Disorder of Golgi Apparatus Precedes Anoxia-Induced Pathology of Mitochondria
Abstract
1. Introduction
2. Results
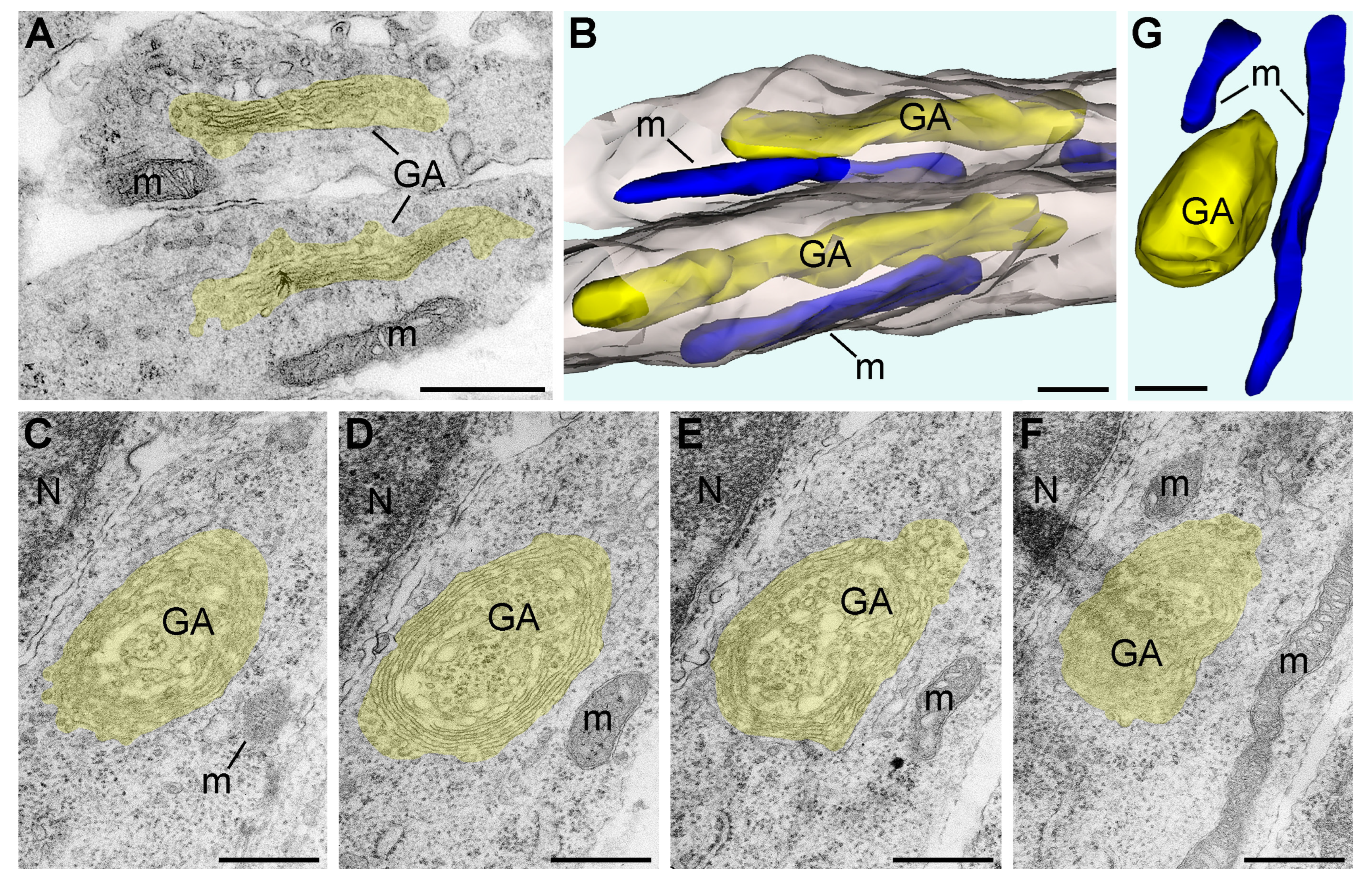
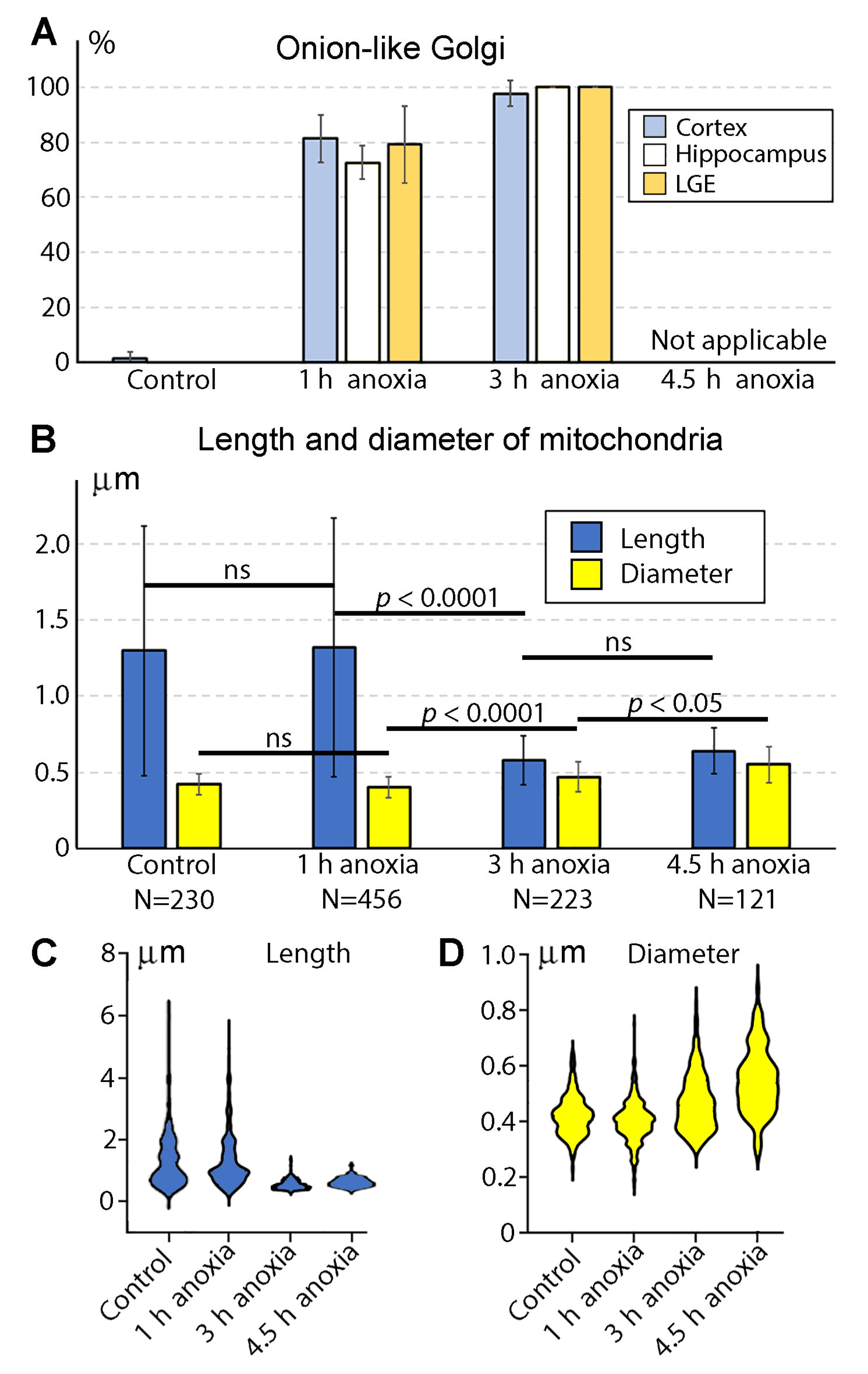
2.1. Morphology of GA in Mouse Embryo Brains in Anoxic Conditions
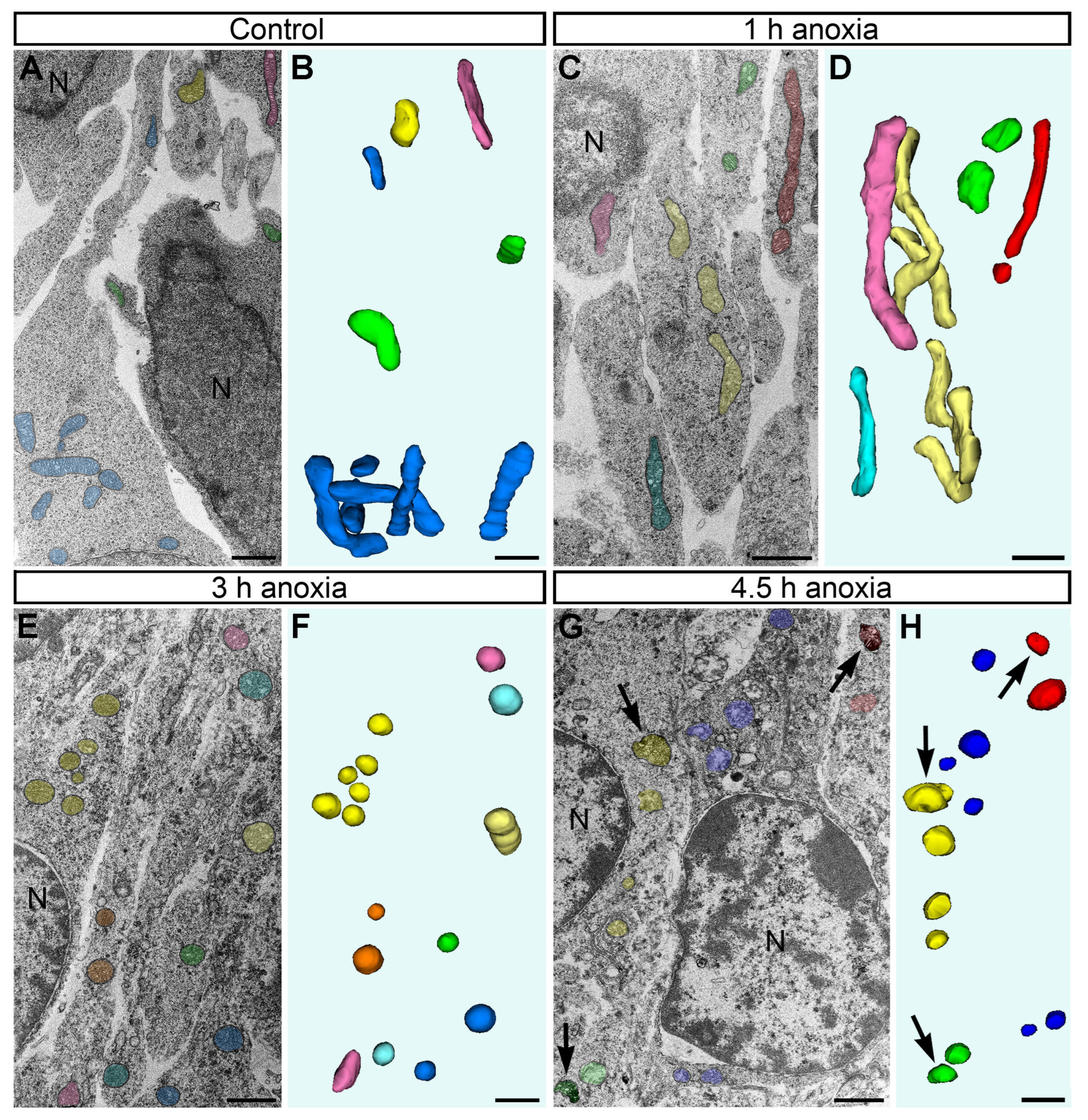
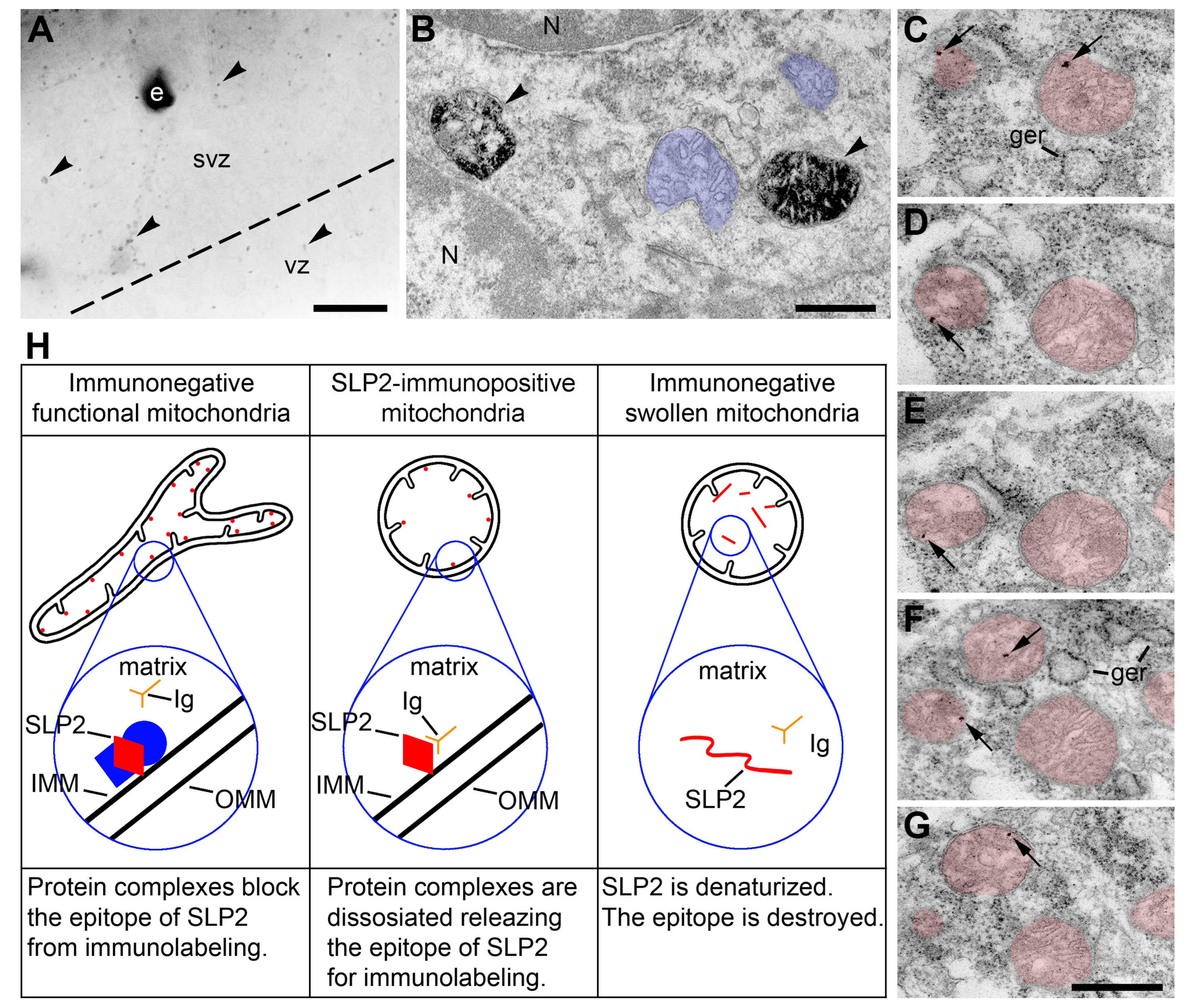
2.2. Morphology of Mitochondria in Anoxic Conditions
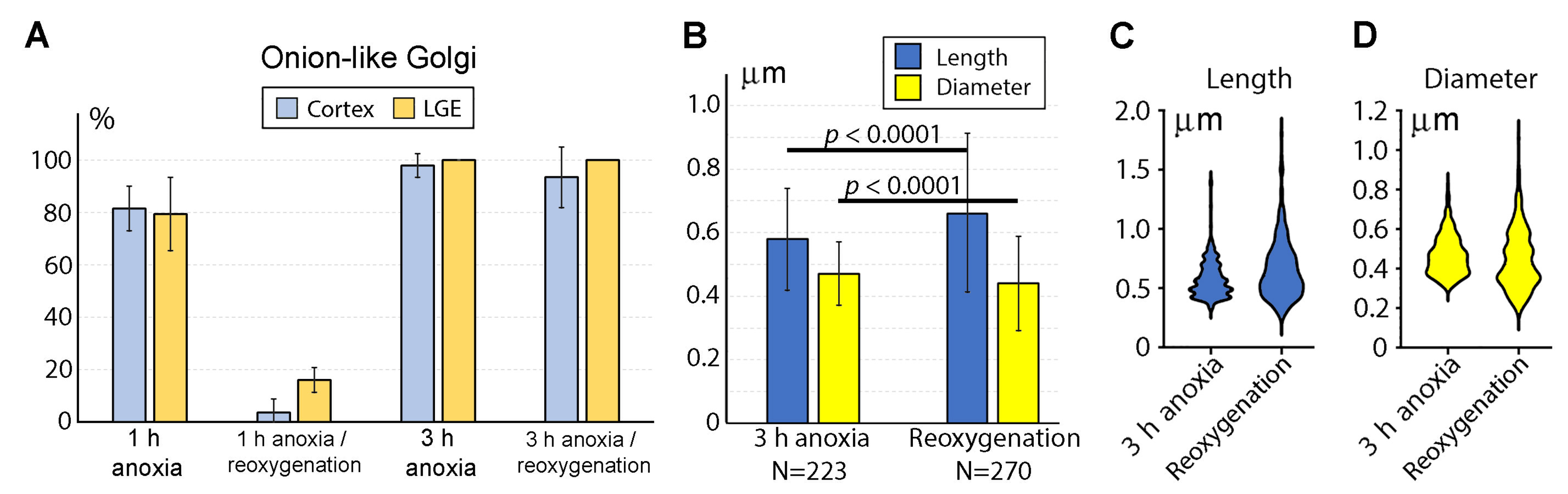
2.3. Reaction of GA and Mitochondria to Reoxygenation
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hara, Y.; Yuk, F.; Puri, R.; Janssen, W.G.; Rapp, P.R.; Morrison, J.H. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl. Acad. Sci. USA 2014, 111, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Langer, T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrinol. Metab. 2016, 27, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, J.; Li, C.; Cao, G.; Wang, X. Prohibitins: A Key Link between Mitochondria and Nervous System Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7494863. [Google Scholar] [CrossRef] [PubMed]
- Morozov, Y.M.; Sun, Y.Y.; Kuan, C.Y.; Rakic, P. Alteration of SLP2-like immunolabeling in mitochondria signifies early cellular damage in developing and adult mouse brain. Eur. J. Neurosci. 2016, 43, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Solenski, N.J.; diPierro, C.G.; Trimmer, P.A.; Kwan, A.L.; Helm, G.A. Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke 2002, 33, 816–824. [Google Scholar] [CrossRef]
- Kirov, S.A.; Fomitcheva, I.V.; Sword, J. Rapid Neuronal Ultrastructure Disruption and Recovery during Spreading Depolarization-Induced Cytotoxic Edema. Cereb. Cortex 2020, 30, 5517–5531. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Delerue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, M.; Arnaune-Pelloquin, L.; Davezac, N.; Mils, V.; Miquel, M.C.; et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016, 90, 3–19. [Google Scholar] [CrossRef]
- Santos, D.; Esteves, A.R.; Silva, D.F.; Januário, C.; Cardoso, S.M. The Impact of Mitochondrial Fusion and Fission Modulation in Sporadic Parkinson’s Disease. Mol. Neurobiol. 2015, 52, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Trushin, S.; Christensen, T.A.; Bachmeier, B.V.; Gateno, B.; Schroeder, A.; Yao, J.; Itoh, K.; Sesaki, H.; Poon, W.W.; et al. Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s Disease. Sci. Rep. 2016, 6, 18725. [Google Scholar] [CrossRef] [PubMed]
- Kislin, M.; Sword, J.; Fomitcheva, I.V.; Croom, D.; Pryazhnikov, E.; Lihavainen, E.; Toptunov, D.; Rauvala, H.; Ribeiro, A.S.; Khiroug, L.; et al. Reversible Disruption of Neuronal Mitochondria by Ischemic and Traumatic Injury Revealed by Quantitative Two-Photon Imaging in the Neocortex of Anesthetized Mice. J. Neurosci. 2017, 37, 333–348. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Datta, D.; Paspalas, C.D.; Arnsten, A.F.T. Ultrastructural evidence for impaired mitochondrial fission in the aged rhesus monkey dorsolateral prefrontal cortex. Neurobiol. Aging 2017, 51, 9–18. [Google Scholar] [CrossRef]
- Ahmad, T.; Aggarwal, K.; Pattnaik, B.; Mukherjee, S.; Sethi, T.; Tiwari, B.K.; Kumar, M.; Micheal, A.; Mabalirajan, U.; Ghosh, B.; et al. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013, 4, e461. [Google Scholar] [CrossRef]
- Mironov, A.A.; Sesorova, I.S.; Seliverstova, E.V.; Beznoussenko, G.V. Different Golgi ultrastructure across species and tissues: Implications under functional and pathological conditions, and an attempt at classification. Tissue Cell 2017, 49, 186–201. [Google Scholar] [CrossRef]
- Clermont, Y.; Rambourg, A.; Hermo, L. Trans-Golgi network (TGN) of different cell types: Three-dimensional structural characteristics and variability. Anat. Rec. 1995, 242, 289–301. [Google Scholar] [CrossRef]
- Petrosyan, A. Onco-Golgi: Is Fragmentation a Gate to Cancer Progression? Biochem. Mol. Biol. J. 2015, 1, 16. [Google Scholar] [CrossRef]
- Baloyannis, S.J. Golgi apparatus and protein trafficking in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42 (Suppl. S3), S153–S162. [Google Scholar] [CrossRef]
- Baloyannis, S.J.; Mavroudis, I.; Mitilineos, D.; Baloyannis, I.S.; Costa, V.G. The hypothalamus in Alzheimer’s disease: A Golgi and electron microscope study. Am. J. Alzheimer’s Dis. Other Dement. 2015, 30, 478–487. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, L.; Guan, Y.; Xu, H.; Niu, Y.; Han, L.; Wei, Y.P.; Lin, L.; Chu, J.; Wang, Q.; et al. Golgin-84-associated Golgi fragmentation triggers tau hyperphosphorylation by activation of cyclin-dependent kinase-5 and extracellular signal-regulated kinase. Neurobiol. Aging 2014, 35, 1352–1363. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Zhang, B.; Bruce, J.; Trojanowski, J.Q.; Lee, V.M. Reduction of detyrosinated microtubules and Golgi fragmentation are linked to tau-induced degeneration in astrocytes. J. Neurosci. 2003, 23, 10662–10671. [Google Scholar] [CrossRef]
- Liazoghli, D.; Perreault, S.; Micheva, K.D.; Desjardins, M.; Leclerc, N. Fragmentation of the Golgi apparatus induced by the overexpression of wild-type and mutant human tau forms in neurons. Am. J. Pathol. 2005, 166, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.I.; Morozov, Y.M.; Micali, N.; Rakic, P. Radial Glial Cells: New Views on Old Questions. Neurochem. Res. 2021, 46, 2512–2524. [Google Scholar] [CrossRef] [PubMed]
- Morozov, Y.M.; Mackie, K.; Rakic, P. Cannabinoid Type 1 Receptor is Undetectable in Rodent and Primate Cerebral Neural Stem Cells but Participates in Radial Neuronal Migration. Int. J. Mol. Sci. 2020, 21, 8657. [Google Scholar] [CrossRef] [PubMed]
- Morozov, Y.M.; Dominguez, M.H.; Varela, L.; Shanabrough, M.; Koch, M.; Horvath, T.L.; Rakic, P. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur. J. Neurosci. 2013, 38, 2341–2348. [Google Scholar] [CrossRef]
- Bouybayoune, I.; Mantovani, S.; Del Gallo, F.; Bertani, I.; Restelli, E.; Comerio, L.; Tapella, L.; Baracchi, F.; Fernández-Borges, N.; Mangieri, M.; et al. Transgenic fatal familial insomnia mice indicate prion infectivity-independent mechanisms of pathogenesis and phenotypic expression of disease. PLoS Pathog. 2015, 11, e1004796. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Ulmer, J.B.; Palade, G.E. Effects of Brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. Eur. J. Cell Biol. 1991, 54, 38–54. [Google Scholar]
- Rambourg, A.; Clermont, Y.; Jackson, C.L.; Képès, F. Effects of brefeldin A on the three-dimensional structure of the Golgi apparatus in a sensitive strain of Saccharomyces cerevisiae. Anat. Rec. 1995, 241, 1–9. [Google Scholar] [CrossRef]
- Han, S.; Nandy, P.; Austria, Q.; Siedlak, S.L.; Torres, S.; Fujioka, H.; Wang, W.; Zhu, X. Mfn2 Ablation in the Adult Mouse Hippocampus and Cortex Causes Neuronal Death. Cells 2020, 9, 116. [Google Scholar] [CrossRef]
- Harland, M.; Torres, S.; Liu, J.; Wang, X. Neuronal Mitochondria Modulation of LPS-Induced Neuroinflammation. J. Neurosci. 2020, 40, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Morozov, Y.M.; Koch, M.; Rakic, P.; Horvath, T.L. Cannabinoid type 1 receptor-containing axons innervate NPY/AgRP neurons in the mouse arcuate nucleus. Mol. Metab. 2017, 6, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Christie, D.A.; Lemke, C.D.; Elias, I.M.; Chau, L.A.; Kirchhof, M.G.; Li, B.; Ball, E.H.; Dunn, S.D.; Hatch, G.M.; Madrenas, J. Stomatin-like protein 2 binds cardiolipin and regulates mitochondrial biogenesis and function. Mol. Cell Biol. 2011, 31, 3845–3856. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, P.; Lapohos, O.; Weraarpachai, W.; Antonicka, H.; Chang, Y.H.; Madrenas, J. Stomatin-like protein 2 deficiency results in impaired mitochondrial translation. PLoS ONE 2017, 12, e0179967. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Y.; Chen, W.; Qiu, Z. Stomatin-Like Protein-2: A Potential Target to Treat Mitochondrial Cardiomyopathy. Heart Lung Circ. 2021, 30, 1449–1455. [Google Scholar] [CrossRef]
- Da Cruz, S.; Parone, P.A.; Gonzalo, P.; Bienvenut, W.V.; Tondera, D.; Jourdain, A.; Quadroni, M.; Martinou, J.C. SLP-2 interacts with prohibitins in the mitochondrial inner membrane and contributes to their stability. Biochim. Biophys. Acta 2008, 1783, 904–911. [Google Scholar] [CrossRef]
- Wai, T.; Saita, S.; Nolte, H.; Müller, S.; König, T.; Richter-Dennerlein, R.; Sprenger, H.G.; Madrenas, J.; Mühlmeister, M.; Brandt, U.; et al. The membrane scaffold SLP2 anchors a proteolytic hub in mitochondria containing PARL and the i-AAA protease YME1L. EMBO Rep. 2016, 17, 1844–1856. [Google Scholar] [CrossRef]
- Fiala, J.C. Reconstruct: A free editor for serial section microscopy. J. Microsc. 2005, 218 Pt 1, 52–61. [Google Scholar] [CrossRef]
- Fiala, J.C.; Harris, K.M. Extending unbiased stereology of brain ultrastructure to three-dimensional volumes. J. Am. Med. Inform. Assoc. 2001, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Sample and Embryo # | Normal Golgi | Onion-Like Golgi | ||
|---|---|---|---|---|
| Number | % | Number | % | |
| Control | ||||
| Cortex #1 | 20 | 95.24 | 1 | 4.76 |
| Cortex #2 | 26 | 100.00 | 0 | 0.00 |
| Cortex #3 | 29 | 96.67 | 1 | 3.33 |
| Cortex #4 | 15 | 100.00 | 0 | 0.00 |
| Cortex total, sum | 90 | 2 | ||
| Cortex total, mean ± SD | 97.98 ± 2.41 | 2.02 ± 2.41 | ||
| Hippocampus #1 | 9 | 100.00 | 0 | 0.00 |
| Hippocampus #2 | 14 | 100.00 | 0 | 0.00 |
| Hippocampus #3 | 28 | 100.00 | 0 | 0.00 |
| Hippocampus #4 | 19 | 100.00 | 0 | 0.00 |
| Hippocampus total, sum | 70 | 0 | ||
| Hippocampus total, mean | 100.00 | 0.00 | ||
| LGE #1 | 19 | 100.00 | 0 | 0.00 |
| LGE #2 | 40 | 100.00 | 0 | 0.00 |
| LGE total, sum | 59 | 0 | ||
| LGE total, mean | 100.00 | 0.00 | ||
| 1 h anoxia | ||||
| Cortex #1 | 7 | 31.82 | 15 | 68.18 |
| Cortex #2 | 2 | 14.29 | 12 | 85.71 |
| Cortex #3 | 7 | 22.58 | 24 | 77.42 |
| Cortex #4 | 3 | 12.00 | 22 | 88.00 |
| Cortex #5 | 1 | 12.50 | 7 | 87.50 |
| Cortex total, sum | 20 | 80 | ||
| Cortex total, mean ± SD | 18.64 ± 8.51 | 81.36 ± 8.51 | ||
| Hippocampus #1 | 19 | 31.67 | 41 | 68.33 |
| Hippocampus #2 | 12 | 23.53 | 39 | 76.47 |
| Hippocampus #3 | 12 | 33.33 | 24 | 66.67 |
| Hippocampus #4 | 5 | 20.83 | 19 | 79.17 |
| Hippocampus total, sum | 48 | 123 | ||
| Hippocampus total, mean ± SD | 27.34 ± 6.10 | 72.66 ± 6.10 | ||
| LGE #1 | 10 | 25.64 | 29 | 74.36 |
| LGE #2 | 7 | 30.43 | 16 | 69.57 |
| LGE #3 | 7 | 26.92 | 19 | 73.08 |
| LGE #4 | 0 | 0.00 | 15 | 100.00 |
| LGE total, sum | 24 | 79 | ||
| LGE total, mean ± SD | 20.75 ± 13.98 | 79.25 ± 13.98 | ||
| 1 h anoxia/reoxygenation | ||||
| Cortex #1 | 29 | 100.00 | 0 | 0.00 |
| Cortex #2 | 39 | 92.86 | 3 | 7.14 |
| Cortex total, sum | 68 | 3 | ||
| Cortex total, mean ± SD | 96.43 ± 5.05 | 3.57 ± 5.05 | ||
| LGE #1 | 21 | 87.50 | 3 | 12.50 |
| LGE #2 | 21 | 80.77 | 5 | 19.23 |
| LGE total, sum | 42 | 8 | ||
| LGE total, mean ± SD | 84.13 ± 4.76 | 15.87 ± 4.76 | ||
| 3 h anoxia | ||||
| Cortex #1 | 0 | 0.00 | 4 | 100.00 |
| Cortex #2 | 1 | 9.09 | 10 | 90.91 |
| Cortex #3 | 0 | 0.00 | 25 | 100.00 |
| Cortex #4 | 0 | 0.00 | 18 | 100.00 |
| Cortex total, sum | 1 | 57 | ||
| Cortex total, mean ± SD | 2.27 ± 4.55 | 97.73 ± 4.55 | ||
| Hippocampus #1 | 0 | 0.00 | 31 | 100.00 |
| Hippocampus #2 | 0 | 0.00 | 29 | 100.00 |
| Hippocampus total, sum | 0 | 60 | ||
| Hippocampus total, mean | 0.00 | 100.00 | ||
| LGE #1 | 0 | 0.00 | 14 | 100.00 |
| LGE #2 | 0 | 0.00 | 11 | 100.00 |
| LGE total, sum | 0 | 25 | ||
| LGE total, mean | 0.00 | 100.00 | ||
| 3 h anoxia/reoxygenation | ||||
| Cortex #1 | 0 | 0.00 | 12 | 100.00 |
| Cortex #2 | 2 | 20.00 | 8 | 80.00 |
| Cortex #3 | 0 | 0.00 | 7 | 100.00 |
| Cortex total, sum | 2 | 27 | ||
| Cortex total, mean ± SD | 6.67 ± 11.55 | 93.33 ± 11.55 | ||
| LGE #1 | 0 | 0.00 | 8 | 100.00 |
| LGE #2 | 0 | 0.00 | 14 | 100.00 |
| LGE #3 | 0 | 0.00 | 7 | 100.00 |
| LGE total, sum | 0 | 29 | ||
| LGE total, mean | 0.00 | 100.00 | ||
| Embryo Analyzed | Ellipsoid | Branched | Donut-Like | MOAS | Total | Mean Length ± SD, µm | Mean Diameter ± SD, µm |
|---|---|---|---|---|---|---|---|
| Control #1 | 100 | 0 | 5 | 0 | 105 | 1.28 ± 0.71 | 0.43 ± 0.08 |
| Control #2 | 53 | 0 | 0 | 2 | 55 | 1.20 ± 1.00 | 0.43 ± 0.05 |
| Control #3 | 65 | 0 | 5 | 0 | 70 | 1.39 ± 0.82 | 0.40 ± 0.07 |
| Control total | 218 | 0 | 10 | 2 | 230 | 1.30 ± 0.82 | 0.42 ± 0.07 |
| 1 h anoxia #1 | 121 | 2 | 3 | 0 | 126 | 1.04 ± 0.61 | 0.40 ± 0.07 |
| 1 h anoxia #2 | 116 | 1 | 1 | 1 | 119 | 1.42 ± 0.92 | 0.38 ± 0.07 |
| 1 h anoxia #3 | 148 | 1 | 0 | 2 | 151 | 1.36 ± 0.79 | 0.41 ± 0.06 |
| 1 h anoxia #4 | 57 | 2 | 0 | 1 | 60 | 1.60 ± 1.09 | 0.44 ± 0.08 |
| 1 h total | 442 | 6 | 4 | 4 | 456 | 1.32 ± 0.85 | 0.40 ± 0.07 |
| 3 h anoxia #1 | 55 | 0 | 0 | 0 | 55 | 0.69 ± 0.21 | 0.52 ± 0.11 |
| 3 h anoxia #2 | 168 | 0 | 0 | 0 | 168 | 0.54 ± 0.12 | 0.45 ± 0.09 |
| 3 h total | 223 | 0 | 0 | 0 | 223 | 0.58 ± 0.16 | 0.47 ± 0.10 |
| 3 h anoxia/reoxygenation #1 | 91 | 0 | 0 | 0 | 91 | 0.70 ± 0.19 | 0.57 ± 0.14 |
| 3 h anoxia/reoxygenation #2 | 35 | 0 | 0 | 0 | 35 | 0.79 ± 0.32 | 0.44 ± 0.11 |
| 3 h anoxia/reoxygenation #3 | 141 | 0 | 3 | 0 | 144 | 0.59 ± 0.24 | 0.37 ± 0.10 |
| 3 h anoxia/reoxygenation total | 267 | 0 | 3 | 0 | 270 | 0.66 ± 0.25 | 0.44 ± 0.15 |
| 4.5 h anoxia #1 | 72 | 0 | 0 | 0 | 72 | 0.65 ± 0.17 | 0.54 ± 0.12 |
| 4.5 h anoxia #2 | 49 | 0 | 0 | 0 | 49 | 0.63 ± 0.13 | 0.56 ± 0.11 |
| 4.5 h total | 121 | 0 | 0 | 0 | 121 | 0.64 ± 0.15 | 0.55 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozov, Y.M.; Rakic, P. Disorder of Golgi Apparatus Precedes Anoxia-Induced Pathology of Mitochondria. Int. J. Mol. Sci. 2023, 24, 4432. https://doi.org/10.3390/ijms24054432
Morozov YM, Rakic P. Disorder of Golgi Apparatus Precedes Anoxia-Induced Pathology of Mitochondria. International Journal of Molecular Sciences. 2023; 24(5):4432. https://doi.org/10.3390/ijms24054432
Chicago/Turabian StyleMorozov, Yury M., and Pasko Rakic. 2023. "Disorder of Golgi Apparatus Precedes Anoxia-Induced Pathology of Mitochondria" International Journal of Molecular Sciences 24, no. 5: 4432. https://doi.org/10.3390/ijms24054432
APA StyleMorozov, Y. M., & Rakic, P. (2023). Disorder of Golgi Apparatus Precedes Anoxia-Induced Pathology of Mitochondria. International Journal of Molecular Sciences, 24(5), 4432. https://doi.org/10.3390/ijms24054432







