Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture
3.3. Microgravity Simulation
3.4. Sample Preparation for UHPLC-IM-QTOF-MS Analysis
3.5. UHPLC-IM-QTOF-MS/MS Analysis
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Riwaldt, S.; Corydon, T.J.; Pantalone, D.; Sahana, J.; Wise, P.; Wehland, M.; Krüger, M.; Melnik, D.; Kopp, S.; Infanger, M.; et al. Role of Apoptosis in Wound Healing and Apoptosis Alterations in Microgravity. Front. Bioeng. Biotechnol. 2021, 9, 1–22. [Google Scholar] [CrossRef]
- Bizzarri, M.; Fedeli, V.; Piombarolo, A.; Angeloni, A. Space Biomedicine: A Unique Opportunity to Rethink the Relationships between Physics and Biology. Biomedicines 2022, 10, 2633. [Google Scholar] [CrossRef]
- Thirsk, R.; Kuipers, A.; Mukai, C.; Williams, D. The space-flight environment: The International Space Station and beyond. CMAJ 2009, 180, 1216–1220. [Google Scholar] [CrossRef]
- Sheyn, D.; Pelled, G.; Netanely, D.; Domany, E.; Gazit, D. The Effect of Simulated Microgravity on Human Mesenchymal Stem Cells Cultured in an Osteogenic Differentiation System: A Bioinformatics Study. Tissue Eng.-Part A 2010, 16, 3403–3412. [Google Scholar] [CrossRef]
- Hughes-Fulford, M.; Tjandrawinata, R.; Fitzgerald, J.; Gasuad, K.; Gilbertson, V. Effects of microgravity on osteoblast growth. Gravit. Space Biol. Bull. 1998, 11, 51–60. [Google Scholar]
- Han, X.; Qiu, L.; Zhang, Y.; Kong, Q.; Wang, H.; Wang, H.; Li, H.; Duan, C.; Wang, Y.; Song, Y.; et al. Transplantation of Sertoli-Islet Cell Aggregates Formed by Microgravity: Prolonged Survival in Diabetic Rats. Exp. Biol. Med. 2009, 234, 595–603. [Google Scholar] [CrossRef]
- NSBRI. National Space Biomedical Research Institute Strategic Plan 2010. Biomed Res. 2010. Available online: http://nsbri.org/wp-content/uploads/2015/08/NSBRI_strategic_plan1 (accessed on 18 January 2022).
- Dinarelli, S.; Longo, G.; Dietler, G.; Francioso, A.; Mosca, L.; Pannitteri, G.; Boumis, G.; Bellelli, A.; Girasole, M. Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci. Rep. 2018, 8, 5277. [Google Scholar] [CrossRef]
- Poon, C. Factors implicating the validity and interpretation of mechanobiology studies in simulated microgravity environments. Eng. Rep. 2020, 2, 1–18. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Navas-Iglesias, N.; Cuadros-Rodríguez, L. From lipid analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part I: Modern lipid analysis. TrAC-Trends Anal. Chem. 2009, 28, 263–278. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Ivanova, S.M.; Morukov, B.V.; Labetskaia, O.I.; IuV, I.; Levina, A.A.; Kozinets, G.I. Morphobiochemical assay of the red blood system in members of the prime crews of the International Space Station. Hum. Physiol. 2009, 43, 43–47. [Google Scholar]
- Girasole, M.; Pompeo, G.; Cricenti, A.; Congiu-Castellano, A.; Andreola, F.; Serafino, A.; Frazer, B.; Boumis, G.; Amiconi, G. Roughness of the plasma membrane as an independent morphological parameter to study RBCs: A quantitative atomic force microscopy investigation. Biochim. Biophys. Acta (BBA)-Biomembr. 2007, 1768, 1268–1276. [Google Scholar] [CrossRef]
- Manis, C.; Manca, A.; Murgia, A.; Uras, G.; Caboni, P.; Congiu, T.; Faa, G.; Pantaleo, A.; Cao, G. Understanding the Behaviour of Human Cell Types under Simulated Microgravity Conditions: The Case of Erythrocytes. Int. J. Mol. Sci. 2022, 23, 6876. [Google Scholar] [CrossRef]
- Alfrey, C.P.; Udden, M.M.; Leach-Huntoon, C.; Driscoll, T.; Pickett, M.H. Control of red blood cell mass in spaceflight. J. Appl. Physiol. 1996, 81, 98–104. [Google Scholar] [CrossRef]
- Taylor, G.R. Advances in experimental medicine. In Advances in Experimental Medicine and Biology; Plenum Press: New York, NY, USA; London, UK, 1997; Volume 225, pp. 269–271. Available online: http://linkinghub.elsevier.com/retrieve/pii/0020711X84902064 (accessed on 18 January 2022).
- Meehan, R.T.; Neale, L.S.; Kraus, E.T.; Stuart, C.A.; Smith, M.L.; Cintron, N.M.; Sams, C.F. Alteration in human mononuclear leucocytes following space flight. Immunology 1992, 76, 491–497. [Google Scholar]
- Diedrich, A.; Paranjape, S.Y.; Robertson, D. Plasma and Blood Volume in Space. Am. J. Med. Sci. 2007, 334, 80–86. [Google Scholar] [CrossRef]
- Erslev, A.J. Erythropoietin. N. Engl. J. Med. 1991, 324, 1339–1344. [Google Scholar] [CrossRef]
- Koury, M.J.; Bondurant, M.C. Erythropoietin Retards DNA Breakdown and Prevents Programmed Death in Erythroid Progenitor Cells. Science 1990, 248, 378–381. [Google Scholar] [CrossRef]
- Trudel, G.; Shahin, N.; Ramsay, T.; Laneuville, O.; Louati, H. Hemolysis contributes to anemia during long-duration space flight. Nat. Med. 2022, 28, 59–62. [Google Scholar] [CrossRef]
- Crimi, M.; Degli Esposti, M. Apoptosis-induced changes in mitochondrial lipids. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 551–557. [Google Scholar] [CrossRef]
- Sprong, H.; Van Der Sluijs, P.; van Meer, G. How proteins move lipids and lipids move proteins. Nat. Rev. Mol. Cell Biol. 2001, 2, 504–513. [Google Scholar] [CrossRef]
- Robertson, J.L. The lipid bilayer membrane and its protein constituents. J. Gen. Physiol. 2018, 150, 1472–1483. [Google Scholar] [CrossRef]
- Koeberle, A.; Shindou, H.; Koeberle, S.C.; Laufer, S.A.; Shimizu, T.; Werz, O. Arachidonoyl-phosphatidylcholine oscillates during the cell cycle and counteracts proliferation by suppressing Akt membrane binding. Proc. Natl. Acad. Sci. USA 2013, 110, 2546–2551. [Google Scholar] [CrossRef]
- Picache, J.A.; Rose, B.S.; Balinski, A.; Leaptrot, K.L.; Sherrod, S.D.; May, J.C.; McLean, J.A. Collision cross section compendium to annotate and predict multi-omic compound identities. Chem. Sci. 2019, 10, 983–993. [Google Scholar] [CrossRef]
- Subbanagounder, G.; Leitinger, N.; Schwenke, D.C.; Wong, J.W.; Lee, H.; Rizza, C.; Watson, A.D.; Faull, K.F.; Fogelman, A.M.; Berliner, J.A. Determinants of bioactivity of oxidized phospholipids: Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2248–2254. [Google Scholar] [CrossRef]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef]
- Ashraf, M.Z.; Kar, N.S.; Podrez, E.A. Oxidized phospholipids: Biomarker for cardiovascular diseases. Int. J. Biochem. Cell Biol. 2009, 41, 1241–1244. [Google Scholar] [CrossRef]
- Huber, J.; Vales, A.; Mitulović, G.; Blumer, M.; Schmid, R.; Witztum, J.L.; Binder, B.R.; Leitinger, N. Oxidized Membrane Vesicles and Blebs from Apoptotic Cells Contain Biologically Active Oxidized Phospholipids That Induce Monocyte-Endothelial Interactions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 101–107. [Google Scholar] [CrossRef]
- Chang, M.-K.; Binder, C.J.; Torzewski, M.; Witztum, J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2002, 99, 13043–13048. [Google Scholar] [CrossRef]
- Rouhanizadeh, M.; Hwang, J.; Clempus, R.E.; Marcu, L.; Lassègue, B.; Sevanian, A.; Hsiai, T.K. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: Implication of NADPH oxidase. Free Radic. Biol. Med. 2005, 39, 1512–1522. [Google Scholar] [CrossRef]
- Patwardhan, G.A.; Beverly, L.J.; Siskind, L.J. Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 2016, 48, 153–168. [Google Scholar] [CrossRef]
- Ulmer, C.Z.; Jones, C.M.; Yost, R.A.; Garrett, T.J.; Bowden, J.A. Optimization of Folch, Bligh-Dyer, and Matyash sample-to-extraction solvent ratios for human plasma-based lipidomics studies. Anal. Chim. Acta 2018, 1037, 351–357. [Google Scholar] [CrossRef]
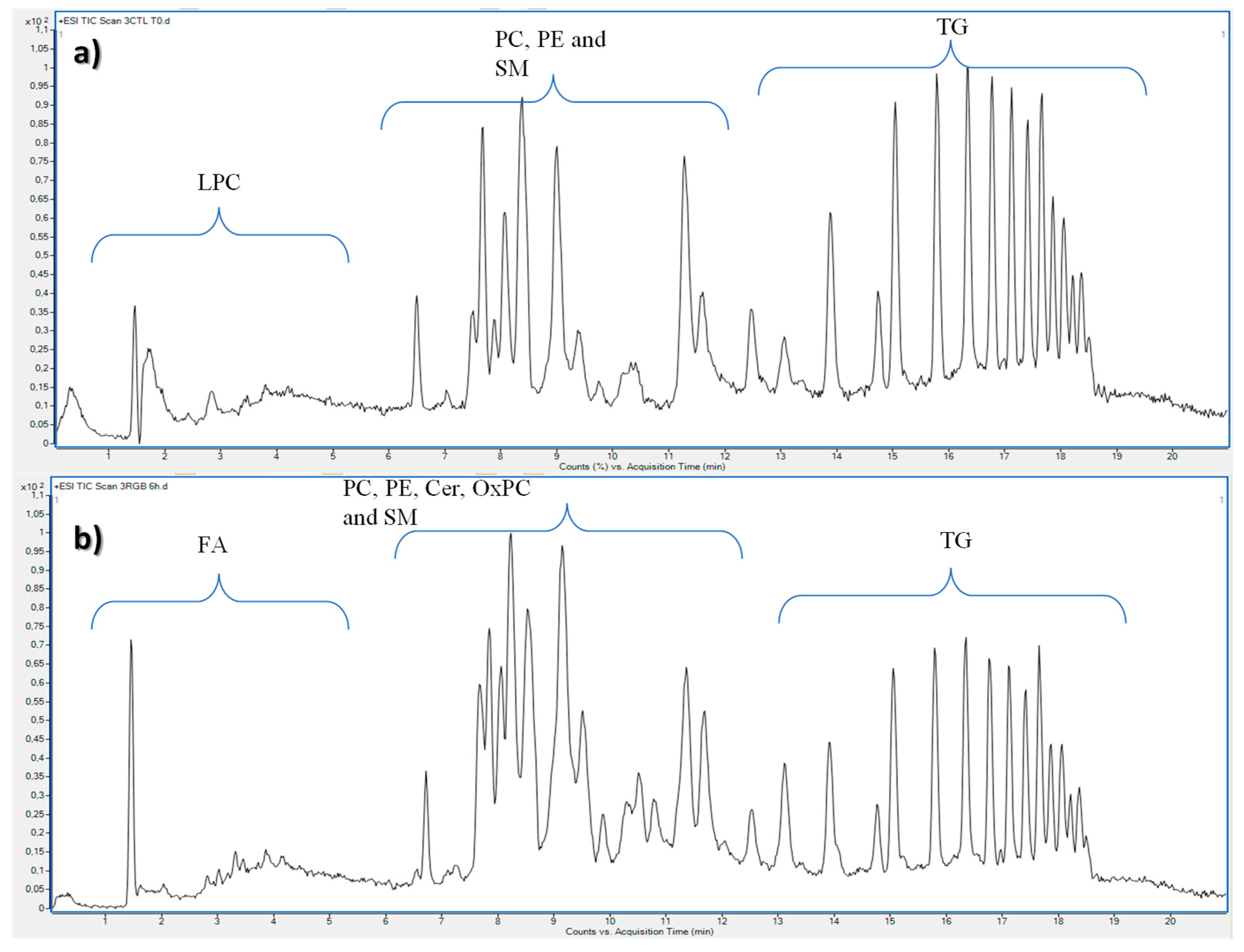
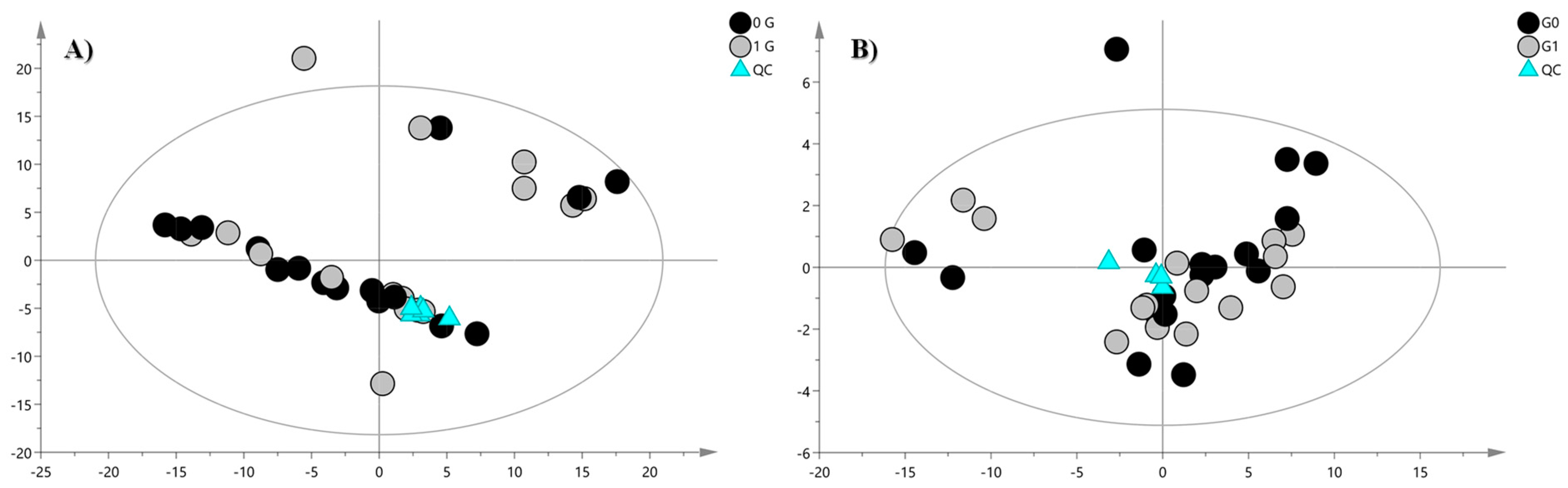
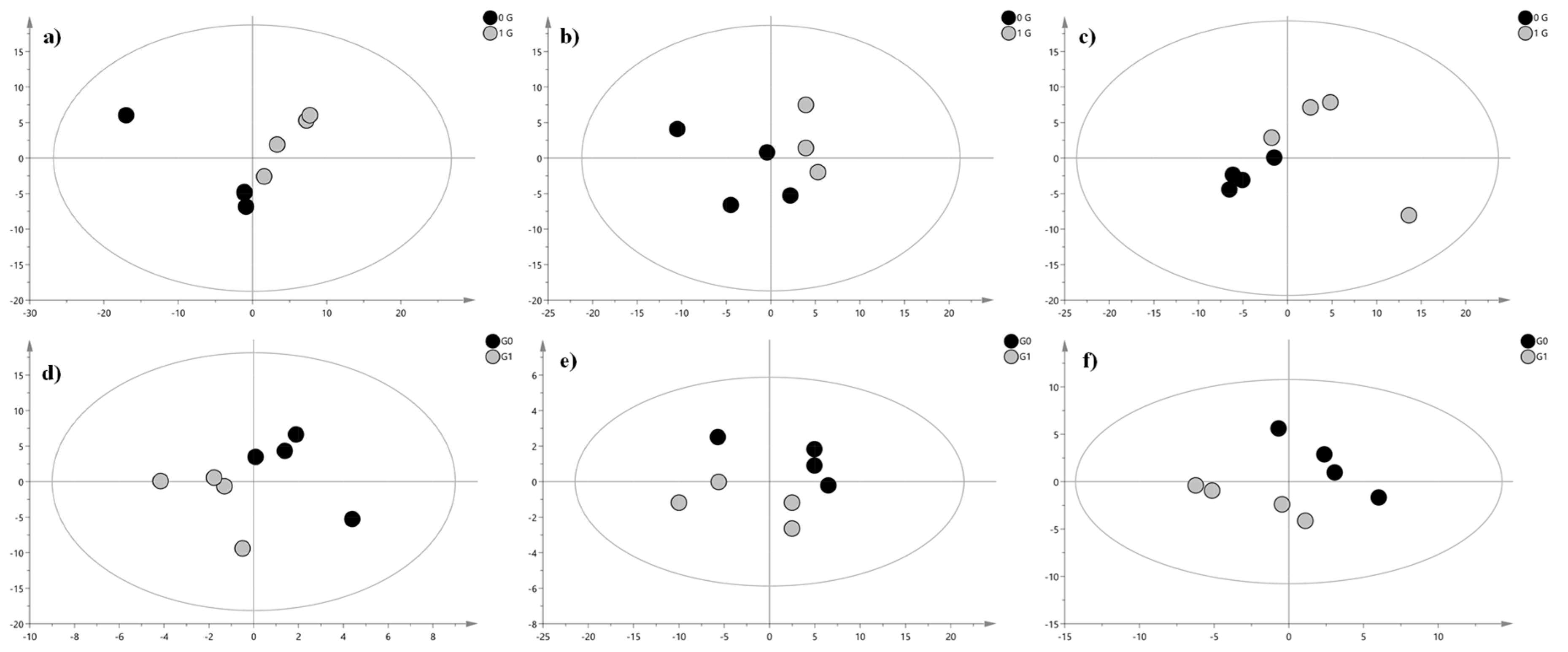
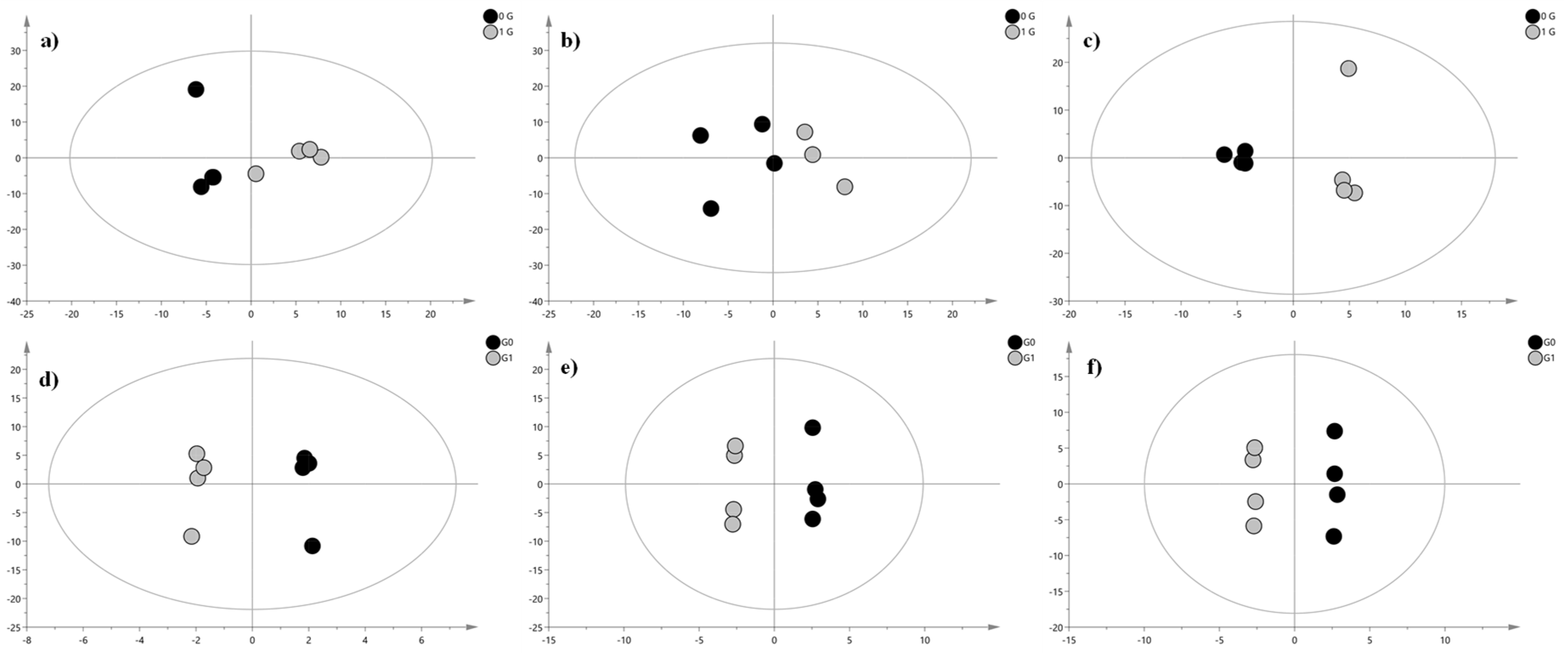
| Lipid | Adduct | m/z Experimental | m/z Theoretical | Δ (ppm) | RT (min) | Fatty Acid Composition | DTCCSN2 (Å2) | VIP | Significance Level | Regulation in G0 Cells |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 h | ||||||||||
| LysoPC16:1 | +H+ | 494.3216 | 494.3241 | 5.1 | 6.55 | 16:1 | 231.58 | 1.73 | ns | up |
| PC 32:2 | +H+ | 730.5394 | 730.5381 | 1.3 | 7.77 | 32:2 | 286.75 | 1.71 | ** | up |
| PC 38:5 | +H+ | 808.5871 | 808.5851 | 2.0 | 8.05 | 18:1, 20:4 | 292.87 | 1.50 | ** | up |
| PC 33:5 | +H+ | 738.5070 | 738.5068 | 0.3 | 7.74 | 287.33 | 1.26 | ns | down | |
| SM d34:0 | +H+ | 705.5930 | 705.5905 | 3.5 | 8.01 | 16:0, 18:0 | 285.87 | 1.10 | * | down |
| PC 38:4 | +H+ | 810.6021 | 810.6007 | 1.7 | 9.23 | 18:0, 20:4 | 295.11 | 1.05 | ** | up |
| PC 36:2 | +H+ | 786.6031 | 786.6007 | 3.0 | 9.43 | 18:1, 18:1 | 291.56 | 1.00 | * | up |
| SM d 38:1 | +H+ | 759.6328 | 759.6375 | 6.0 | 11.31 | 297.55 | 1.00 | ** | down | |
| PC 36:1 | +H+ | 788.6192 | 788.6164 | 3.2 | 10.43 | 293.88 | 0.99 | * | up | |
| FA 16:2 | -H− | 251.2021 | 251.2017 | 1.6 | 3.59 | 16:2 | 114.95 | 1.38 | ** | down |
| HexCer_AP t37:1 | +(C2H3O2)− | 832.6152 | 832.6164 | 1.5 | 10.43 | 22:1, 15:0 | 296.27 | 1.46 | ** | up |
| Etn-1-P-Cer 32:1 | -H− | 687.5469 | 687.5446 | 3.4 | 7.51 | 14:1, 18:0 | 265.47 | 1.13 | *** | up |
| EtherOxPC 36:4e + 1O | +(CHO2)− | 828.5761 | 828.5781 | 2.4 | 8.3 | 16:0, 20:4 | 293.50 | 1.07 | * | up |
| SM d40:1 | +(CHO2)− | 831.6632 | 831.6597 | 4 | 11.66 | 301.49 | 1.06 | ns | up | |
| PE 36:3 | -H− | 740.5249 | 740.5236 | 2 | 7.50 | 18:2, 18:1 | 270.58 | 1.02 | ns | up |
| 9 h | ||||||||||
| PC 38:6 | +H+ | 806.5700 | 806.5694 | 0.74 | 8.48 | 18:2, 20:4 | 293.14 | 1.79 | * | up |
| SM d 38:1 | +H+ | 759.6328 | 759.6375 | 6.0 | 11.31 | 297.55 | 1.75 | ** | down | |
| PC 35:5 | +H+ | 766.5399 | 766.5381 | 2.3 | 8.33 | 15:1, 20:4 | 280.17 | 1.73 | *** | up |
| PE 36:1 | +H+ | 746.5712 | 746.5694 | 2.1 | 10.71 | 18:0, 18:1 | 281.87 | 1.71 | ** | up |
| PC 36:5 | +H+ | 780.5541 | 780.5538 | 0.3 | 8.11 | 16:1, 20:4 | 228.33 | 1.70 | ** | up |
| PC 32:0 | +H+ | 734.572 | 734.5694 | 3.5 | 8.92 | 16:0, 16:0 | 284.83 | 1.64 | ** | up |
| PC 36:2 | +H+ | 786.6031 | 786.6007 | 3.0 | 9.25 | 18:1, 18:1 | 291.56 | 1.63 | ** | up |
| SM d34:0 | +H+ | 705.5930 | 705.5905 | 3.5 | 8.01 | 16:0, 18:0 | 285.87 | 1.61 | ** | up |
| PC 35:5 | +H+ | 766.5407 | 766.5381 | 3.4 | 8.51 | 288.55 | 1.60 | ** | down | |
| PE 38:6 | +H+ | 764.524 | 764.5225 | 2.0 | 7.89 | 16:0, 22:6 | 279.42 | 1.59 | ** | up |
| PC 36:3 | +H+ | 784.5872 | 784.5851 | 2.7 | 8.25 | 18:1, 18:2 | 289.42 | 1.57 | ** | up |
| PC 35:4 | +H+ | 768.5593 | 768.5538 | 7.0 | 8.73 | 15:0, 20:4 | 289.52 | 1.56 | ** | up |
| SM d44:5 | +H+ | 835.6691 | 835.6688 | 1.0 | 11.61 | 14:3, 30:2 | 305.48 | 1.52 | ** | up |
| SM 42:3 | +H+ | 811.6713 | 811.6688 | 3.1 | 10.49 | 302.65 | 1.48 | ** | up | |
| PC 38:7 | +H+ | 804.554 | 804.5 538 | 0.3 | 7.93 | 18:3, 20:4 | 292.2 | 1.45 | ns | up |
| PC 34:0 | +H+ | 762.6032 | 762.6007 | 3.3 | 10.27 | 290.86 | 1.38 | ns | up | |
| PC 36:5 | +H+ | 780.5541 | 780.5538 | 0.3 | 8.11 | 289.42 | 1.37 | * | up | |
| PC 36:4 | +H+ | 782.5701 | 782.5694 | 1.0 | 9.08 | 16:0, 20:4 | 229.67 | 1.36 | ** | up |
| PC 34:3 | +H+ | 756.554 | 759.5538 | 0.3 | 8.92 | 18:1, 16:2 | 286.35 | 1.33 | * | up |
| PE 34:2 | +H+ | 716.5251 | 716.5225 | 3.5 | 8.37 | 16:0, 18:2 | 273.06 | 1.23 | ns | up |
| PC 36:1 | +H+ | 788.6192 | 788.6164 | 3.2 | 10.43 | 293.88 | 1.21 | * | up | |
| SM d42:2 | +H+ | 813.6870 | 813.6844 | 3.2 | 11.61 | 305.35 | 1.21 | ** | up | |
| PC 38:5 | +H+ | 808.5870 | 808.5851 | 2.3 | 8.04 | 16:0, 22:5 | 292.87 | 1.20 | ns | up |
| PC 33:5 | +H+ | 738.5070 | 738.5068 | 0.3 | 7.74 | 287.33 | 1.07 | * | down | |
| PE 36:3 | +H+ | 742.5399 | 742.5381 | 2.5 | 8.51 | 16:0, 20:3 | 276.61 | 1.04 | ns | up |
| PE 36:4 | +H+ | 740.5239 | 740.5225 | 2.0 | 8.19 | 16:0, 20:4 | 276.93 | 1.03 | ** | up |
| SM d42:2 | +H+ | 813.6870 | 813.6844 | 3.2 | 11.61 | 305.03 | 1.00 | ** | up | |
| EtherOxPC 36:4e + 1O | +(CHO2)− | 828.5761 | 828.5781 | 2.4 | 8.3 | 16:0, 20:4 | 293.50 | 1.56 | * | down |
| PE 36:2 | -H− | 742.5403 | 742.5392 | 1.5 | 9.55 | 18:1, 18:1 | 271.52 | 1.16 | ** | up |
| PC 38:6 | +(CHO2)− | 850.5613 | 850.5604 | 1.1 | 7.62 | 18:2, 20:4 | 296.34 | 1.01 | ns | up |
| 24 h | ||||||||||
| PC 35:4 | +H+ | 768.5593 | 768.5538 | 7.0 | 8.73 | 15:0, 20:4 | 289.52 | 1.84 | ** | up |
| PE 36:4 | +H+ | 740.5239 | 740.5225 | 2.0 | 8.19 | 16:0, 20:4 | 276.93 | 1.84 | ** | up |
| PC 36:2 | +H+ | 786.6031 | 786.6007 | 3.0 | 9.25 | 18:1, 18:1 | 291.56 | 1.64 | ** | up |
| PC 32:0 | +H+ | 734.572 | 734.5694 | 3.5 | 8.92 | 16:0, 16:0 | 284.83 | 1.61 | ns | up |
| SM d42:2 | +H+ | 813.6870 | 813.6844 | 3.2 | 11.98 | 305.03 | 1.46 | * | up | |
| SM 42:3 | +H+ | 811.6713 | 811.6688 | 3.1 | 10.49 | 302.65 | 1.40 | ** | up | |
| PC 36:3 | +H+ | 784.5872 | 784.5851 | 2.7 | 8.25 | 18:1, 18:2 | 289.42 | 1.35 | * | up |
| PE 36:1 | +H+ | 746.5712 | 746.5694 | 2.1 | 10.71 | 18:0, 18:1 | 281.87 | 1.34 | ns | up |
| PE 38:6 | +H+ | 764.524 | 764.5225 | 2.0 | 7.89 | 18:2, 20:4 | 279.42 | 1.21 | ** | up |
| PC 36:4 | +H+ | 782.5701 | 782.5694 | 1.0 | 9.08 | 16:0, 20:4 | 229.67 | 1.20 | *** | up |
| PC 36:5 | +H+ | 780.5541 | 780.5538 | 0.3 | 8.11 | 289.42 | 1.20 | * | up | |
| PC 38:4 | +H+ | 810.6021 | 810.6007 | 1.7 | 9.23 | 18:0, 20:4 | 295.11 | 1.19 | ** | up |
| PC 34:0 | +H+ | 762.6032 | 762.6007 | 3.3 | 10.27 | 290.86 | 1.13 | * | up | |
| PC 35:5 | +H+ | 766.5399 | 766.5381 | 2.3 | 8.33 | 15:1, 20:4 | 280.17 | 1.11 | * | down |
| PE 36:3 | +H+ | 742.5399 | 742.5381 | 2.5 | 8.52 | 16:0, 20:3 | 276.61 | 1.11 | ns | up |
| SM d44:5 | +H+ | 835.6691 | 835.6688 | 1.0 | 11.61 | 14:3, 30:2 | 305.48 | 1.09 | ** | up |
| SM d42:2 | +H+ | 813.6870 | 813.6844 | 3.2 | 11.62 | 305.35 | 1.05 | ns | up | |
| PC 35:5 | +H+ | 766.5407 | 766.5381 | 3.4 | 8.51 | 288.55 | 1.03 | * | down | |
| LysoPC16:1 | +H+ | 494.3216 | 494.3241 | 5.1 | 6.55 | 16:1 | 231.58 | 1.01 | * | up |
| FA 16:2 | -H− | 251.2021 | 251.2017 | 1.6 | 3.59 | 16:2 | 114.95 | 1.99 | ns | up |
| Cer 42:1 | +(CHO2)− | 694.6369 | 694.6355 | 2.1 | 15.27 | 18:1, 24:0 | 278.31 | 1.02 | * | down |
| HexCer_AP t37:2 | +(C2H3O2)− | 830.5979 | 830.5999 | 2.5 | 9.42 | 22:1, 15:1 | 295.07 | 1.01 | ns | down |
| HexCer_AP t37:1 | +(C2H3O2)− | 832.6152 | 832.6164 | 1.5 | 10.43 | 22:1, 15:0 | 296.27 | 1.01 | ns | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manis, C.; Murgia, A.; Manca, A.; Pantaleo, A.; Cao, G.; Caboni, P. Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions. Int. J. Mol. Sci. 2023, 24, 4379. https://doi.org/10.3390/ijms24054379
Manis C, Murgia A, Manca A, Pantaleo A, Cao G, Caboni P. Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions. International Journal of Molecular Sciences. 2023; 24(5):4379. https://doi.org/10.3390/ijms24054379
Chicago/Turabian StyleManis, Cristina, Antonio Murgia, Alessia Manca, Antonella Pantaleo, Giacomo Cao, and Pierluigi Caboni. 2023. "Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions" International Journal of Molecular Sciences 24, no. 5: 4379. https://doi.org/10.3390/ijms24054379
APA StyleManis, C., Murgia, A., Manca, A., Pantaleo, A., Cao, G., & Caboni, P. (2023). Untargeted Lipidomics of Erythrocytes under Simulated Microgravity Conditions. International Journal of Molecular Sciences, 24(5), 4379. https://doi.org/10.3390/ijms24054379







