Biodegradable Polymeric Nanoparticles Loaded with Flavonoids: A Promising Therapy for Inflammatory Bowel Disease
Abstract
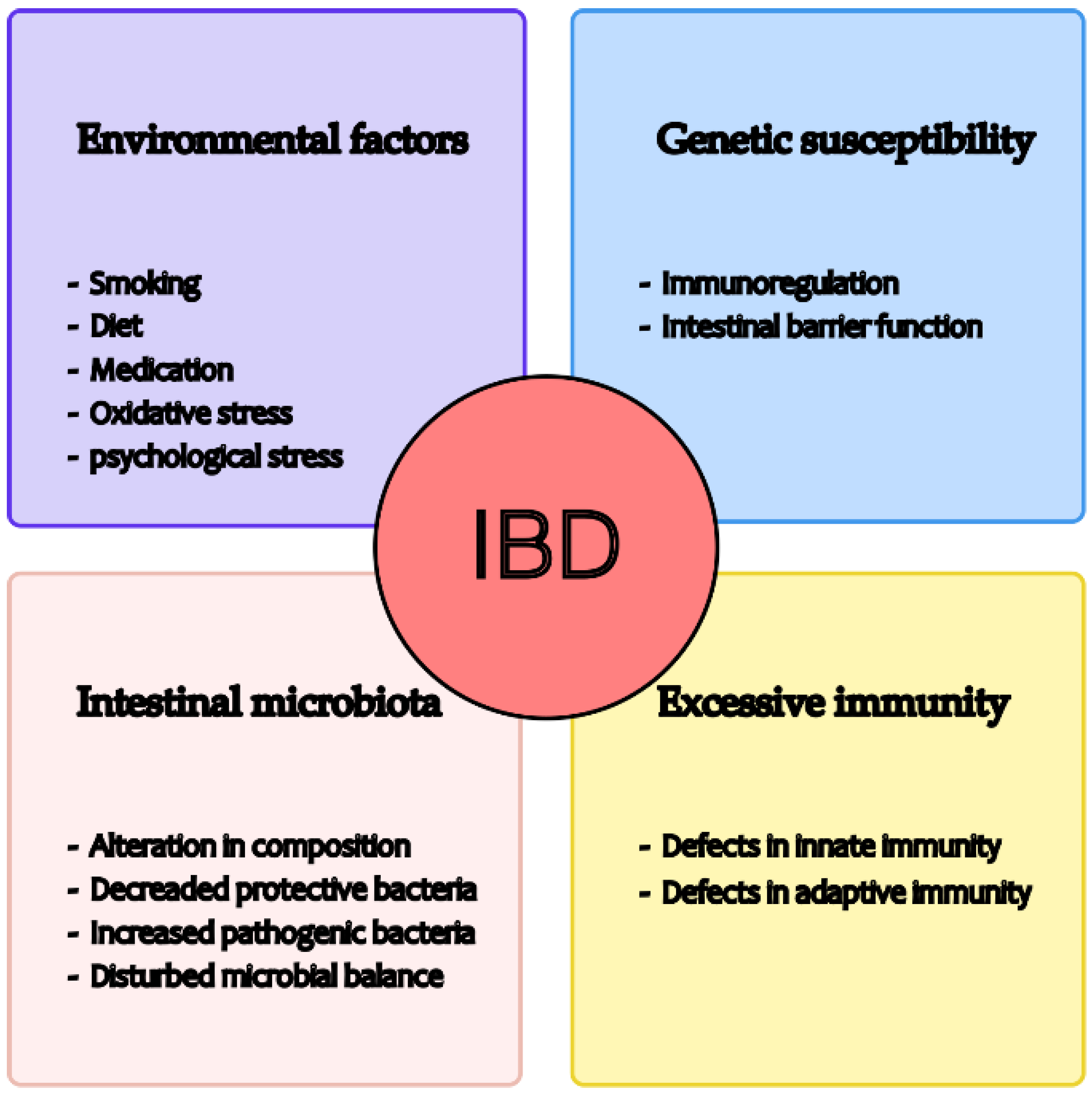
1. Introduction
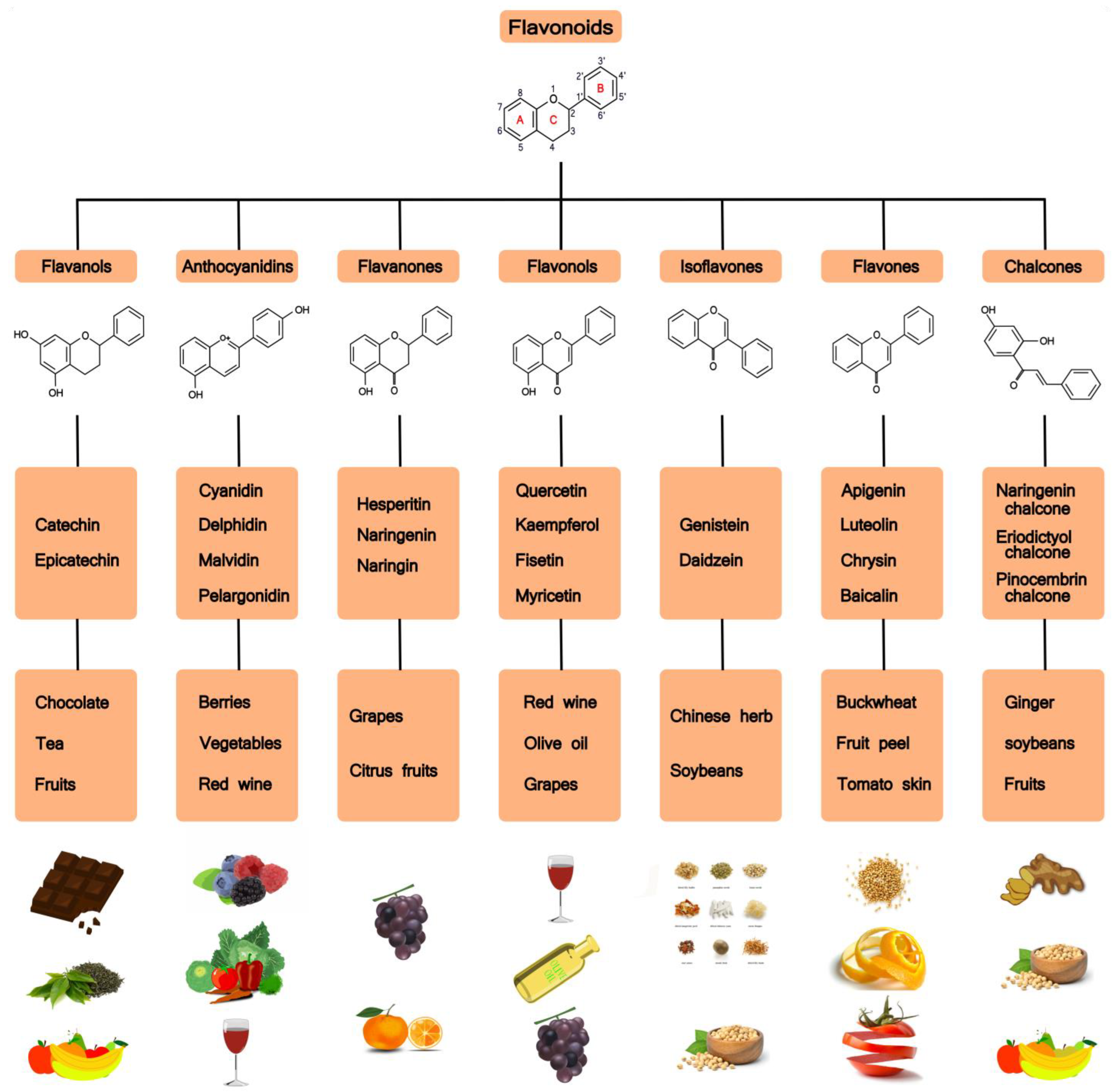
2. Flavonoids
2.1. Classification, Metabolism and Absorption of Flavonoids
2.2. Mechanisms of Flavonoids Regulating IBD
2.2.1. Antioxidant Property
2.2.2. Preservation of Epithelial Barrier
2.2.3. Shaping Microbiota
2.2.4. Immunomodulatory Function
2.2.5. The Modulation of Enteroendocrine System
2.2.6. Molecular Mechanisms of Flavonoids Modulating IBD
2.3. Bioavailability of Flavonoids
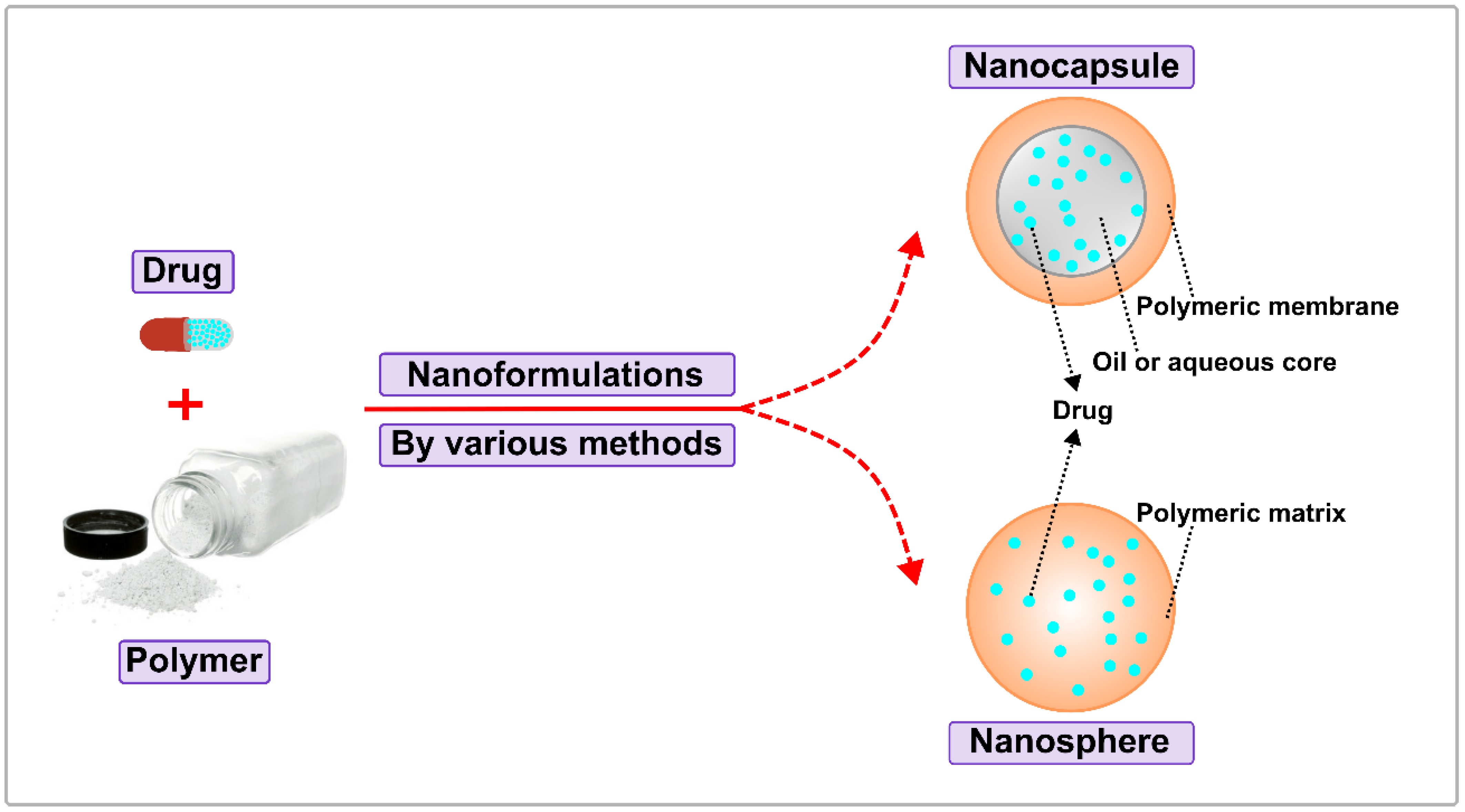
3. Biodegradable Polymeric Nanoparticles
3.1. Formulations
3.2. Natural Biodegradable Polymers
3.2.1. Chitosan
3.2.2. Zein
3.2.3. Alginate
3.2.4. Hyaluronic Acid
3.3. Synthetic Biodegradable Polymers
3.3.1. Poly(Lactide-Co-Glycolide Acid) (PLGA)
3.3.2. Poly(ε-Caprolactone) (PCL)
3.3.3. Dendrigraft Poly-L-Lysine (DGL)
3.3.4. Polyanhydride
| Biodegradable Polymers | Name | Sources | Merits | Demerits |
|---|---|---|---|---|
| Natural biodegradable polymers | Chitosan | Exoskeleton of crustaceans | Biodegradability, low cost biocompatibility, mucosal immune [103]. | Soluble in acidic solutions, limited application [130]. |
| Zein | Corn | Biodegradability, low toxicity, biocompatibility, low cost [107]. | Soluble in water containing organic solvents, limited application [106]. | |
| Alginate | Algae | Low toxicity, low cost, used in mucous membranes and traverse body [111]. | Tedious preparation process, no targeting [131]. | |
| Hyaluronic acid | Animal tissue, microbial | Biocompatibility, low toxicity [132]. | Mass-production may lead to impurity, high price of biological extraction [133]. | |
| Synthetic biodegradable polymers | Poly(lactide-co- glycolide acid) | Polymerization of lactic acid and glycolic acid | Loading multiple antigens and immune modulators, used in mucous membranes and traverse body [134]. | Organic solvents are required, lack of stability, mucosal administration is ineffective [135]. |
| Poly (ε-caprolactone) | Polymerization of ε-caprolactone | Biodegradability, colloidal stable, low toxicity, facile celluar uptake [122]. | Slow degradation rate, poor mechanical properties, low cell adhesion [123]. | |
| Dendrigraft poly-L-lysine | Lysine polycondensation synthesis | Low toxicity, targeted [136]. | Preparation requires complex coupling processes, immunogenicity may interfere with booster immunity [125]. | |
| Polyanhydride | Methyl vinyl ether-maleic hydride synthesis | Sustained release, surface erosion [137]. | Highly sensitive to hydrolysis, limited application [138]. |
3.4. Delivery Routes of Biodegradable Polymeric NPs in IBD
3.4.1. pH-Sensitive Polymeric NPs
3.4.2. ROS-Responsive Polymeric NPs
3.4.3. Targeted Polymeric NPs
3.4.4. Multi-Responsive Polymeric NPs
4. Applications of Biodegradable Polymeric Nanoparticles in IBD
4.1. Quercetin Nanoparticles
4.2. Apigenin Nanoparticles
4.3. Epigallocatechin Gallate (EGCG) Nanoparticles
4.4. Naringenin Nanoparticles
4.5. Synergic Effects of Multiple Flavonoids Nanoparticles
5. Challenge and Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Pal, P.; Mak, J.W.Y.; Ng, S.C. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol. Hepatol. 2020, 5, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Ogata, H. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol. 2006, 41, 10–16. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi Porro, G. Biologic therapy for inflammatory bowel disease. Drugs 2005, 65, 2253–2286. [Google Scholar] [CrossRef]
- Ho, S.M.; Lewis, J.D.; Mayer, E.A.; Plevy, S.E.; Chuang, E.; Rappaport, S.M.; Croitoru, K.; Korzenik, J.R.; Krischer, J.; Hyams, J.S.; et al. Challenges in IBD Research: Environmental Triggers. Inflamm. Bowel Dis. 2019, 25 (Suppl. S2), S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L. Environmental factors affecting inflammatory bowel disease: Have we made progress? Dig. Dis. 2009, 27, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Ince, M.N.; Elliott, D.E. Immunologic and molecular mechanisms in inflammatory bowel disease. Surg. Clin. N. Am. 2007, 87, 681–696. [Google Scholar] [CrossRef]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Annese, V. Genetics and epigenetics of IBD. Pharmacol. Res. 2020, 159, 104892. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef]
- Machado, A.; Geraldi, M.V.; do Nascimento, R.P.; Moya, A.; Vezza, T.; Diez-Echave, P.; Galvez, J.J.; Cazarin, C.B.B.; Marostica Junior, M.R. Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-kappaB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, L.; Ruan, Z. Dietary Supplementation with Epicatechin Improves Intestinal Barrier Integrity in Mice. Foods 2022, 11, 3301. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S29–S45. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.Y.; Qin, Y.; Liu, H.J.; Cui, Z.H.; Li, M.; Yang, J.H.; Zhong, W.L.; Liu, Y.R.; Chen, S.; Sun, T.; et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-kappaB signaling. Oncotarget 2017, 8, 100216–100226. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and Its Flavonoids Regulate TNBS-Induced Inflammatory Bowel Disease through Anti-Inflammation and Suppressing Isolated Jejunum Contraction. Int. J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef]
- Lyu, Y.L.; Zhou, H.F.; Yang, J.; Wang, F.X.; Sun, F.; Li, J.Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediat. Inflamm. 2022, 2022, 5665778. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, S.; Li, T.; Li, N.; Han, D.; Zhang, B.; Xu, Z.Z.; Zhang, S.; Pang, J.; Wang, S.; et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome 2021, 9, 184. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Ververidis, F.; Trantas, E.; Douglas, C.; Vollmer, G.; Kretzschmar, G.; Panopoulos, N. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2007, 2, 1214–1234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Proenca, C.; Rocha, S.; Lima, J.; Carvalho, F.; Fernandes, E.; Freitas, M. Immunomodulatory Effects of Flavonoids in the Prophylaxis and Treatment of Inflammatory Bowel Diseases: A Comprehensive Review. Curr. Med. Chem. 2018, 25, 3374–3412. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- Galsanov, S.B.; Tourova, A.D.; Klimenko, E.D. Effect of quercitrin on structural changes in the large and small intestines in experimental enterocolitis. Biull. Eksp. Biol. Med. 1976, 81, 623–625. [Google Scholar] [CrossRef]
- Li, M.; Weigmann, B. A Novel Pathway of Flavonoids Protecting against Inflammatory Bowel Disease: Modulating Enteroendocrine System. Metabolites 2022, 12, 31. [Google Scholar] [CrossRef]
- Rezaie, A.; Parker, R.D.; Abdollahi, M. Oxidative stress and pathogenesis of inflammatory bowel disease: An epiphenomenon or the cause? Dig. Dis. Sci. 2007, 52, 2015–2021. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547. [Google Scholar] [CrossRef]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Veljaca, M.; Lesch, C.A.; Pllana, R.; Sanchez, B.; Chan, K.; Guglietta, A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1995, 272, 417–422. [Google Scholar] [PubMed]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Al-Enazi, M.M.; Al-Assaf, A.H.; Parmar, M.Y.; Ahmed, M.M. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J. Gastroenterol. 2013, 19, 5633–5644. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Sandoval, M. Nitric Oxide. III. A molecular prelude to intestinal inflammation. Am. J. Physiol. 1999, 276, G795–G799. [Google Scholar] [CrossRef] [PubMed]
- Camuesco, D.; Comalada, M.; Rodriguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Galvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.; Wiendl, M.; Muller, T.M.; Heidbreder, K.; Voskens, C.; Neurath, M.F.; Zundler, S. Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players. Front. Med. 2021, 8, 643973. [Google Scholar] [CrossRef]
- Bian, Y.; Dong, Y.; Sun, J.; Sun, M.; Hou, Q.; Lai, Y.; Zhang, B. Protective Effect of Kaempferol on LPS-Induced Inflammation and Barrier Dysfunction in a Coculture Model of Intestinal Epithelial Cells and Intestinal Microvascular Endothelial Cells. J. Agric. Food Chem. 2020, 68, 160–167. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef]
- Kikut, J.; Konecka, N.; Zietek, M.; Kulpa, D.; Szczuko, M. Diet supporting therapy for inflammatory bowel diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef]
- Ren, J.; Yue, B.; Wang, H.; Zhang, B.; Luo, X.; Yu, Z.; Zhang, J.; Ren, Y.; Mani, S.; Wang, Z.; et al. Acacetin Ameliorates Experimental Colitis in Mice via Inhibiting Macrophage Inflammatory Response and Regulating the Composition of Gut Microbiota. Front. Physiol. 2020, 11, 577237. [Google Scholar] [CrossRef]
- Mu, J.; Xu, J.; Wang, L.; Chen, C.; Chen, P. Anti-inflammatory effects of purple sweet potato anthocyanin extract in DSS-induced colitis: Modulation of commensal bacteria and attenuated bacterial intestinal infection. Food Funct. 2021, 12, 11503–11514. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Targeting cytokines in inflammatory bowel disease. Sci. Transl. Med. 2022, 14, eabq4473. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shao, F.; Yang, Y.; Gu, L.; Zheng, W.; Wu, X.; Gu, Y.; Shu, Y.; Sun, Y.; Xu, Q. Epigallocatechin-3-gallate sensitizes IFN-gamma-stimulated CD4+ T cells to apoptosis via alternative activation of STAT1. Int. Immunopharmacol. 2014, 23, 434–441. [Google Scholar] [CrossRef]
- Tao, F.; Qian, C.; Guo, W.; Luo, Q.; Xu, Q.; Sun, Y. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem. Pharmacol. 2013, 85, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.; Salem, M.; Pedersen, J.; Nielsen, O.H. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013, 76, 1–8. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Pedersen, J.; Coskun, M.; Soendergaard, C.; Salem, M.; Nielsen, O.H. Inflammatory pathways of importance for management of inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 64–77. [Google Scholar] [CrossRef]
- Roy, P.K.; Rashid, F.; Bragg, J.; Ibdah, J.A. Role of the JNK signal transduction pathway in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 200–202. [Google Scholar] [CrossRef]
- Al Shukor, N.; Ravallec, R.; Van Camp, J.; Raes, K.; Smagghe, G. Flavonoids stimulate cholecystokinin peptide secretion from the enteroendocrine STC-1 cells. Fitoterapia 2016, 113, 128–131. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Margalef, M.; Blay, M.; Arola-Arnal, A.; Muguerza, B.; Ardevol, A.; Pinent, M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014, 5, 2357–2364. [Google Scholar] [CrossRef]
- Grau-Bove, C.; Gonzalez-Quilen, C.; Terra, X.; Blay, M.T.; Beltran-Debon, R.; Jorba-Martin, R.; Espina, B.; Pinent, M.; Ardevol, A. Effects of Flavanols on Enteroendocrine Secretion. Biomolecules 2020, 10, 844. [Google Scholar] [CrossRef]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar] [CrossRef]
- Matvienko, O.A.; Alekel, D.L.; Genschel, U.; Ritland, L.; Van Loan, M.D.; Koehler, K.J. Appetitive hormones, but not isoflavone tablets, influence overall and central adiposity in healthy postmenopausal women. Menopause 2010, 17, 594–601. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, X.; Zhang, Y.; Li, L.; Zhao, X.; Cui, H. Isoflavone reduces body weight by decreasing food intake in ovariectomized rats. Ann. Nutr. Metab. 2009, 54, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, A.N.; Thaqi, M.; Priyamvada, S.; Jayawardena, D.; Kumar, A.; Gujral, T.; Chatterjee, I.; Mugarza, E.; Saksena, S.; Onyuksel, H.; et al. GLP-1 nanomedicine alleviates gut inflammation. Nanomedicine 2017, 13, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Bang-Berthelsen, C.H.; Holm, T.L.; Pyke, C.; Simonsen, L.; Sokilde, R.; Pociot, F.; Heller, R.S.; Folkersen, L.; Kvist, P.H.; Jackerott, M.; et al. GLP-1 Induces Barrier Protective Expression in Brunner’s Glands and Regulates Colonic Inflammation. Inflamm. Bowel Dis. 2016, 22, 2078–2097. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Katz, S.; Fang, J.C.; Bernstein, C.N.; Abou-Assi, S.G.; Teduglutide Study, G. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 962–973. [Google Scholar] [CrossRef]
- Ivory, C.P.; Wallace, L.E.; McCafferty, D.M.; Sigalet, D.L. Interleukin-10-independent anti-inflammatory actions of glucagon-like peptide 2. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1202–G1210. [Google Scholar] [CrossRef]
- Qi, K.K.; Lv, J.J.; Wu, J.; Xu, Z.W. Therapeutic effects of different doses of polyethylene glycosylated porcine glucagon-like peptide-2 on ulcerative colitis in male rats. BMC Gastroenterol. 2017, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Zou, H.; Lee, C.; Muppidi, A.; Chao, E.; Fu, Q.; Luo, X.; Wang, D.; Schultz, P.G.; Shen, W. Stapled, Long-Acting Glucagon-like Peptide 2 Analog with Efficacy in Dextran Sodium Sulfate Induced Mouse Colitis Models. J. Med. Chem. 2018, 61, 3218–3223. [Google Scholar] [CrossRef] [PubMed]
- De Smet, B.; Thijs, T.; Moechars, D.; Colsoul, B.; Polders, L.; Ver Donck, L.; Coulie, B.; Peeters, T.L.; Depoortere, I. Endogenous and exogenous ghrelin enhance the colonic and gastric manifestations of dextran sodium sulphate-induced colitis in mice. Neurogastroenterol. Motil. 2009, 21, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar]
- Jia, X.; Cong, B.; Zhang, J.; Li, H.; Liu, W.; Chang, H.; Dong, M.; Ma, C. CCK8 negatively regulates the TLR9-induced activation of human peripheral blood pDCs by targeting TRAF6 signaling. Eur J. Immunol. 2014, 44, 489–499. [Google Scholar] [CrossRef]
- Lubbers, T.; De Haan, J.J.; Hadfoune, M.; Zhang, Y.; Luyer, M.D.; Grundy, D.; Buurman, W.A.; Greve, J.W. Lipid-enriched enteral nutrition controls the inflammatory response in murine Gram-negative sepsis. Crit. Care Med. 2010, 38, 1996–2002. [Google Scholar] [CrossRef]
- Lubbers, T.; Kox, M.; de Haan, J.J.; Greve, J.W.; Pompe, J.C.; Ramakers, B.P.; Pickkers, P.; Buurman, W.A. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit. Care Med. 2013, 41, 1258–1265. [Google Scholar] [CrossRef]
- Saia, R.S.; Ribeiro, A.B.; Giusti, H. Cholecystokinin Modulates the Mucosal Inflammatory Response and Prevents the Lipopolysaccharide-Induced Intestinal Epithelial Barrier Dysfunction. Shock 2020, 53, 242–251. [Google Scholar] [CrossRef]
- Hoensch, H.P.; Weigmann, B. Regulation of the intestinal immune system by flavonoids and its utility in chronic inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 877–881. [Google Scholar] [CrossRef]
- Li, A.; Gan, Y.; Wang, R.; Liu, Y.; Ma, T.; Huang, M.; Cui, X. IL-22 Up-Regulates beta-Defensin-2 Expression in Human Alveolar Epithelium via STAT3 but Not NF-kappaB Signaling Pathway. Inflammation 2015, 38, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Di Pizio, A.; Niv, M.Y. Promiscuity and selectivity of bitter molecules and their receptors. Bioorg. Med. Chem. 2015, 23, 4082–4091. [Google Scholar] [CrossRef] [PubMed]
- Englander, E.W.; Gomez, G.A.; Greeley, G.H., Jr. Alterations in stomach ghrelin production and in ghrelin-induced growth hormone secretion in the aged rat. Mech. Ageing Dev. 2004, 125, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Marti, A.; Depoortere, I.; Blay, M.T.; Terra, X.; Pinent, M.; Ardevol, A. Subchronic treatment with grape-seed phenolics inhibits ghrelin production despite a short-term stimulation of ghrelin secretion produced by bitter-sensing flavanols. Mol. Nutr. Food Res. 2016, 60, 2554–2564. [Google Scholar] [CrossRef]
- Shen, Q.; Li, X.; Li, W.; Zhao, X. Enhanced intestinal absorption of daidzein by borneol/menthol eutectic mixture and microemulsion. AAPS Pharm. Sci. Tech. 2011, 12, 1044–1049. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones greatly improves their hepatic metabolic stability and intestinal absorption. Mol. Pharm. 2007, 4, 826–832. [Google Scholar] [CrossRef]
- Nielsen, I.L.; Chee, W.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Kou, L.; Bhutia, Y.D.; Yao, Q.; He, Z.; Sun, J.; Ganapathy, V. Transporter-Guided Delivery of Nanoparticles to Improve Drug Permeation across Cellular Barriers and Drug Exposure to Selective Cell Types. Front. Pharmacol. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef]
- Zelmer, C.; Zweifel, L.P.; Kapinos, L.E.; Craciun, I.; Guven, Z.P.; Palivan, C.G.; Lim, R.Y.H. Organelle-specific targeting of polymersomes into the cell nucleus. Proc. Natl. Acad. Sci. USA 2020, 117, 2770–2778. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Wang, L.; Jungels, R.R.; Sharma, B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2020, 101, 469–483. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yue, H.; Xu, L.; Liu, Y.; Song, Y.; Tang, C.; Yin, C. siRNA release kinetics from polymeric nanoparticles correlate with RNAi efficiency and inflammation therapy via oral delivery. Acta Biomater. 2020, 103, 213–222. [Google Scholar] [CrossRef]
- Le, Z.; Chen, Y.; Han, H.; Tian, H.; Zhao, P.; Yang, C.; He, Z.; Liu, L.; Leong, K.W.; Mao, H.Q.; et al. Hydrogen-Bonded Tannic Acid-Based Anticancer Nanoparticle for Enhancement of Oral Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 42186–42197. [Google Scholar] [CrossRef]
- Zhang, C.X.; Cheng, Y.; Liu, D.Z.; Liu, M.; Cui, H.; Zhang, B.L.; Mei, Q.B.; Zhou, S.Y. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J. Nanobiotechnol. 2019, 17, 18. [Google Scholar] [CrossRef]
- Zhang, L.; Beatty, A.; Lu, L.; Abdalrahman, A.; Makris, T.M.; Wang, G.; Wang, Q. Microfluidic-assisted polymer-protein assembly to fabricate homogeneous functionalnanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110768. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesaro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, J.; Cui, S.; Qian, Z.; Gu, Y. Enhanced tumor targeting and antitumor efficacy via hydroxycamptothecin-encapsulated folate-modified N-succinyl-N’-octyl chitosan micelles. J. Pharm. Sci. 2013, 102, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Buheazah, T.M.; AlHomoud, H.S.; Al-Nasif, H.A.; Alam, M.A. A Chitosan-PLGA based catechin hydrate nanoparticles used in targeting of lungs and cancer treatment. Saudi J. Biol. Sci. 2020, 27, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- De Marco, I. Zein Microparticles and Nanoparticles as Drug Delivery Systems. Polymers 2022, 14, 2172. [Google Scholar] [CrossRef]
- Berardi, A.; Bisharat, L.; AlKhatib, H.S.; Cespi, M. Zein as a Pharmaceutical Excipient in Oral Solid Dosage Forms: State of the Art and Future Perspectives. AAPS Pharm. Sci. Tech. 2018, 19, 2009–2022. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, J.M.; Yang, X.Q.; Guo, J.; Lin, Y. Amphiphilic zein hydrolysate as a novel nano-delivery vehicle for curcumin. Food Funct. 2015, 6, 2636–2645. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Wang, Y.; Yang, X.; Sun, C.; Mao, L.; Gao, Y. Zein-hyaluronic acid binary complex as a delivery vehicle of quercetagetin: Fabrication, structural characterization, physicochemical stability and in vitro release property. Food Chem. 2019, 276, 322–332. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des. 2016, 22, 3399–3410. [Google Scholar] [CrossRef]
- Borges, O.; Cordeiro-da-Silva, A.; Romeijn, S.G.; Amidi, M.; de Sousa, A.; Borchard, G.; Junginger, H.E. Uptake studies in rat Peyer’s patches, cytotoxicity and release studies of alginate coated chitosan nanoparticles for mucosal vaccination. J. Control. Release 2006, 114, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.A.; Bourbon, A.I.; Vicente, A.A.; Cerqueira, M.A. Alginate/chitosan nanoparticles for encapsulation and controlled release of vitamin B2. Int. J. Biol. Macromol. 2014, 71, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, M.; Yu, S.; Jin, Z.; Zhao, K. An overview of biodegradable nanomaterials and applications in vaccines. Vaccine 2020, 38, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Wang, Y.; Wan, J.; Yuan, P.; Chen, H.; Zhang, L. Facile preparation of hyaluronic acid-based quercetin nanoformulation for targeted tumor therapy. Int. J. Biol. Macromol. 2020, 147, 937–945. [Google Scholar] [CrossRef]
- Lai, P.; Daear, W.; Lobenberg, R.; Prenner, E.J. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly(d,l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf. B Biointerfaces 2014, 118, 154–163. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887. [Google Scholar] [CrossRef]
- Wu, Y.R.; Choi, H.J.; Kang, Y.G.; Kim, J.K.; Shin, J.W. In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int. J. Nanomed. 2017, 12, 7007–7013. [Google Scholar] [CrossRef]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface modification of PLGA nanoparticles with biotinylated chitosan for the sustained in vitro release and the enhanced cytotoxicity of epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Palama, I.E.; Cortese, B.; D’Amone, S.; Gigli, G. mRNA delivery using non-viral PCL nanoparticles. Biomater. Sci. 2015, 3, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Quercetin-biapigenin nanoparticles are effective to penetrate the blood-brain barrier. Drug Deliv. Transl. Res. 2022, 12, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; He, Y.; Feng, X.; Fu, D. epsilon-Polylysine and next-generation dendrigraft poly-L-lysine: Chemistry, activity, and applications in biopharmaceuticals. J. Biomater. Sci. Polym. Ed. 2015, 26, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Francoia, J.P.; Rossi, J.C.; Monard, G.; Vial, L. Digitizing Poly-l-lysine Dendrigrafts: From Experimental Data to Molecular Dynamics Simulations. J. Chem. Inf. Model. 2017, 57, 2173–2180. [Google Scholar] [CrossRef]
- Zhao, K.; Li, D.; Cheng, G.; Zhang, B.; Han, J.; Chen, J.; Wang, B.; Li, M.; Xiao, T.; Zhang, J.; et al. Targeted Delivery Prodigiosin to Choriocarcinoma by Peptide-Guided Dendrigraft Poly-l-lysines Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5458. [Google Scholar] [CrossRef]
- Kumar, N.; Langer, R.S.; Domb, A.J. Polyanhydrides: An overview. Adv. Drug Deliv. Rev. 2002, 54, 889–910. [Google Scholar] [CrossRef]
- Darling, R.; Senapati, S.; Christiansen, J.; Liu, L.; Ramer-Tait, A.E.; Narasimhan, B.; Wannemuehler, M. Polyanhydride Nanoparticles Induce Low Inflammatory Dendritic Cell Activation Resulting in CD8(+) T Cell Memory and Delayed Tumor Progression. Int. J. Nanomed. 2020, 15, 6579–6592. [Google Scholar] [CrossRef]
- Hakimi, S.; Mortazavian, E.; Mohammadi, Z.; Samadi, F.Y.; Samadikhah, H.; Taheritarigh, S.; Tehrani, N.R.; Rafiee-Tehrani, M. Thiolated methylated dimethylaminobenzyl chitosan: A novel chitosan derivative as a potential delivery vehicle. Int. J. Biol. Macromol. 2017, 95, 574–581. [Google Scholar] [CrossRef]
- Lopes, M.; Abrahim, B.; Veiga, F.; Seica, R.; Cabral, L.M.; Arnaud, P.; Andrade, J.C.; Ribeiro, A.J. Preparation methods and applications behind alginate-based particles. Expert Opin. Drug Deliv. 2017, 14, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahdev, P.; Ochyl, L.J.; Akerberg, J.; Moon, J.J. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J. Control. Release 2015, 208, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Babo, P.S.; Reis, R.L.; Gomes, M.E. Production and characterization of hyaluronic acid microparticles for the controlled delivery of growth factors using a spray/dehydration method. J. Biomater. Appl. 2016, 31, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Soema, P.C.; Slutter, B.; Ossendorp, F.; Jiskoot, W. PLGA particulate delivery systems for subunit vaccines: Linking particle properties to immunogenicity. Hum. Vaccin. Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Sah, H.; Thoma, L.A.; Desu, H.R.; Sah, E.; Wood, G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomed. 2013, 8, 747–765. [Google Scholar] [CrossRef]
- Collet, H.; Souaid, E.; Cottet, H.; Deratani, A.; Boiteau, L.; Dessalces, G.; Rossi, J.C.; Commeyras, A.; Pascal, R. An expeditious multigram-scale synthesis of lysine dendrigraft (DGL) polymers by aqueous N-carboxyanhydride polycondensation. Chemistry 2010, 16, 2309–2316. [Google Scholar] [CrossRef]
- Phanse, Y.; Carrillo-Conde, B.R.; Ramer-Tait, A.E.; Roychoudhury, R.; Broderick, S.; Pohl, N.; Rajan, K.; Narasimhan, B.; Wannemuehler, M.J.; Bellaire, B.H. Functionalization promotes pathogen-mimicking characteristics of polyanhydride nanoparticle adjuvants. J. Biomed. Mater. Res. A 2017, 105, 2762–2771. [Google Scholar] [CrossRef]
- Basu, A.; Domb, A.J. Recent Advances in Polyanhydride Based Biomaterials. Adv. Mater. 2018, 30, e1706815. [Google Scholar] [CrossRef]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Petkau, K.; Kaeser, A.; Fischer, I.; Brunsveld, L.; Schenning, A.P. Pre- and postfunctionalized self-assembled pi-conjugated fluorescent organic nanoparticles for dual targeting. J. Am. Chem. Soc. 2011, 133, 17063–17071. [Google Scholar] [CrossRef] [PubMed]
- Jacob, E.M.; Borah, A.; Pillai, S.C.; Kumar, D.S. Inflammatory Bowel Disease: The Emergence of New Trends in Lifestyle and Nanomedicine as the Modern Tool for Pharmacotherapy. Nanomaterials 2020, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Bahramsoltani, R.; Marques, A.M.; Naseri, R.; Rahimi, R.; Haratipour, P.; Iranpanah, A.; Panah, A.I.; Farzaei, M.H.; Abdollahi, M. A systematic review of nano formulation of natural products for the treatment of inflammatory bowel disease: Drug delivery and pharmacological targets. Daru 2018, 26, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control. Release 2014, 183, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Huang, Y.; Canup, B.S.B.; Gou, S.; Chen, N.; Dai, F.; Xiao, B.; Li, C. Oral nanotherapeutics with enhanced mucus penetration and ROS-responsive drug release capacities for delivery of curcumin to colitis tissues. J. Mater. Chem. B 2021, 9, 604–1615. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Zhang, S.; Langer, R.; Traverso, G. Nanoparticulate Drug Delivery Systems Targeting Inflammation for Treatment of Inflammatory Bowel Disease. Nano Today 2017, 16, 82–96. [Google Scholar] [CrossRef]
- He, W.; Kapate, N.; Shields CWt Mitragotri, S. Drug delivery to macrophages: A review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 165, 15–40. [Google Scholar] [CrossRef]
- Lamprecht, A. IBD: Selective nanoparticle adhesion can enhance colitis therapy. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 311–312. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Merlin, D. Nanoparticle-Mediated Drug Delivery Systems For The Treatment Of IBD: Current Perspectives. Int. J. Nanomed. 2019, 14, 8875–8889. [Google Scholar] [CrossRef] [PubMed]
- Diez-Echave, P.; Ruiz-Malagon, A.J.; Molina-Tijeras, J.A.; Hidalgo-Garcia, L.; Vezza, T.; Cenis-Cifuentes, L.; Rodriguez-Sojo, M.J.; Cenis, J.L.; Rodriguez-Cabezas, M.E.; Rodriguez-Nogales, A.; et al. Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int. J. Pharm. 2021, 606, 120935. [Google Scholar] [CrossRef]
- Khater, S.I.; Lotfy, M.M.; Alandiyjany, M.N.; Alqahtani, L.S.; Zaglool, A.W.; Althobaiti, F.; Ismail, T.A.; Soliman, M.M.; Saad, S.; Ibrahim, D. Therapeutic Potential of Quercetin Loaded Nanoparticles: Novel Insights in Alleviating Colitis in an Experimental DSS Induced Colitis Model. Biomedicines 2022, 10, 1654. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, L.; Du, X.; Tian, J.; Yuan, Y.; Jia, M.; He, Y.; Zeng, R.; Qiao, R.; Li, C. Smart Responsive Quercetin-Conjugated Glycol Chitosan Prodrug Micelles for Treatment of Inflammatory Bowel Diseases. Mol. Pharm. 2021, 18, 1419–1430. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, Y.; Peng, Q.; Zhao, X.; Hu, D.; Wen, J.; Liu, K.; Li, R.; Wang, K.; Sun, J. Apigenin-Mn(II) loaded hyaluronic acid nanoparticles for ulcerative colitis therapy in mice. Front. Chem. 2022, 10, 969962. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (-)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Li, Z.; Gu, L. Fabrication of self-assembled (-)-epigallocatechin gallate (EGCG) ovalbumin-dextran conjugate nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2014, 62, 1301–1309. [Google Scholar] [CrossRef]
- Liu, S.; Cao, Y.; Ma, L.; Sun, J.; Ramos-Mucci, L.; Ma, Y.; Yang, X.; Zhu, Z.; Zhang, J.; Xiao, B. Oral antimicrobial peptide-EGCG nanomedicines for synergistic treatment of ulcerative colitis. J. Control. Release 2022, 347, 544–560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.P.; Abraham, A. PVP- coated naringenin nanoparticles for biomedical applications - In vivo toxicological evaluations. Chem. Biol. Interact. 2016, 257, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Yoon, Y.H.; Choi, K.; Kwon, M.; Goo, S.H.; Cha, J.S.; Choi, M.K.; Lee, H.S.; Song, I.S. Enhanced oral bioavailability of morin administered in mixed micelle formulation with PluronicF127 and Tween80 in rats. Biol. Pharm. Bull. 2015, 38, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 201700447. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lei, J.; Zhang, B. Dietary Quercetin Alleviated DSS-induced Colitis in Mice Through Several Possible Pathways by Transcriptome Analysis. Curr. Pharm. Biotechnol. 2020, 21, 1666–1673. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Venigalla, M.; Gyengesi, E.; Munch, G. Curcumin and Apigenin—Novel and promising therapeutics against chronic neuroinflammation in Alzheimer’s disease. Neural. Regen. Res. 2015, 10, 1181–1185. [Google Scholar]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Ganjare, A.B.; Nirmal, S.A.; Patil, A.N. Use of apigenin from Cordia dichotoma in the treatment of colitis. Fitoterapia 2011, 82, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Chaen, Y.; Yamamoto, Y.; Suzuki, T. Naringenin promotes recovery from colonic damage through suppression of epithelial tumor necrosis factor-alpha production and induction of M2-type macrophages in colitic mice. Nutr. Res. 2019, 64, 82–92. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kwon, S.J.; Ju, J.; Bose, M.; Lee, M.J.; Hong, J.; Hao, X.; Yang, C.S. Effect of genistein on the bioavailability and intestinal cancer chemopreventive activity of (-)-epigallocatechin-3-gallate. Carcinogenesis 2008, 29, 2019–2024. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boque, N.; Macarulla, M.T.; Portillo, M.P.; Martinez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2022, 1, 24. [Google Scholar] [CrossRef]
- Li, T.; Li, F.; Liu, X.; Liu, J.; Li, D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4-MyD88-mediated NF-kappaB and MAPK signaling pathways. Phytother. Res. 2019, 33, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Kumar, N. Nanonization of curcumin by antisolvent precipitation: Process development, characterization, freeze drying and stability performance. Int. J. Pharm. 2014, 477, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Brusini, R.; Varna, M.; Couvreur, P. Advanced nanomedicines for the treatment of inflammatory diseases. Adv. Drug Deliv. Rev. 2020, 157, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fang, S.; Liang, X. Natural deep eutectic solvents as eco-friendly and sustainable dilution medium for the determination of residual organic solvents in pharmaceuticals with static headspace-gas chromatography. J. Pharm. Biomed. Anal. 2018, 158, 262–268. [Google Scholar] [CrossRef]
- Woodley, J.M. New opportunities for biocatalysis: Making pharmaceutical processes greener. Trends. Biotechnol. 2008, 26, 321–327. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Hou, T.; Zeng, H.; Kalambhe, D.; Wang, B.; Shen, X.; Huang, Y. Macrophage-based nanotherapeutic strategies in ulcerative colitis. J. Control. Release 2020, 320, 363–380. [Google Scholar] [CrossRef]
- Sekharan, T.R.; Katari, O.; Ruhina Rahman, S.N.; Pawde, D.M.; Goswami, A.; Chandira, R.M.; Shunmugaperumal, T. Neoteric solvents for the pharmaceutical industry: An update. Drug Discov. Today 2021, 26, 1702–1711. [Google Scholar] [CrossRef]
 down;
down;  up).
up).
 down;
down;  up).
up).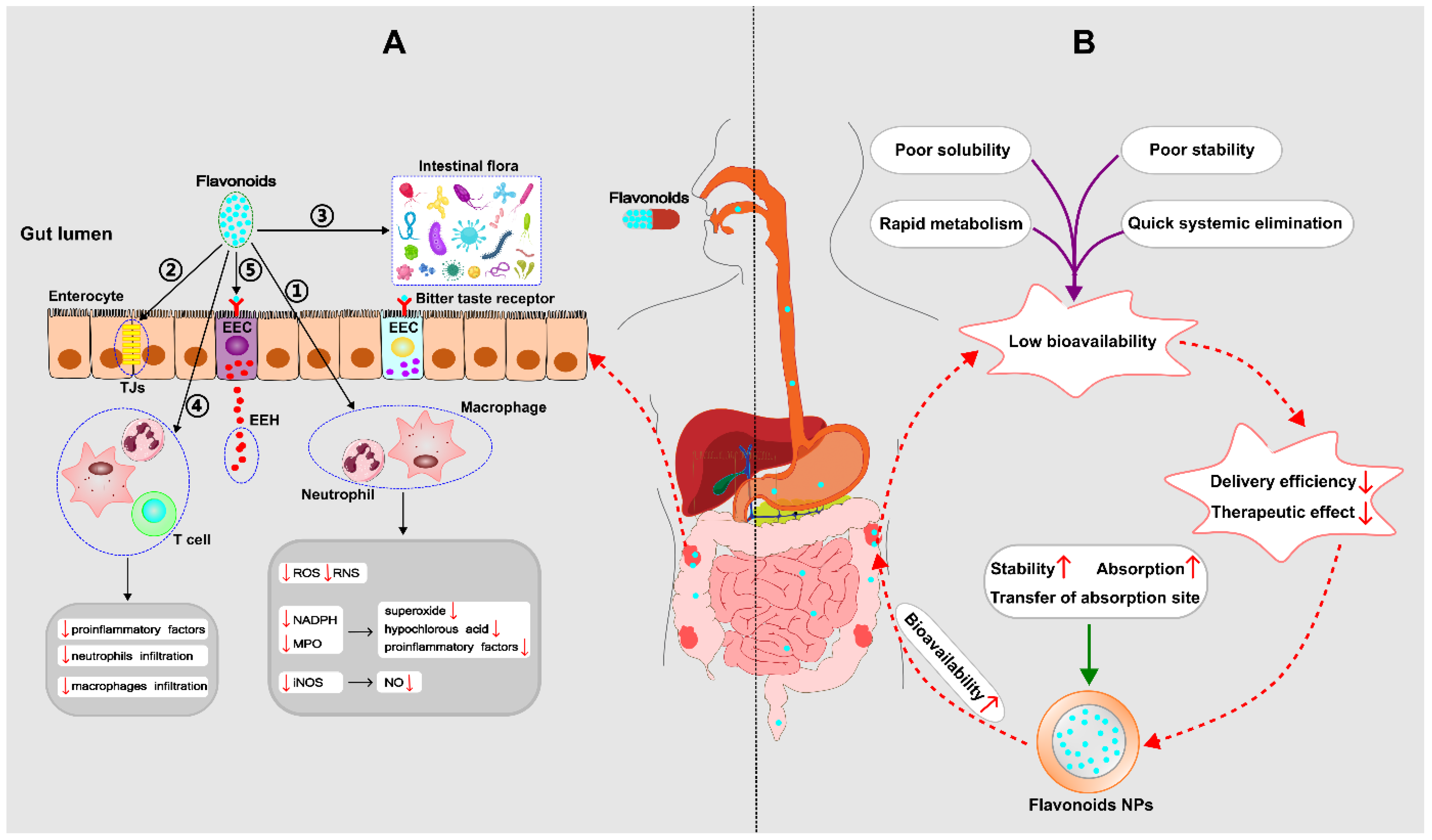

 decrease;
decrease;  increase).
increase).
 decrease;
decrease;  increase).
increase).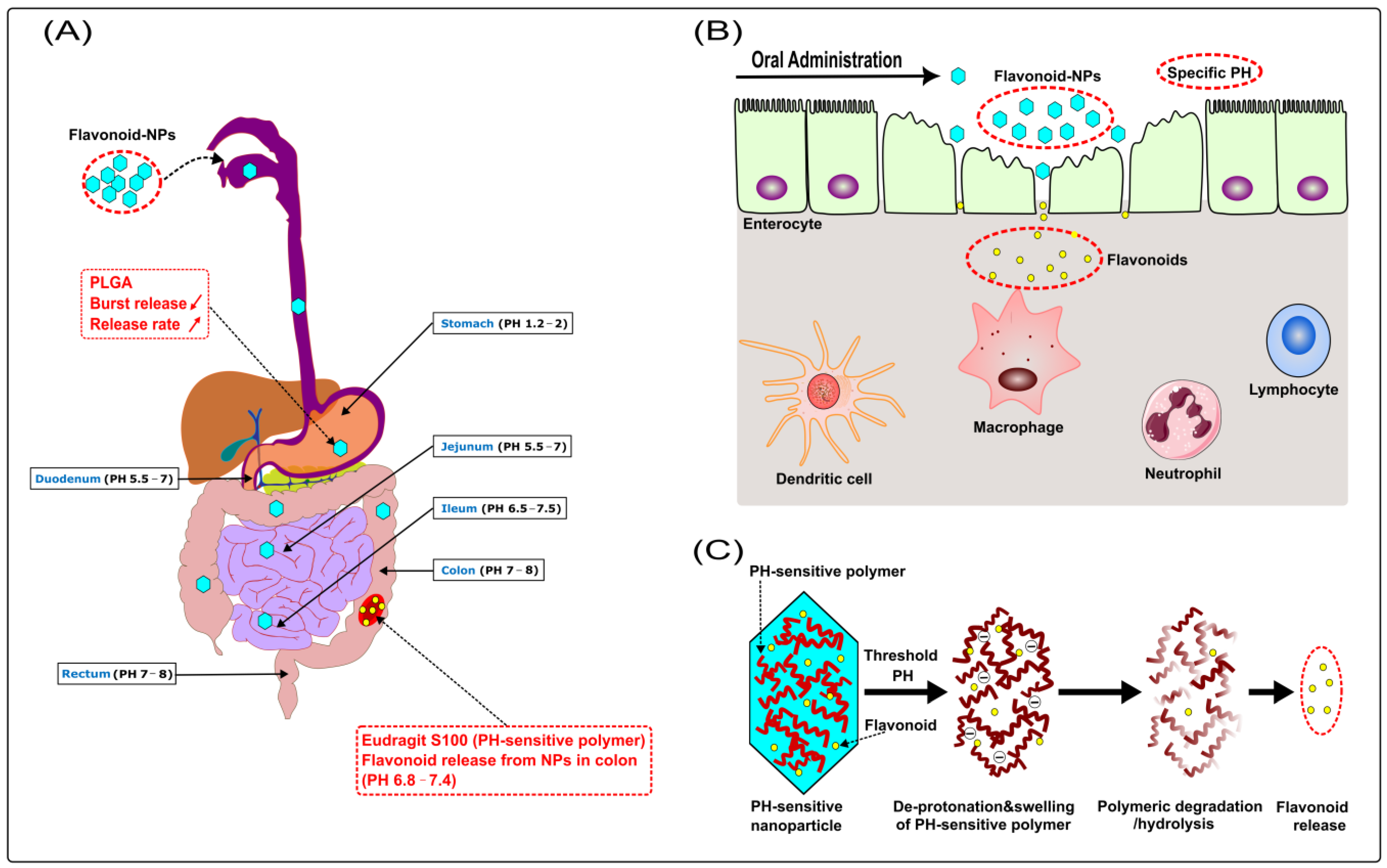
| Flavonoid | Nanometerial | Model | Effects | Reference |
|---|---|---|---|---|
| Quercetin | Silk fibroin | DSS mice | Reduced disease activity index, histological damage and proinflammatory cytokines. | [154] |
| Chitosan | DSS rats | Reduced disease activity index, fecal calprotectin marker and proinflammatory cytokines, upregulated genes expressing tight junction proteins, prevented mucosal damage. | [155] | |
| Conjugated glycol chitosan prodrug micelles | DSS mice | Better therapeutic efficacy than free quercetin. | [156] | |
| Apigenin | Sodium hyaluronate | DSS mice | More powerful anti-colitis effect, higher solubility and bioavailability compared with free apigenin. | [157] |
| EGCG | Chitosan | Excised mouse jejunum | Enhanced intestinal absorption of EGCG. | [158] |
| Chitosan | Mice | Improved plasma exposure of EGCG by enhancing the intestinal stability. | [159] | |
| Ovalbumin-dextran | Caco-2 cells | More stable and higher absorbance than free EGCG. | [160] | |
| Silk fibroin, surface functionalization of antimicrobial peptides, hydrogel (chitosan/alginate) | DSS mice | Repaired epithelial barrier, downregulated proinflammatory factors, upregulated anti-inflammatory factors, regulated gut microbiota. | [161] | |
| Naringenin | PVP | SD rats | Increased bioavailability. | [162] |
| Mixed micelle of Pluronic F127 and Tween 80 | SD rats | Increased bioavailability, solubility and intestinal permeability. | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liu, Y.; Weigmann, B. Biodegradable Polymeric Nanoparticles Loaded with Flavonoids: A Promising Therapy for Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 4454. https://doi.org/10.3390/ijms24054454
Li M, Liu Y, Weigmann B. Biodegradable Polymeric Nanoparticles Loaded with Flavonoids: A Promising Therapy for Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2023; 24(5):4454. https://doi.org/10.3390/ijms24054454
Chicago/Turabian StyleLi, Mingrui, Ying Liu, and Benno Weigmann. 2023. "Biodegradable Polymeric Nanoparticles Loaded with Flavonoids: A Promising Therapy for Inflammatory Bowel Disease" International Journal of Molecular Sciences 24, no. 5: 4454. https://doi.org/10.3390/ijms24054454
APA StyleLi, M., Liu, Y., & Weigmann, B. (2023). Biodegradable Polymeric Nanoparticles Loaded with Flavonoids: A Promising Therapy for Inflammatory Bowel Disease. International Journal of Molecular Sciences, 24(5), 4454. https://doi.org/10.3390/ijms24054454






