Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut–Liver Axis
Abstract
:1. Introduction
2. Results
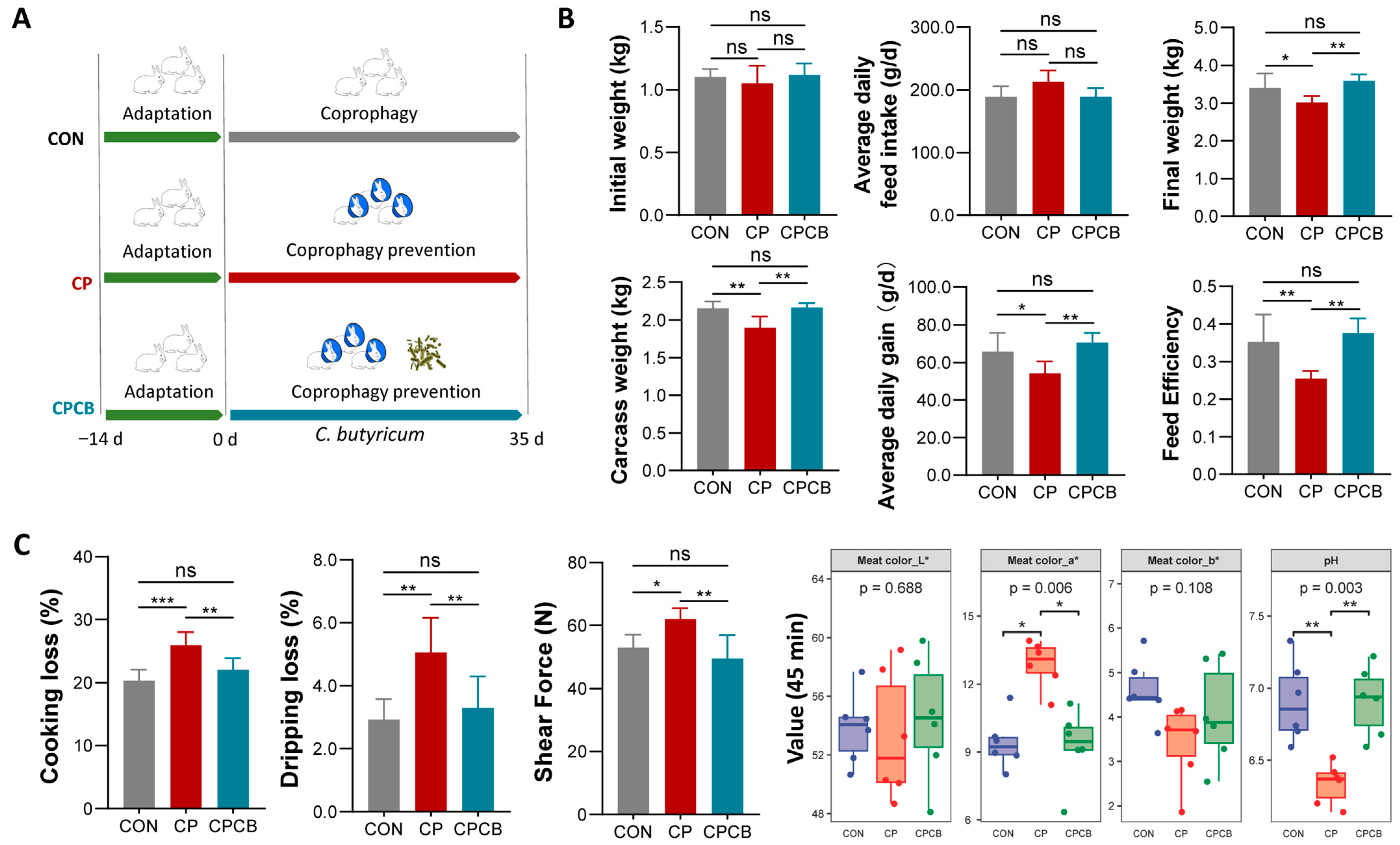
2.1. Growth Performance and Meat Quality
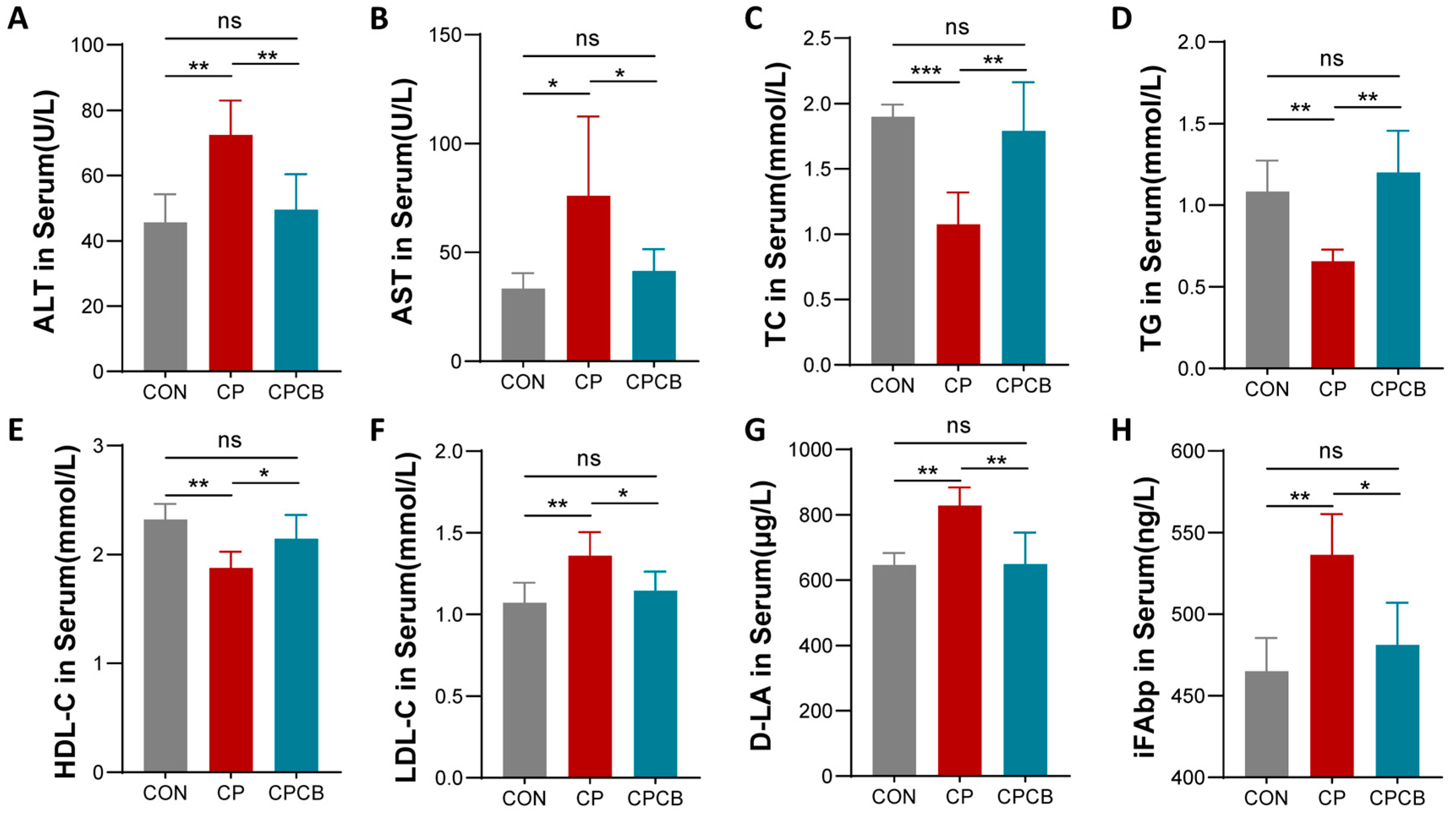
2.2. Serum Biochemistry Analysis
2.3. Metabolite Analysis in the Cecal Contents
2.4. Metabolite Analysis in Liver
2.5. Microbiome Analysis of the Cecal Contents
3. Discussion
4. Materials and Methods
4.1. Animal Ethics
4.2. Animals and Samples
4.3. Growth Performance Analysis
4.4. Histological Analysis
4.5. Serum Biochemical Analyses
4.6. Cytokine and Metabolite Assays
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Western Blot
4.9. Immunofluorescence Analysis
4.10. 16S rRNA Gene Sequencing
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Thacker, E.J.; Brandt, C.S. Coprophagy in the rabbit. J. Nutr. 1955, 55, 375–385. [Google Scholar] [CrossRef]
- Soave, O.; Brand, C.D. Coprophagy in animals: A review. Cornell Vet. 1991, 81, 357–364. [Google Scholar] [PubMed]
- Bo, T.B.; Zhang, X.Y.; Kohl, K.D.; Wen, J.; Tian, S.J.; Wang, D.H. Coprophagy prevention alters microbiome, metabolism, neurochemistry, and cognitive behavior in a small mammal. ISME J. 2020, 14, 2625–2645. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Sun, G.; Xue, M.; Sun, S.; Huang, T.; Zhou, J.; Loor, J.J.; Li, M. Transcriptome Analysis of the Effects of Fasting Caecotrophy on Hepatic Lipid Metabolism in New Zealand Rabbits. Animals 2019, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, H.; Chen, M.; Ni, M.; Yuan, D.; Cai, H.; Chen, Z.; Li, M.; Xu, H. Impact of coprophagy prevention on the growth performance, serum biochemistry, and intestinal microbiome of rabbits. BMC Microbiol. 2023, 23, 125. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Huang, W.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021, 13, 1104. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Schaap, F.G.; Trauner, M.; Jansen, P.L. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 55–67. [Google Scholar] [CrossRef]
- Ahmad, T.R.; Haeusler, R.A. Bile acids in glucose metabolism and insulin signalling—Mechanisms and research needs. Nat. Rev. Endocrinol. 2019, 15, 701–712. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef]
- Kohl, K.D.; Carey, H.V. A place for host-microbe symbiosis in the comparative physiologist’s toolbox. J. Exp. Biol. 2016, 219, 3496–3504. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Li, Y.P.; Zhu, Q.; Qiao, J.Y.; Wang, W.J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 2018, 125, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Cui, X.; Wang, Z.; Xiao, C.; Ji, Q.; Wei, Q.; Huang, Y.; Bao, G.; Liu, Y. Effects of Clostridium butyricum and a Bacteriophage Cocktail on Growth Performance, Serum Biochemistry, Digestive Enzyme Activities, Intestinal Morphology, Immune Responses, and the Intestinal Microbiota in Rabbits. Antibiotics 2021, 10, 1347. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Dong, H.; Wang, Y.; Zhang, J. Effect of the dietary probiotic Clostridium butyricum on growth, intestine antioxidant capacity and resistance to high temperature stress in kuruma shrimp Marsupenaeus japonicus. J. Therm. Biol. 2017, 66, 93–100. [Google Scholar] [CrossRef]
- Stellwag, E.J.; Hylemon, P.B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J. Lipid Res. 1979, 20, 325–333. [Google Scholar] [CrossRef]
- Kitahara, M.; Takamine, F.; Imamura, T.; Benno, Y. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha-dehydroxylating activity. Int. J. Syst. Evol. Microbiol. 2001, 51, 39–44. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and efficacy of the feed additive consisting of Clostridium butyricum FERM BP-2789 (Miya-Gold® S) for chickens for fattening, chickens reared for laying, turkeys for fattening, turkeys reared for breeding, minor avian species (excluding laying birds), piglets (suckling and weaned) and minor porcine species (Miyarisan Pharmaceutical Co. Ltd.). EFSA J. Eur. Food Saf. Auth. 2021, 19, e06450. [Google Scholar] [CrossRef]
- Molnár, A.; Such, N.; Farkas, V.; Pál, L.; Menyhárt, L.; Wágner, L.; Husvéth, F.; Dublecz, K. Effects of Wheat Bran and Clostridium butyricum Supplementation on Cecal Microbiota, Short-Chain Fatty Acid Concentration, pH and Histomorphometry in Broiler Chickens. Animals 2020, 10, 2230. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Lv, J.; Dou, X.; Zhang, Y. Effects of Dietary Supplementation with Clostridium butyricum on the Amelioration of Growth Performance, Rumen Fermentation, and Rumen Microbiota of Holstein Heifers. Front. Nutr. 2021, 8, 763700. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transplant. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Sinal, C.J.; Tohkin, M.; Miyata, M.; Ward, J.M.; Lambert, G.; Gonzalez, F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000, 102, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Edwards, P.A. FXR signaling in metabolic disease. FEBS Lett. 2008, 582, 10–18. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 1999, 3, 543–553. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.A.; Laubitz, D.; Ohland, C.L.; Midura-Kiela, M.T.; Patil, K.; Besselsen, D.G.; Jamwal, D.R.; Jobin, C.; Ghishan, F.K.; Kiela, P.R. Microbial dysbiosis associated with impaired intestinal Na+/H+ exchange accelerates and exacerbates colitis in ex-germ free mice. Mucosal Immunol. 2018, 11, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, H.; Ni, M.; Wang, Z.; Guo, C.; Niu, Y.; Xing, S.; Song, M.; Wang, Y.; Jiang, Y.; et al. Microbiome-Metabolome Analysis of the Immune Microenvironment of the Cecal Contents, Soft Feces, and Hard Feces of Hyplus Rabbits. Oxidative Med. Cell. Longev. 2022, 2022, 5725442. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Z.; Ni, M.; Xing, S.; Yu, L.; Xu, H.; Zhuo, D.; Li, M. Screening and stability analysis of reference genes in fasting caecotrophy model in rabbits. Mol. Biol. Rep. 2022, 49, 1057–1065. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]




| Gene ID | Name | Primer Sequence (5′-3′) | bp | Amplification Efficiency (%) | R2 (%) |
|---|---|---|---|---|---|
| XM_017344772.1 | OCLN | F: TCCGACTTCGTGGAGAGAGT R: TACTGCTGCTGCTCAAACGA | 181 | 102.5 | 99.7 |
| XM_017348359.1 | ZO-1 | F: TCCATAGAGACCGGCGTCA R: GGTTTTAGGATCACAGTGTGGC | 222 | 103.2 | 99.1 |
| NM_001089316.1 | CLDN1 | F: AGATGCGGATGGCTGTCAT R: AAGTAGGGCACCTCCCAGAA | 203 | 107.5 | 98.6 |
| XM_051858582.1 | MUC1 | F: TTCGGCACTGATTTCACAGA R: CAGAGGAGGGAGACAGAACATC | 227 | 105.4 | 99.3 |
| NM_001082253 | GAPDH | F: CGATGCCCCCATGTTTGTGA R: TCATGAGCCCCTCCACAATG | 149 | 106.8 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Chen, M.; Zhang, R.; Wang, Z.; He, H.; Wan, Z.; Li, H.; Cai, H.; Chen, Z.; Li, M.; et al. Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut–Liver Axis. Int. J. Mol. Sci. 2023, 24, 17554. https://doi.org/10.3390/ijms242417554
Li Z, Chen M, Zhang R, Wang Z, He H, Wan Z, Li H, Cai H, Chen Z, Li M, et al. Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut–Liver Axis. International Journal of Molecular Sciences. 2023; 24(24):17554. https://doi.org/10.3390/ijms242417554
Chicago/Turabian StyleLi, Zhichao, Mengjuan Chen, Ran Zhang, Zhitong Wang, Hui He, Zhiyi Wan, Hengjian Li, Hanfang Cai, Zhi Chen, Ming Li, and et al. 2023. "Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut–Liver Axis" International Journal of Molecular Sciences 24, no. 24: 17554. https://doi.org/10.3390/ijms242417554
APA StyleLi, Z., Chen, M., Zhang, R., Wang, Z., He, H., Wan, Z., Li, H., Cai, H., Chen, Z., Li, M., & Xu, H. (2023). Clostridium butyricum Ameliorates the Effect of Coprophagy Prevention on Hepatic Lipid Synthesis in Rabbits via the Gut–Liver Axis. International Journal of Molecular Sciences, 24(24), 17554. https://doi.org/10.3390/ijms242417554







