Neuroprotective Strategies for Ischemic Stroke—Future Perspectives
Abstract
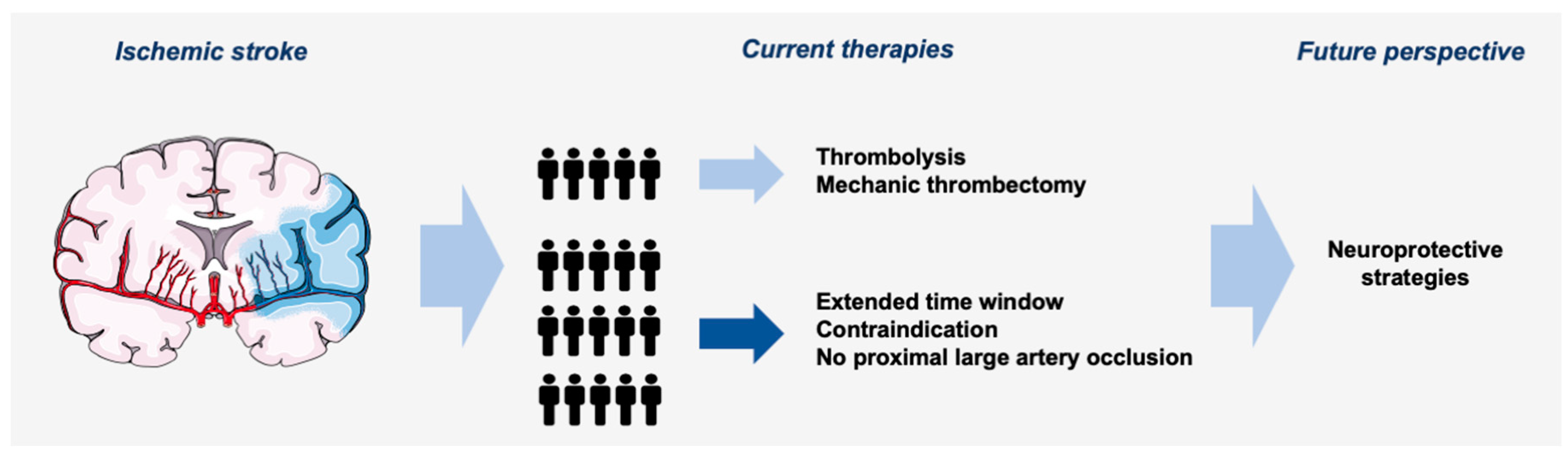
1. Introduction
2. Ischemic Stroke Pathophysiology
3. Current Stroke Treatment
3.1. Systemic Thrombolysis
3.2. Mechanical Thrombectomy
3.3. Supportive Care
3.4. Limitations
4. Neuroprotective Strategies and Approaches
4.1. Neuroprotectants
4.2. Stem Cells and EVs
4.3. Microbiota–Gut–Brain Axis
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef]
- Vajda, F.J.E. Neuroprotection and neurodegenerative disease. J. Clin. Neurosci. 2002, 9, 4–8. [Google Scholar] [CrossRef]
- Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Quest for Quality in Translational Stroke Research—A New Dawn for Neuroprotection? Int. J. Mol. Sci. 2022, 23, 5281. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.Q.; Ma, X.T.; Hu, Z.W.; Yang, S.; Chen, M.; Bosco, D.B.; Wu, L.J.; Tian, D.S. Dual Functions of Microglia in Ischemic Stroke. Neurosci. Bull. 2019, 35, 921–933. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Ma, H.; Campbell, B.C.V.; Parsons, M.W.; Churilov, L.; Levi, C.R.; Hsu, C.; Kleinig, T.J.; Wijeratne, T.; Curtze, S.; Dewey, H.M.; et al. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N. Engl. J. Med. 2019, 380, 1795–1803. [Google Scholar] [CrossRef]
- Chugh, C. Acute Ischemic Stroke: Management Approach. Indian J. Crit. Care Med. 2019, 23, S140. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Desai, S.M.; Jovin, T.G. Indications for Mechanical Thrombectomy for Acute Ischemic Stroke: Current Guidelines and Beyond. Neurology 2021, 97, S126–S136. [Google Scholar] [CrossRef]
- Marko, M.; Posekany, A.; Szabo, S.; Scharer, S.; Kiechl, S.; Knoflach, M.; Serles, W.; Ferrari, J.; Lang, W.; Sommer, P.; et al. Trends of r-tPA (Recombinant Tissue-Type Plasminogen Activator) Treatment and Treatment-Influencing Factors in Acute Ischemic Stroke. Stroke 2020, 51, 1240–1247. [Google Scholar] [CrossRef]
- Lahr, M.M.H.; Luijckx, G.J.; Vroomen, P.C.A.J.; Van Der Zee, D.J.; Buskens, E. Proportion of patients treated with thrombolysis in a centralized versus a decentralized acute stroke care setting. Stroke 2012, 43, 1336–1340. [Google Scholar] [CrossRef]
- Jadhav, A.P.; Desai, S.M.; Kenmuir, C.L.; Rocha, M.; Starr, M.T.; Molyneaux, B.J.; Gross, B.A.; Jankowitz, B.T.; Jovin, T.G. Eligibility for Endovascular Trial Enrollment in the 6- to 24-Hour Time Window: Analysis of a Single Comprehensive Stroke Center. Stroke 2018, 49, 1015–1017. [Google Scholar] [CrossRef]
- Andrew Josephson, S.; Kamel, H. The Acute Stroke Care Revolution: Enhancing Access to Therapeutic Advances. JAMA 2018, 320, 1239–1240. [Google Scholar] [CrossRef]
- Ballarin, B.; Tymianski, M. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol. Sin. 2018, 39, 661–668. [Google Scholar] [CrossRef]
- Sun, H.S.; Doucette, T.A.; Liu, Y.; Fang, Y.; Teves, L.; Aarts, M.; Ryan, C.L.; Bernard, P.B.; Lau, A.; Forder, J.P.; et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke 2008, 39, 2544–2553. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Nunez, D.M.; Ji, Z.; Sun, X.; Teves, L.; Garman, J.D.; Tymianski, M. Plasmin-resistant PSD-95 inhibitors resolve effect-modifying drug-drug interactions between alteplase and nerinetide in acute stroke. Sci. Transl. Med. 2021, 13, eabb1498. [Google Scholar] [CrossRef]
- Leonard, M.G.; Briyal, S.; Gulati, A. Endothelin B receptor agonist, IRL-1620, provides long-term neuroprotection in cerebral ischemia in rats. Brain Res. 2012, 1464, 14–23. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Briyal, S.; Gulati, A. Sovateltide (IRL-1620) activates neuronal differentiation and prevents mitochondrial dysfunction in adult mammalian brains following stroke. Sci. Rep. 2020, 10, 12737. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Briyal, S.; Khandekar, D.; Gulati, A. Sovateltide (IRL-1620) affects neuronal progenitors and prevents cerebral tissue damage after ischemic stroke. Can. J. Physiol. Pharmacol. 2020, 98, 659–666. [Google Scholar] [CrossRef]
- Gulati, A.; Agrawal, N.; Vibha, D.; Misra, U.K.; Paul, B.; Jain, D.; Pandian, J.; Borgohain, R. Safety and Efficacy of Sovateltide (IRL-1620) in a Multicenter Randomized Controlled Clinical Trial in Patients with Acute Cerebral Ischemic Stroke. CNS Drugs 2021, 35, 85–104. [Google Scholar] [CrossRef]
- Shibata, M.; Kumar, S.R.; Amar, A.; Fernandez, J.A.; Hofman, F.; Griffin, J.H.; Zlokovic, B.V. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 2001, 103, 1799–1805. [Google Scholar] [CrossRef]
- Williams, P.D.; Zlokovic, B.V.; Griffin, J.H.; Pryor, K.E.; Davis, T.P. Preclinical Safety and Pharmacokinetic Profile of 3K3A-APC, a Novel, Modified Activated Protein C for Ischemic Stroke. Curr. Pharm. Des. 2012, 18, 4215. [Google Scholar] [CrossRef]
- Lyden, P.; Levy, H.; Weymer, S.; Pryor, K.; Kramer, W.; Griffin, J.; Davis, T.P.; Zlokovic, B. Phase 1 safety, tolerability and pharmacokinetics of 3K3A-APC in healthy adult volunteers. Curr. Pharm. Des. 2013, 19, 7479–7485. [Google Scholar] [CrossRef]
- Lyden, P.; Pryor, K.E.; Coffey, C.S.; Cudkowicz, M.; Conwit, R.; Jadhav, A.; Sawyer Jr, R.N.; Claassen, J.; Adeoye, O.; Song, S.; et al. Final Results of the RHAPSODY Trial: A Multi-Center, Phase 2 Trial Using a Continual Reassessment Method to Determine the Safety and Tolerability of 3K3A-APC, A Recombinant Variant of Human Activated Protein C, in Combination with Tissue Plasminogen Activator, Mechanical Thrombectomy or both in Moderate to Severe Acute Ischemic Stroke. Ann. Neurol. 2019, 85, 125–136. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Wang, L.; Liu, J. Urinary Kallidinogenase plus rt-PA Intravenous Thrombolysis for Acute Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Comput. Math. Methods Med. 2022, 2022, 1500669. [Google Scholar] [CrossRef]
- Garwood, C.J.; Cooper, J.D.; Hanger, D.P.; Noble, W. Anti-Inflammatory Impact of Minocycline in a Mouse Model of Tauopathy. Front. Psychiatry 2010, 1, 136. [Google Scholar] [CrossRef]
- Tikka, T.M.; Koistinaho, J.E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 2001, 166, 7527–7533. [Google Scholar] [CrossRef]
- Naderi, Y.; Panahi, Y.; Barreto, G.E.; Sahebkar, A. Neuroprotective effects of minocycline on focal cerebral ischemia injury: A systematic review. Neural. Regen. Res. 2020, 15, 773. [Google Scholar] [CrossRef]
- Malhotra, K.; Chang, J.J.; Khunger, A.; Blacker, D.; Switzer, J.A.; Goyal, N.; Hernandez, A.V.; Pasupuleti, V.; Alexandrov, A.V.; Tsivgoulis, G. Minocycline for acute stroke treatment: A systematic review and meta-analysis of randomized clinical trials. J. Neurol. 2018, 265, 1871–1879. [Google Scholar] [CrossRef]
- Ortiz, J.F.; Ruxmohan, S.; Saxena, A.; Morillo Cox, Á.; Bashir, F.; Tambo, W.; Ghani, M.R.; Moya, G.; Córdova, I. Minocycline and Magnesium as Neuroprotective Agents for Ischemic Stroke: A Systematic Review. Cureus 2020, 12, e1233. [Google Scholar] [CrossRef]
- Gwag, B.J.; Lee, Y.A.; Ko, S.Y.; Lee, M.J.; Im, D.S.; Yun, B.S.; Lim, H.R.; Park, S.M.; Byun, H.Y.; Son, S.J.; et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J. Cereb. Blood Flow Metab. 2007, 27, 1142–1151. [Google Scholar] [CrossRef]
- Hong, J.M.; Lee, J.S.; Lee, Y.B.; Shin, D.H.; Shin, D.I.; Hwang, Y.H.; Ahn, S.H.; Kim, J.G.; Sohn, S.I.; Kwon, S.U.; et al. Nelonemdaz for Patients with Acute Ischemic Stroke Undergoing Endovascular Reperfusion Therapy: A Randomized Phase II Trial. Stroke 2022, 53, 3250–3259. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.S.; Gwag, B.J.; Choi, D.W.; An, C.S.; Kang, H.G.; Song, T.J.; Ahn, S.H.; Kim, C.H.; Shin, D.I.; et al. The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz (RODIN) Trial: Protocol for a Double-Blinded Clinical Trial of Nelonemdaz in Patients with Hyperacute Ischemic Stroke and Endovascular Thrombectomy. J. Stroke 2023, 25, 160–168. [Google Scholar] [CrossRef]
- Li, R.; Huang, C.; Chen, J.; Guo, Y.; Tan, S. The role of uric acid as a potential neuroprotectant in acute ischemic stroke: A review of literature. Neurol. Sci. 2015, 36, 1097–1103. [Google Scholar] [CrossRef]
- Romanos, E.; Planas, A.M.; Amaro, S.; Chamorro, Á. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J. Cereb. Blood Flow Metab. 2007, 27, 14–20. [Google Scholar] [CrossRef]
- Chamorro, Á.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Martí-Fábregas, J.; Gállego, J.; Krupinski, J.; Gomis, M.; Cánovas, D.; et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014, 13, 453–460. [Google Scholar] [CrossRef]
- Amaro, S.; Laredo, C.; Renú, A.; Llull, L.; Rudilosso, S.; Obach, V.; Urra, X.; Planas, A.M.; Chamorro, Á. Uric Acid Therapy Prevents Early Ischemic Stroke Progression: A Tertiary Analysis of the URICO-ICTUS Trial (Efficacy Study of Combined Treatment with Uric Acid and r-tPA in Acute Ischemic Stroke). Stroke 2016, 47, 2874–2876. [Google Scholar] [CrossRef]
- Sabroe, I.; Read, R.C.; Whyte, M.K.B.; Dockrell, D.H.; Vogel, S.N.; Dower, S.K. Toll-like receptors in health and disease: Complex questions remain. J. Immunol. 2003, 171, 1630–1635. [Google Scholar] [CrossRef]
- Fernández, G.; Moraga, A.; Cuartero, M.I.; García-Culebras, A.; Peña-Martínez, C.; Pradillo, J.M.; Hernández-Jiménez, M.; Sacristán, S.; Ayuso, M.I.; Gonzalo-Gobernado, R.; et al. TLR4-Binding DNA Aptamers Show a Protective Effect against Acute Stroke in Animal Models. Mol. Ther. 2018, 26, 2047. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Martín-Vílchez, S.; Ochoa, D.; Mejía-Abril, G.; Román, M.; Camargo-Mamani, P.; Luquero-Bueno, S.; Jilma, B.; Moro, M.A.; Fernández, G.; et al. First-in-human phase I clinical trial of a TLR4-binding DNA aptamer, ApTOLL: Safety and pharmacokinetics in healthy volunteers. Mol. Ther. Nucleic Acids 2022, 28, 124. [Google Scholar] [CrossRef]
- Lapchak, P.A. A Critical assessment of edaravone acute ischemic stroke efficacy trials: Is edaravone an effective neuroprotective therapy? Expert Opin. Pharm. 2010, 11, 1753. [Google Scholar] [CrossRef]
- Fidalgo, M.; Ricardo Pires, J.; Viseu, I.; Magalhães, P.; Gregório, H.; Afreixo, V.; Gregório, T. Edaravone for acute ischemic stroke—Systematic review with meta-analysis. Clin. Neurol. Neurosurg. 2022, 219, 107299. [Google Scholar] [CrossRef]
- Xu, J.; Wang, A.; Meng, X.; Yalkun, G.; Xu, A.; Gao, Z.; Chen, H.; Ji, Y.; Xu, J.; Geng, D.; et al. Edaravone Dexborneol Versus Edaravone Alone for the Treatment of Acute Ischemic Stroke: A Phase III, Randomized, Double-Blind, Comparative Trial. Stroke 2021, 52, 772–780. [Google Scholar] [CrossRef]
- Chrostek, M.R.; Fellows, E.G.; Crane, A.T.; Grande, A.W.; Low, W.C. Efficacy of stem cell-based therapies for stroke. Brain Res. 2019, 1722, 146362. [Google Scholar] [CrossRef]
- Fukunaga, A.; Uchida, K.; Hara, K.; Kuroshima, Y.; Kawase, T. Differentiation and angiogenesis of central nervous system stem cells implanted with mesenchyme into ischemic rat brain. Cell Transplant. 1999, 8, 435–441. [Google Scholar] [CrossRef]
- Daadi, M.M.; Li, Z.; Arac, A.; Grueter, B.A.; Sofilos, M.; Malenka, R.C.; Wu, J.C.; Steinberg, G.K. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol. Ther. 2009, 17, 1282–1291. [Google Scholar] [CrossRef]
- Pollock, K.; Stroemer, P.; Patel, S.; Stevanato, L.; Hope, A.; Miljan, E.; Dong, Z.; Hodges, H.; Price, J.; Sinden, J.D. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp. Neurol. 2006, 199, 143–155. [Google Scholar] [CrossRef]
- Stroemer, P.; Patel, S.; Hope, A.; Oliveira, C.; Pollock, K.; Sinden, J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil. Neural Repair 2009, 23, 895–908. [Google Scholar] [CrossRef]
- Smith, E.J.; Stroemer, R.P.; Gorenkova, N.; Nakajima, M.; Crum, W.R.; Tang, E.; Stevanato, L.; Sinden, J.D.; Modo, M. Implantation site and lesion topology determine efficacy of a human neural stem cell line in a rat model of chronic stroke. Stem Cells 2012, 30, 785–796. [Google Scholar] [CrossRef]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef]
- Brazelton, T.R.; Rossi, F.M.V.; Keshet, G.I.; Blau, H.M. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science 2000, 290, 1775–1779. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Lu, M.; Zhang, X.; Chopp, M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J. Neurol. Sci. 2001, 189, 49–57. [Google Scholar] [CrossRef]
- Iihoshi, S.; Honmou, O.; Houkin, K.; Hashi, K.; Kocsis, J.D. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004, 1007, 1–9. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef]
- Wislet-Gendebien, S.; Hans, G.; Leprince, P.; Rigo, J.; Moonen, G.; Rogister, B. Plasticity of cultured mesenchymal stem cells: Switch from nestin-positive to excitable neuron-like phenotype. Stem Cells 2005, 23, 392–402. [Google Scholar] [CrossRef]
- Wang, F.; Tang, H.; Zhu, J.; Zhang, J.H. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018, 27, 1825. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Wang, L.; Lu, M.; Chopp, M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 2001, 56, 1666–1672. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Vu, Q.; Xie, K.; Eckert, M.; Zhao, W.; Cramer, S.C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology 2014, 82, 1277. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Dabrowska, S.; Lukomska, B.; Janowski, M. Mesenchymal Stem Cells for Neurological Disorders. Adv. Sci. 2021, 8, 2002944. [Google Scholar] [CrossRef]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [CrossRef]
- Chung, J.W.; Chang, W.H.; Bang, O.Y.; Moon, G.J.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H.; STARTING-2 Collaborators; et al. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96, e1012–e1023. [Google Scholar] [CrossRef]
- Zheng, X.; Hermann, D.M.; Bähr, M.; Doeppner, T.R. The role of small extracellular vesicles in cerebral and myocardial ischemia-Molecular signals, treatment targets, and future clinical translation. Stem Cells 2021, 39, 403–413. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; de Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res. Ther. 2020, 11, 313. [Google Scholar] [CrossRef]
- Zheng, X.; Haupt, M.; Bähr, M.; Tatenhorst, L.; Doeppner, T.R. Treating Cerebral Ischemia: Novel Therapeutic Strategies from Experimental Stroke Research. Cereb. Ischemia 2021, 165–186. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Kuang, Y.; Venkataramani, V.; Jin, F.; Hein, K.; Zafeiriou, M.P.; Lenz, C.; Moebius, W.; Kilic, E.; et al. Extracellular Vesicles Derived from Neural Progenitor Cells—A Preclinical Evaluation for Stroke Treatment in Mice. Transl. Stroke Res. 2021, 12, 185–203. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C.; Huang, Y.; Wang, Y.; Xia, X.; Zheng, J.C. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun. Signal 2019, 17, 96. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Zhang, W.; Chen, Y.; Bihl, J.C. Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci. Ther. 2020, 26, 1255–1265. [Google Scholar] [CrossRef]
- Haupt, M.; Zheng, X.; Kuang, Y.; Lieschke, S.; Janssen, L.; Bosche, B.; Jin, F.; Hein, K.; Kilic, E.; Venkataramani, V.; et al. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl. Med. 2020, 10, 357–373. [Google Scholar] [CrossRef]
- Alehossein, P.; Taheri, M.; Tayefeh Ghahremani, P.; Dakhlallah, D.; Brown, C.M.; Ishrat, T.; Nasoohi, S. Transplantation of Exercise-Induced Extracellular Vesicles as a Promising Therapeutic Approach in Ischemic Stroke. Transl. Stroke Res. 2022. [Google Scholar] [CrossRef]
- Arya, A.K.; Hu, B. Brain-gut axis after stroke. Brain Circ. 2018, 4, 165. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Zhang, Y.; Wang, Z.; Peng, J.; Gerner, S.T.; Yin, S.; Jiang, Y. Gut microbiota-brain interaction: An emerging immunotherapy for traumatic brain injury. Exp. Neurol. 2021, 337, 113585. [Google Scholar] [CrossRef]
- Wen, S.W.; Wong, C.H.Y. An unexplored brain-gut microbiota axis in stroke. Gut Microbes 2017, 8, 601. [Google Scholar] [CrossRef]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef]
- Winek, K.; Engel, O.; Koduah, P.; Heimesaat, M.M.; Fischer, A.; Bereswill, S.; Dames, C.; Kershaw, O.; Gruber, A.D.; Curato, C.; et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke 2016, 47, 1354–1363. [Google Scholar] [CrossRef]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef]
- Xu, K.; Gao, X.; Xia, G.; Chen, M.; Zeng, N.; Wang, S.; You, C.; Tian, X.; Di, H.; Tang, W.; et al. Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut 2021, 70, 1486–1494. [Google Scholar] [CrossRef]
- Xia, G.H.; You, C.; Gao, X.X.; Zeng, X.L.; Zhu, J.J.; Xu, K.Y.; Tan, C.H.; Xu, R.T.; Wu, Q.H.; Zhou, H.W.; et al. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated with Brain Injury and Prognosis of Stroke. Front. Neurol. 2019, 10, 397. [Google Scholar] [CrossRef]
- Wang, H.; Song, W.; Wu, Q.; Gao, X.; Li, J.; Tan, C.; Zhou, H.; Zhu, J.; He, Y.; Yin, J. Fecal Transplantation from db/db Mice Treated with Sodium Butyrate Attenuates Ischemic Stroke Injury. Microbiol. Spectr. 2021, 9, e00042-21. [Google Scholar] [CrossRef]
| Substance | Mechanism | Evidence | Limitations | Perspective |
|---|---|---|---|---|
| NA-1 | - Prevent PDS-95 from attaching to NMDAR, thereby inhibiting excessive Ca2+ influx and generation of nitric oxide. | - Reduced infarct size and improved neurological outcome have been observed in preclinical stroke models. | - An RCT (ESCAPE-NA1) showed that the neurological outcome did not improve. - There is a hypothesis that the drug-drug interaction of alteplase and NA-1 led to a decreased plasma level of NA-1. | - An ongoing RCT is investigating NA-1 treatment in patients without thrombolysis treatment (ESCAPE-NEXT). |
| Sovateltide | - Agonist of ETBR. - Improve NPC differentiation and improve mitochondrial shape as well as biogenesis, in ischemic brains. | - A Phase III RCT revealed improved neurological outcome 90 days post-treatment (NCT04046484). | - RCT only involved a sample size of 40 participants. | - A larger-sized randomized controlled trial RCT is needed to confirm the findings. |
| 3K3A-APC | - Recombinant APC with lower anticoagulant properties has antiapoptotic and anti-inflammatory effects. | - A Phase II RCT showed that patients treated intravenously with 3K3A-APC combined with thrombectomy, thrombolysis, or both showed a trend towards a lower hemorrhage rate (RHAPSODY). | - The RCT only included a small sample size of 110 participants. | - A Phase III trial with an estimated enrollment of 1400 participants is currently ongoing (RHAPSODY-2). |
| HUK | - Regulates the kallikrein–kinin system. | - Several RCT has been conducted. - A meta-analysis of 18 RCTs revealed that HUK combined with rt-PA significantly improved the neurological recovery and quality of life in stroke patients. | - Small sample size and methodological weaknesses in the studies. - Only two studies documented the mortality rate during the follow-up period. | - More studies, especially those documenting the mortality rate, are necessary to investigate the drug’s safety. |
| Minocycline | - Antibiotic drug from the group of tetracyclines. - Has been shown to be a potent inhibitor of microglia activation, thereby suppressing the production of anti-inflammatory cytokines and mediators. | - Several RCTs revealed a neuroprotective effect, indicated by improvement in 3-month functional independence, Barthel index, and NIHSS score. | - several RCTs had small sample sizes and were underpowered. - The combination with magnesium showed no clear results. | - Additional clinical trials employing minocycline alone and in combination with magnesium are now being conducted to expand the clinical evidence (NCT05512910; NCT05032781). |
| Neu2000 | - Aims to prevent damage mediated by the NMDA receptor and free radicals. | - A phase II RCT involving 208 stroke patients who underwent endovascular reperfusion was conducted. - The results showed a trend towards better scores on the modified Rankin Scale 12 weeks after stroke onset, but there was no significant difference. | - No significant neuroprotection has been found in RCTs to date. | - A phase III clinical trial is currently underway to clarify the efficacy in hyperacute ischemic stroke and endovascular thrombectomy patients (RODIN). |
| Uric Acid | - Byproduct of purine metabolism and acts as an endogenous antioxidant. | - A clinical phase IIb/III study involving 411 stroke patients showed that the therapy did not result in a higher proportion of patients with excellent outcomes at 90 days post-stroke. - A secondary analysis revealed that there was an improvement in early ischemic worsening. | - There was no significant difference in the primary endpoint of the RCT (NCT00860366). | - Further clinical trials are necessary to determine the potential benefits. |
| ApoTOLL | - TLR4 antagonist. - TLR4 plays a role in activating the innate immune response and triggering the inflammatory response. | - Preclinic studies have shown neuroprotective effects. - A Phase I RCT demonstrated safety. | - No official results from a Phase II RCT have been released till date. | - A Phase Ib/IIa RCT was completed in 2022, but the official results of the study have not yet been made public (NCT04734548). |
| Edaravone | - Free radical scavenger that has been shown to regulate apoptosis, microglia activation, and long-term neuroinflammation, as well as exhibiting antioxidant activity. | - A meta-analysis of 19 RCTs revealed improved neurological outcomes and decreased mortality. - The combination of Edaravone and Dexborneol was found to be even more effective than Edaravone alone in terms of outcome. | - The majority of studies on Edaravone were conducted in Asia, particularly in Japan. | - Further studies are underway to test the efficacy of Edaravone alone and in combination with Dexborneol (NCT05024526; NCT05032781). |
| NCT Number; Name | Drug | Phase | Route | Estimated Enrollment | Country |
|---|---|---|---|---|---|
| NCT04462536 (ESCAPE-NEXT) | NA-1 (nerinetide) | III | intravenous | 1020 | Canada |
| NCT02315443 (FRONTIER) | NA-1 (nerinetide) | III | intravenous | 586 | Canada |
| NCT05484154 (RHAPSODY-2) | 3K3A-APC | III | intravenous | 1400 | United States |
| NCT03320018 (H2M) | Hydrogen and Minocycline | II and III | intravenous and oral | 100 | United States |
| NCT03347786 | Verapamil | I | intravenous | 20 | United States |
| NCT05032781 | Minocycline and magnesium | I | intra-arterially | 24 | United States |
| NCT05041010 (RODIN) | Neu2000 | III | intravenous | 496 | Korea |
| NCT05512910 (MIST-B) | Minocycline | IV | oral | 90 | China |
| NCT05124353 | Cerebrolysin | II | intravenous | 100 | Poland |
| NCT05024526 | Edaravone | N/A | intravenous | 80 | China |
| NCT05249920 (TASTE-2) | Edaravone Dexborneol | III | intravenous | 1362 | China |
| NCT Number | Cell Line | Phase | Administration | Estimated Enrollment | Treatment after Stroke Onset | Country |
|---|---|---|---|---|---|---|
| NCT04811651 | UC-MSC | II | Intravenous | 200 | within 6 months | China |
| NCT05292625 | UC-MSC | I | Intravenous | 48 | 72 h and 3 months | Vietnam |
| UC-MSC | II | intrathecal | 72 h and 3 months | |||
| NCT04280003 | Alogenic adipose tissue-derived MSC | II | Intravenous | 30 | within 4 days | Spain |
| NCT04097652 | UC-MSC (UMC119-06) | I | Intravenous | 9 | 48 to 168 h | Taiwan |
| NCT04434768 | UC-MSC (UMSC01) | I | intraarterial and intravenous | 14 | N/A | Taiwan |
| NCT04093336 | UC-MSC | I and II | Intravenous | 120 | within 7 days | China |
| NCT Number; Name | Cell Line | Phase | Route | Participants | Time Point | Result |
|---|---|---|---|---|---|---|
| NCT01678534 (AMASCIS-01) | allogeneic stem cells from adipose tissue | II | intravenous | 19 | Within 2 weeks | No end points were statistically significant. |
| NCT01501773 (InVeST) | autologous bone marrow mononuclear cells | II | intravenous | 120 | median of 18.5 days | No beneficial effect on stroke outcome. |
| NCT02117635 (PISCES-II) | hNPC (CTX0E03) | II | intracerebral | 23 | 2–13 months | Improvements in upper limb functions. |
| NCT01468064 (AMETIS) | EPCs | II | intracerebral | 20 | 7 days | No significant difference in neurological or functional improvement, but fewer serious adverse events. |
| NCT00875654 (ISIS) | MSC | II | intravenous | 31 | Within 6 weeks | Improved motor recovery. |
| NCT03004976 (CoBIS 2) | UCB stem cells | II | intravenous | 79 | 3–10 days | Not yet published |
| NCT01436487 (MASTERS) | multipotent adult progenitor cells | II | intravenous | 134 | 1–2 days | No beneficial effect on stroke outcome. |
| NCT01716481 (STARTING-2) | MSC | III | intravenous | 54 | 90 days | No improved outcome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haupt, M.; Gerner, S.T.; Bähr, M.; Doeppner, T.R. Neuroprotective Strategies for Ischemic Stroke—Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4334. https://doi.org/10.3390/ijms24054334
Haupt M, Gerner ST, Bähr M, Doeppner TR. Neuroprotective Strategies for Ischemic Stroke—Future Perspectives. International Journal of Molecular Sciences. 2023; 24(5):4334. https://doi.org/10.3390/ijms24054334
Chicago/Turabian StyleHaupt, Matteo, Stefan T. Gerner, Mathias Bähr, and Thorsten R. Doeppner. 2023. "Neuroprotective Strategies for Ischemic Stroke—Future Perspectives" International Journal of Molecular Sciences 24, no. 5: 4334. https://doi.org/10.3390/ijms24054334
APA StyleHaupt, M., Gerner, S. T., Bähr, M., & Doeppner, T. R. (2023). Neuroprotective Strategies for Ischemic Stroke—Future Perspectives. International Journal of Molecular Sciences, 24(5), 4334. https://doi.org/10.3390/ijms24054334






