Prognostic Value of EMT Gene Signature in Malignant Mesothelioma
Abstract
1. Introduction
2. Results
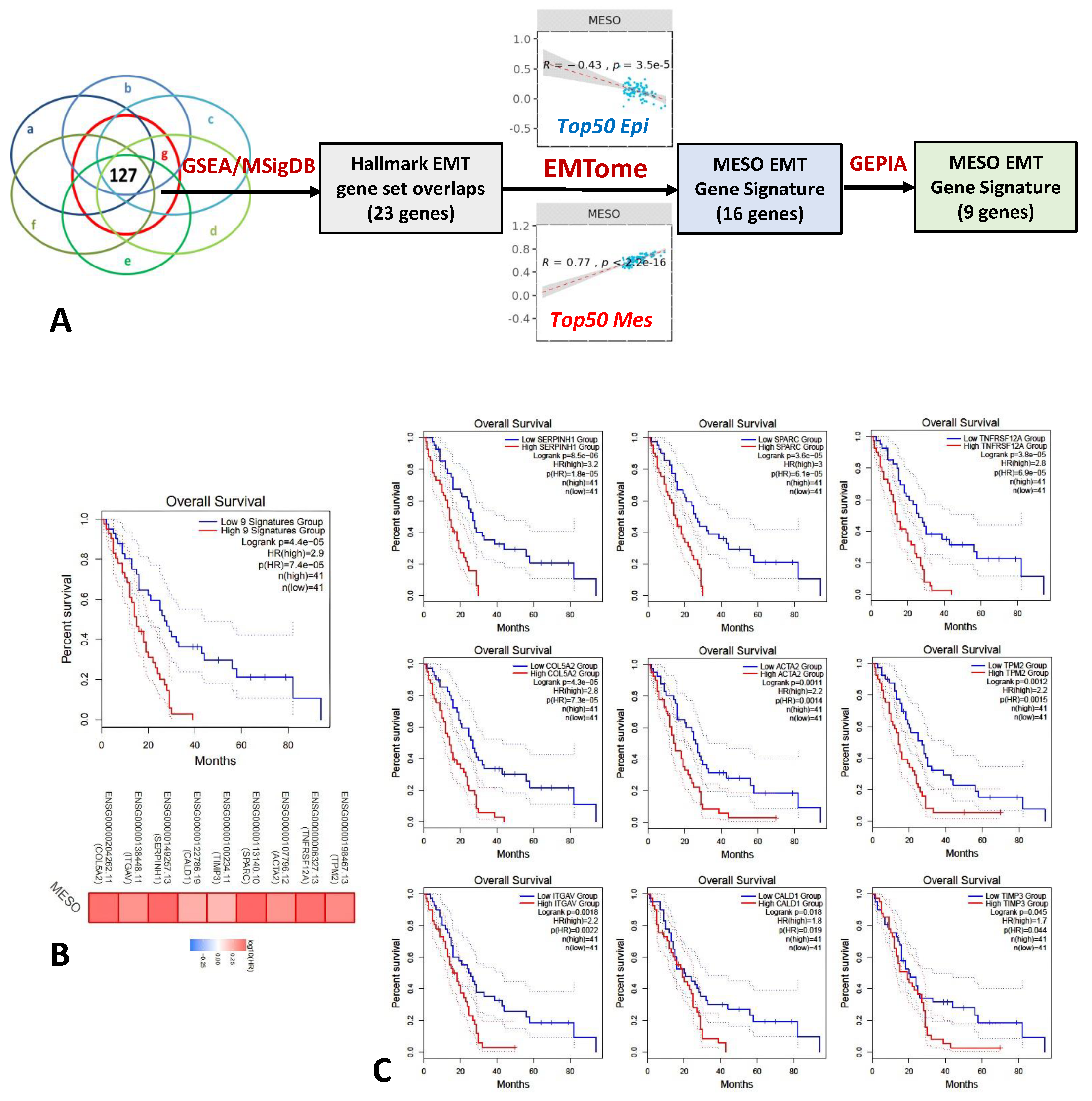
2.1. EMT Gene Signature Associated with Overall Survival in Mesothelioma
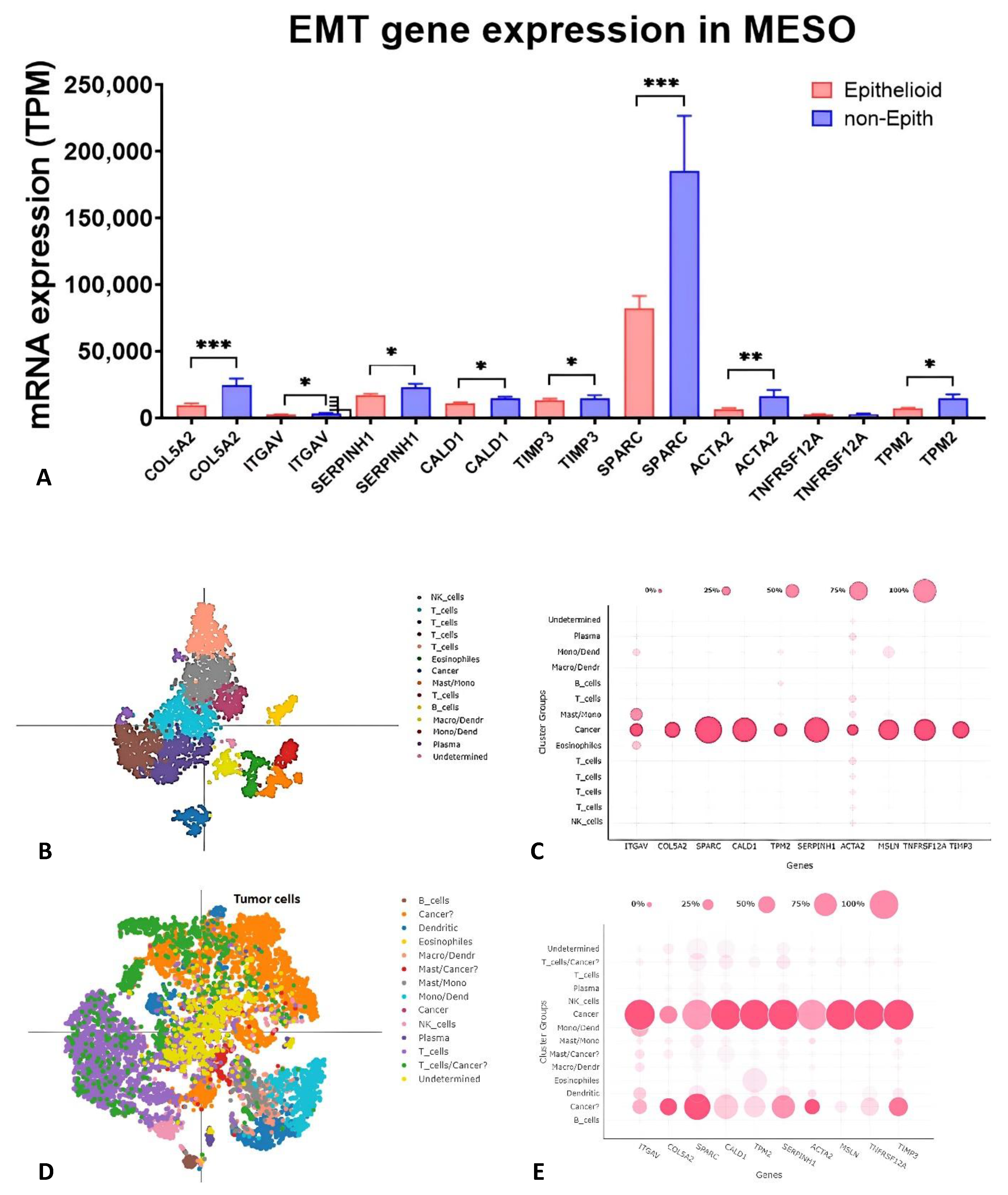
2.2. Gene Expression of Nine EMT Gene Signatures in Tumors, Pleural Effusion, and TCGA Database
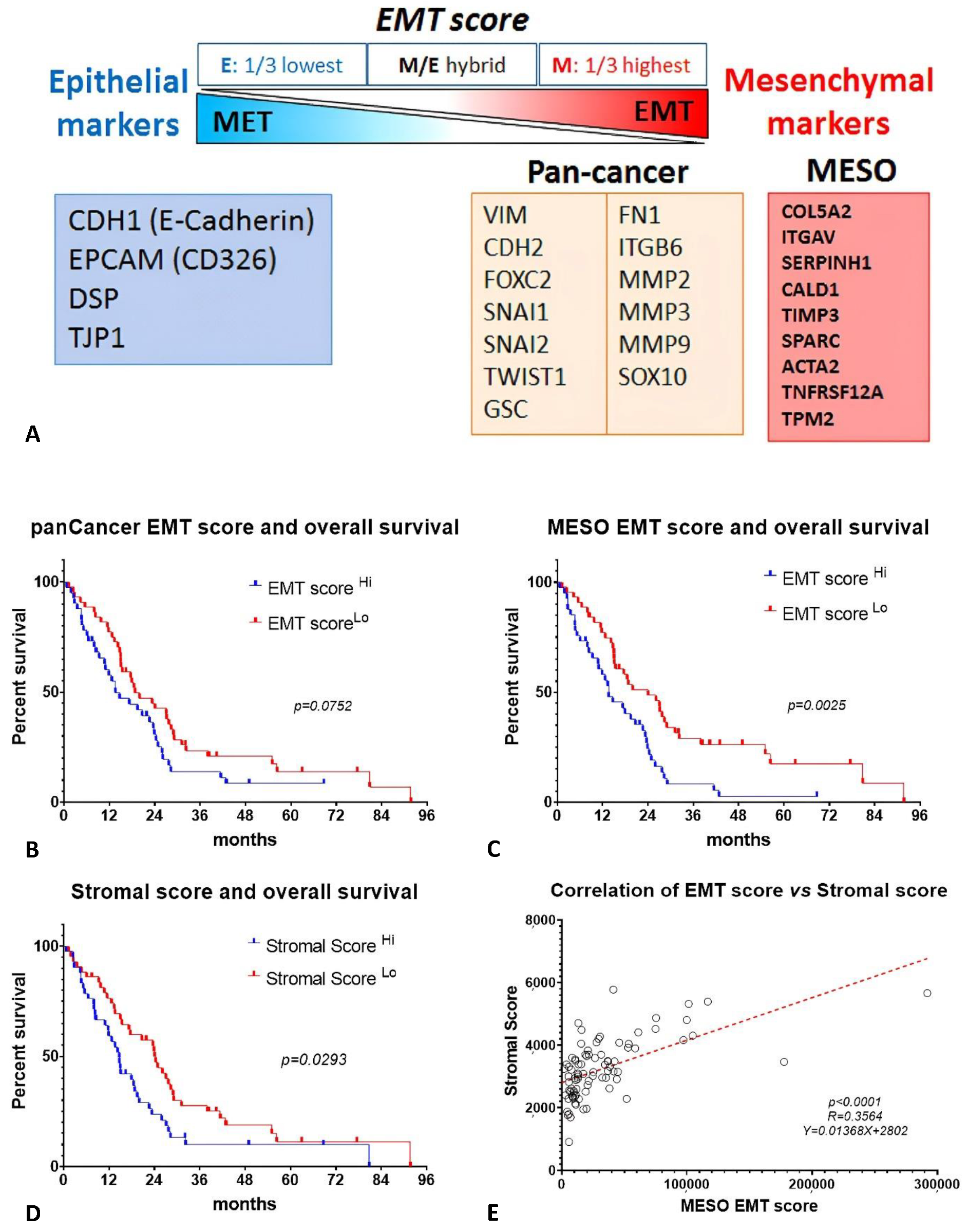
2.3. EMT Score and Stromal Score Are Associated with Survival in MESO TCGA Database
2.4. Proteomic Analysis and Signaling Pathway Networks of MESO EMT Genes
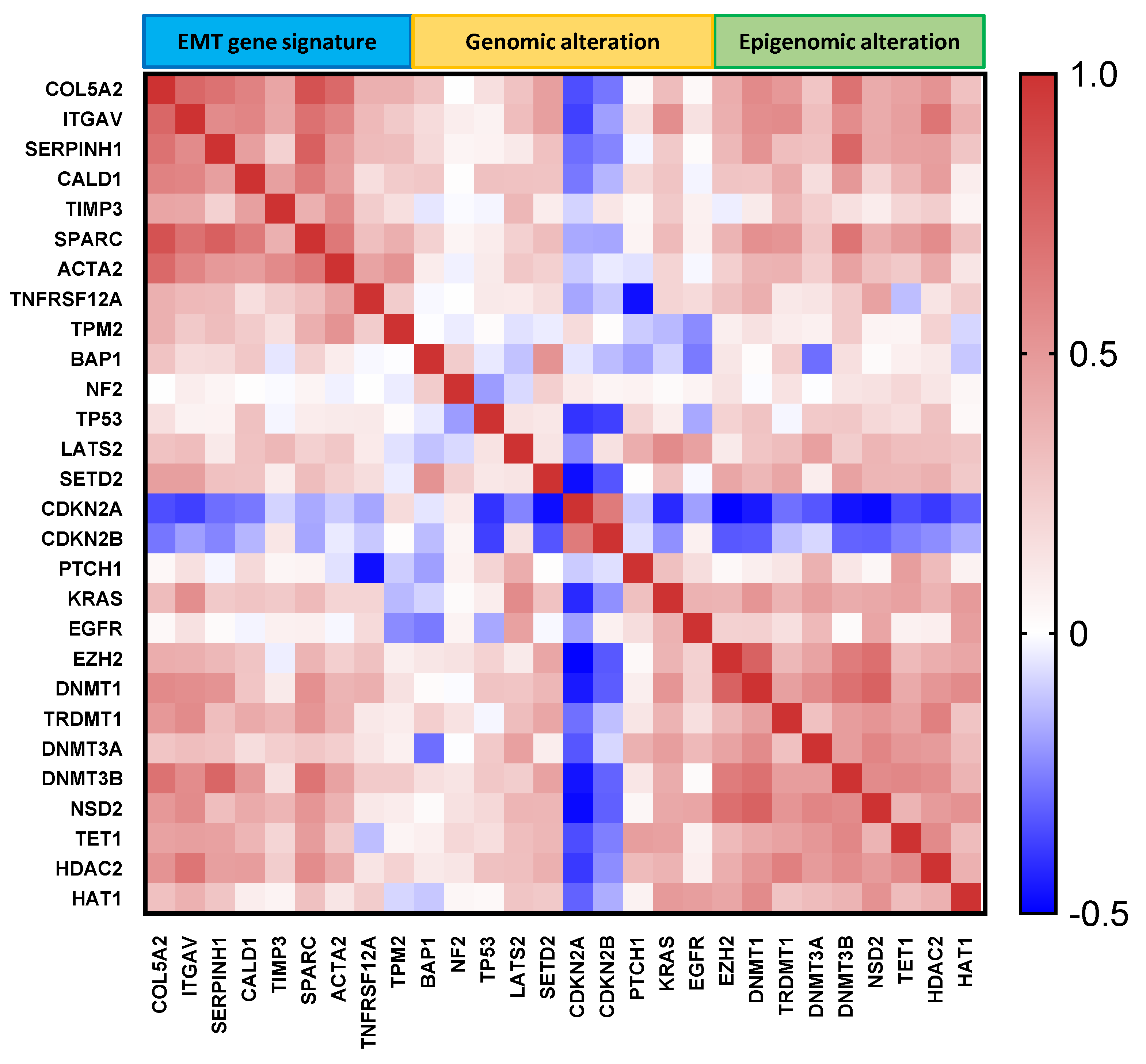
2.5. Correlation of MESO EMT Genes with Genomic/Epigenomic Alterations
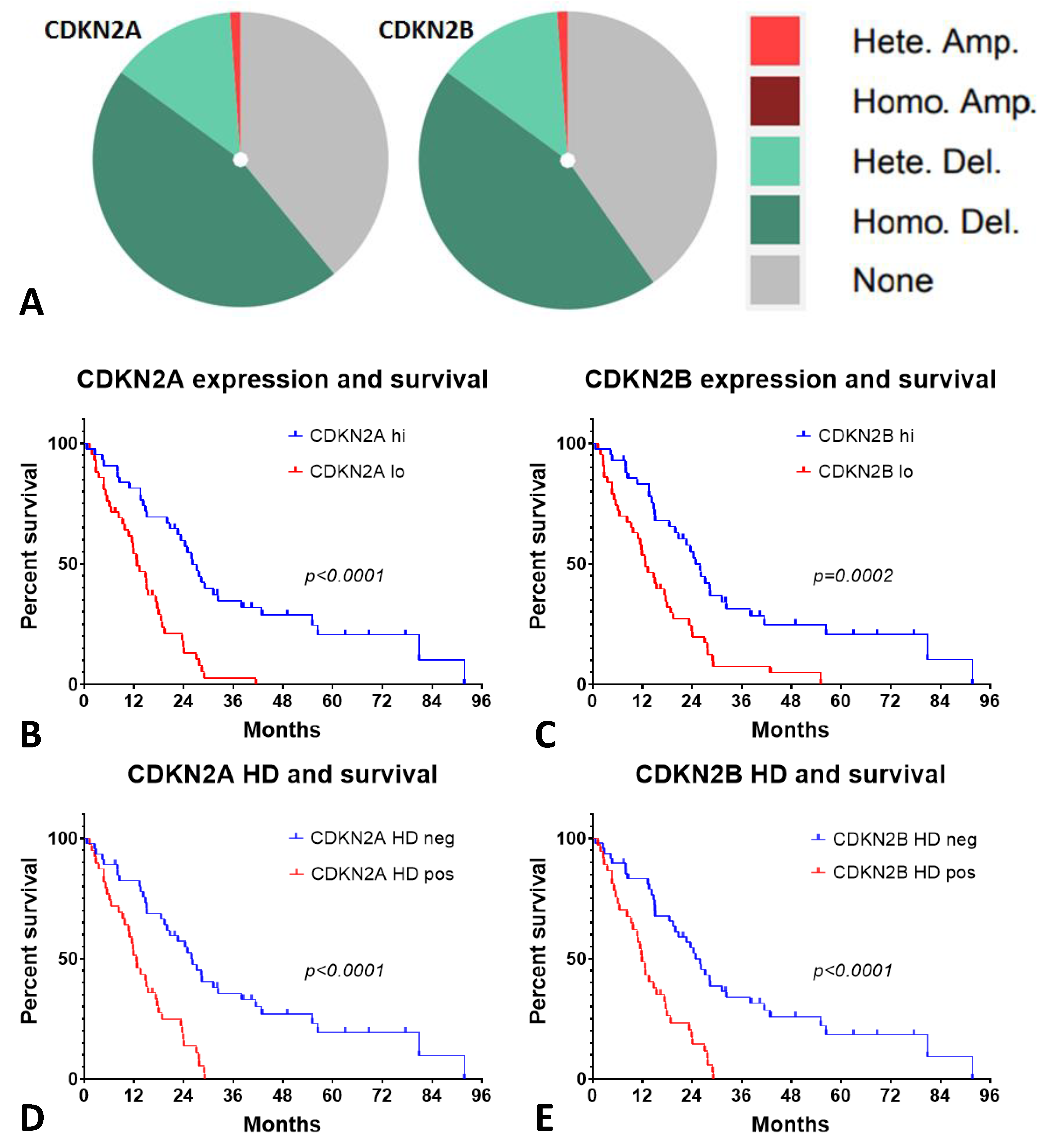
2.6. Low CDKN2A/B Expression and Homozygous Deletion (HD) of p16 Had an Inverse Outcome on the Patients with MESO
3. Discussion
4. Materials and Methods
4.1. Murine Mesothelioma Cells and Mouse Model
4.2. Patients with Mesothelioma
4.3. Identification of MESO EMT Gene Signature
4.4. Online Resources for Multiomics Analysis
4.5. Calculation of EMT Score and Stromal Score
4.6. Selection of Genes of Interest with Genomic/Epigenomic Alterations
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mossman, B.T.; Gee, J.B.L. Asbestos-Related Diseases. New Engl. J. Med. 1989, 320, 1721–1730. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Sun, X.-M.; Wu, L. High Time for Complete Ban on Asbestos Use in Developing Countries. JAMA Oncol. 2019, 5, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.J.; Donahoe, L.; Bradbury, P.A.; Leighl, N.; Keshavjee, S.; Hope, A.; Pal, P.; Cabanero, M.; Czarnecka, K.; McRae, K.; et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): Final results from a single-centre, phase 2 trial. Lancet Oncol. 2021, 22, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Murakami, J.; Wu, L.; Kohno, M.; Chan, M.-L.; Zhao, Y.; Yun, Z.; Cho, B.C.J.; de Perrot, M. Triple-modality therapy maximizes antitumor immune responses in a mouse model of mesothelioma. Sci. Transl. Med. 2021, 13, eabd9882. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Wu, L.; Murakami, J.; Zhao, Y.; Yun, H.; de Perrot, M. IRF3 Knockout Results in Partial or Complete Rejection of Murine Mesothelioma. J. Clin. Med. 2021, 10, 5196. [Google Scholar] [CrossRef]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef]
- Nowak, A.K.; Jackson, A.; Sidhu, C. Management of Advanced Pleural Mesothelioma—At the Crossroads. JCO Oncol. Prac. 2022, 18, 116–124. [Google Scholar] [CrossRef]
- Brcic, L.; Kern, I. Clinical significance of histologic subtyping of malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020, 9, 924–933. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Adachi, Y.; Ito, K.; Hayashi, Y.; Kimura, R.; Tan, T.Z.; Yamaguchi, R.; Ebi, H. Epithelial-to-Mesenchymal Transition is a Cause of Both Intrinsic and Acquired Resistance to KRAS G12C Inhibitor in KRAS G12C–Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 5962–5973. [Google Scholar] [CrossRef]
- Cai, Z.; Poulos, R.C.; Liu, J.; Zhong, Q. Machine learning for multi-omics data integration in cancer. Iscience 2022, 25, 103798. [Google Scholar] [CrossRef] [PubMed]
- Assum, I.; Krause, J.; Scheinhardt, M.O.; Müller, C.; Hammer, E.; Börschel, C.S.; Völker, U.; Conradi, L.; Geelhoed, B.; Zeller, T.; et al. Tissue-specific multi-omics analysis of atrial fibrillation. Nat. Commun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salji, M.J.; Blomme, A.; Däbritz, J.H.M.; Repiscak, P.; Lilla, S.; Patel, R.; Sumpton, D.; Broek, N.J.V.D.; Daly, R.; Zanivan, S.; et al. Multi-omics & pathway analysis identify potential roles for tumor N-acetyl aspartate accumulation in murine models of castration-resistant prostate cancer. Iscience 2022, 25, 104056. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Deshmukh, A.P.; Hollander, P.D.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2020, 124, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Amjad, S.; Yun, H.; Mani, S.; de Perrot, M. A panel of emerging EMT genes identified in malignant mesothelioma. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, K.; Chen, X.; Li, Y.; Chen, Y. Analysis of Immune–Stromal Score-Based Gene Signature and Molecular Subtypes in Osteosarcoma: Implications for Prognosis and Tumor Immune Microenvironment. Front. Genet. 2021, 12, 699385. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.P.; Tong, P.; Diao, L.; Cardnell, R.J.; Gibbons, D.L.; William, W.N.; Skoulidis, F.; Parra, E.R.; Rodriguez-Canales, J.; Wistuba, I.I.; et al. A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin. Cancer Res. 2016, 22, 609–620. [Google Scholar] [CrossRef]
- Obacz, J.; Yung, H.; Shamseddin, M.; Linnane, E.; Liu, X.; Azad, A.A.; Rassl, D.M.; Fairen-Jimenez, D.; Rintoul, R.C.; Nikolić, M.Z.; et al. Biological basis for novel mesothelioma therapies. Br. J. Cancer 2021, 125, 1039–1055. [Google Scholar] [CrossRef]
- Fassina, A.; Cappellesso, R.; Guzzardo, V.; Via, L.D.; Piccolo, S.; Ventura, L.; Fassan, M. Epithelial–mesenchymal transition in malignant mesothelioma. Mod. Pathol. 2012, 25, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Wirawan, A.; Tajima, K.; Takahashi, F.; Mitsuishi, Y.; Winardi, W.; Hidayat, M.; Hayakawa, D.; Matsumoto, N.; Izumi, K.; Asao, T.; et al. A Novel Therapeutic Strategy Targeting the Mesenchymal Phenotype of Malignant Pleural Mesothelioma by Suppressing LSD1. Mol. Cancer Res. 2022, 20, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.-H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K.; et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; Hollander, P.D.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. 2021, 118, e2102050118. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Negi, S.; Kashyap, M.; Nizamuddin, S.; Singh, A.; Khattri, A. Pan-Cancer Analysis Shows Enrichment of Macrophages, Overexpression of Checkpoint Molecules, Inhibitory Cytokines, and Immune Exhaustion Signatures in EMT-High Tumors. Front. Oncol. 2022, 11, 793881. [Google Scholar] [CrossRef]
- Morrison, C.D.; Parvani, J.G.; Schiemann, W.P. The relevance of the TGF-β Paradox to EMT-MET programs. Cancer Lett. 2013, 341, 30–40. [Google Scholar] [CrossRef]
- Creaney, J.; Patch, A.-M.; Addala, V.; Sneddon, S.A.; Nones, K.; Dick, I.M.; Lee, Y.C.G.; Newell, F.; Rouse, E.J.; Naeini, M.M.; et al. Comprehensive genomic and tumour immune profiling reveals potential therapeutic targets in malignant pleural mesothelioma. Genome Med. 2022, 14, 1–18. [Google Scholar] [CrossRef]
- Jung, A.R.; Jung, C.-H.; Noh, J.K.; Lee, Y.C.; Eun, Y.-G. Epithelial-mesenchymal transition gene signature is associated with prognosis and tumor microenvironment in head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 3652. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P.T. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Fujimoto, N.; Matsumoto, T.; Tsukahara, S.; Nagakawa, S.; Ueda, S.; Ushijima, M.; Kashiwagi, E.; Takeuchi, A.; Inokuchi, J.; et al. Differential Impact of TGFB1 Variation by Metastatic Status in Androgen-Deprivation Therapy for Prostate Cancer. Front. Oncol. 2021, 11, 697955. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Yu, B.; Qin, H.; Xue, L.; Liang, Y.-J.; Quan, Z.-X. EMT-related gene expression is positively correlated with immunity and may be derived from stromal cells in osteosarcoma. PeerJ 2020, 8, e8489. [Google Scholar] [CrossRef]
- Giannoni, E.; Bianchini, F.; Masieri, L.; Serni, S.; Torre, E.; Calorini, L.; Chiarugi, P. Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness. Cancer Res. 2010, 70, 6945–6956. [Google Scholar] [CrossRef]
- Czekay, R.-P.; Cheon, D.-J.; Samarakoon, R.; Kutz, S.M.; Higgins, P.J. Cancer-Associated Fibroblasts: Mechanisms of Tumor Progression and Novel Therapeutic Targets. Cancers 2022, 14, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Nishida, N.; Fujino, S.; Ogino, T.; Takahashi, H.; Miyoshi, N.; Uemura, M.; Satoh, T.; Yamamoto, H.; Mizushima, T.; et al. Comprehensive profiling of novel epithelial–mesenchymal transition mediators and their clinical significance in colorectal cancer. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, W.; Xu, M.; Song, J.; Mao, J.; Li, C.; Du, X.; Jiang, Y.; Li, E.; Zhang, R.; et al. Cancer-associated fibroblast-derived SDF-1 induces epithelial-mesenchymal transition of lung adenocarcinoma via CXCR4/β-catenin/PPARδ signalling. Cell Death Dis. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.P.; Martinez, V.D.; Minatel, B.C.; Pewarchuk, M.E.; Marshall, E.A.; MacAulay, G.M.; Hubaux, R.; Pearson, D.D.; Goodarzi, A.A.; Dellaire, G.; et al. Genomics and Epigenetics of Malignant Mesothelioma. High Throughput. 2018, 7, 20. [Google Scholar] [CrossRef]
- Bueno, R.; Stawiski, E.W.; Goldstein, L.D.; Durinck, S.; De Rienzo, A.; Modrusan, Z.; Gnad, F.; Nguyen, T.T.; Jaiswal, B.S.; Chirieac, L.R.; et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat. Genet. 2016, 48, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Yang, S.-R.; Egger, J.; Jayakumaran, G.; Spencer, R.S.; Lopardo, J.; Nash, G.M.; Cercek, A.; Travis, W.D.; Kris, M.G.; et al. Molecular Characterization of Peritoneal Mesotheliomas. J. Thorac. Oncol. 2022, 17, 455–460. [Google Scholar] [CrossRef]
- Klebe, S.; Salle, F.G.; Bruno, R.; Brcic, L.; Chen-Yost, H.I.; Jaurand, M.-C. The highlights of the 15th international conference of the international mesothelioma interest group–Do molecular concepts challenge the traditional approach to pathological mesothelioma diagnosis? Lung Cancer 2021, 163, 1–6. [Google Scholar] [CrossRef]
- McLoughlin, K.C.; Kaufman, A.S.; Schrump, D.S. Targeting the epigenome in malignant pleural mesothelioma. Transl. Lung Cancer Res. 2017, 6, 350–365. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, K. Epigenetics, environment and epidemiology: An interview with Karl Kelsey. Epigenomics 2022, 14, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Feng, M.; Bai, L.; Liao, W.; Zhou, K.; Zhang, M.; Wu, Q.; Wen, F.; Lei, W.; Zhang, P.; et al. Comprehensive analysis of EMT-related genes and lncRNAs in the prognosis, immunity, and drug treatment of colorectal cancer. J. Transl. Med. 2021, 19, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Holdgate, G.A.; Bardelle, C.; Lanne, A.; Read, J.; O′Donovan, D.H.; Smith, J.M.; Selmi, N.; Sheppard, R. Drug discovery for epigenetics targets. Drug Discov. Today 2022, 27, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Worthmüller-Rodriguez, J.; Wu, L.; Vrugt, B.; De Perrot, M.; Schwaller, B. Establishment of immortalized murine mesothelial cells and a novel mesothelioma cell line. In Vitro Cell Dev. Biol. Anim. 2015, 51, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.; Pecze, L.; Felley-Bosco, E.; Wu, L.; de Perrot, M.; Schwaller, B. Stem Cell Factor-Based Identification and Functional Properties of In Vitro-Selected Subpopulations of Malignant Mesothelioma Cells. Stem Cell Rep. 2017, 8, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Janes, S.M.; Alrifai, D.; Fennell, D.A. Perspectives on the Treatment of Malignant Pleural Mesothelioma. New Engl. J. Med. 2021, 385, 1207–1218. [Google Scholar] [CrossRef]
- Quetel, L.; Meiller, C.; Assié, J.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; de Wolf, J.; Caruso, S.; Copin, M.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223. [Google Scholar] [CrossRef]
- Hiltbrunner, S.; Fleischmann, Z.; Sokol, E.S.; Zoche, M.; Felley-Bosco, E.; Curioni-Fontecedro, A. Genomic landscape of pleural and peritoneal mesothelioma tumors. Lancet 2022, 127, 1997–2005. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, J.-L.; Sun, Q.; Harber, J.; Dawson, A.G.; Nakas, A.; Busacca, S.; Sharkey, A.J.; Waller, D.; Sheaff, M.T.; et al. Clonal architecture in mesothelioma is prognostic and shapes the tumour microenvironment. Nat. Commun. 2021, 12, 1751. [Google Scholar] [CrossRef] [PubMed]
- Al Emran, A.; Chatterjee, A.; Rodger, E.J.; Tiffen, J.C.; Gallagher, S.J.; Eccles, M.R.; Hersey, P. Targeting DNA Methylation and EZH2 Activity to Overcome Melanoma Resistance to Immunotherapy. Trends Immunol. 2019, 40, 328–344. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Yoshihara, K.; Yun, H.; Karim, S.; Shokri, N.; Zaeimi, F.; Man, H.S.J.; Zia, A.; Felley-Bosco, E.; de Perrot, M. Prognostic Value of EMT Gene Signature in Malignant Mesothelioma. Int. J. Mol. Sci. 2023, 24, 4264. https://doi.org/10.3390/ijms24054264
Wu L, Yoshihara K, Yun H, Karim S, Shokri N, Zaeimi F, Man HSJ, Zia A, Felley-Bosco E, de Perrot M. Prognostic Value of EMT Gene Signature in Malignant Mesothelioma. International Journal of Molecular Sciences. 2023; 24(5):4264. https://doi.org/10.3390/ijms24054264
Chicago/Turabian StyleWu, Licun, Kosuke Yoshihara, Hana Yun, Saraf Karim, Nastaran Shokri, Fatemeh Zaeimi, H. S. Jeffrey Man, Amin Zia, Emanuela Felley-Bosco, and Marc de Perrot. 2023. "Prognostic Value of EMT Gene Signature in Malignant Mesothelioma" International Journal of Molecular Sciences 24, no. 5: 4264. https://doi.org/10.3390/ijms24054264
APA StyleWu, L., Yoshihara, K., Yun, H., Karim, S., Shokri, N., Zaeimi, F., Man, H. S. J., Zia, A., Felley-Bosco, E., & de Perrot, M. (2023). Prognostic Value of EMT Gene Signature in Malignant Mesothelioma. International Journal of Molecular Sciences, 24(5), 4264. https://doi.org/10.3390/ijms24054264







