Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies
Abstract
SIGNIFICANCE STATEMENT
1. Introduction
2. Results
2.1. Search Results
2.2. Characteristics of Included Studies
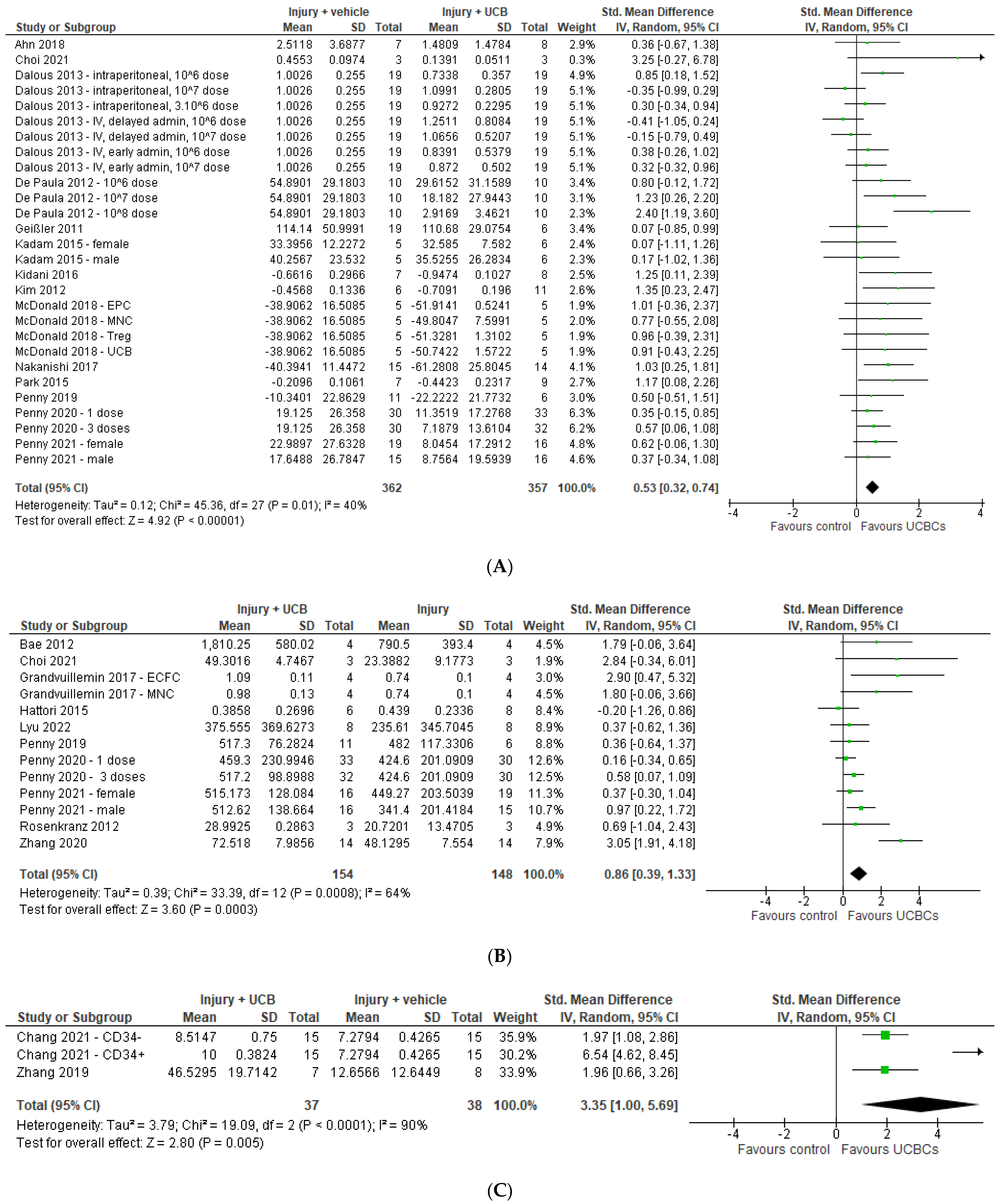
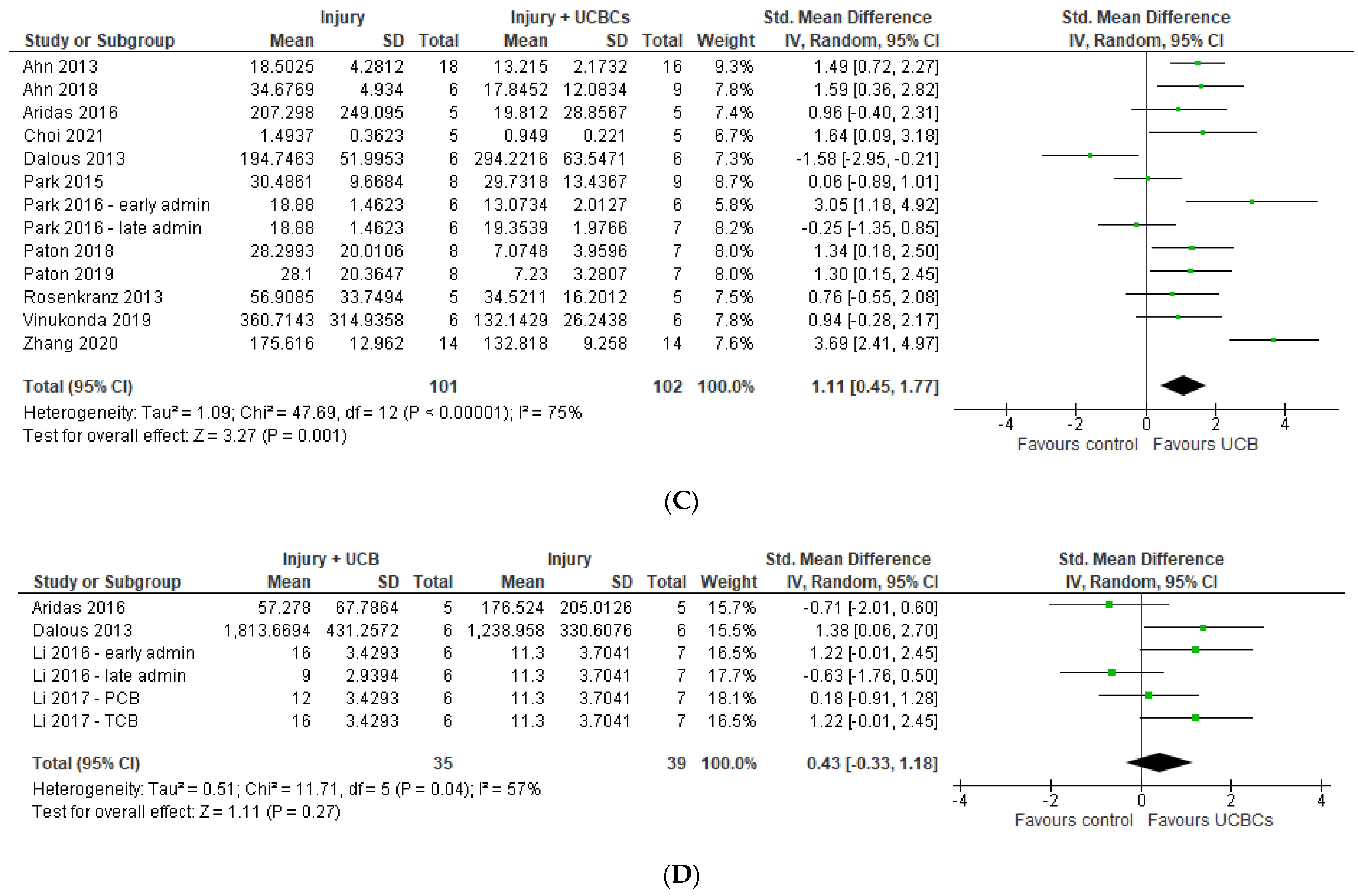
2.3. Markers of Brain Injury
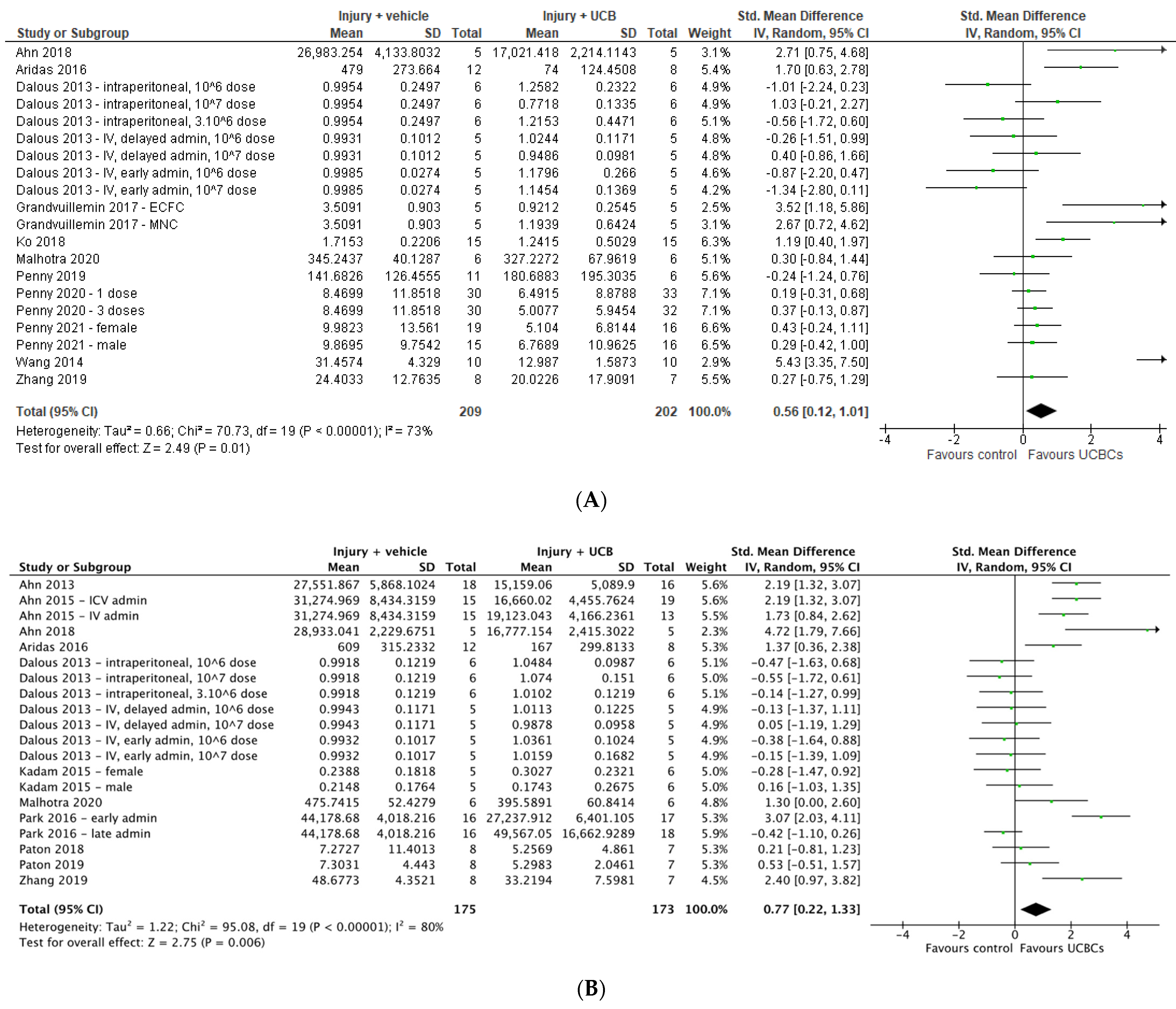
2.4. Effect of Ucb-Derived Cell Therapy on Infarct Size, Neuron Number, Oligodendrocyte Number & Apoptosis
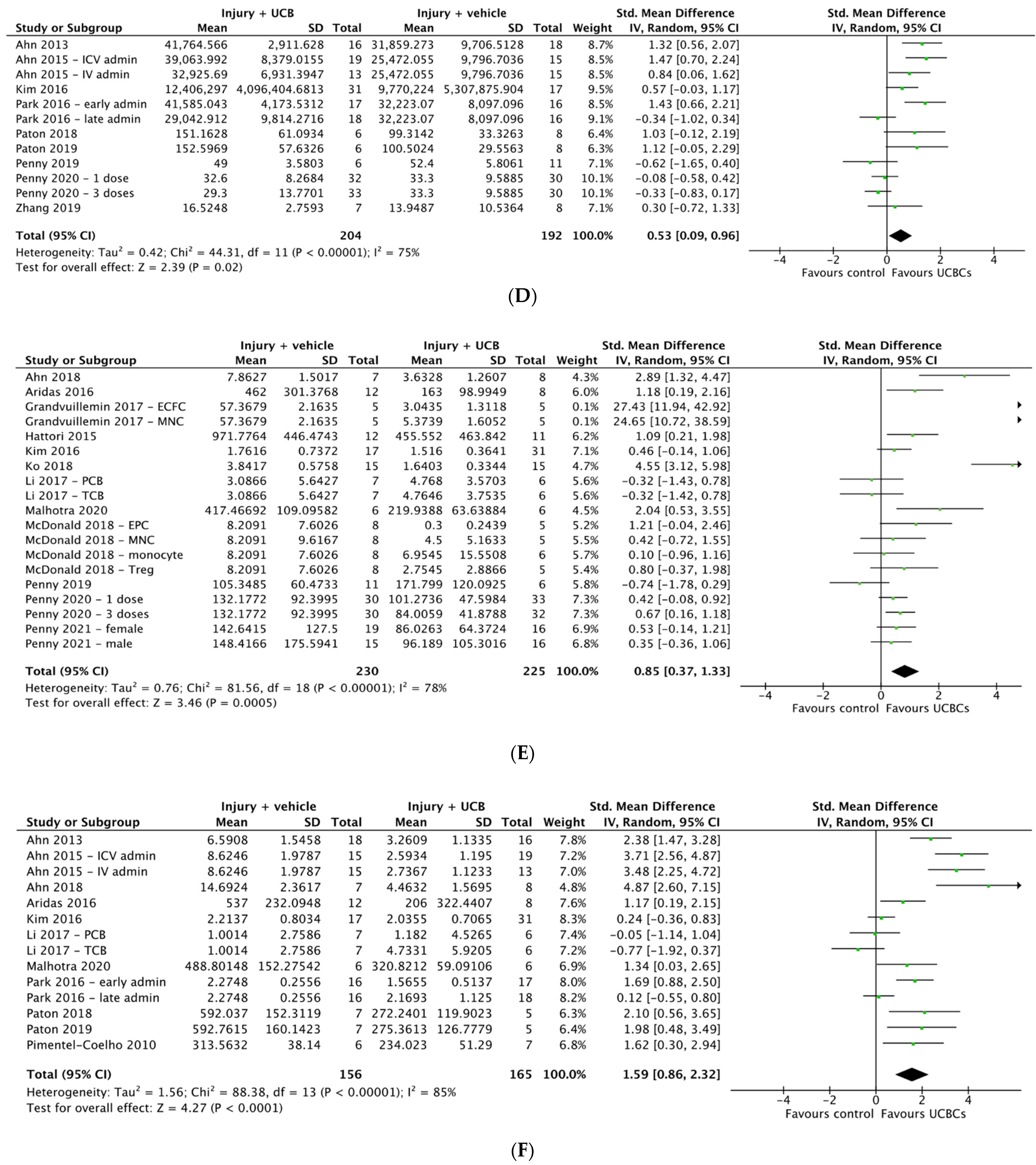
2.5. Effect of Ucb-Derived Cell Therapy on Astrogliosis & Microglia
2.6. Effect of Ucb-Derived Cell Therapy on Neuroinflammation
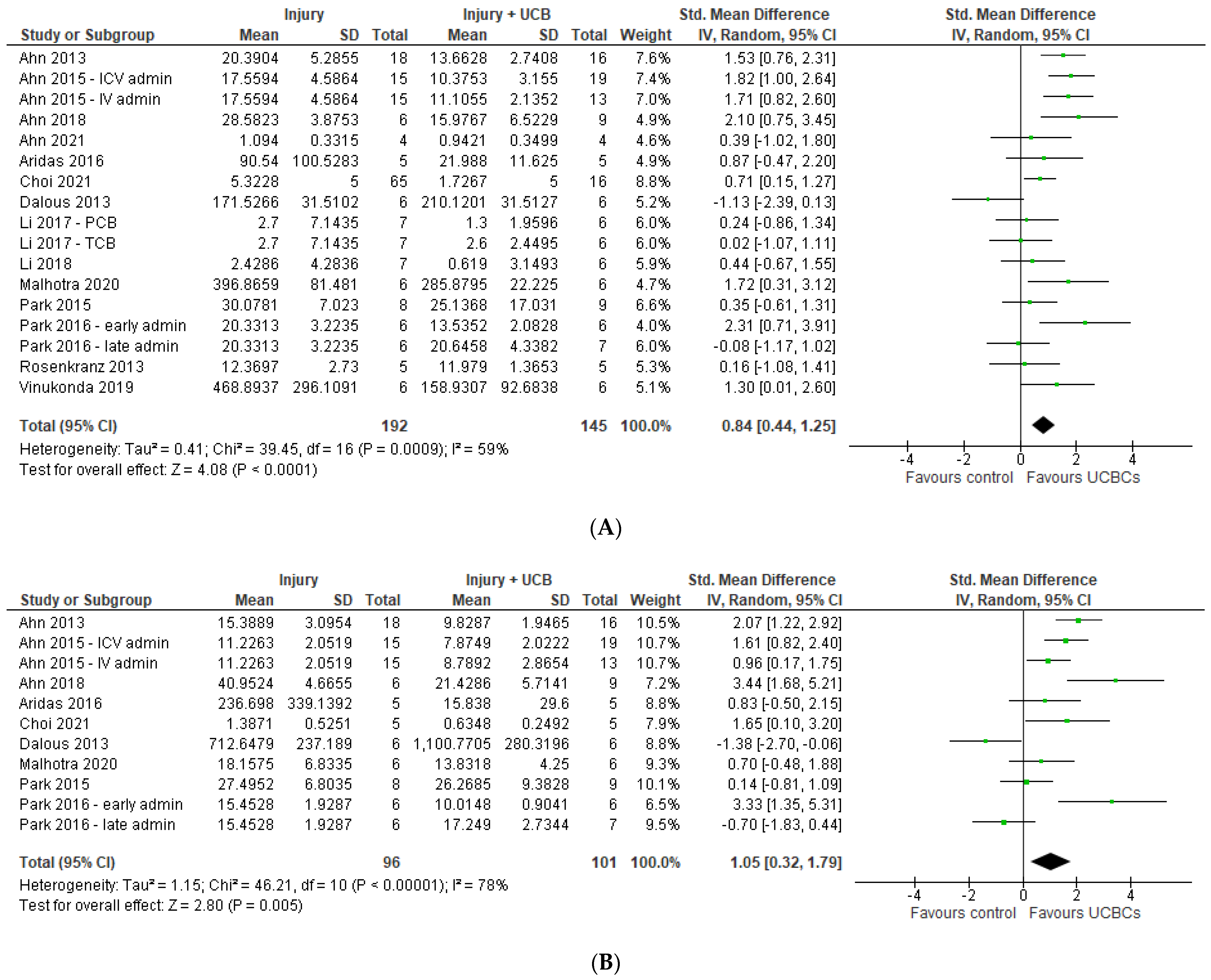
2.7. Effect of Ucb-Derived Cell Therapy on Motor Function
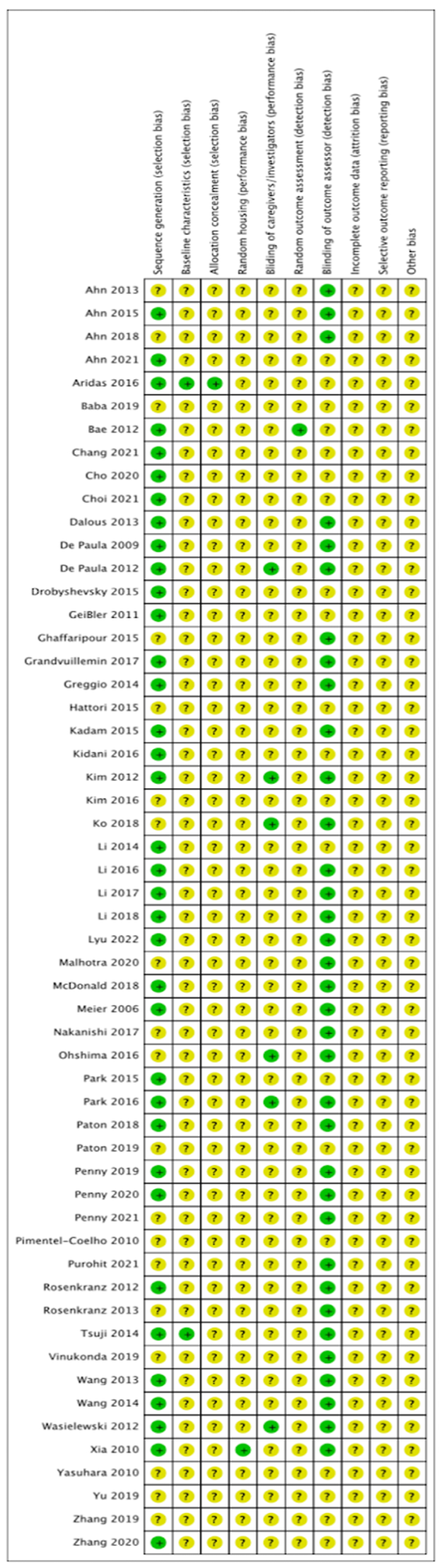
2.8. Quality Assessment
3. Discussion
3.1. Effect of Ucb-Derived Cell Therapy on Brain Outcomes of Perinatal Brain Injury
3.2. Knowledge Gaps Identified
3.3. Limitations
3.4. Future Directions
4. Methods
4.1. Selection Criteria
4.2. Search Strategy
4.3. Study Selection
4.4. Data Extraction
4.5. Data Synthesis and Statistical Analysis
4.6. Quality Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Novak, C.M.M.D.; Ozen, M.M.D.; Burd, I.M.D.P. Perinatal Brain Injury. Clin. Perinatol. 2018, 45, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Sizonenko, S.V.; Baud, O. Editorial: Preventing developmental brain injury-from animal models to clinical trials. Front. Neurol. 2019, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J.M.D. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009, 8, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Glass, H.C. Hypoxic-Ischemic Encephalopathy and Other Neonatal Encephalopathies. Continuum (Minneap. Minn.) 2018, 24, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Vincer, M.J.; Allen, A.C.; Allen, V.M.; Baskett, T.F.; O’Connell, C.M. Trends in the prevalence of cerebral palsy among very preterm infants (<31 weeks’ gestational age). Paediatr. Child Health 2014, 19, 185–189. [Google Scholar] [CrossRef]
- Leavy, A.; Jimenez Mateos, E.M. Perinatal Brain Injury and Inflammation: Lessons from Experimental Murine Models. Cells 2020, 9, 2640. [Google Scholar] [CrossRef]
- Lea, C.L.; Smith-Collins, A.; Luyt, K. Protecting the premature brain: Current evidence-based strategies for minimising perinatal brain injury in preterm infants. Arch. Dis. Childhood Fetal Neonatal Ed. 2017, 102, F176–F182. [Google Scholar] [CrossRef]
- Tagin, M.A.; Woolcott, C.G.; Vincer, M.J.; Whyte, R.K.; Stinson, D.A. Hypothermia for Neonatal Hypoxic Ischemic Encephalopathy: An Updated Systematic Review and Meta-analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 558–566. [Google Scholar] [CrossRef]
- Vogel, J.P.; Souza, J.P.; Metin Gülmezoglu, A.; Mori, R.; Lumbiganon, P.; Qureshi, Z.; Carroli, G.; Laopaiboon, M.; Fawole, B.; Ganchimeg, T.; et al. Use of Antenatal Corticosteroids and Tocolytic Drugs in Preterm Births in 29 Countries: An Analysis of the WHO Multicountry Survey on Maternal and Newborn Health. Obstet. Gynecol. Surv. 2015, 70, 79–81. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G.; Jacobs, S.E. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Libr. Cochrane Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010, 340, 409. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Sahebkar, A.; Sichani, L.S.; Moridikia, A.; Nazari, S.; Sadri Nahand, J.; Salehi, H.; Stenvang, J.; Masoudifar, A.; Mirzaei, H.R.; et al. Therapeutic application of multipotent stem cells. J. Cell. Physiol. 2018, 233, 2815–2823. [Google Scholar] [CrossRef]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2018, 15, 47. [Google Scholar] [CrossRef]
- Malhotra, A.; Castillo-Melendez, M.; Allison, B.J.; Sutherland, A.E.; Nitsos, I.; Pham, Y.; McDonald, C.A.; Fahey, M.C.; Polglase, G.R.; Jenkin, G.; et al. Neurovascular effects of umbilical cord blood-derived stem cells in growth-restricted newborn lambs. Stem Cell Res. Ther. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nieda, M.; Nicol, A.; Denning-Kendall, P.; Sweetenham, J.; Bradley, B.; Hows, J. Endothelial cell precursors are normal components of human umbilical cord blood. Br. J. Haematol. 1997, 98, 775–777. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Horecka, A. Characteristics of hematopoietic stem cells of umbilical cord blood. Cytotechnology 2014, 67, 387–396. [Google Scholar] [CrossRef]
- Tolar, J.; Hippen, K.L.; Blazar, B.R. Immune regulatory cells in umbilical cord blood: T regulatory cells and mesenchymal stromal cells. Br. J. Haematol. 2009, 147, 200–206. [Google Scholar] [CrossRef]
- Tsuji, M.; Sawada, M.; Watabe, S.; Sano, H.; Kanai, M.; Tanaka, E.; Ohnishi, S.; Sato, Y.; Sobajima, H.; Hamazaki, T.; et al. Autologous cord blood cell therapy for neonatal hypoxic-ischaemic encephalopathy: A pilot study for feasibility and safety. Sci. Rep. 2020, 10, 4603. [Google Scholar] [CrossRef]
- Zhou, L.; McDonald, C.; Yawno, T.; Jenkin, G.; Miller, S.; Malhotra, A. Umbilical Cord Blood and Cord Tissue-Derived Cell Therapies for Neonatal Morbidities: Current Status and Future Challenges. Stem Cells Transl. Med. 2022, 11, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.; Nguyen, T.; Smith, M.; Penny, T.; Paton, M.C.; Zhou, L.; Jenkin, G.; Miller, S.L.; McDonald, C.A.; Malhotra, A. Umbilical cord blood-derived cell therapy for perinatal brain injury: A systematic review & meta-analysis of preclinical studies—Part B. bioRxiv 2022. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells Prevent Hydrocephalus After Severe Intraventricular Hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Im, G.H.; Choi, S.J.; Park, W.S. Optimal Route for Mesenchymal Stem Cells Transplantation after Severe Intraventricular Hemorrhage in Newborn Rats. PLoS ONE 2015, 10, e0132919. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Kim, Y.E.; Sung, S.I.; Sung, D.K.; Park, W.S. Mesenchymal stem cells transplantation attenuates brain injury and enhances bacterial clearance in Escherichia coli meningitis in newborn rats. Pediatr. Res. 2018, 84, 778–785. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Jie, H.; Jung, W.-B.; Jeong, J.-H.; Ko, S.; Im, G.H.; Park, W.S.; Lee, J.H.; Chang, Y.S.; Chung, S. Stem cell restores thalamocortical plasticity to rescue cognitive deficit in neonatal intraventricular hemorrhage. Exp. Neurol. 2021, 342, 113736. [Google Scholar] [CrossRef]
- Aridas, J.D.S.; McDonald, C.A.; Paton, M.C.B.; Yawno, T.; Sutherland, A.E.; Nitsos, I.; Pham, Y.; Ditchfield, M.; Fahey, M.C.; Wong, F.; et al. Cord blood mononuclear cells prevent neuronal apoptosis in response to perinatal asphyxia in the newborn lamb: Umbilical cord blood cells for treatment of perinatal asphyxia. J. Physiol. 2016, 594, 1421–1435. [Google Scholar] [CrossRef]
- Baba, N.; Wang, F.; Iizuka, M.; Shen, Y.; Yamashita, T.; Takaishi, K.; Tsuru, E.; Matsushima, S.; Miyamura, M.; Fujieda, M.; et al. Induction of regional chemokine expression in response to human umbilical cord blood cell infusion in the neonatal mouse ischemia-reperfusion brain injury model. PLoS ONE 2019, 14, e0221111. [Google Scholar] [CrossRef]
- Bae, S.-H.; Kong, T.-H.; Lee, H.-S.; Kim, K.-S.; Hong, K.S.; Chopp, M.; Kang, M.-S.; Moon, J. Long-Lasting Paracrine Effects of Human Cord Blood Cells on Damaged Neocortex in an Animal Model of Cerebral Palsy. Cell Transpl. 2012, 21, 2497–2515. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, S.; Li, Y.; Liu, S.; Ma, T.; Wei, W. Umbilical cord blood CD34+ cells administration improved neurobehavioral status and alleviated brain injury in a mouse model of cerebral palsy. Childs Nerv. Syst. 2021, 37, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Choi, J.I.; Kim, J.-O.; Jung, J.E.; Kim, D.-W.; Kim, M. Therapeutic mechanism of cord blood mononuclear cells via the IL-8-mediated angiogenic pathway in neonatal hypoxic-ischaemic brain injury. Sci. Rep. 2020, 10, 4446. [Google Scholar] [CrossRef]
- Choi, J.I.; Choi, J.-W.; Shim, K.-H.; Choung, J.S.; Kim, H.-J.; Sim, H.R.; Suh, M.R.; Jung, J.E.; Kim, M. Synergistic effect in neurological recovery via anti-apoptotic akt signaling in umbilical cord blood and erythropoietin combination therapy for neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci. 2021, 22, 11995. [Google Scholar] [CrossRef] [PubMed]
- Dalous, J.; Pansiot, J.; Pham, H.; Chatel, P.; Nadaradja, C.; D’Agostino, I.; Vottier, G.; Schwendimann, L.; Vanneaux, V.; Charriaut-Marlangue, C.; et al. Use of Human Umbilical Cord Blood Mononuclear Cells to Prevent Perinatal Brain Injury: A Preclinical Study. Stem Cells Dev. 2013, 22, 169–179. [Google Scholar] [CrossRef]
- De Paula, S.; Santos Vitola, A.; Greggio, S.; De Paula, D.; Billig Mello, P.; Mistrello Lubianca, J.; Leal Xavier, L.; Holmer Fiori, U.; Dacosta, J.C. Hemispheric Brain Injury and Behavioral Deficits Induced by Severe Neonatal Hypoxia-Ischemia in Rats Are Not Attenuated by Intravenous Administration of Human Umbilical Cord Blood Cells. Pediatr. Res. 2009, 65, 631–635. [Google Scholar] [CrossRef] [PubMed]
- de Paula, S.; Greggio, S.; Marinowic, D.R.; Machado, D.C.; DaCosta, J.C. The dose-response effect of acute intravenous transplantation of human umbilical cord blood cells on brain damage and spatial memory deficits in neonatal hypoxia-ischemia. Neuroscience 2012, 210, 431–441. [Google Scholar] [CrossRef]
- Drobyshevsky, A.; Cotten, C.M.; Shi, Z.; Luo, K.; Jiang, R.; Derrick, M.; Tracy, E.T.; Gentry, T.; Goldberg, R.N.; Kurtzberg, J.; et al. Human Umbilical Cord Blood Cells Ameliorate Motor Deficits in Rabbits in a Cerebral Palsy Model. Dev. Neurosci. 2015, 37, 349–362. [Google Scholar] [CrossRef]
- Geissler, M.; Dinse, H.R.; Neuhoff, S.; Kreikemeier, K.; Meier, C. Human umbilical cord blood cells restore brain damage induced changes in rat somatosensory cortex. PLoS ONE 2011, 6, e20194. [Google Scholar] [CrossRef]
- Ghaffaripour, H.A.; Jalali, M.; Nikravesh, M.R.; Seghatoleslam, M.; Sanchooli, J. Neuronal cell reconstruction with umbilical cord blood cells in the brain hypoxia-ischemia. Iran. Biomed. J. 2015, 19, 29–34. [Google Scholar] [CrossRef]
- Grandvuillemin, I.; Garrigue, P.; Ramdani, A.; Boubred, F.; Simeoni, U.; Dignat-George, F.; Sabatier, F.; Guillet, B. Long-Term Recovery After Endothelial Colony-Forming Cells or Human Umbilical Cord Blood Cells Administration in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy. Stem Cells Transl. Med. 2017, 6, 1987–1996. [Google Scholar] [CrossRef]
- Greggio, S.; de Paula, S.; Azevedo, P.N.; Venturin, G.T.; DaCosta, J.C. Intra-arterial transplantation of human umbilical cord blood mononuclear cells in neonatal hypoxic–ischemic rats. Life Sci. 2014, 96, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Sato, Y.; Kondo, T.; Ichinohashi, Y.; Sugiyama, Y.; Yamamoto, M.; Kotani, T.; Hirata, H.; Hirakawa, A.; Suzuki, S.; et al. Administration of Umbilical Cord Blood Cells Transiently Decreased Hypoxic-Ischemic Brain Injury in Neonatal Rats. Dev. Neurosci. 2015, 37, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.D.; Chen, H.; Markowitz, G.J.; Raja, S.; George, S.; Verina, T.; Shotwell, E.; Loechelt, B.; Johnston, M.V.; Kamani, N.; et al. Systemic Injection of CD34+-Enriched Human Cord Blood Cells Modulates Poststroke Neural and Glial Response in a Sex-Dependent Manner in CD1 Mice. Stem Cells Dev. 2015, 24, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Kidani, Y.; Miki, Y.; Nomimura, N.; Minakawa, S.; Tanaka, N.; Miyoshi, H.; Wakabayashi, K.; Kudo, Y. The therapeutic effect of CD133+ cells derived from human umbilical cord blood on neonatal mouse hypoxic-ischemic encephalopathy model. Life Sci. 2016, 157, 108–115. [Google Scholar] [CrossRef]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef]
- Kim, Y.E.; Park, W.S.; Sung, D.K.; Ahn, S.Y.; Sung, S.I.; Yoo, H.S.; Chang, Y.S. Intratracheal transplantation of mesenchymal stem cells simultaneously attenuates both lung and brain injuries in hyperoxic newborn rats. Pediatr. Res. 2016, 80, 415–424. [Google Scholar] [CrossRef]
- Ko, H.R.; Ahn, S.Y.; Chang, Y.S.; Hwang, I.; Yun, T.; Sung, D.K.; Sung, S.I.; Park, W.S.; Ahn, J.-Y. Human UCB-MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF-CREB signaling. Stem Cell Res. Ther. 2018, 9, 326. [Google Scholar] [CrossRef]
- Li, X.; Shang, Q.; Zhang, L. Comparison of the Efficacy of Cord Blood Mononuclear Cells (MNCs) and CD34+ Cells for the Treatment of Neonatal Mice with Cerebral Palsy. Cell Biochem. Biophys. 2014, 70, 1539–1544. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.; Loose, J.; Nitsos, I.; Bischof, R.; Castillo-Melendez, M.; McDonald, C.A.; Wong, F.Y.; Jenkin, G.; et al. Preterm white matter brain injury is prevented by early administration of umbilical cord blood cells. Exp. Neurol. 2016, 283, 179–187. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.; Loose, J.; Nitsos, I.; Allison, B.J.; Bischof, R.; McDonald, C.A.; Jenkin, G.; Miller, S.L. Term vs. preterm cord blood cells for the prevention of preterm brain injury. Pediatr. Res. 2017, 82, 1030–1038. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.E.; Gurung, S.; Paton, M.; McDonald, C.; Tiwari, A.; Pham, Y.; Castillo-Melendez, M.; Jenkin, G.; et al. Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia. Exp. Neurol. 2018, 308, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Sun, D.M.; Ng, C.P.; Cheng, W.S.; Chen, J.F.; He, Y.Z.; Lam, S.Y.; Zheng, Z.Y.; Huang, G.D.; Wang, C.C.; et al. Umbilical Cord Blood Mononuclear Cell Treatment for Neonatal Rats With Hypoxic Ischemia. Front. Cell. Neurosci. 2022, 16, 823320. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Middelanis, J.; Wasielewski, B.; Neuhoff, S.; Roth-Haerer, A.; Gantert, M.; Dinse, H.R.; Dermietzel, R.; Jensen, A. Spastic paresis after perinatal brain damage in rats is reduced by human cord blood mononuclear cells. Pediatr. Res. 2006, 59, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Sato, Y.; Mizutani, Y.; Ito, M.; Hirakawa, A.; Higashi, Y. Rat umbilical cord blood cells attenuate hypoxic-ischemic brain injury in neonatal rats. Sci. Rep. 2017, 7, 44111. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Taguchi, A.; Sato, Y.; Ogawa, Y.; Saito, S.; Yamahara, K.; Ihara, M.; Harada-Shiba, M.; Ikeda, T.; Matsuyama, T.; et al. Evaluations of Intravenous Administration of CD34+ Human Umbilical Cord Blood Cells in a Mouse Model of Neonatal Hypoxic-Ischemic Encephalopathy. Dev. Neurosci. 2016, 38, 331–341. [Google Scholar] [CrossRef]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Yoo, H.S.; Sung, D.K.; Im, G.H.; Choi, S.J.; Chang, Y.S. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 2015, 10, e0120893. [Google Scholar] [CrossRef]
- Park, W.S.; Sung, S.I.; Ahn, S.Y.; Sung, D.K.; Im, G.H.; Yoo, H.S.; Choi, S.J.; Chang, Y.S. Optimal Timing of Mesenchymal Stem Cell Therapy for Neonatal Intraventricular Hemorrhage. Cell Transpl. 2016, 25, 1131–1144. [Google Scholar] [CrossRef]
- Paton, M.C.; Allison, B.J.; Li, J.; Fahey, M.C.; Sutherland, A.E.; Nitsos, I.; Bischof, R.J.; Dean, J.M.; Moss, T.J.; Polglase, G.R.; et al. Human Umbilical Cord Blood Therapy Protects Cerebral White Matter from Systemic LPS Exposure in Preterm Fetal Sheep. Dev. Neurosci. 2018, 40, 258–270. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Allison, B.J.; Fahey, M.C.; Li, J.; Sutherland, A.E.; Pham, Y.; Nitsos, I.; Bischof, R.J.; Moss, T.J.; Polglase, G.R.; et al. Umbilical cord blood versus mesenchymal stem cells for inflammation-induced preterm brain injury in fetal sheep. Pediatr. Res. 2019, 86, 165–173. [Google Scholar] [CrossRef]
- Penny, T.R.; Sutherland, A.E.; Mihelakis, J.G.; Paton, M.C.B.; Pham, Y.; Lee, J.; Jones, N.M.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; et al. Human Umbilical Cord Therapy Improves Long-Term Behavioral Outcomes Following Neonatal Hypoxic Ischemic Brain Injury. Front. Physiol. 2019, 10, 283. [Google Scholar] [CrossRef]
- Penny, T.; Pham, Y.; Sutherland, A.; Mihelakis, J.; Lee, J.; Jenkin, G.; Fahey, M.; Miller, S.; McDonald, C. Multiple Doses of Umbilical Cord Blood Cells Improve Long-Term Perinatal Brain Injury. Stem Cells Transl. Med. 2020, 9, S3. [Google Scholar] [CrossRef]
- Penny, T.R.; Pham, Y.; Sutherland, A.E.; Lee, J.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; McDonald, C.A. Umbilical cord blood therapy modulates neonatal hypoxic ischemic brain injury in both females and males. Sci. Rep. 2021, 11, 15788. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Coelho, P.M.; Magalhães, E.S.; Lopes, L.M.; Deazevedo, L.C.; Santiago, M.F.; Mendez-Otero, R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: Functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2010, 19, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Purohit, D.; Finkel, D.A.; Malfa, A.; Liao, Y.; Ivanova, L.; Kleinman, G.M.; Hu, F.; Shah, S.; Thompson, C.; Joseph, E.; et al. Human Cord Blood Derived Unrestricted Somatic Stem Cells Restore Aquaporin Channel Expression, Reduce Inflammation and Inhibit the Development of Hydrocephalus After Experimentally Induced Perinatal Intraventricular Hemorrhage. Front. Cell. Neurosci. 2021, 15, 633185. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, K.; Kumbruch, S.; Tenbusch, M.; Marcus, K.; Marschner, K.; Dermietzel, R.; Meier, C. Transplantation of human umbilical cord blood cells mediated beneficial effects on apoptosis, angiogenesis and neuronal survival after hypoxic-ischemic brain injury in rats. Cell Tissue Res. 2012, 348, 429–438. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Tenbusch, M.; May, C.; Marcus, K.; Meier, C. Changes in Interleukin-1 alpha serum levels after transplantation of umbilical cord blood cells in a model of perinatal hypoxic-ischemic brain damage. Ann. Anat. 2013, 195, 122–127. [Google Scholar] [CrossRef]
- Tsuji, M.; Taguchi, A.; Ohshima, M.; Kasahara, Y.; Sato, Y.; Tsuda, H.; Otani, K.; Yamahara, K.; Ihara, M.; Harada-Shiba, M.; et al. Effects of intravenous administration of umbilical cord blood CD34+ cells in a mouse model of neonatal stroke. Neuroscience 2014, 263, 148–158. [Google Scholar] [CrossRef]
- Vinukonda, G.; Liao, Y.; Hu, F.; Ivanova, L.; Purohit, D.; Finkel, D.A.; Giri, P.; Bapatla, L.; Shah, S.; Zia, M.T.; et al. Human Cord Blood-Derived Unrestricted Somatic Stem Cell Infusion Improves Neurobehavioral Outcome in a Rabbit Model of Intraventricular Hemorrhage. Stem Cells Transl. Med. 2019, 8, 1157–1169. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhao, Y.-S.; Hu, M.-y.; Sun, Y.-Q.; Chen, Y.-X.; Bi, X.-H. Umbilical cord blood cells regulate endogenous neural stem cell proliferation via hedgehog signaling in hypoxic ischemic neonatal rats. Brain Res. 2013, 1518, 26–35. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, X. Umbilical cord blood cells regulate the differentiation of endogenous neural stem cells in hypoxic ischemic neonatal rats via the hedgehog signaling pathway. Brain Res. 2014, 1560, 18–26. [Google Scholar] [CrossRef]
- Wasielewski, B.; Jensen, A.; Roth-Härer, A.; Dermietzel, R.; Meier, C. Neuroglial activation and Cx43 expression are reduced upon transplantation of human umbilical cord blood cells after perinatal hypoxic-ischemic injury. Brain Res. 2012, 1487, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Hong, X.; Chen, X.; Lan, F.; Zhang, G.; Liao, L. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J. Perinat. Med. 2010, 38, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Hara, K.; Maki, M.; Xu, L.; Yu, G.; Ali, M.M.; Masuda, T.; Yu, S.J.; Bae, E.K.; Hayashi, T.; et al. Mannitol facilitates neurotrophic factor up-regulation and behavioural recovery in neonatal hypoxic-ischaemic rats with human umbilical cord blood grafts. J. Cell. Mol. Med. 2010, 14, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Y.; Luo, Z.; Luo, P.; Xiao, N.; Sun, X.; Cheng, L. Effects of human umbilical cord blood CD34+ cell transplantation in neonatal hypoxic-ischemia rat model. Brain Dev. 2019, 41, 173–181. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Chen, J.; Luo, M.; Qu, Y.; Mu, D.; Chen, Q. Umbilical cord mesenchymal stem cells and umbilical cord blood mononuclear cells improve neonatal rat memory after hypoxia-ischemia. Behav. Brain Res. 2019, 362, 56–63. [Google Scholar] [CrossRef]
- Zhang, M.-B.; Song, C.-C.; Li, G.-Z.; Chen, L.-F.; Ma, R.; Yu, X.-H.; Gong, P.; Wang, X.-L. Transplantation of umbilical cord blood mononuclear cells attenuates the expression of IL-1β via the TLR4/NF-κB pathway in hypoxic-ischemic neonatal rats. J. Neurorestoratol. 2020, 8, 122–130. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; de Vries, R.B.M.; Ritskes-Hoitinga, M.; Rovers, M.M.; Leeflang, M.M.; IntHout, J.; Wever, K.E.; Hooft, L.; de Beer, H.; Kuijpers, T.; et al. Facilitating healthcare decisions by assessing the certainty in the evidence from preclinical animal studies. PLoS ONE 2018, 13, e0187271. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Johnston, M.V.; Trescher, W.H.; Ishida, A.; Nakajima, W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr. Res. 2001, 49, 735–741. [Google Scholar] [CrossRef]
- Serrenho, I.; Rosado, M.; Dinis, A.; Cardoso, C.M.; Graos, M.; Manadas, B.; Baltazar, G. Stem Cell Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 3142. [Google Scholar] [CrossRef]
- Archambault, J.; Moreira, A.; McDaniel, D.; Winter, L.; Sun, L.; Hornsby, P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS ONE 2017, 12, e0189895. [Google Scholar] [CrossRef]
- Blencowe, H.M.; Cousens, S.P.; Oestergaard, M.Z.P.; Chou, D.M.D.; Moller, A.-B.M.; Narwal, R.M.D.; Adler, A.P.; Vera Garcia, C.M.P.H.; Rohde, S.M.P.H.; Say, L.M.D.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Ghafghazi, S. Cell Therapy Needs Rigorous Translational Studies in Large Animal Models. J. Am. Coll. Cardiol. 2015, 66, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Chang, Y.-H.; Shyu, W.-C.; Lin, S.-Z. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transpl. 2015, 24, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.-F.; Zhang, H.; Ding, H.-F.; Li, D.; Yi, X.-H.; Gao, X.-Y.; Mou, W.-W.; Ju, X.-L. Therapeutic effect of placenta-derived mesenchymal stem cells on hypoxic-ischemic brain damage in rats. World J. Pediatr. 2014, 11, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Serrenho, I.; Cardoso, C.M.; Grãos, M.; Dinis, A.; Manadas, B.; Baltazar, G. Hypothermia Does Not Boost the Neuroprotection Promoted by Umbilical Cord Blood Cells in a Neonatal Hypoxia-Ischemia Rat Model. Int. J. Mol. Sci. 2022, 24, 257. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Melendez, M.; Yawno, T.; Jenkin, G.; Miller, S.L. Stem cell therapy to protect and repair the developing brain: A review of mechanisms of action of cord blood and amnion epithelial derived cells. Front. Neurosci. 2013, 7, 194. [Google Scholar] [CrossRef]
- Hirst, J.A.; Howick, J.; Aronson, J.K.; Roberts, N.; Perera, R.; Koshiaris, C.; Heneghan, C. The need for randomization in animal trials: An overview of systematic reviews. PLoS ONE 2014, 9, e98856. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Malhotra, A.; Novak, I.; Miller, S.L.; Jenkin, G. Autologous transplantation of umbilical cord blood-derived cells in extreme preterm infants: Protocol for a safety and feasibility study. BMJ Open 2020, 10, e036065. [Google Scholar] [CrossRef]



| Study | Strain and Species | Brain Injury Model | Term vs Pre-term Model | Age Injury Induced | UCB Cell Source & Type | Route of Administration | Total Cells per Dose | Cell Administration Time Post-injury | Comparator |
|---|---|---|---|---|---|---|---|---|---|
| Ahn 2013 [24] | Sprague-Dawley rats | IVH | Pre-term | PND4 | Human MSCs | Intraventricular | 1 × 105 | 2 days | Injury + PBS |
| Ahn 2015 [25] | Sprague-Dawley rats | IVH | Pre-term | PND4 | Human MSCs | IC or IV | 1 × 105 (IC) or 5 × 105 (IV) | 2 days | Injury + NS |
| Ahn 2018 [26] | Sprague-Dawley rats | Meningitis | Term | PND11 | Human MSCs | Intraventricular | 1 × 105 | 6 h | Injury + NS |
| Ahn 2021 [27] | Sprague-Dawley rats | IVH | Pre-term | PND4 | Human MSCs | Intraventricular | 1 × 105 | 2 days | Injury + PBS |
| Aridas 2016 [28] | Merino Border Leicester cross sheep | HI | Term | 139-141 days of gestation | Sheep MNCs | Arterial | 1 × 108 | 12 h after birth | Injury + no vehicle |
| Baba 2019 [29] | NOD/SCID mice | HI | Term | PND9 | Human MNCs | IV | 5 × 106 | 21 days | Injury + PBS |
| Bae 2012 [30] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | IV | 1 × 107 | 1 day | Injury + PBS |
| Chang 2021 [31] | NOD/SCID mice | HI | Term | PND9 | Human CD34+ or CD34- HSCs | IC | 1 × 105 | 12 h | Injury + no vehicle |
| Cho 2020 [32] | ICR mice | HI | Pre-term | PND7 | Human MNCs | IP | 3 × 107 | 7 days | Injury + no vehicle |
| Choi 2021 [33] | ICR mice | HI | Pre-term | PND7 | Human MNCs | IP | 3 × 107 | 7 days | Injury + PBS |
| Dalous 2013 [34] | Sprague-Dawley rats | Excitotoxic brain injury | Pre-term | PND5 | Human MNCs | IP or IV | 106, 3 × 106 or 107 (IP) 106 or 107 (IV) | 1 or 24 h (IP), 6 or 24 h (IV) | Injury + saline |
| De Paula 2009 | Wistar rats | HI | Term | PND7 | Human MNCs | IV | 1 × 107 | 1 day | Injury + saline soln |
| De Paula 2012 [36] | Wistar rats | HI | Term | PND7 | Human MNCs | IV | 1 × 106, 1 × 107 or 1 × 108 | 1 day | Injury + vehicle |
| Drobyshevsky 2015 [37] | New Zealand white rabbits | HI | Pre-term | 22 days of gestation | Human MNCs | IV | 2.5 × 106 or 5 × 106 | 4 h after birth | Injury + saline |
| Geissler 2011 [38] | Wistar rats | HI | Term | PND7 | Human MNCs | IP | 1 × 107 | 1 day | Injury + NS |
| Ghaffaripour 2015 [39] | Wistar rats | HI | Term | PND14 | Human MNCs | IV | 2 × 105 | 7 days | Injury + saline soln |
| Grandvuillemin 2017 [40] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs or ECFCs | IP | 1 × 107 (MNC) or 5 × 105 (ECFC) | 2 days | Injury + saline soln |
| Greggio 2014 [41] | Wistar rats | HI | Term | PND7 | Human MNCs | Arterial | 1 × 106 or 1 × 107 | 1 day | Injury + vehicle |
| Hattori 2015 [42] | Wistar rats | HI | Term | PND7 | Human MNCs | IP | 1 × 107 | 6 h | Injury + vehicle |
| Kadam 2015 [43] | CD1 mice | HI | Term | PND12 | Human CD34+ enriched MNCs | IP | 1 × 105 | 2 days | Injury + vehicle |
| Kidani 2016 [44] | SCID mice | HI | Pre-term | PND7 | Human CD133+ cells | IP | 1 × 105 | 1 day | Injury + NS |
| Kim 2012 [45] | Sprague-Dawley rats | HI | Term | PND10 | Human MSCs | Intraventricular | 1 × 105 | 6 h | Injury + PBS |
| Kim 2016 [46] | Sprague-Dawley rats | Hyperoxia | Pre-term | PND0-14 | Human MSCs | Intratracheal | 1 × 105 | PND5 | Injury + NS |
| Ko 2018 [47] | Sprague-Dawley rats | IVH | Pre-term | PND4 | Human MSCs | Intracerebroventricular | 1 × 105 | 2 days | Injury + NS |
| Li 2014 [48] | Sprague-Dawley rats | HI | Pre-term | PND7 | Human MNCs or CD34+ cells | IV | 1.5 × 106 | 7 days | Injury + saline |
| Li 2016 [49] | Merino-Border Leicester cross sheep | HI | Pre-term | 102.2 ± 0.3 days of gestation | Sheep MNCs | IV | 5 × 107 | 12 h or 5 days | Injury + saline |
| Li 2017 [50] | Merino-Border Leicester cross sheep | HI | Pre-term | 102.2 ± 0.3 days of gestation | Sheep MNCs | IV | 5 × 107 | 12 h | Injury + saline |
| Li 2018 [51] | Merino-Border Leicester cross sheep | HI | Pre-term | 102.2 ± 0.2 days of gestation | Sheep MNCs | IV | 5 × 107 | 12 h | Injury + saline |
| Lyu 2022 [52] | Unspecified rats | HI | Term | PND7 | Human MNCs | IV | 1 × 107 | 1 day | Injury + NS |
| Malhotra 2020 [15] | Border Leicester- Merino cross sheep | FGR | Pre-term | 88 days of gestation | Sheep MNCs | IV | 2.5 × 107 | 1 h after birth | Injury + saline |
| McDonald 2018 [14] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs, Tregs, monocytes, EPCs | IP | 1 × 106 (MNCs) or 2 × 105 (other) | 1 day | Injury + PBS |
| Meier 2006 [53] | Wistar rats | HI | Term | PND7 | Human MNCs | IP | 1 × 107 | 1 day | Injury + NS |
| Nakanishi 2017 [54] | Sprague-Dawley rats | HI | Term | PND7 | Rat MNCs | IP | 2 × 106 | 3 days | Injury + PBS |
| Ohshima 2016 [55] | CB-17 SCID mice | HI | Pre-term | PND8 | Human CD34+ cells | IV | 1 × 105 | 2 days | Injury + PBS |
| Park 2015 [56] | Sprague-Dawley rats | HI | Pre-term | PND7 | Human MSCs | Intraventricular | 1 × 105 | 6 h | Injury + no vehicle |
| Park 2016 [57] | Sprague-Dawley rats | IVH | Term | PND4 | Human MSCs | Intraventricular | 1 × 105 | 2 or 7 days | Injury + PBS |
| Paton 2018 [58] | Border Leicester-Merino cross sheep | Chorioamnionitis | Pre-term | 95 days of gestation | Human MNCs | IV | 1 × 108 | 6 h | Injury + saline |
| Paton 2019 [59] | Border Leicester-Merino cross sheep | Chorioamnionitis | Pre-term | 95 days of gestation | Human MNCs | IV | 1 × 108 | 6 h | Injury + saline |
| Penny 2019 [60] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | IP | 1 × 106 | 1 day | Injury + PBS |
| Penny 2020 [61] | Sprague-Dawley rats | HI | Term | PND10 | Human MNCs | Intranasal or IP | 1 × 106 | 1 day (1 dose group) or 1, 3 and 10 days (3 dose group) | Injury + saline |
| Penny 2021 [62] | Sprague-Dawley rats | HI | Term | PND10 | Human MNCs | Intranasal or IP | 1 × 106 | 1, 3 and 10 days | Injury + saline |
| Pimentel-Coelho 2010 [63] | Lister-Hooded rats | HI | Term | PND7 | Human MNCs | IP | 2 × 106 | 3 h | Injury + vehicle |
| Purohit 2021 [64] | New Zealand white rabbits | IVH | Pre-term | 3–4 h after birth | Human unrestricted somatic stem cells | Intraventricular | 2 × 106 | 18 h | Injury + saline |
| Rosenkranz 2012 [65] | Wistar rats | HI | Term | PND7 | Human MNCs | IP | 1 × 107 | 1 day | Injury + vehicle |
| Rosenkranz 2013 [66] | Wistar rats | HI | Term | PND7 | Human MNCs | IP | 1 × 107 | 1 day | Injury + vehicle |
| Tsuji 2014 [67] | CB-17 SCID mice | Ischaemic stroke | Term | PND12 | Human CD34+ cells | IV | 1 × 105 | 2 days | Injury + PBS |
| Vinukonda 2019 [68] | New Zealand white rabbits | IVH | Pre-term | 3-4 h after birth | Human unrestricted somatic stem cells | Intraventricular or IV | 2 × 106 (intraventricular) or 1 × 106 (IV) | 18 h | Injury + saline |
| Wang 2013 [69] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | Intraventricular | 3 × 106 | 1 day | Injury + PBS |
| Wang 2014 [70] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | Intraventricular | 3 × 106 | 1 day | Injury + PBS |
| Wasielewski 2012 [71] | Wistar rats | HI | Term | PND7 | Human MNCs | IP or intrathecal | 1 × 107 | 1 day | Injury + saline |
| Xia 2010 [72] | Sprague-Dawley rats | HI | Term | PND7 | Human MSCs | IC | 1 × 105 | 3 days | Injury + vehicle |
| Yasuhara 2010 [73] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | IV | 1.5 × 106 | 7 days | Injury + PBS |
| Yu 2019 [74] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCS or CD34+ cells | IV | 1 × 106 (MNCs) or 1.5 × 104 (CD34+) | 7 days | Injury + PBS |
| Zhang 2019 [75] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | Intraventricular | 1 × 107 | 1 day | Injury + PBS |
| Zhang 2020 [76] | Sprague-Dawley rats | HI | Term | PND7 | Human MNCs | Intraventricular | 3 × 106 | 1 day | Injury + NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Purcell, E.; Smith, M.J.; Penny, T.R.; Paton, M.C.B.; Zhou, L.; Jenkin, G.; Miller, S.L.; McDonald, C.A.; Malhotra, A. Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies. Int. J. Mol. Sci. 2023, 24, 4351. https://doi.org/10.3390/ijms24054351
Nguyen T, Purcell E, Smith MJ, Penny TR, Paton MCB, Zhou L, Jenkin G, Miller SL, McDonald CA, Malhotra A. Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies. International Journal of Molecular Sciences. 2023; 24(5):4351. https://doi.org/10.3390/ijms24054351
Chicago/Turabian StyleNguyen, Timothy, Elisha Purcell, Madeleine J. Smith, Tayla R. Penny, Madison C. B. Paton, Lindsay Zhou, Graham Jenkin, Suzanne L. Miller, Courtney A. McDonald, and Atul Malhotra. 2023. "Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies" International Journal of Molecular Sciences 24, no. 5: 4351. https://doi.org/10.3390/ijms24054351
APA StyleNguyen, T., Purcell, E., Smith, M. J., Penny, T. R., Paton, M. C. B., Zhou, L., Jenkin, G., Miller, S. L., McDonald, C. A., & Malhotra, A. (2023). Umbilical Cord Blood-Derived Cell Therapy for Perinatal Brain Injury: A Systematic Review & Meta-Analysis of Preclinical Studies. International Journal of Molecular Sciences, 24(5), 4351. https://doi.org/10.3390/ijms24054351









