.; Togni, S.; Dugall, M.; Cesarone, M.R.; Feragalli, B.; Ippolito, E.; Errichi, B.M.; et al. Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy. A pilot study. Panminerva Med. 2011, 53, 43–49. [Google Scholar]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Khamoshian, K.; Sotoudeh, G. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double-blind placebo- controlled clinical trial. Complement. Ther. Med. 2019, 43, 253–260. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Ebrahimkhani, S.; Ghavamzadeh, S.; Mehdizadeh, A. The effects of vitamin D and curcuminoids supplementation on anthropometric measurements and blood pressure in type 2 diabetic patients with coexisting hypovitaminosis D: A double-blind, placebo-controlled randomized clinical trial. Clin. Nutr. ESPEN 2020, 37, 178–186. [Google Scholar] [CrossRef]
- Funamoto, M.; Shimizu, K.; Sunagawa, Y.; Katanasaka, Y.; Miyazaki, Y.; Kakeya, H.; Yamakage, H.; Satoh-Asahara, N.; Wada, H.; Hasegawa, K.; et al. Effects of Highly Absorbable Curcumin in Patients with Impaired Glucose Tolerance and Non-Insulin-Dependent Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 8208237. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Wahlqvist, M.L.; Chou, Y.C.; Fang, W.H.; Lee, J.T.; Kuan, J.C.; Liu, H.Y.; Lu, T.M.; Xiu, L.; Hsu, C.C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Efficacy of Turmeric as Adjuvant Therapy in Type 2 Diabetic Patients. Indian J. 2015, 30, 180–186. [Google Scholar] [CrossRef]
- Mokhtari, M.; Razzaghi, R.; Momen-Heravi, M. The effects of curcumin intake on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2020, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Li, Y.; Pan, H.Z.; Zhou, X.L.; Sun, D.J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Na, L.X.; Yan, B.L.; Jiang, S.; Cui, H.L.; Li, Y.; Sun, C.H. Curcuminoids Target Decreasing Serum Adipocyte-fatty Acid Binding Protein Levels in Their Glucose-lowering Effect in Patients with Type 2 Diabetes. Biomed. Environ. Sci. 2014, 27, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Neerati, P.; Devde, R.; Gangi, A.K. Evaluation of the effect of curcumin capsules on glyburide therapy in patients with type-2 diabetes mellitus. Phytother. Res. 2014, 28, 1796–1800. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Curcuminoids Plus Piperine Modulate Adipokines in Type 2 Diabetes Mellitus. Curr. Clin. Pharmacol. 2017, 12, 253–258. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Reiner, Ž.; Majeed, M.; Sahebkar, A. Curcuminoids modify lipid profile in type 2 diabetes mellitus: A randomized controlled trial. Complement. Ther. Med. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Pingali, U.; Mateen, A. Evaluation of curcuminoids, atorvastatin and placebo on endothelial dysfunction and biomarkers in elderly diabetic patients. Basic Clin. Pharmacol. Toxicol. 2014, 115, 261. [Google Scholar]
- Rahimi, H.R.; Mohammadpour, A.H.; Dastani, M.; Jaafari, M.R.; Abnous, K.; Mobarhan, M.G.; Oskuee, R.K. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: A randomized clinical trial. Avicenna J. 2016, 6, 567–577. [Google Scholar]
- Shafabakhsh, R.; Asemi, Z.; Reiner, Z.; Soleimani, A.; Aghadavod, E.; Bahmani, F. The Effects of Nano-curcumin on Metabolic Status in Patients With Diabetes on Hemodialysis, a Randomized, Double Blind, Placebo-controlled Trial. Iran. J. Kidney Dis. 2020, 14, 290–299. [Google Scholar]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef]
- Sousa, D.F.; Araujo, M.F.M.; de Mello, V.D.; Damasceno, M.M.C.; Freitas, R. Cost-Effectiveness of Passion Fruit Albedo versus Turmeric in the Glycemic and Lipaemic Control of People with Type 2 Diabetes: Randomized Clinical Trial. J. Am. Coll. Nutr. 2020, 40, 679–688. [Google Scholar] [CrossRef]
- Srinivasan, A.; Selvarajan, S.; Kamalanathan, S.; Kadhiravan, T.; Prasanna Lakshmi, N.C.; Adithan, S. Effect of Curcuma longa on vascular function in native Tamilians with type 2 diabetes mellitus: A randomized, double-blind, parallel arm, placebo-controlled trial. Phytother. Res. 2019, 33, 1898–1911. [Google Scholar] [CrossRef]
- Steigerwalt, R.; Nebbioso, M.; Appendino, G.; Belcaro, G.; Ciammaichella, G.; Cornelli, U.; Luzzi, R.; Togni, S.; Dugall, M.; Cesarone, M.R.; et al. Meriva, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012, 54, 11–16. [Google Scholar]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Usharani, P.; Mateen, A.A.; Naidu, M.U.R.; Raju, Y.S.N.; Chandra, N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus—A randomized, parallel-group, placebo-controlled, 8-week study. Drugs RD 2008, 9, 243–250. [Google Scholar] [CrossRef]
- Yang, H.; Xu, W.; Zhou, Z.; Liu, J.; Li, X.; Chen, L.; Weng, J.; Yu, Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp. Clin. Endocrinol. Diabetes 2015, 123, 360–367. [Google Scholar] [CrossRef]
- Basu, P.; Shah, N.; Siriki, R.; Rahaman, M.; Farhat, S. Curcumin, antioxidant, and pioglitazone therapy with inclusion of vitamin e in non-alcoholic fatty liver disease: A randomized, open-label, placebo-controlled clinical prospective trial (captive). Am. J. Gastroenterol. 2013, 108, S149–S150. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht, M.G.B.F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Chirapongsathorn, S.; Jearjesdakul, J.; Sanpajit, T.; Juthaputthi, A. Curcumin trend to improve alanine transaminase (ALT) in non-alcoholic fatty liver disease (NAFLD) with abnormal ALT. J. Gastroenterol. Hepatol. 2012, 27, 231–232. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: A double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2019, 22, 22. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Asghari-Jafarabadi, M. Effects of turmeric and chicory seed supplementation on antioxidant and inflammatory biomarkers in patients with non-alcoholic fatty liver disease (NAFLD). Adv. Integr. Med. 2018, 5, 89–95. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S.M. Effects of turmeric on homocysteine and Fetuin-A in patients with nonalcoholic fatty liver disease: A randomized Double-Blind placebo-controlled study. Iran. Red Crescent Med. J. 2017, 19, e43193. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S.M. Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int. J. Vitam. Nutr. Res. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Gholami, A.; Hooshmand, E.; Tavallaie, S.; Nobakht, M.G.B.F. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug Metab. Pers. Ther. 2019, 34, 30. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht, M.G.B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Moradi Kelardeh, B.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M.A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef]
- Navekar, R.; Rafraf, M.; Ghaffari, A.; Asghari-Jafarabadi, M.; Khoshbaten, M. Turmeric Supplementation Improves Serum Glucose Indices and Leptin Levels in Patients with Nonalcoholic Fatty Liver Diseases. J. Am. Coll. Nutr. 2017, 36, 261–267. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendia, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Soflaei, S.S.; Sahebkar, A. Efficacy of phospholipidated curcumin in nonalcoholic fatty liver disease: A clinical study. J. Asian Nat. Prod. Res. 2019, 21, 798–805. [Google Scholar] [CrossRef]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-Pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hekmatdoost, A.; Hatami, B.; Mansour, A.; Zahra, Z.; Hedayati, M.; Sadeghi, A. Comparing different non-invasive methods in assessment of the effects of curcumin on hepatic fibrosis in patients with non-alcoholic fatty liver disease. Gastroenterology 2018, 11, S8–S13. [Google Scholar]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 133. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 6. [Google Scholar] [CrossRef]
- Selmanovic, S.; Beganlic, A.; Salihefendic, N.; Ljuca, F.; Softic, A.; Smajic, E. Therapeutic Effects of Curcumin on Ultrasonic Morphological Characteristics of Liver in Patients with Metabolic Syndrome. Acta Inform. Med. 2017, 25, 169–174. [Google Scholar] [CrossRef]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease—An Evolving View. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Atabaki, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. Int. Immunopharmacol. 2020, 85, 106607. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Calderon-Perez, L.; Llaurado, E.; Companys, J.; Pla-Paga, L.; Boque, N.; Puiggros, F.; Valls, R.M.; Pedret, A.; Llabres, J.M.; Arola, L.; et al. Acute Effects of Turmeric Extracts on Knee Joint Pain: A Pilot, Randomized Controlled Trial. J. Med. Food 2020, 29, 29. [Google Scholar] [CrossRef]
- Di Pierro, F.; Zacconi, P.; Bertuccioli, A.; Togni, S.; Eggenhoffner, R.; Giacomelli, L.; Scaltrini, S. A naturally-inspired, curcumin-based lecithin formulation (Meriva formulated as the finished product Algocur) alleviates the osteo-muscular pain conditions in rugby players. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4935–4940. [Google Scholar]
- Gupte, P.A.; Giramkar, S.A.; Harke, S.M.; Kulkarni, S.K.; Deshmukh, A.P.; Hingorani, L.L.; Mahajan, M.P.; Bhalerao, S.S. Evaluation of the efficacy and safety of Capsule Longvida® Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: A pilot clinical study. J. Inflamm. Res. 2019, 12, 145–152. [Google Scholar] [CrossRef]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, K.; Davoudian, N.; Jaafari, M.R.; Mirfeizi, Z. The Effect of Nanocurcumin in Improvement of Knee Osteoarthritis: A Randomized Clinical Trial. Curr. Rheumatol. Rev. 2020, 16, 158–164. [Google Scholar] [CrossRef]
- Henrotin, Y.; Gharbi, M.; Dierckxsens, Y.; Priem, F.; Marty, M.; Seidel, L.; Albert, A.; Heuse, E.; Bonnet, V.; Castermans, C. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complement. Altern. Med. 2014, 14, 159. [Google Scholar] [CrossRef]
- Henrotin, Y.; Malaise, M.; Wittoek, R.; de Vlam, K.; Brasseur, J.P.; Luyten, F.P.; Jiangang, Q.; Van den Berghe, M.; Uhoda, R.; Bentin, J.; et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: A double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res. Ther. 2019, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Kertia, N.; Ahmad Husain Asdie, A.; Wasilah Rochmah, W.; Marsetyawan, M. Anti-inflammatory activities and the safety of curcuminoid compared to diclofenac sodium for the treatment of osteoarthritis. Int. J. Rheum. Dis. 2013, 16, 37. [Google Scholar]
- Kertia, N.; Asdie, A.H.; Rochmah, W. Ability of curcuminoid compared to diclofenac sodium in reducing the secretion of cycloxygenase-2 enzyme by synovial fluid’s monocytes of patients with osteoarthritis. Acta Med. 2012, 44, 105–113. [Google Scholar]
- Khanna, A.; Das, S.S.; Smina, T.P.; Thomas, J.V.; Kunnumakkara, A.B.; Maliakel, B.; Krishnakumar, I.M.; Mohanan, R. Curcumagalactomannoside/Glucosamine Combination Improved Joint Health Among Osteoarthritic Subjects as Compared to Chondroitin Sulfate/Glucosamine: Double-Blinded, Randomized Controlled Study. J. Altern. Complement. Med. 2020, 26, 945–955. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Murata, S.; Yabumoto, H.; Maeda, T.; Akamatsu, S. The Efficacy and Safety of Highly-Bioavailable Curcumin for Treating Knee Osteoarthritis: A 6-Month Open-Labeled Prospective Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120948471. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J. Diet. Suppl. 2016, 13, 209–220. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Panda, S.K.; Nirvanashetty, S.; Parachur, V.A.; Mohanty, N.; Swain, T. A Randomized, Double Blind, Placebo Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Curene versus Placebo in Reducing Symptoms of Knee OA. Biomed. Res. Int. 2018, 2018, 5291945. [Google Scholar] [CrossRef]
- Pinsornsak, P.; Niempoog, S. The efficacy of Curcuma Longa, L. extract as an adjuvant therapy in primary knee osteoarthritis: A randomized control trial. J. Med. Assoc. Thai 2012, 95 (Suppl. S1), S51–S58. [Google Scholar]
- Rahimnia, A.R.; Panahi, Y.; Alishiri, G.; Sharafi, M.; Sahebkar, A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2015, 65, 521–525. [Google Scholar] [CrossRef]
- Raj, J.P.; Venkatachalam, S.; Racha, P.; Bhaskaran, S.; Amaravati, R.S. Effect of Turmacin supplementation on joint discomfort and functional outcome among healthy participants—A randomized placebo-controlled trial. Complement. Ther. Med. 2020, 53, 102522. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M. Turmeric (Curcuma longa): Effects of Curcuma longa Extracts Compared With Ibuprofen for Reduction of Pain and Functional Improvement in Patients With Knee Osteoarthritis. Holist. Nurs. Pract. 2016, 30, 183–186. [Google Scholar] [CrossRef]
- Sahebkar, A.H.; Panahi, Y.; Sabouri, A.; Rahimnia, A.; Sharafi, M.; Alishiri, G.H. Efficacy and safety of supplementation with curcuminoids in the treatment of patients with osteoarthritis: A randomized controlled trial. Avicenna J. 2015, 5, 15–16. [Google Scholar]
- Saksena, A.K.; Srivastava, S.; Khattri, S.; Kumar, S. Efficacy of Curcuma longa in osteoarthritis: Association of IL-1beta, IL-10 and MMP-9 with severity of disease. J. Immunol. 2016, 196, 124-13. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis: A randomized trial. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef]
- Thomas, J.V.; Smina, T.P.; Khanna, A.; Kunnumakkara, A.B.; Maliakel, B.; Mohanan, R.; Krishnakumar, I.M. Influence of a low-dose supplementation of curcumagalactomannoside complex (CurQfen) in knee osteoarthritis: A randomized, open-labeled, active-controlled clinical trial. Phytother. Res. 2020, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jones, G.; Winzenberg, T.; Cai, G.; Laslett, L.L.; Aitken, D.; Hopper, I.; Singh, A.; Jones, R.; Fripp, J.; et al. Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion-Synovitis of Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef]
- Abbas, A.A.; Ali, A.H.; Ali, H.F.A.; Abbas, R.A. Effect of dietary supplement (Turmeric) in the level of concentration of lactic acid and lactic acid dehydrogenase in the players of the University of Babylon Futsal. Indian J. Public Health Res. Dev. 2020, 11, 1323–1327. [Google Scholar]
- Ali, R.H.; Ray, H.R.D. The Effect of Turmeric Consumption to VO(2)Max and Lactate Threshold. In 1st Annual Applied Science and Engineering Conference; Abdullah, A.G., Nandiyanto, A.B.D., Danuwijaya, A.A., Eds.; IOP Publishing: Bristol, UK, 2017; Volume 180. [Google Scholar]
- Amalraj, A.; Divya, C.; Gopi, S. The Effects of Bioavailable Curcumin (Cureit) on Delayed Onset Muscle Soreness Induced By Eccentric Continuous Exercise: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2020, 23, 545–553. [Google Scholar] [CrossRef]
- Basham, S.A.; Waldman, H.S.; Krings, B.M.; Lamberth, J.; Smith, J.W.; McAllister, M.J. Effect of Curcumin Supplementation on Exercise-Induced Oxidative Stress, Inflammation, Muscle Damage, and Muscle Soreness. J. Diet. Suppl. 2019, 17, 401–414. [Google Scholar] [CrossRef]
- Cardaci, T.D.; Machek, S.B.; Wilburn, D.T.; Hwang, P.S.; Willoughby, D.S. Ubiquitin Proteasome System Activity is Suppressed by Curcumin following Exercise-Induced Muscle Damage in Human Skeletal Muscle. J. Am. Coll. Nutr. 2020, 40, 401–411. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva): A randomised, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Faria, F.R.; Gomes, A.C.; Antunes, A.; Rezende, K.R.; Pimentel, G.D.; Oliveira, C.L.P.; Antunes, B.M.; Lira, F.S.; Aoki, M.S.; Mota, J.F. Effects of turmeric extract supplementation on inflammation and muscle damage after a half-marathon race: A randomized, double-blind, placebo-controlled trial. Eur. J. Appl. Physiol. 2020, 120, 1531–1540. [Google Scholar] [CrossRef]
- Franceschi, F.; Feregalli, B.; Togni, S.; Cornelli, U.; Giacomelli, L.; Eggenhoffner, R.; Belcaro, G. A novel phospholipid delivery system of curcumin (Meriva) preserves muscular mass in healthy aging subjects. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 762–766. [Google Scholar] [PubMed]
- Gerchman, A.; Hillman, A.; O’Hora, E. The Effect of Curcumin on Inflammation and Exercise Induced Muscle Damage in Healthy Adults. Med. Sci. Sport. Exerc. 2018, 50, 721. [Google Scholar] [CrossRef]
- Herrick, L.P.; Goh, J.; Menke, W.; Campbell, M.S.; Fleenor, B.S.; Abel, M.G.; Bergstrom, H.C. Effects of Curcumin and Fenugreek Soluble Fiber on the Physical Working Capacity at the Fatigue Threshold, Peak Oxygen Consumption, and Time to Exhaustion. J. Strength Cond. Res. 2020, 34, 3346–3355. [Google Scholar] [CrossRef]
- Jager, R.; Caldwell, A.R.; Sanders, E.; Mitchell, J.B.; Rogers, J.; Purpura, M.; Oliver, J.M. Curcumin reduces muscle damage and soreness following muscle-damaging exercise. FASEB J. 2017, 31, lb766. [Google Scholar]
- Jager, R.; Purpura, M.; Kerksick, C.M. Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise. Nutrients 2019, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- Mallard, A.R.; Briskey, D.; Richards, B.A.; Rao, A. Curcumin Improves Delayed Onset Muscle Soreness and Postexercise Lactate Accumulation. J. Diet. Suppl. 2020, 18, 531–542. [Google Scholar] [CrossRef]
- McAllister, M.J.; Basham, S.A.; Waldman, H.S.; Smith, J.W.; Butawan, M.B.; Bloomer, R.J. Effects of Curcumin on the Oxidative Stress Response to a Dual Stress Challenge in Trained Men. J. Diet. Suppl. 2018, 17, 261–272. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef]
- Sciberras, J.N.; Galloway, S.D.; Fenech, A.; Grech, G.; Farrugia, C.; Duca, D.; Mifsud, J. The effect of turmeric (Curcumin) supplementation on cytokine and inflammatory marker responses following 2 hours of endurance cycling. J. Int. Soc. Sport. Nutr. 2015, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sport. Med. 2014, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Chino, K.; Akazawa, N.; Imaizumi, A.; Ozawa, H.; Sumi, Y.; Maeda, S.; Takahashi, H. Curcumin intake after eccentric exercise effectively reduces muscle damage and enables faster recovery. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, S1–S4. [Google Scholar] [CrossRef]
- Tanabe, Y.; Chino, K.; Ohnishi, T.; Ozawa, H.; Sagayama, H.; Maeda, S.; Takahashi, H. Effects of oral curcumin ingested before or after eccentric exercise on markers of muscle damage and inflammation. Scand. J. Med. Sci. Sport. 2019, 29, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Chino, K.; Sagayama, H.; Lee, H.J.; Ozawa, H.; Maeda, S.; Takahashi, H. Effective Timing of Curcumin Ingestion to Attenuate Eccentric Exercise-Induced Muscle Soreness in Men. J. Nutr. Sci. Vitaminol. 2019, 65, 82–89. [Google Scholar] [CrossRef]
- Tanabe, Y.; Maeda, S.; Akazawa, N.; Zempo-Miyaki, A.; Choi, Y.; Ra, S.G.; Imaizumi, A.; Otsuka, Y.; Nosaka, K. Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur. J. Appl. Physiol. 2015, 115, 1949–1957. [Google Scholar] [CrossRef]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The Efficacy of the Novel Bioavailable Curcumin (Cureit) in the Management of Sarcopenia in Healthy Elderly Subjects: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2020, 24, 40–49. [Google Scholar] [CrossRef]
- Wang, I.L.; Hsiao, C.Y.; Li, Y.H.; Meng, F.B.; Huang, C.C.; Chen, Y.M. Nanobubbles Water Curcumin Extract Reduces Injury Risks on Drop Jumps in Women: A Pilot Study. Evid. Based Complement. Altern. Med. 2019, 2019, 8647587. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef]
- Chandran, B.; Goel, A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Amalraj, A.; Raj, K.K.J.; Divya, C.; Kunnumakkara, A.B.; Gopi, S. A novel bioavailable hydrogenated curcuminoids formulation (CuroWhite TM) improves symptoms and diagnostic indicators in rheumatoid arthritis patients–A randomized, double blind and placebo controlled study. J. Tradit. Complement. Med. 2019, 9, 346–352. [Google Scholar] [CrossRef]
- Javadi, M.; Khadem Haghighian, H.; Goodarzy, S.; Abbasi, M.; Nassiri-Asl, M. Effect of curcumin nanomicelle on the clinical symptoms of patients with rheumatoid arthritis: A randomized, double-blind, controlled trial. Int. J. Rheum. Dis. 2019, 22, 1857–1862. [Google Scholar] [CrossRef]
- Schulman, R. Special Curcumin Extract from Turmeric Shows Promise in Rheumatoid Arthritis Patients in Pilot Trial. HerbalGram 2012, 95, 33. Available online: https://www.herbalgram.org/resources/herbalgram/issues/95/table-of-contents/hg95-resrvw-curcumin/ (accessed on 22 February 2023).
- Wahono, C.S.; Kalim, H.; Saveria, I.; Setyorini, C.D.; Wahyuni, Z.; Dimpudus, R.A.; Kusworini, H. Effect of curcumin and vitamin d on disease activity, fatigue, and cytokine profile in systemic lupus erythematosus patients with deficiency vitamin d. Lupus Sci. Med. 2017, 4, A38–A39. [Google Scholar] [CrossRef]
- Wahono, C.S.; Saveria, I.; Setyorini, C.D.; Wahyuni, Z.D.; Handono, K.; Kalim, H. The effect of adding curcumin on vitamin D3 supplementation on cytokines balance, in sle patients with hypovitamin D. Lupus Sci. Med. 2017, 4, A120. [Google Scholar] [CrossRef]
- Wahono, C.S.; Susianti, H.; Wahyuni, Z.D.; Saveria, I.; Setyorini, C.D.; Handono, K.; Kalim, H. The effect of adding curcumin on vitamin D3 supplementation on anti-DSDNA levels and proteinuria, in SLE patients with hypovitamin D. Lupus Sci. Med. 2017, 4, A119–A120. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hajialilo, M.; Dolati, S.; Eghbal-Fard, S.; Heydarlou, H.; Ghaebi, M.; Ghassembaglou, A.; Aghebati-Maleki, L.; Samadi Kafil, H.; Kamrani, A.; et al. The effects of nanocurcumin on Treg cell responses and treatment of ankylosing spondylitis patients: A randomized, double-blind, placebo-controlled clinical trial. J. Cell. Biochem. 2019, 09, 09. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, M.; Ahmadi, M.R.H.; Rahmani, A.; Dastjerdi, M.M.; Asadollahi, K. Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg. 2018, 114, e785–e791. [Google Scholar] [CrossRef] [PubMed]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch. Endocrinol. Metab. 2018, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Togni, S.; Giacomelli, L.; Franceschi, F.; Eggenhoffner, R.; Feragalli, B.; Belcaro, G.; Cacchio, M.; Shu, H.; Dugall, M. Effects of a curcumin-based supplementation in asymptomatic subjects with low bone density: A preliminary 24-week supplement study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1684–1689. [Google Scholar]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The Neuromodulatory Effects of omega-3 Fatty Acids and Nano-Curcumin on the COX-2/iNOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 12. [Google Scholar] [CrossRef]
- Abdolahi, M.; Sarraf, P.; Javanbakht, M.H.; Honarvar, N.M.; Hatami, M.; Soveyd, N.; Tafakhori, A.; Sedighiyan, M.; Djalali, M.; Jafarieh, A.; et al. A Novel Combination of omega-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study. CNS Neurol. Disord. Drug Targets 2018, 17, 430–438. [Google Scholar] [CrossRef]
- Abdolahi, M.; Tafakhori, A.; Togha, M.; Okhovat, A.A.; Siassi, F.; Eshraghian, M.R.; Sedighiyan, M.; Djalali, M.; Mohammadzadeh Honarvar, N.; Djalali, M. The synergistic effects of omega-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-alpha gene expression and serum level in migraine patients. Immunogenetics 2017, 69, 371–378. [Google Scholar] [CrossRef]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2020, 41, 101256. [Google Scholar] [CrossRef]
- Djalali, M.; Djalali, M.; Abdolahi, M.; Mohammadi, H.; Heidari, H.; Hosseini, S.; Sadeghizadeh, M. The Effect of Nano-Curcumin Supplementation on Pentraxin 3 Gene Expression and Serum Level in Migraine Patients. Rep. Biochem. Mol. Biol. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Honarvar, N.M.; Soveid, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Karzar, N.H. Anti-Neuroinflammatory Properties of n-3 Fatty Acids and Nano-Curcumin on Migraine Patients, from Cellular to Clinical Insight: A Randomized, Double Blind, and Placebo-Controlled Trial. Endocr. Metab. Immune Disord. Drug Targets 2020, 29, 29. [Google Scholar] [CrossRef]
- Chiu, S.S.; Woodbury-Farina, M.; Terpstra, K.; Badmaev, V.; Cernovsky, Z.; Jirui Hou, J.; Raheb, H.; Husni, M.; Copen, J.; Shad, M.; et al. Translating curry extract to novel therapeutic approach in schizophrenia: The emerging role of epigenetics signaling. Planta Med. Int. Open 2018, 5, DM02. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Guloksuz, S.; Tek, C. Effects of Curcumin on Cognitive Functioning and Inflammatory State in Schizophrenia: A Double-Blind, Placebo-Controlled Pilot Trial. J. Clin. Psychopharmacol. 2019, 39, 182–184. [Google Scholar] [CrossRef]
- Miodownik, C.; Lerner, V.; Kudkaeva, N.; Lerner, P.P.; Pashinian, A.; Bersudsky, Y.; Eliyahu, R.; Kreinin, A.; Bergman, J. Curcumin as Add-On to Antipsychotic Treatment in Patients With Chronic Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Neuropharmacol. 2019, 30, 30. [Google Scholar] [CrossRef]
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Karunaratne, K.; Davis, M.C.; Marder, S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr Res. 2018, 195, 572–573. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients With Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurother 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Caldarazzo Ienco, E.; Bisordi, C.; Chico, L.; Lo Gerfo, A.; Fabbrini, M.; Rossi, M.; Petrozzi, L.; Rocchi, A.; Siciliano, G. High bioavailability curcumin and motor neuron degeneration: Results of a pilot therapeutic trial in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 236. [Google Scholar] [CrossRef]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Simoncini, C.; Schirinzi, E.; Ricci, G.; Chico, L.; Govoni, A. Amyotrophic lateral sclerosis and Curcumin: A double-blind, placebo-controlled clinical trial. Eur. J. Neurol. 2020, 27, 874. [Google Scholar]
- Dolati, S.; Aghebati-Maleki, L.; Ahmadi, M.; Marofi, F.; Babaloo, Z.; Ayramloo, H.; Jafarisavari, Z.; Oskouei, H.; Afkham, A.; Younesi, V.; et al. Nanocurcumin restores aberrant miRNA expression profile in multiple sclerosis, randomized, double-blind, placebo-controlled trial. J. Cell. Physiol. 2018, 233, 5222–5230. [Google Scholar] [CrossRef]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Schettino, C.; Polverino, P.; Allocca, S.; Adelfi, L.; D’Amico, A.; Capaldo, G.; Varriale, B.; Di Salle, A.; Peluso, G.; et al. Synergistic interplay between curcumin and polyphenol-rich foods in the mediterranean diet: Therapeutic prospects for neurofibromatosis 1 patients. Nutrients 2017, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Shadnoush, M.; Zahedi, H.; Norouzy, A.; Sahebkar, A.; Sadeghi, O.; Najafi, A.; Hosseini, S.; Qorbani, M.; Ahmadi, A.; Ardehali, S.H.; et al. Effects of supplementation with curcuminoids on serum adipokines in critically ill patients: A randomized double-blind placebo-controlled trial. Phytother. Res. 2020, 34, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Gholami, M.S.; Siassi, F.; Qorbani, M.; Sotoudeh, G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2020, 34, 896–903. [Google Scholar] [CrossRef]
- Bergman, J.; Miodownik, C.; Bersudsky, Y.; Sokolik, S.; Lerner, P.P.; Kreinin, A.; Polakiewicz, J.; Lerner, V. Curcumin as an add-on to antidepressive treatment: A randomized, double-blind, placebo-controlled, pilot clinical study. Clin. Neuropharmacol. 2013, 36, 73–77. [Google Scholar] [CrossRef]
- Decker, C. Curcumin Comparable to Fluoxetine for Treatment of Major Depressive Disorder. Intern. Med. Alert 2014, 17, 23. [Google Scholar]
- Esmaily, H.; Sahebkar, A.; Iranshahi, M.; Ganjali, S.; Mohammadi, A.; Ferns, G.; Ghayour-Mobarhan, M. An investigation of the effects of curcumin on anxiety and depression in obese individuals: A randomized controlled trial. Chin. J. Integr. Med. 2015, 21, 332–338. [Google Scholar] [CrossRef]
- Kanchanatawan, B.; Tangwongchai, S.; Sughondhabhirom, A.; Suppapitiporn, S.; Hemrunrojn, S.; Carvalho, A.F.; Maes, M. Add-on Treatment with Curcumin Has Antidepressive Effects in Thai Patients with Major Depression: Results of a Randomized Double-Blind Placebo-Controlled Study. Neurotox. Res. 2018, 33, 621–633. [Google Scholar] [CrossRef]
- Kawasaki, K.; Muroyama, K.; Murosaki, S. Effect of a water extract of Curcuma longa on emotional states in healthy participants. Bmfh 2018, 37, 25–29. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. An Exploratory Analysis of Changes in Mental Wellbeing Following Curcumin and Fish Oil Supplementation in Middle-Aged and Older Adults. Nutrients 2020, 12, 2902. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Efficacy of curcumin, and a saffron/curcumin combination for the treatment of major depression: A randomised, double-blind, placebo-controlled study. J. Affect. Disord. 2017, 207, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maes, M.; Meddens, M.J.; Maker, G.L.; Arnoldussen, E.; Drummond, P.D. Curcumin and major depression: A randomised, double-blind, placebo-controlled trial investigating the potential of peripheral biomarkers to predict treatment response and antidepressant mechanisms of change. Eur. Neuropsychopharmacol. 2015, 25, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Badeli, R.; Karami, G.R.; Sahebkar, A. Investigation of the efficacy of adjunctive therapy with bioavailability-boosted curcuminoids in major depressive disorder. Phytother. Res. 2015, 29, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res. 2014, 28, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150, 3190–3199. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Ross, S.M. Curcuma longa (Theracumin): A Bioavailable Form of Curcumin and Its Cognitive Benefits. Holist. Nurs. Pract. 2018, 32, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Lubieniecki, K.L.; Rossman, M.J.; Van Ark, H.J.; Bassett, C.J.; Strahler, T.R.; Chonchol, M.B.; Justice, J.N.; Seals, D.R. Curcumin supplementation and motor-cognitive function in healthy middle-aged and older adults. Nutr. Healthy Aging 2018, 4, 323–333. [Google Scholar] [CrossRef]
- Seen, W.P.; Mun, T.Y.; Mohanty, B.K.; Ebenezer, E.; Sugathan, S. Curcumin consumption and cognitive function in elderly. Int. J. Pharm. Sci. Res. 2017, 8, 5367–5372. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Abbas, S.H.; Abdulridha, M.K.; Najeb, A.A. Potential benefit of curcumin adjuvant therapy to the standard Helicobacter pylori eradication therapy in patients with peptic ulcer disease. Asian J. Pharm. Clin. Res. 2017, 10, 313–317. [Google Scholar] [CrossRef]
- Judaki, A.; Rahmani, A.; Feizi, J.; Asadollahi, K.; Hafezi Ahmadi, M.R. Curcumin in Combination with Triple Therapy Regimes Ameliorates Oxidative Stress and Histopathologic Changes in Chronic Gastritis-Associated Helicobacter Pylori Infection. Arq. De Gastroenterol. 2017, 54, 177–182. [Google Scholar] [CrossRef]
- Khonche, A.; Biglarian, O.; Panahi, Y.; Valizadegan, G.; Soflaei, S.S.; Ghamarchehreh, M.E.; Majeed, M.; Sahebkar, A. Adjunctive Therapy with Curcumin for Peptic Ulcer: A Randomized Controlled Trial. Drug Res. 2016, 66, 444–448. [Google Scholar] [CrossRef]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Kositchaiwat, C.; Kositchaiwat, S.; Havanondha, J. Curcuma longa Linn. in the treatment of gastric ulcer comparison to liquid antacid: A controlled clinical trial. J. Med. Assoc. Thail. 1993, 76, 601–605. [Google Scholar]
- Patcharatrakul, T.; Vutrapongwatana, U.; Phromchampa, W.; Chaiwatanarat, T.; Werawatganon, D.; Gonlachanvit, S. Effects of 4-week curcuminoids on clinical and gastric functions in patients with overlapping gastroesophageal reflux disease (GERD) and functional dyspepsia (FD): A randomized control study. Gastroenterology 2020, 158, S1148. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Vutrapongwatana, U.; Phromchampa, W.; Chaiwatanarat, T.; Werawatganon, D.; Gonlachanvit, S. Acute effect of curcuminoid on esophageal and gastric physiology: A randomized corss-over trial in gastroesophageal reflux disease (GERD) patients with positive pH monitoring. Gastroenterology 2020, 158, S1075–S1076. [Google Scholar] [CrossRef]
- Prucksunand, C.; Indrasukhsri, B.; Leethochawalit, M.; Hungspreugs, K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J. Trop. Med. Public Health 2001, 32, 208–215. [Google Scholar]
- Puttapitakpong, C.; Jearjesdakul, J. Effectiveness of curcuma longa linn compared with omeprazole on treatment of functional dyspepsia. Gastroenterology 2016, 150, S43. [Google Scholar] [CrossRef]
- Rawat, N.; McAdam, E.; Alhamdani, A.; Cronin, J.G.; Lewis, P.D.; Griffiths, P.; Manson, J.M.; Caplin, S.; Brown, T.H.; Baxter, J.; et al. Oral curcumin suppresses NF-κB activity in Barrett’s esophagus: A pilot study. Gastroenterology 2009, 136, A297. [Google Scholar] [CrossRef]
- Szymanski, M.C.; Gillum, T.L.; Gould, L.M.; Morin, D.S.; Kuennen, M.R. Short-term dietary curcumin supplementation reduces gastrointestinal barrier damage and physiological strain responses during exertional heat stress. J. Appl. Physiol. 2018, 124, 330–340. [Google Scholar] [CrossRef]
- Van Dau, N.; Ham, N.N.; Khac, D.H.; Lam, N.T.; Son, P.T.; Tan, N.T.; Van, D.D.; Dahlgren, S.; Grabe, M.; Johansson, R.; et al. The effects of a traditional drug, turmeric (Curcuma longa), and placebo on the healing of duodenal ulcer. Phytomedicine 1998, 5, 29–34. [Google Scholar] [CrossRef]
- Yongwatana, K.; Harinwan, K.; Chirapongsathorn, S.; Opuchar, K.; Sanpajit, T.; Piyanirun, W.; Puttapitakpong, C. Curcuma long Linn versus omeprazole in treatment of functonal dyspepsia, a randomized, double-blind, pacebo-controlled trial. Gastroenterology 2019, 156, S171. [Google Scholar] [CrossRef]
- Bommelaer, G.; Laharie, D.; Nancey, S.; Hebuterne, X.; Roblin, X.; Nachury, M.; Peyrin-Biroulet, L.; Fumery, M.; Richard, D.; Pereira, B.; et al. Oral Curcumin No More Effective Than Placebo in Preventing Recurrence of Crohn’s Disease After Surgery in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ikeya, K.; Bamba, S.; Andoh, A.; Yamasaki, H.; Mitsuyama, K.; Nasuno, M.; Tanaka, H.; Matsuura, A.; Kato, M.; et al. Highly bioavailable curcumin derivative ameliorates Crohn’s disease symptoms: A randomized, double-blind, multicenter study. J. Crohn Colitis 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Pitisuttithum, P.; Patcharatrakul, T.; Werawatganon, D.; Gonlachanvit, S. A randomized controlled study on the effects of curcuminoid on instestinal permeability evaluated by urine lactulose mannitol ratio (LNR) after aspirin ingestion. Gastroenterology 2019, 156, S504. [Google Scholar] [CrossRef]
- Tuntipopipat, S.; Judprasong, K.; Zeder, C.; Wasantwisut, E.; Winichagoon, P.; Charoenkiatkul, S.; Hurrell, R.; Walczyk, T. Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J. Nutr. 2006, 136, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Booth, J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J. Altern. Complement. Med. 2004, 10, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.J.; Hunter, J.O. A double blind, placebo controlled randomised trial of Curcuma extract in the treatment of steroid dependent inflammatory bowel disease. Gastroenterology 2003, 124, A205. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Penmetsa, A.; Kathi, P.; Girish, G.; Goren, I.; Reddy, D.N. Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-controlled Pilot Study. J. Clin. Gastroenterol. 2020, 55, 702–708. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef]
- Kedia, S.; Bhatia, V.; Thareja, S.; Garg, S.; Mouli, V.P.; Bopanna, S.; Tiwari, V.; Makharia, G.; Ahuja, V. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 147–154. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, U.; Shah, J.; Singh, P.; Vaishnavi, C.; Prasad, K.K.; Singh, K. Impact of curcuma longa on clinical activity and inflammatory markers in patients with active ulcerative colitis: A double-blind randomised placebo-controlled trial. J. Crohns Colitis 2019, 13, S322–S323. [Google Scholar] [CrossRef]
- Masoodi, M.; Mahdiabadi, M.A.; Mokhtare, M.; Agah, S.; Kashani, A.H.F.; Rezadoost, A.M.; Sabzikarian, M.; Talebi, A.; Sahebkar, A. The efficacy of curcuminoids in improvement of ulcerative colitis symptoms and patients’ self-reported well-being: A randomized double-blind controlled trial. J. Cell. Biochem. 2018, 119, 9552–9559. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Mansoori, A.; Shayesteh, A.; Hashemi, S.J. The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytother. Res. 2020, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Salomon, N.; Lang, A.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Wu, J.; Ching, J.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin Add-on Therapy for Remission Induction in Mild-moderate Active Ulcerative Colitis: A Multi-center, Randomized, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1381–1382. [Google Scholar] [CrossRef]
- Agarwal, K.A.; Tripathi, C.D.; Agarwal, B.B.; Saluja, S. Efficacy of turmeric (curcumin) in pain and postoperative fatigue after laparoscopic cholecystectomy: A double-blind, randomized placebo-controlled study. Surg. Endosc. 2011, 25, 3805–3810. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.E.; Nelson, K.M.; Gossard, A.A.; Carey, E.J.; Tabibian, J.H.; Lindor, K.D.; LaRusso, N.F. Efficacy and safety of curcumin in primary sclerosing cholangitis: An open label pilot study. Scand. J. Gastroenterol. 2019, 54, 633–639. [Google Scholar] [CrossRef]
- Ghaffarzadegan, T.; Zanzer, Y.C.; Ostman, E.; Hallenius, F.; Essen, S.; Sandahl, M.; Nyman, M. Postprandial Responses of Serum Bile Acids in Healthy Humans after Ingestion of Turmeric before Medium/High-Fat Breakfasts. Mol. Nutr. Food Res. 2019, 63, e1900672. [Google Scholar] [CrossRef] [PubMed]
- Rasyid, A.; Lelo, A. The effect of curcumin and placebo on human gall-bladder function: An ultrasound study. Aliment. Pharmacol. Ther. 1999, 13, 245–249. [Google Scholar] [CrossRef]
- Rasyid, A.; Rahman, A.R.; Jaalam, K.; Lelo, A. The chronopharmacological effect of curcumin on human gall-bladder. Med. J. Indones. 2001, 10, 219–223. [Google Scholar] [CrossRef]
- Rasyid, A.; Rahman, A.R.; Jaalam, K.; Lelo, A. Effect of different curcumin dosages on human gall bladder. Asia Pac. J. Clin. Nutr. 2002, 11, 314–318. [Google Scholar] [CrossRef]
- Abd El-Ghany, M.A.; Emad, A.S.; Amani, M.I. Nutraceutical effects of some Egyptian herbs on liver failure patients. World J. Med. Sci. 2014, 10, 1–11. [Google Scholar] [CrossRef]
- Kertia, N.; Asdie, A.H.; Rochmah, W.; Marsetyawan. Comparison of the effects of curcuminoid from Curcuma domestica Val. rhizome extract and diclofenac sodium on the liver function of patients with osteoarthritis. J. Pharmacogn. Phytother. 2012, 4, 62–65. [Google Scholar] [CrossRef]
- Kim, S.W.; Ha, K.C.; Choi, E.K.; Jung, S.Y.; Kim, M.G.; Kwon, D.Y.; Yang, H.J.; Kim, M.J.; Kang, H.J.; Back, H.I.; et al. The effectiveness of fermented turmeric powder in subjects with elevated alanine transaminase levels: A randomised controlled study. BMC Complement. Altern. Med. 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- N, T.K.; Thomas, J.V.; Nair, S.S.; J, N.M.; Maliakel, B.P.; Krishnakumar, I.M. A Novel Curcumin-Galactomannoside Complex Delivery System Improves Hepatic Function Markers in Chronic Alcoholics: A Double-Blinded, randomized, Placebo-Controlled Study. BioMed Res. Int. 2018, 2018, 9159281. [Google Scholar] [CrossRef]
- Nouri-Vaskeh, M.; Afshan, H.; Malek Mahdavi, A.; Alizadeh, L.; Fan, X.; Zarei, M. Curcumin ameliorates health-related quality of life in patients with liver cirrhosis: A randomized, double-blind placebo-controlled trial. Complement. Ther. Med. 2020, 49, 102351. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Vaskeh, M.; Malek Mahdavi, A.; Afshan, H.; Alizadeh, L.; Zarei, M. Effect of curcumin supplementation on disease severity in patients with liver cirrhosis: A randomized controlled trial. Phytother. Res. 2020, 34, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Ehtejab, G. A pilot clinical trial of radioprotective effects of curcumin supplementation in patients with prostate cancer. J. Cancer Sci. Ther. 2013, 5, 320–324. [Google Scholar] [CrossRef]
- Hejazi, J.; Rastmanesh, R.; Taleban, F.A.; Molana, S.H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85. [Google Scholar] [CrossRef]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Scoccianti, M.; Togni, S.; Appendino, G.; Ciammaichella, G. Meriva, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: A pilot, product evaluation registry study. Panminerva Med. 2012, 54, 17–22. [Google Scholar]
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother. Res. 2019, 33, 370–378. [Google Scholar] [CrossRef]
- Garg, M.; Chintamani; Tandon, M.; Bamal, R. To study the role of curcumin in LABC patients undergoing NACT. Indian J. Surg. Oncol. 2013, 4, 174–175. [Google Scholar] [CrossRef]
- Kalluru, H.; Kondaveeti, S.S.; Telapolu, S.; Kalachaveedu, M. Turmeric supplementation improves the quality of life and hematological parameters in breast cancer patients on paclitaxel chemotherapy: A case series. Complement. Ther. Clin. Pract. 2020, 41, 101247. [Google Scholar] [CrossRef] [PubMed]
- Nct. Phase II Study of Curcumin vs. Placebo for Chemotherapy-Treated Breast Cancer Patients Undergoing Radiotherapy. 2012. Available online: https://clinicaltrials.gov/show/nct01740323 (accessed on 20 February 2023).
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G.R. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ryan Wolf, J.; Heckler, C.E.; Guido, J.J.; Peoples, A.R.; Gewandter, J.S.; Ling, M.; Vinciguerra, V.P.; Anderson, T.; Evans, L.; Wade, J.; et al. Oral curcumin for radiation dermatitis: A URCC NCORP study of 686 breast cancer patients. Support Care Cancer 2018, 26, 1543–1552. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L., Jr.; et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Correa, M.; Hylind, L.M.; Marrero, J.H.; Zahurak, M.L.; Murray-Stewart, T.; Casero, R.A., Jr.; Montgomery, E.A.; Iacobuzio-Donahue, C.; Brosens, L.A.; Offerhaus, G.J.; et al. Efficacy and Safety of Curcumin in Treatment of Intestinal Adenomas in Patients With Familial Adenomatous Polyposis. Gastroenterology 2018, 155, 668–673. [Google Scholar] [CrossRef]
- Gunther, J.R.; Chadha, A.S.; Yang, P.Y.; Munsell, M.F.; Das, P.; Delclos, M.E.; Foo, W.C.; Kaur, H.; Clemons, M.; Mathew, G.G.; et al. A Phase 2 Randomized Double Blinded Study Evaluating the Efficacy of Curcumin With Pre-Operative Chemoradiation for Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, E7. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Santosa, D.; Suharti, C.; Riwanto, I.; Dharmana, E.; Pangarsa, E.A.; Setiawan, B.; Suyono, S.; Lumban, T.M. The effects of curcumin addition on remission status of multiple myeloma patients. Hemasphere 2019, 3, 975. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Badmaev, V.; Manoharan, A.; Ramakrishna, R. The potential role of curcumin in patients with monoclonal gammopathy of undefined significance--its effect on paraproteinemia and the urinary N-telopeptide of type I collagen bone turnover marker. Clin. Cancer Res. 2009, 15, 5917–5922. [Google Scholar] [CrossRef]
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am. J. Hematol. 2012, 87, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Nct. Curcumin (Diferuloylmethane Derivative) With or Without Bioperine in Patients With Multiple Myeloma. 2005. Available online: https://clinicaltrials.gov/show/nct00113841 (accessed on 20 February 2023).
- Arun, P.; Sagayaraj, A.; Azeem Mohiyuddin, S.M.; Santosh, D. Role of turmeric extract in minimising mucositis in patients receiving radiotherapy for head and neck squamous cell cancer: A randomised, placebo-controlled trial. J. Laryngol. Otol. 2020, 134, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral administration of nanomicelle curcumin in the prevention of radiotherapy-induced mucositis in head and neck cancers. Spec. Care Dent. 2019, 39, 166–172. [Google Scholar] [CrossRef]
- Neetha, M.C.; Panchaksharappa, M.G.; Pattabhiramasastry, S.; Shivaprasad, N.V.; Venkatesh, U.G. Chemopreventive Synergism between Green Tea Extract and Curcumin in Patients with Potentially Malignant Oral Disorders: A Double-blind, Randomized Preliminary Study. J. Contemp. Dent. Pract. 2020, 21, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.V.; Paradkar, P.H.; Jagtap, S.S.; Agashe, S.V.; Soman, G.; Vaidya, A.B. Chemopreventive potential and safety profile of a Curcuma longa extract in women with cervical low-grade squamous intraepithelial neoplasia. Asian Pac. J. Cancer Prev. 2011, 12, 3305–3311. [Google Scholar]
- Purbadi, S.; Rustamadji, P.; Prijanti, A.R.; Sekarutami, S.M.; Sutrisna, B.; Suyatna, F.D.; Andrijono, A. Biocurcumin as Radiosensitiser for Cervical Cancer Study (BRACES): A Double-Blind Randomised Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2020, 2020, 10. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2018, 5, 138. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Parsons, H.A.; Baracos, V.E.; Hong, D.S.; Abbruzzese, J.; Bruera, E.; Kurzrock, R. The effects of curcumin (diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget 2016, 7, 20293–20304. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, D.; Tewari, M.; Shukla, H.S.; Roy, B.K. Impact of turmeric as dietary approach on HER2 status in blood of gastric cancer patients. Int. J. Phytomedicine 2014, 6, 293–299. [Google Scholar]
- Farhadi, M.; Bakhshandeh, M.; Shafiei, B.; Mahmoudzadeh, A.; Hosseinimehr, S.J. The radioprotective effects of nano-curcumin against genotoxicity induced by iodine-131 in patients with differentiated thyroid carcinoma (DTC) by micronucleus assay. Int. J. Cancer Manag. 2018, 11, e14193. [Google Scholar] [CrossRef]
- Sandoughdaran, S.; Razzaghdoust, A.; Tabibi, A.; Basiri, A.; Simforoosh, N.; Mofid, B. Randomized, Double-blind Pilot Study of Nanocurcumin in Bladder Cancer Patients Receiving Induction Chemotherapy. Urol. J. 2020, 04, 30. [Google Scholar] [CrossRef]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C.; et al. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother. Res. PTR 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, A. Curcumin in cancer supportive care: Hype or hope? Eur. J. Integr. Med. 2012, 4, 18–19. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Nouzari, S.M.H.; Jalalian, H.R.; Sahebkar, A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Sahebkar, A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1461–1467. [Google Scholar] [CrossRef]
- Prasongsook, N.; Sitalarom, K.; Saichaemchan, S.; Peechatanan, K.; Chaiworramukkul, A. A double-blind, placebo-controlled randomized phase II study: Evaluating the effect of curcumin for treatment of cancer anorexia-cachexia syndrome in solid cancer patients. J. Clin. Oncol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Tanabe, Y.; Sugawara, J.; Ajisaka, R. Effects of curcumin intake and aerobic exercise training on arterial compliance in postmenopausal women. Artery Res. 2013, 7, 67–72. [Google Scholar] [CrossRef]
- Aslanabadi, N.; Entezari-Maleki, T.; Rezaee, H.; Jafarzadeh, H.R.; Vahedpour, R. Curcumin for the prevention of myocardial injury following elective percutaneous coronary intervention; a pilot randomized clinical trial. Eur. J. Pharmacol. 2019, 858, 172471. [Google Scholar] [CrossRef] [PubMed]
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.A.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial variability across individuals in the vascular response and nutrigenomic response to an acute intake of curcumin: A randomised controlled trial. J. Hypertens. 2018, 36, e97. [Google Scholar] [CrossRef]
- Campbell, M.S.; Berrones, A.J.; Krishnakumar, I.M.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Responsiveness to curcumin intervention is associated with reduced aortic stiffness in young, obese men with higher initial stiffness. J. Funct. Foods 2017, 29, 154–160. [Google Scholar] [CrossRef]
- Choi, Y.; Tanabe, Y.; Akazawa, N.; Zempo-Miyaki, A.; Maeda, S. Curcumin supplementation attenuates the decrease in endothelial function following eccentric exercise. J. Exerc. Nutr. Biochem. 2019, 23, 7–12. [Google Scholar] [CrossRef]
- Kuszewski, J.C.; Wong, R.H.X.; Wood, L.G.; Howe, P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Pour, A.H.; Dastani, M.; Salari, R.; Radbin, S.; Mehri, S.; Ghorbani, M.; Karimani, A.; Salari, M. Curcumin effects on myeloperoxidase, interleukin-18 and matrix metalloproteinase-9 inflammatory biomarkers in patients with unstable angina: A randomized clinical trial. Avicenna J. 2019, 9, 428–435. [Google Scholar]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jager, R. Novel Form of Curcumin Improves Endothelial Function in Young, Healthy Individuals: A Double-Blind Placebo Controlled Study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Akazawa, N.; Miyaki, A.; Choi, Y.; Tanabe, Y.; Imai, T.; Maeda, S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: Pilot study. Am. J. Hypertens. 2012, 25, 651–656. [Google Scholar] [CrossRef]
- Sukardi, R.; Sastroasmoro, S.; Siregar, N.C.; Djer, M.M.; Suyatna, F.D.; Sadikin, M.; Ibrahim, N.; Rahayuningsih, S.E.; Witarto, A.B. The role of curcumin as an inhibitor of oxidative stress caused by ischaemia re-perfusion injury in tetralogy of Fallot patients undergoing corrective surgery. Cardiol. Young 2016, 26, 431–438. [Google Scholar] [CrossRef]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Tanabe, Y.; Sugawara, J.; Ajisaka, R.; Maeda, S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 2012, 32, 795–799. [Google Scholar] [CrossRef]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med. 2008, 40, 201–210. [Google Scholar]
- Dastani, M.; Bigdelu, L.; Hoseinzadeh, M.; Rahimi, H.R.; Karimani, A.; Hooshang Mohammadpour, A.; Salari, M. The effects of curcumin on the prevention of atrial and ventricular arrhythmias and heart failure in patients with unstable angina: A randomized clinical trial. Avicenna J. 2019, 9, 1–9. [Google Scholar]
- Garg, A.X.; Devereaux, P.J.; Hill, A. Oral curcumin in elective abdominal aortic aneurysm repair: A multicentre randomized controlled trial. Can. Med. Assoc. J. 2018, 190, E1425. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Wada, H.; Sunagawa, Y.; Fujita, M.; Kakeya, H.; Imaizumi, A.; Hashimoto, T.; Akao, M.; Katanasaka, Y.; Osakada, G.; et al. Highly absorptive curcumin improves left ventricular diastolic function regardless of blood pressure in hypertensive patients. J. Am. Coll. Cardiol. 2012, 59, E987. [Google Scholar] [CrossRef]
- Phrommintikul, A.; Chanchai, R.; Wongcharoen, W. Effects of Curcuminoids on Myocardial Injury After Percutaneous Coronary Intervention. J. Med. Food 2019, 22, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Bosca, A.; Carrion Gutierrez, M.A.; Soler, A.; Puerta, C.; Diez, A.; Quintanilla, E.; Bernd, A.; Miquel, J. Effects of the antioxidant turmeric on lipoprotein peroxides: Implications for the prevention of atherosclerosis. Age 1997, 20, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Jia, H.; Li, Y.; Li, J. Curcumin improves treatment outcome of Takayasu arteritis patients by reducing TNF-alpha: A randomized placebo-controlled double-blind clinical trial. Immunol. Res. 2017, 65, 969–974. [Google Scholar] [CrossRef]
- Wongcharoen, W.; Jai-Aue, S.; Phrommintikul, A.; Nawarawong, W.; Woragidpoonpol, S.; Tepsuwan, T.; Sukonthasarn, A.; Apaijai, N.; Chattipakorn, N. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am. J. Cardiol. 2012, 110, 40–44. [Google Scholar] [CrossRef]
- Ara, S.A.; Mudda, J.; Lingappa, A.; Rao, P.; Zakaullah, S. Efficacy of curcumin in oral submucous fibrosis-A randomized controlled clinical trial. Int. J. Pharm. Sci. Res. 2018, 9, 5277–5286. [Google Scholar]
- Chainani-Wu, N.; Collins, K.; Silverman, S., Jr. Use of curcuminoids in a cohort of patients with oral lichen planus, an autoimmune disease. Phytomedicine 2012, 19, 418–423. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Madden, E.; Lozada-Nur, F.; Silverman, S., Jr. High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 2012, 66, 752–760. [Google Scholar] [CrossRef]
- Chainani-Wu, N.; Silverman, S., Jr.; Reingold, A.; Bostrom, A.; Mc Culloch, C.; Lozada-Nur, F.; Weintraub, J. A randomized, placebo-controlled, double-blind clinical trial of curcuminoids in oral lichen planus. Phytomedicine 2007, 14, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Diana, H.; Cahyanto, A.; Amaliya, A.; Hardianto, A.; Maulina, T. The efficacy of curcuminoid in treating acute inflammation pain. Pain Pract. 2018, 18, 80. [Google Scholar]
- Kia, S.J.; Basirat, M.; Mortezaie, T.; Moosavi, M.S. Comparison of oral Nano-Curcumin with oral prednisolone on oral lichen planus: A randomized double-blinded clinical trial. BMC Complement. Med. Ther. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; R, P.; et al. A Randomized Double-Blind Placebo-Controlled Phase IIB Trial of Curcumin in Oral Leukoplakia. Cancer Prev. Res. 2016, 9, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lutfikadila, G.; Nurwiadh, A.; Cahyanto, A.; Amaliya, A.; Maulina, T. Effectiveness of curcuminoid dosage on inflammatory pain in acute periapical abscess. Pain Pract. 2018, 18, 83. [Google Scholar]
- Majeed, M.; Majeed, S.; Nagabhushanam, K. Efficacy and Safety of Tetrahydrocurcuminoids for the Treatment of Canker Sore and Gingivitis. Evid. Based Complement. Altern. Med. 2020, 2020, 6611877. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Kia, S.J.; Mashaei, L.; Moosavi, M.S. Oral nano-curcumin on gingival inflammation in patients with gingivitis and mild periodontitis. Clin. Exp. Dent. Res. 2020, 21, 21. [Google Scholar] [CrossRef]
- Maulina, T.; Diana, H.; Cahyanto, A.; Amaliya, A. The efficacy of curcumin in managing acute inflammation pain on the post-surgical removal of impacted third molars patients: A randomised controlled trial. J. Oral Rehabil. 2018, 45, 677–683. [Google Scholar] [CrossRef]
- Nct. A Clinical Study of Curcuminoids in the Treatment of Oral Lichen Planus. 2007. Available online: https://clinicaltrials.gov/show/nct00525421 (accessed on 20 February 2023).
- Piyush, P.; Mahajan, A.; Singh, K.; Ghosh, S.; Gupta, S. Comparison of therapeutic response of lycopene and curcumin in oral submucous fibrosis: A randomized controlled trial. Oral Dis. 2019, 25, 73–79. [Google Scholar] [CrossRef]
- Rai, A.; Kaur, M.; Gombra, V.; Hasan, S.; Kumar, N. Comparative evaluation of curcumin and antioxidants in the management of oral submucous fibrosis. J. Investig. Clin. Dent. 2019, 10, e12464. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Jacobs, R.; Singh, J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J. Oral Sci. 2010, 52, 251–256. [Google Scholar] [CrossRef]
- Saran, G.; Umapathy, D.; Misra, N.; Channaiah, S.G.; Singh, P.; Srivastava, S.; Shivakumar, S. A comparative study to evaluate the efficacy of lycopene and curcumin in oral submucous fibrosis patients: A randomized clinical trial. Indian J. Dent. Res. 2018, 29, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Aravinda, K.; Saxena, V.S.; Srinivas, K.; Ratnakar, P.; Gupta, J.; Sachdev, A.S.; Shivhare, P. Comparison of curcumin with intralesional steroid injections in Oral Submucous Fibrosis—A randomized, open-label interventional study. J. Oral Biol. Craniofacial Res. 2014, 4, 169–173. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin. Nutr. 2020, 39, 3594–3600. [Google Scholar] [CrossRef] [PubMed]
- Hami, M.; Bigdeli, A.; Khameneh Bagheri, R.; Rajabi, O.; Salehi, M.; Zahedi Avval, F. The effect of curcumin in prevention of contrast nephropathy following coronary angiography or angioplasty in CKD patients. Iran. J. Kidney Dis. 2019, 13, 304–309. [Google Scholar] [PubMed]
- Jimenez-Osorio, A.S.; Garcia-Nino, W.R.; Gonzalez-Reyes, S.; Alvarez-Mejia, A.E.; Guerra-Leon, S.; Salazar-Segovia, J.; Falcon, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The Effect of Dietary Supplementation With Curcumin on Redox Status and Nrf2 Activation in Patients With Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L.; Dehghanzadeh, G.R. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef]
- Khosravi, A.; Hashemi, H.; Farahani, M.M.; Dolatkhah, M.; Rostami, Z.; Panahi, Y. The effects of curcumin on left ventricular function in patients with chronic renal failure. Arch. Cardiovasc. Imaging 2015, 4, e38087. [Google Scholar] [CrossRef]
- Pakfetrat, M.; Akmali, M.; Malekmakan, L.; Dabaghimanesh, M.; Khorsand, M. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial. Int. 2015, 19, 124–131. [Google Scholar] [CrossRef]
- Pakfetrat, M.; Basiri, F.; Malekmakan, L.; Roozbeh, J. Effects of turmeric on uremic pruritus in end stage renal disease patients: A double-blind randomized clinical trial. J. Nephrol. 2014, 27, 203–207. [Google Scholar] [CrossRef]
- Sabaghian, T.; Gheydari, M.E.; Divani, F. Evaluation of Curcumin (Turmeric Extract) Effect on Prevention of CIN in Patient Under Elective Coronary Angiography, a Randomized Double Blind Placebocontrolled Clinical Trial. Iran. J. Kidney Dis. 2020, 14, 198–205. [Google Scholar]
- Samadian, F.; Dalili, N.; Poor-Reza Gholi, F.; Fattah, M.; Malih, N.; Nafar, M.; Firoozan, A.; Ahmadpoor, P.; Samavat, S.; Ziaie, S. Evaluation of Curcumin’s effect on inflammation in hemodialysis patients. Clin. Nutr. ESPEN 2017, 22, 19–23. [Google Scholar] [CrossRef]
- Trakarnvanich, T. SUN-025 Curcuminoids can prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures: A randomized controlled trial. Kidney Int. Rep. 2020, 5, S215. [Google Scholar] [CrossRef]
- Vafadar Afshar, G.; Rasmi, Y.; Yaghmaei, P.; Khadem-Ansari, M.H.; Makhdomii, K.; Rasooli, J. The Effects of Nano-curcumin Supplementation on Serum Level of hs-CRP, Adhesion Molecules, and Lipid Profiles in Hemodialysis Patients, A Randomized Controlled Clinical Trial. Iran. J. Kidney Dis. 2020, 14, 52–61. [Google Scholar] [PubMed]
- Vanaie, A.; Shahidi, S.; Iraj, B.; Siadat, Z.D.; Kabirzade, M.; Shakiba, F.; Mohammadi, M.; Parvizian, H. Curcumin as a major active component of turmeric attenuates proteinuria in patients with overt diabetic nephropathy. J. Res. Med. Sci. 2019, 24, 77. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, F.; Javadi, M.; Karami, A.A.; Gholaminejad, F.; Kavianpour, M.; Haghighian, H.K. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res. 2018, 32, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Ataei-Almanghadim, K.; Farshbaf-Khalili, A.; Ostadrahimi, A.R.; Shaseb, E.; Mirghafourvand, M. The effect of oral capsule of curcumin and vitamin E on the hot flashes and anxiety in postmenopausal women: A triple blind randomised controlled trial. Complement. Ther. Med. 2020, 48, 102267. [Google Scholar] [CrossRef] [PubMed]
- Fadinie, W.; Lelo, A.; Wijaya, D.W.; Lumbanraja, S.N. Curcumin’s Effect on COX-2 and IL-10 Serum in Preeclampsia’s Patient Undergo Sectio Caesarea with Spinal Anesthesia. Open Access Maced. J. Med. Sci. 2019, 7, 3376–3379. [Google Scholar] [CrossRef] [PubMed]
- Fadinie, W.; Lelo, A.; Wijaya, D.W.; Lumbanraja, S.N. Curcumin and its effect on preeclampsia: As anti-inflammatory, analgesic, and anticoagulant. Int. J. Curr. Pharm. Res. 2020, 12, 43–47. [Google Scholar] [CrossRef]
- Fanaei, H.; Khayat, S.; Kasaeian, A.; Javadimehr, M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Neuropeptides 2016, 56, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hesami, S.; Kavianpour, M.; Rashidi Nooshabadi, M.; Yousefi, M.; Lalooha, F.; Khadem Haghighian, H. Randomized, double-blind, placebo-controlled clinical trial studying the effects of Turmeric in combination with mefenamic acid in patients with primary dysmenorrhoea. J. Gynecol. Obstet. Hum. Reprod. 2020, 50, 101840. [Google Scholar] [CrossRef] [PubMed]
- Rajuddin, R.; Wiweko, B.; Nugroho, L. The effects of curcumin administration on expression patterns of VEGF and COX-2 in fertile endometrium: A randomised clinical trial. Int. J. Appl. Pharm. 2019, 11, 149–152. [Google Scholar] [CrossRef]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor gamma coactivator 1alpha gene expression in polycystic ovarian syndrome (PCOS) patients: A randomized placebo-controlled clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomedicine 2021, 80, 153395. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The effects of curcumin supplementation on glycemic status, lipid profile and hs-CRP levels in overweight/obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef]
- The effect of curcuma on chest x-ray improvement and sputum coversion in patients with intensive phase treatment of pulmonary tuberculosis. Respirology 2019, 24, 146.
- Abidi, A.; Gupta, S.; Agarwal, M.; Bhalla, H.L.; Saluja, M. Evaluation of Efficacy of Curcumin as an Add-on therapy in Patients of Bronchial Asthma. J. Clin. Diagn. Res. 2014, 8, HC19–HC24. [Google Scholar] [CrossRef]
- Jusufovic, E.; Kosnik, M.; Jusufovic, A.; Becarevic, M.; Al-Ahmad, M.; Nurkic, J.; Osmic, M.; Nadarevic, A.; Petrak, F.; Halilovic, D.; et al. Curcumin as an add-on therapy of moderate partially controlled asthma. Eur. Respir. J. 2017, 50, 442. [Google Scholar] [CrossRef]
- Jusufovic, E.; Kosnik, M.; Nurkic, J.; Arifhodzic, N.; Al-Ahmad, M.; Bulat-Kardum, L.; Becarevic, M.; Osmic, M.; Nadarevic, A.; Jusufovic, A.; et al. Curcumin Improves Therapy of Moderate Partially Controlled Asthma: Placebo-controlled, single blind study. Eur. Respir. J. 2019, 54, 2. [Google Scholar] [CrossRef]
- Kim, D.H.; Phillips, J.F.; Lockey, R.F. Oral curcumin supplementation in patients with atopic asthma. Allergy Rhinol Provid. 2011, 2, e51–e53. [Google Scholar] [CrossRef]
- Manarin, G.; Anderson, D.; Silva, J.M.E.; Coppede, J.D.S.; Roxo-Junior, P.; Pereira, A.M.S.; Carmona, F. Curcuma longa L. ameliorates asthma control in children and adolescents: A randomized, double-blind, controlled trial. J. Ethnopharmacol. 2019, 238, 111882. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ghanei, M.; Bashiri, S.; Hajihashemi, A.; Sahebkar, A. Short-term Curcuminoid Supplementation for Chronic Pulmonary Complications due to Sulfur Mustard Intoxication: Positive Results of a Randomized Double-blind Placebo-controlled Trial. Drug Res. 2015, 65, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ghanei, M.; Hajhashemi, A.; Sahebkar, A. Effects of Curcuminoids-Piperine Combination on Systemic Oxidative Stress, Clinical Symptoms and Quality of Life in Subjects with Chronic Pulmonary Complications Due to Sulfur Mustard: A Randomized Controlled Trial. J. Diet. Suppl. 2016, 13, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Bergonzi, M.C.; Isacchi, B.; Antiga, E.; Caproni, M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J. Pharm. Pharmacol. 2018, 70, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: A prospective clinical trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef]
- Panahi, Y.; Sahebkar, A.; Parvin, S.; Saadat, A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann. Clin. Biochem. 2012, 49, 580–588. [Google Scholar] [CrossRef]
- Sahebkar, A.; Panahi, Y.; Amiri, M.; Davoudi, S.M.; Beiraghdar, F.; Hoseininejad, S.L.; Kolivand, M. Promising improvement of sulfur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: Results of a randomized double-blind placebo-controlled trial. Clin. Biochem. 2011, 44, S338. [Google Scholar] [CrossRef]
- Feig, J.L.; Wang, R.; Lim, H.; Wade, K.; Liu, H.; Fahey, J.; Chien, A.L.; Kang, S. The impact of oral phytochemicals on ultraviolet B-induced erythema response in human skin. J. Investig. Dermatol. 2018, 138, S103. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Notay, M.; Sivamani, R.K. Randomized Controlled Pilot Study of Dietary Supplementation with Turmeric or Herbal Combination Tablets on Skin Barrier Function in Healthy Subjects. J. Med. Food 2018, 20, 20. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Pourang, A.; Clark, A.K.; Burney, W.; Sivamani, R.K. Dietary supplementation with turmeric polyherbal formulation decreases facial redness: A randomized double-blind controlled pilot study. J. Integr. Med. 2019, 17, 20–23. [Google Scholar] [CrossRef]
- Da Silva, T.A.L.; de Medeiros, D.C.; da Silva Cunha de Medeiros, R.C.; Medeiros, R.M.V.; de Souza, L.; de Medeiros, J.A.; Dos Santos, R.V.T.; de Alcantara Varela, P.W.; Leite-Lais, L.; Dantas, P.M.S. Influence of curcumin on glycemic profile, inflammatory markers, and oxidative stress in HIV-infected individuals: A randomized controlled trial. Phytother. Res. 2020, 34, 2323–2330. [Google Scholar] [CrossRef]
- Karimi, A.; Mahmoodpoor, A.; Kooshki, F.; Niazkar, H.R.; Shoorei, H.; Tarighat-Esfanjani, A. Effects of nanocurcumin on inflammatory factors and clinical outcomes in critically ill patients with sepsis: A pilot randomized clinical trial. Eur. J. Integr. Med. 2020, 36, 6. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Medeiros, D.C.; Medeiros, G.; Medeiros, R.; de Souza Araujo, J.; Medeiros, J.A.; Ururahy, M.A.G.; Santos, R.V.T.; Medeiros, R.M.V.; Leite-Lais, L.; et al. Influence of curcumin supplementation on metabolic and lipid parameters of people living with HIV/AIDS: A randomized controlled trial. BMC Complement. Altern. Med. 2019, 19, 202. [Google Scholar] [CrossRef]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef]
- Allegri, P.; Rissotto, R.; Rissotto, F.; Masala, A.; Crivelli, M.G.; Blanco, A.R.; Murialdo, U. Evaluation of the anti-inflammatory efficacy of high oral bioavailability Curcumin as addon treatment in non-infectious uveitic cystoid macular edema by SD-OCT and OCT-Angiography: Preliminary results. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5356. [Google Scholar]
- Allegri, P.; Mastromarino, A.; Neri, P. Management of chronic anterior uveitis relapses: Efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin. Ophthalmol. 2010, 4, 1201–1206. [Google Scholar] [CrossRef]
- Ferrara, M.; Allegrini, D.; Sorrentino, T.; Sborgia, G.; Parmeggiani, F.; Borgia, A.; Romano, M.R. Curcumin-Based Treatment for Macular Edema from Uncommon Etiologies: Efficacy and Safety Assessment. J. Med. Food 2020, 23, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Lal, B.; Kapoor, A.K.; Asthana, O.P.; Agrawal, P.K.; Prasad, R.; Kumar, P.; Srimal, R.C. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother. Res. 1999, 13, 318–322. [Google Scholar] [CrossRef]
- Kalpravidh, R.W.; Siritanaratkul, N.; Insain, P.; Charoensakdi, R.; Panichkul, N.; Hatairaktham, S.; Srichairatanakool, S.; Phisalaphong, C.; Rachmilewitz, E.; Fucharoen, S. Improvement in oxidative stress and antioxidant parameters in beta-thalassemia/Hb E patients treated with curcuminoids. Clin. Biochem. 2010, 43, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Tamaddoni, A.; Qujeq, D.; Nasseri, E.; Zayeri, F.; Zand, H.; Gholami, M.; Mir, S.M. An investigation of the effects of curcumin on iron overload, hepcidin level, and liver function in beta-thalassemia major patients: A double-blind randomized controlled clinical trial. Phytother. Res. 2018, 32, 1828–1835. [Google Scholar] [CrossRef]
- Nasseri, E.; Mohammadi, E.; Tamaddoni, A.; Qujeq, D.; Zayeri, F.; Zand, H. Benefits of Curcumin Supplementation on Antioxidant Status in beta-Thalassemia Major Patients: A Double-Blind Randomized Controlled Clinical Trial. Ann. Nutr. Metab. 2017, 71, 136–144. [Google Scholar] [CrossRef]
- Tamaddoni, A.; Nasseri, E.; Mohammadi, E.; Qujeq, D.; Zayeri, F.; Zand, H.; Mir, S.M.; Gholami, M. A Double-blind Randomized Controlled Trial of Curcumin for Improvement in Glycemic Status, Lipid Profile and Systemic Inflammation in β-Thalassemia Major. J. Herb. Med. 2020, 21, 100324. [Google Scholar] [CrossRef]
- Biswas, J.; Sinha, D.; Mukherjee, S.; Roy, S.; Siddiqi, M.; Roy, M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum. Exp. Toxicol. 2010, 29, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Falgiano, P.A.; Gillum, T.L.; Schall, Z.J.; Strag, H.R.; Kuennen, M.R. Dietary curcumin supplementation does not alter peripheral blood mononuclear cell responses to exertional heat stress. Eur. J. Appl. Physiol. 2018, 118, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Klickovic, U.; Doberer, D.; Gouya, G.; Aschauer, S.; Weisshaar, S.; Storka, A.; Bilban, M.; Wolzt, M. Human pharmacokinetics of high dose oral curcumin and its effect on heme oxygenase-1 expression in healthy male subjects. Biomed Res. Int. 2014, 2014, 458592. [Google Scholar] [CrossRef] [PubMed]
- Laine, F.; Laviolle, B.; Bardou-Jacquet, E.; Fatih, N.; Jezequel, C.; Collet, N.; Ropert, M.; Morcet, J.; Hamon, C.; Reymann, J.M.; et al. Curcuma decreases serum hepcidin levels in healthy volunteers: A placebo-controlled, randomized, double-blind, cross-over study. Fundam. Clin. Pharmacol. 2017, 31, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Sut, S.; Dal Ben, S.; Voinovich, D.; Dall’Acqua, S. Untargeted UPLC-MS metabolomics reveals multiple changes of urine composition in healthy adult volunteers after consumption of curcuma longa L. extract. Food Res. Int. Ott. Ont. 2020, 127, 108730. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Kaur, J.; Catalina, M. Anti-oxidation actions of curcumin in two forms of bed rest: Oxidative stress serum and salivary markers. Asian Pac. J. Trop. Med. 2010, 3, 651–654. [Google Scholar] [CrossRef]
- Roy, M.; Sinha, D.; Mukherjee, S.; Biswas, J. Curcumin prevents DNA damage and enhances the repair potential in a chronically arsenic-exposed human population in West Bengal, India. Eur. J. Cancer Prev. 2011, 20, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar] [PubMed]
- Wu, S.; Xiao, D. Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis. Ann. Allergy Asthma Immunol. 2016, 117, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Higgins, J.P.; Clayton, G.; Sterne, J.A.; Hróbjartsson, A.; Savović, J. Empirical Evidence of Study Design Biases in Randomized Trials: Systematic Review of Meta-Epidemiological Studies. PLoS ONE 2016, 11, e0159267. [Google Scholar] [CrossRef]
- Kaptchuk, T.J. The double-blind, randomized, placebo-controlled trial: Gold standard or golden calf? J. Clin. Epidemiol. 2001, 54, 541–549. [Google Scholar] [CrossRef]
- Apridonidze, T.; Essah, P.A.; Iuorno, M.J.; Nestler, J.E. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1929–1935. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Davis, M.A.; Ettinger, W.H.; Neuhaus, J.M. Obesity and osteoarthritis of the knee: Evidence from the National Health and Nutrition Examination Survey (NHANES I). Semin. Arthritis Rheum. 1990, 20, 34–41. [Google Scholar] [CrossRef]
- Rücker, G.; Carpenter, J.R.; Schwarzer, G. Detecting and adjusting for small-study effects in meta-analysis. Biom. J. 2011, 53, 351–368. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Adult Obesity Facts. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 31 January 2023).
- Centers for Disease Control and Prevention. Prevalence of Prediabetes Among Adults. Available online: https://www.cdc.gov/diabetes/data/statistics-report/prevalence-of-prediabetes.html (accessed on 31 January 2023).
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 31 January 2023).
- Xu, Y.; Wu, Q. Trends and disparities in osteoarthritis prevalence among US adults, 2005–2018. Sci. Rep. 2021, 11, 21845. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B 2015, 985, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.; Malhotra Mukhtyar, R.; Theofanous, D.; Pepper, C.; Thomas, A.; Brown, K.; Khan, S. A Systematic Review Assessing Clinical Utility of Curcumin with a Focus on Cancer Prevention. Mol. Nutr. Food Res. 2021, 65, e2000977. [Google Scholar] [CrossRef] [PubMed]
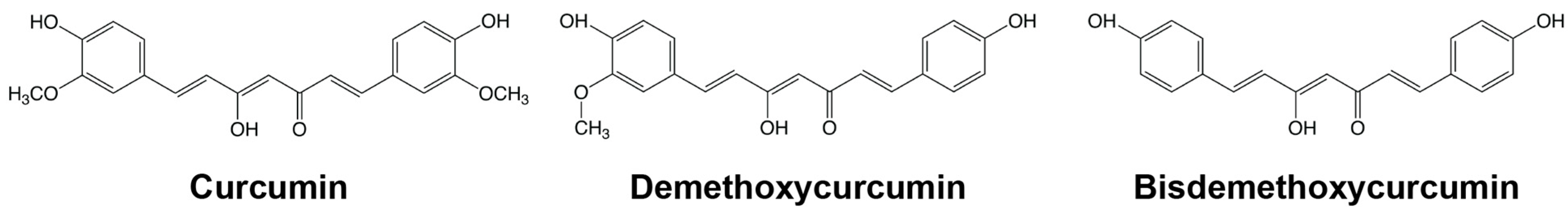
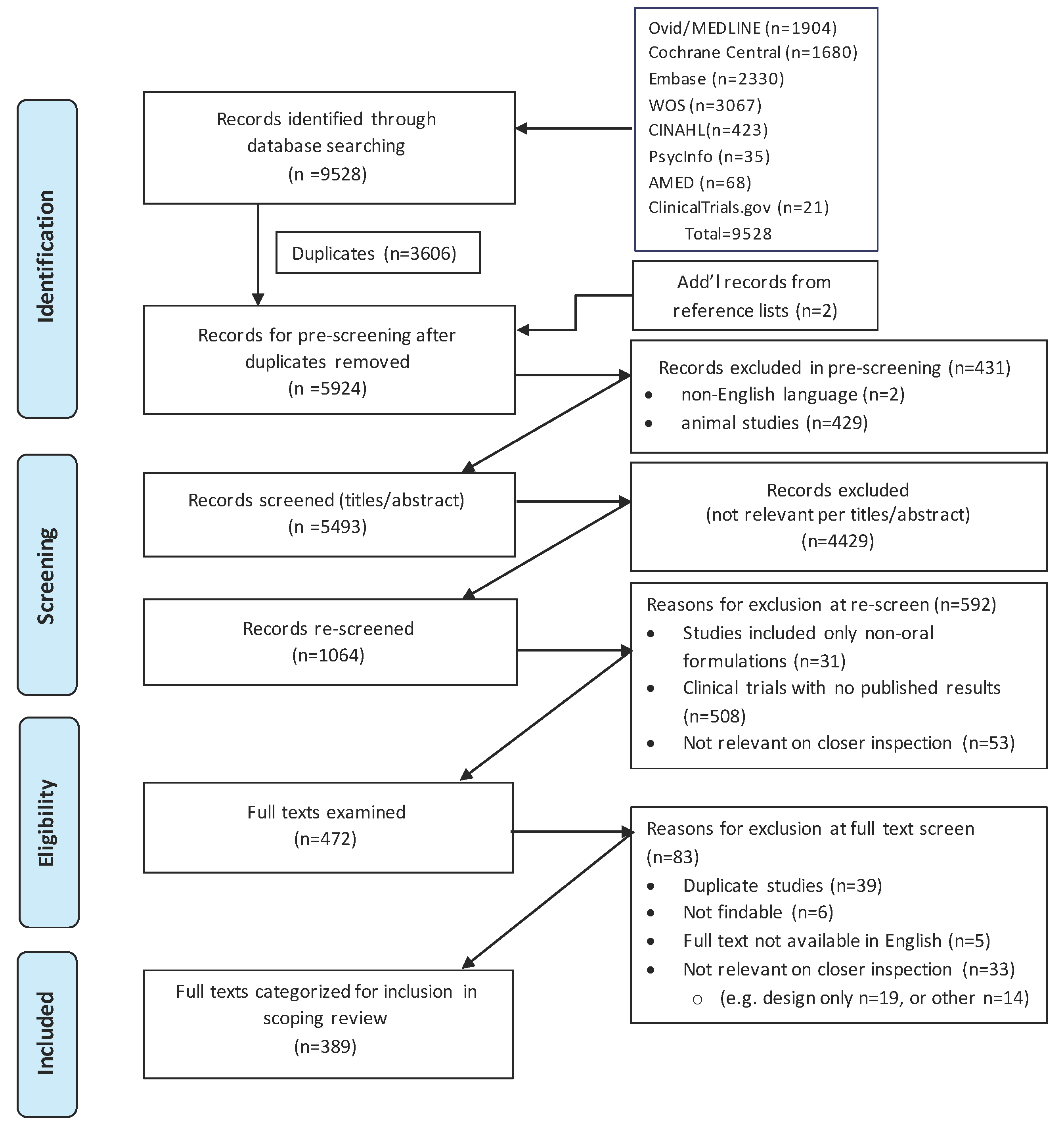
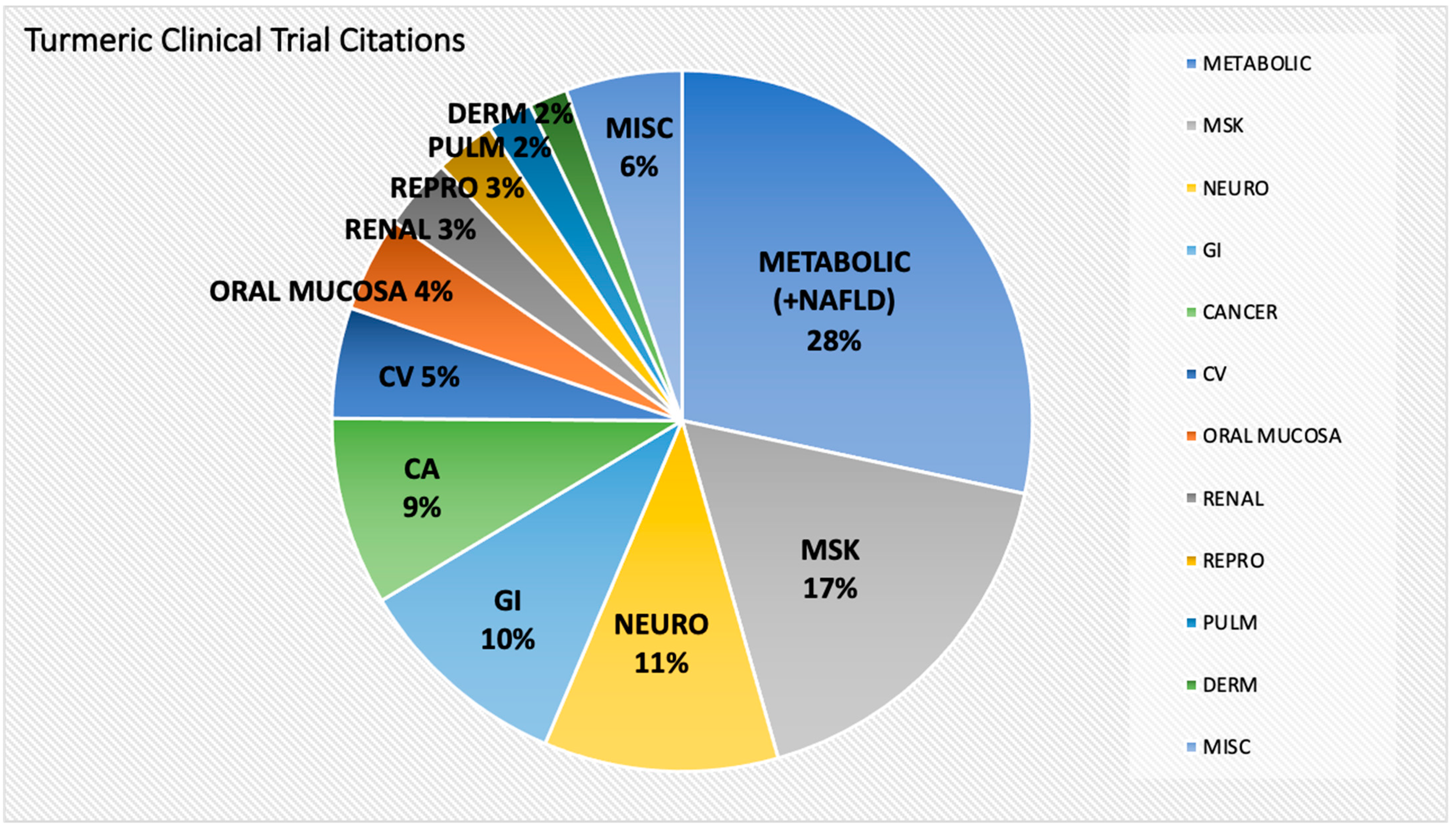
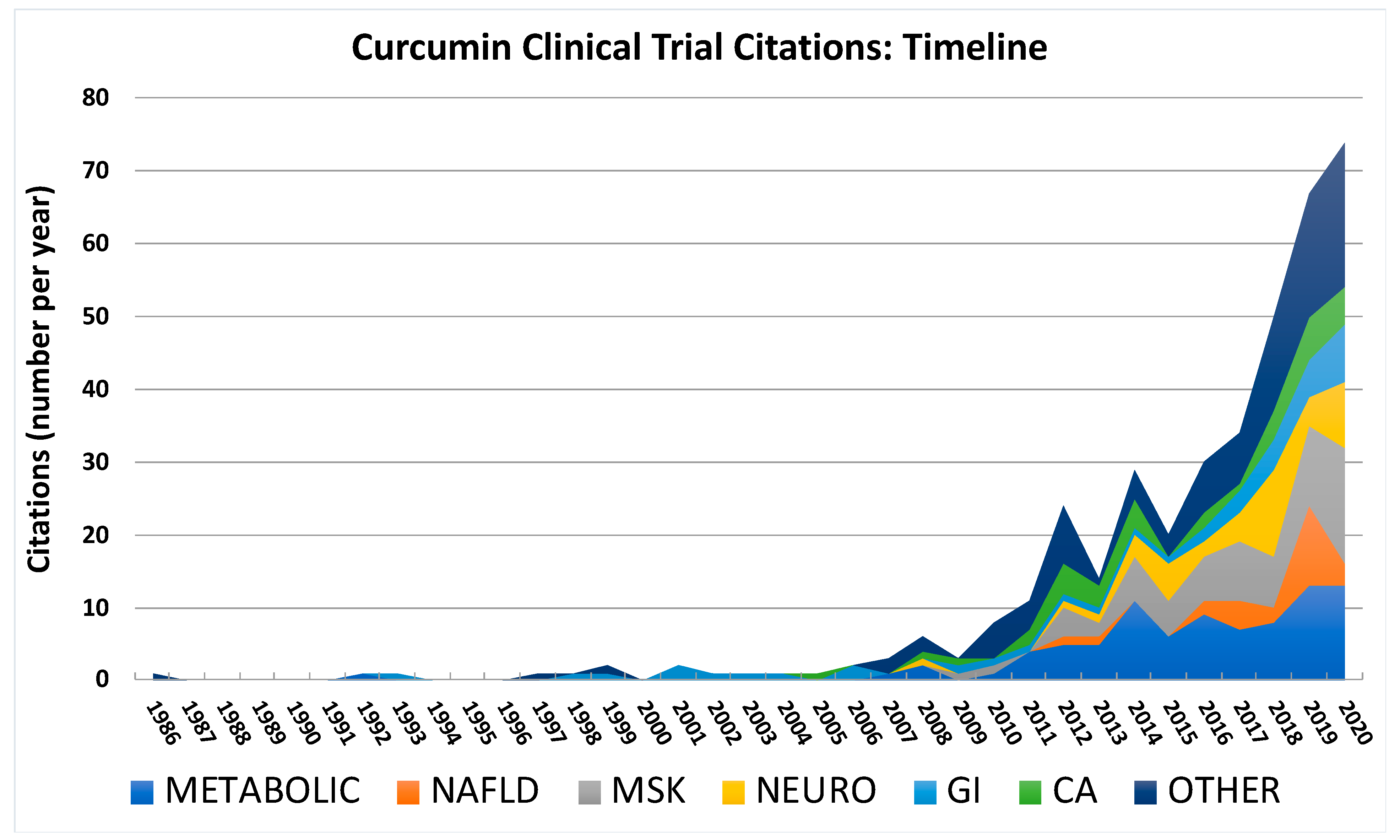
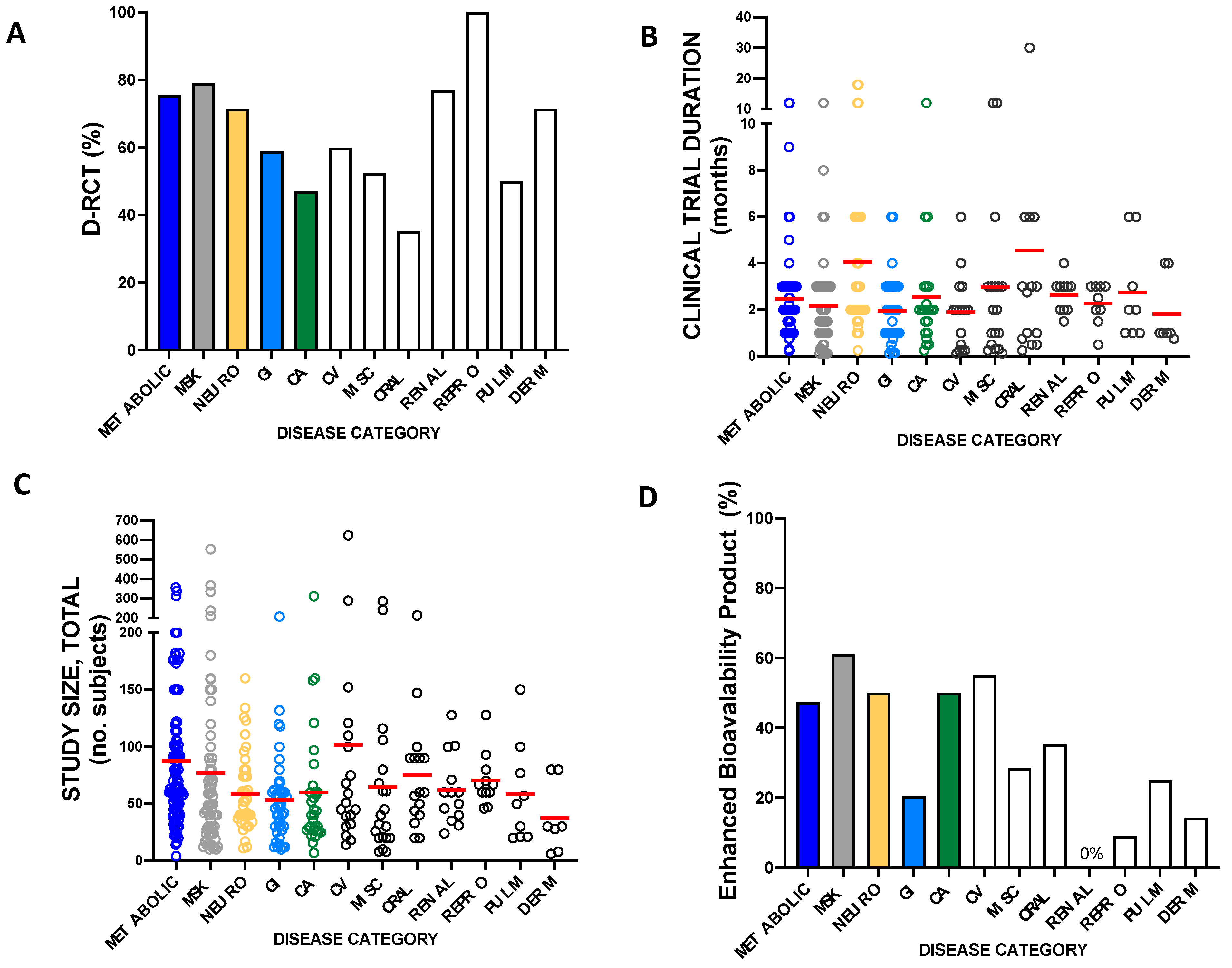

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panknin, T.M.; Howe, C.L.; Hauer, M.; Bucchireddigari, B.; Rossi, A.M.; Funk, J.L. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. Int. J. Mol. Sci. 2023, 24, 4476. https://doi.org/10.3390/ijms24054476
Panknin TM, Howe CL, Hauer M, Bucchireddigari B, Rossi AM, Funk JL. Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. International Journal of Molecular Sciences. 2023; 24(5):4476. https://doi.org/10.3390/ijms24054476
Chicago/Turabian StylePanknin, Timothy M., Carol L. Howe, Meg Hauer, Bhanu Bucchireddigari, Anthony M. Rossi, and Janet L. Funk. 2023. "Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials" International Journal of Molecular Sciences 24, no. 5: 4476. https://doi.org/10.3390/ijms24054476
APA StylePanknin, T. M., Howe, C. L., Hauer, M., Bucchireddigari, B., Rossi, A. M., & Funk, J. L. (2023). Curcumin Supplementation and Human Disease: A Scoping Review of Clinical Trials. International Journal of Molecular Sciences, 24(5), 4476. https://doi.org/10.3390/ijms24054476