Serum Metabolomic Profiling of Patients with Lipedema
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Cohort
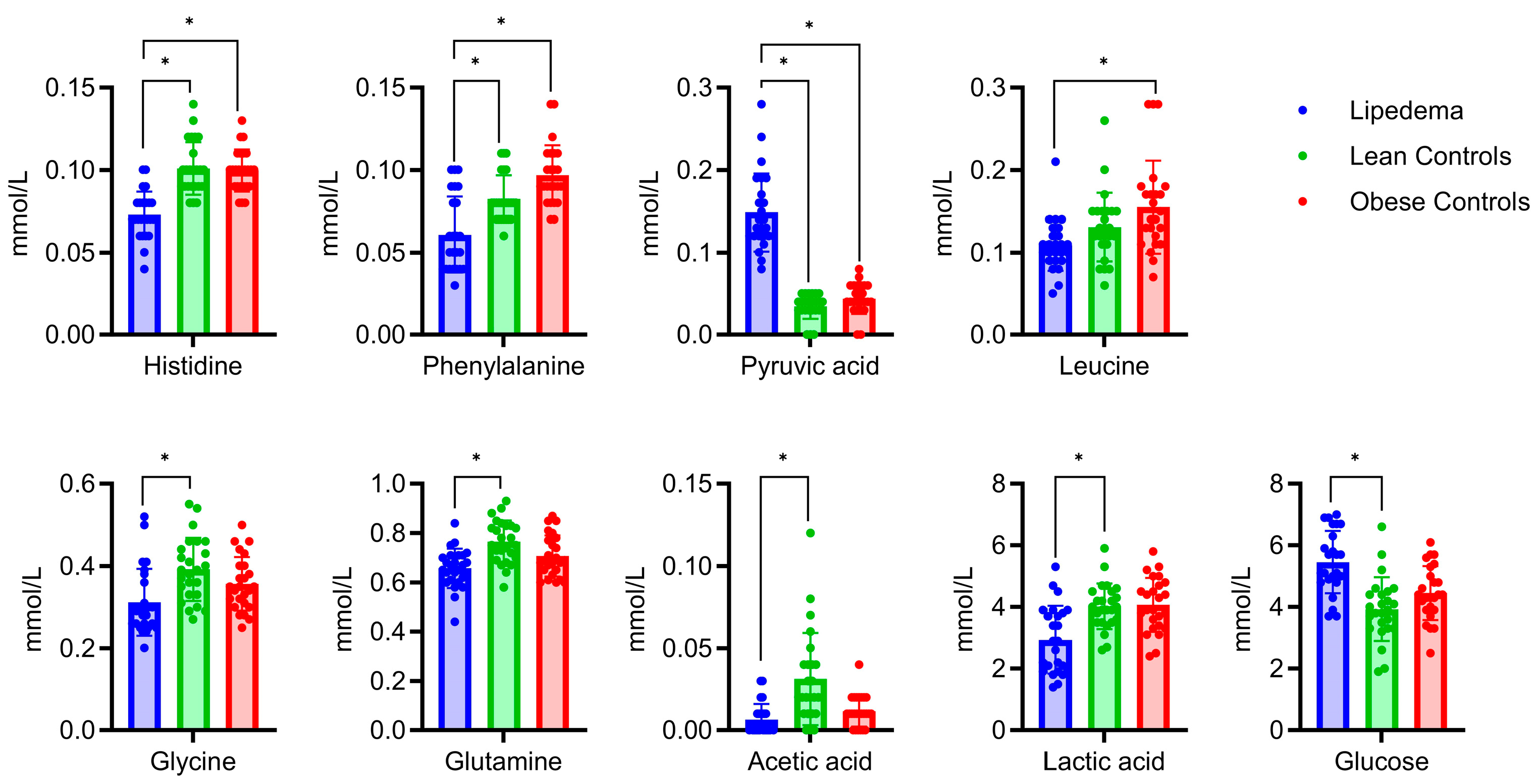
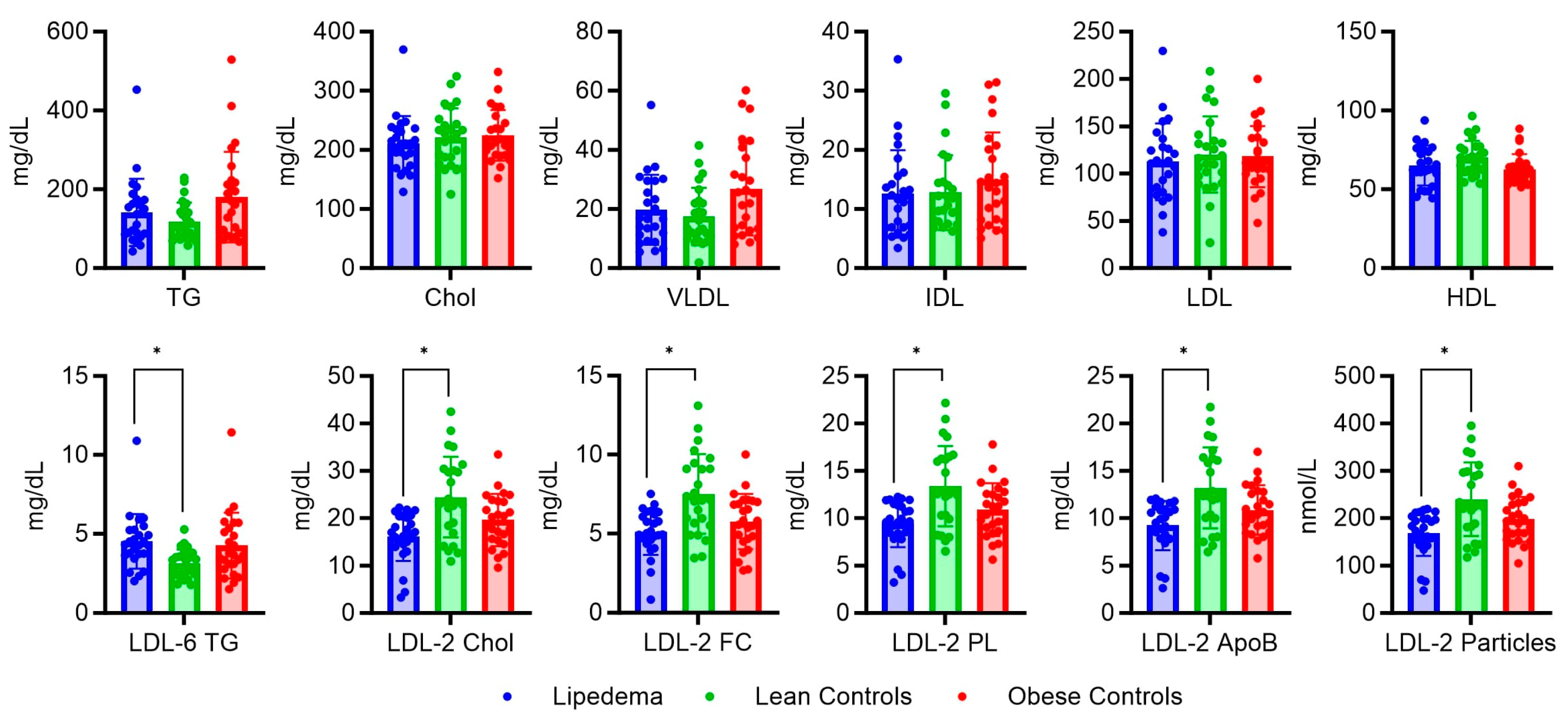
2.2. Serum Metabolic and Lipidomic Profiles
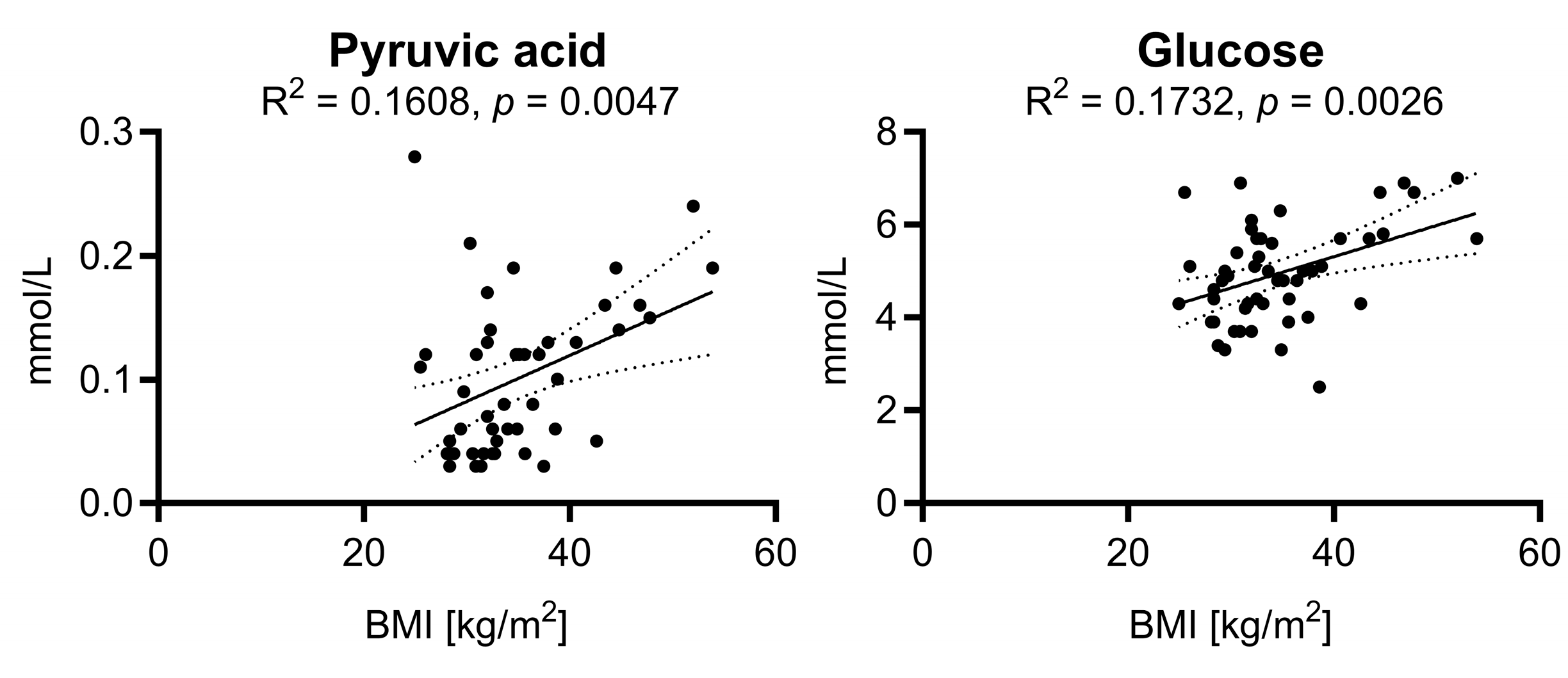
2.3. Analysis of Metabolite Variations in Relation to Body Mass Index: Subgroup Comparison and Correlation Analysis
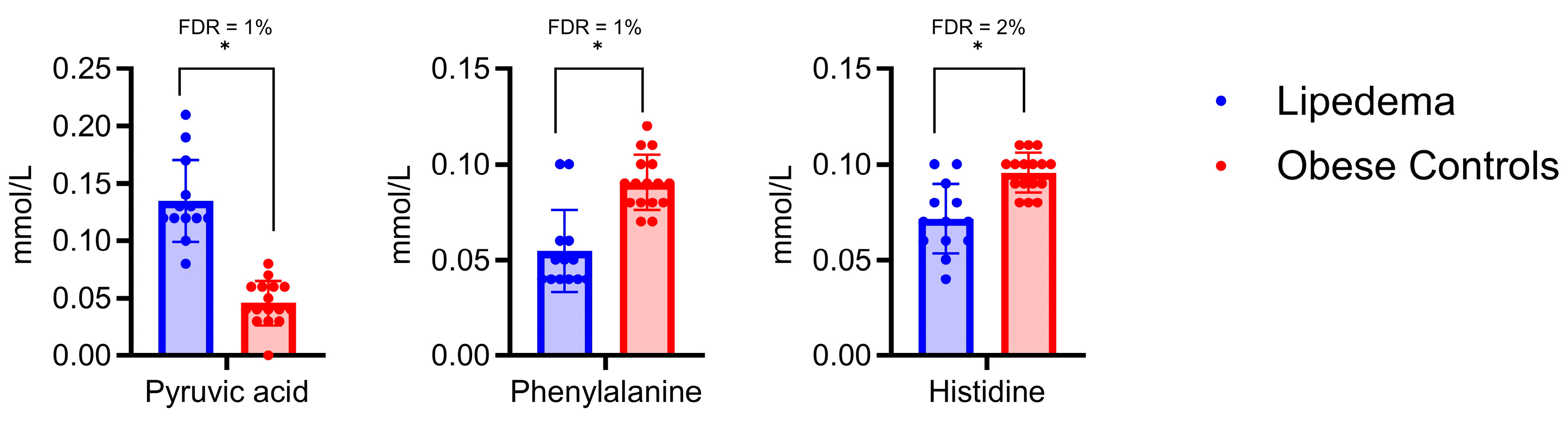
2.4. Diagnostic Accuracy of Pyruvic Acid, Histidine, and Phenylalanine in Predicting Lipedema
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethical Approval
4.2. Selection of Lipedema Patients
4.3. Selection of the Control Groups
4.4. Sample Collection, Preparation, and 1H NMR Spectroscopy
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; Donahue, P.M.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Ishaq, M.; Bandara, N.; Morgan, S.; Nowell, C.; Mehdi, A.M.; Lyu, R.; McCarthy, D.; Anderson, D.; Creek, D.J.; Achen, M.G.; et al. Key signaling networks are dysregulated in patients with the adipose tissue disorder, lipedema. Int. J. Obes. 2022, 46, 502–514. [Google Scholar] [CrossRef]
- Child, A.H.; Gordon, K.D.; Sharpe, P.; Brice, G.; Ostergaard, P.; Jeffery, S.; Mortimer, P.S. Lipedema: An inherited condition. Am. J. Med. Genet. A 2010, 152a, 970–976. [Google Scholar] [CrossRef]
- Katzer, K.; Hill, J.L.; McIver, K.B.; Foster, M.T. Lipedema and the Potential Role of Estrogen in Excessive Adipose Tissue Accumulation. Int. J. Mol. Sci. 2021, 22, 11720. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hypertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef]
- Michelini, S.; Herbst, K.L.; Precone, V.; Manara, E.; Marceddu, G.; Dautaj, A.; Maltese, P.E.; Paolacci, S.; Ceccarini, M.R.; Beccari, T.; et al. A Multi-Gene Panel to Identify Lipedema-Predisposing Genetic Variants by a Next-Generation Sequencing Strategy. J. Pers. Med. 2022, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Falck, J.; Rolander, B.; Nygårdh, A.; Jonasson, L.L.; Mårtensson, J. Women with lipoedema: A national survey on their health, health-related quality of life, and sense of coherence. BMC Women’s Health 2022, 22, 457. [Google Scholar] [CrossRef]
- Danzi, F.; Pacchiana, R.; Mafficini, A.; Scupoli, M.T.; Scarpa, A.; Donadelli, M.; Fiore, A. To metabolomics and beyond: A technological portfolio to investigate cancer metabolism. Signal Transduct. Target. Ther. 2023, 8, 137. [Google Scholar] [CrossRef]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef]
- Nankam, P.A.N.; Cornely, M.; Klöting, N.; Blüher, M. Is subcutaneous adipose tissue expansion in people living with lipedema healthier and reflected by circulating parameters? Front. Endocrinol. 2022, 13, 1000094. [Google Scholar] [CrossRef]
- Wolf, S.; Deuel, J.W.; Hollmén, M.; Felmerer, G.; Kim, B.S.; Vasella, M.; Grünherz, L.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. A Distinct Cytokine Profile and Stromal Vascular Fraction Metabolic Status without Significant Changes in the Lipid Composition Characterizes Lipedema. Int. J. Mol. Sci. 2021, 22, 3313. [Google Scholar] [CrossRef] [PubMed]
- Compan, V.; Pierredon, S.; Vanderperre, B.; Krznar, P.; Marchiq, I.; Zamboni, N.; Pouyssegur, J.; Martinou, J.C. Monitoring Mitochondrial Pyruvate Carrier Activity in Real Time Using a BRET-Based Biosensor: Investigation of the Warburg Effect. Mol. Cell 2015, 59, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Nye, C.K.; Hanson, R.W.; Kalhan, S.C. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J. Biol. Chem. 2008, 283, 27565–27574. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Boschek, C.B.; Hugo, F.; Eigenbrodt, E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005, 15, 300–308. [Google Scholar] [CrossRef]
- Si, Y.; Shi, H.; Lee, K. Impact of perturbed pyruvate metabolism on adipocyte triglyceride accumulation. Metab. Eng. 2009, 11, 382–390. [Google Scholar] [CrossRef][Green Version]
- Nikolau, B.J.; Oliver, D.J.; Schnable, P.S.; Wurtele, E.S. Molecular biology of acetyl-CoA metabolism. Biochem. Soc. Trans. 2000, 28, 591–593. [Google Scholar] [CrossRef]
- Yamashita, H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S171–S175. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Lustgarten, M.S.; Price, L.L.; Phillips, E.M.; Fielding, R.A. Serum glycine is associated with regional body fat and insulin resistance in functionally-limited older adults. PLoS ONE 2013, 8, e84034. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Yang, Y.; Dai, Z.; Wu, Z.; Wu, G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids 2018, 50, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Haüssinger, D. Nitrogen metabolism in liver: Structural and functional organization and physiological relevance. Biochem. J. 1990, 267, 281–290. [Google Scholar] [CrossRef]
- Aldarini, N.; Alhasawi, A.A.; Thomas, S.C.; Appanna, V.D. The role of glutamine synthetase in energy production and glutamine metabolism during oxidative stress. Antonie Van Leeuwenhoek 2017, 110, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Sidoryk-Węgrzynowicz, M.; Zielińska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e311. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e674. [Google Scholar] [CrossRef]
- Lagarde, D.; Jeanson, Y.; Portais, J.C.; Galinier, A.; Ader, I.; Casteilla, L.; Carrière, A. Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective. Front. Physiol. 2021, 12, 689747. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.M.; Steiner, M.; Kainz, V.; Tempfer, H.; Spitzer, G.; Plank, T.; Bauer, H.C.; Bresgen, N.; Habenbacher, A.; Bauer, H.; et al. Lipedema: The Use of Cultured Adipocytes for Identification of Diagnostic Markers. Plast. Reconstr. Surg. 2023, 152, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Michelini, S.; Ricolfi, L.; Caroleo, M.C.; Gallelli, L.; De Sarro, G.; Onorato, A.; Cione, E. Management of Lipedema with Ketogenic Diet: 22-Month Follow-Up. Life 2021, 11, 1402. [Google Scholar] [CrossRef]
- Keith, L.; Seo, C.; Rowsemitt, C.; Pfeffer, M.; Wahi, M.; Staggs, M.; Dudek, J.; Gower, B.; Carmody, M. Ketogenic diet as a potential intervention for lipedema. Med. Hypotheses 2021, 146, 110435. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Zomparelli, S.; De Santis, G.L.; Seraceno, S.; Zuena, C.; Frank, G.; Cianci, R.; Centofanti, D.; De Lorenzo, A. Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study. Nutrients 2023, 15, 3654. [Google Scholar] [CrossRef]
- Cannataro, R.; Cione, E. Nutritional Supplements and Lipedema: Scientific and Rational Use. Nutraceuticals 2022, 2, 270–277. [Google Scholar] [CrossRef]
- Bonetti, G.; Herbst, K.L.; Dhuli, K.; Kiani, A.K.; Michelini, S.; Michelini, S.; Ceccarini, M.R.; Michelini, S.; Ricci, M.; Cestari, M.; et al. Dietary supplements for lipedema. J. Prev. Med. Hyg. 2022, 63, E169. [Google Scholar]
- Yin, P.; Lehmann, R.; Xu, G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem. 2015, 407, 4879–4892. [Google Scholar] [CrossRef]
- Morris, C.; O’Grada, C.M.; Ryan, M.F.; Roche, H.M.; Gibney, M.J.; Gibney, E.R.; Brennan, L. The relationship between BMI and metabolomic profiles: A focus on amino acids. Proc. Nutr. Soc. 2012, 71, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Borsche, M.; Balck, A.; Föh, B.; Rahmöller, J.; Peters, E.; Knickmann, J.; Lane, M.; Vollstedt, E.J.; Elsner, S.A.; et al. One-year surveillance of SARS-CoV-2 transmission of the ELISA cohort: A model for population-based monitoring of infection risk. Sci. Adv. 2022, 8, eabm5016. [Google Scholar] [CrossRef] [PubMed]
- A Healthy Lifestyle. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 11 August 2023).
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Schmelter, F.; Föh, B.; Mallagaray, A.; Rahmöller, J.; Ehlers, M.; Lehrian, S.; von Kopylow, V.; Künsting, I.; Lixenfeld, A.S.; Martin, E.; et al. Metabolic and Lipidomic Markers Differentiate COVID-19 From Non-Hospitalized and Other Intensive Care Patients. Front. Mol. Biosci. 2021, 8, 737039. [Google Scholar] [CrossRef]


| Clinical Characteristics | Lipedema (Lip.) n = 25 | Lean Controls (Lean) n = 25 | Obese Controls (Obese) n = 25 | p-Value |
|---|---|---|---|---|
| Male | 0 | 0 | 0 | - |
| Female | 25 | 25 | 25 | - |
| Age, Years | 48.4 (11.1) | 48.4 (11.0) | 45.0 (12.1) | lip. vs. lean > 0.9999 lip vs. obese 0.3121 |
| Body Mass Index (BMI), kg/m2 | 37.0 (8.0) | 22.7 (1.4) | 32.4 (3.7) | lip vs. lean < 0.0001 lip vs. obese 0.0116 |
| Normal Weight: BMI of 18.5 to 24.9 kg/m2 | 1 | 25 | 0 | - |
| Overweight: BMI of 25 to 29.9 kg/m2 | 3 | 0 | 8 | 0.4606 |
| Moderate Obesity (Class I): BMI of 30 to 34.9 kg/m2 | 8 | 0 | 12 | >0.9999 |
| Severe Obesity (Class II): BMI of 35 to 39.9 kg/m2 | 5 | 0 | 4 | 0.9048 |
| Very Severe Obesity (Class III): BMI of 40 kg/m2 or Greater | 8 | 0 | 1 | - |
| Lipedema Stage (St): | ||||
| St. 1 | 3 | N.A. | N.A. | - |
| St. 2 | 17 | N.A. | N.A. | |
| St. 3 | 5 | N.A. | N.A. | |
| Onset of Lipedema: | ||||
| Puberty | 12 | N.A. | N.A. | - |
| Pregnancy | 3 | N.A. | N.A. | |
| Menopause | 2 | N.A. | N.A. | |
| Other | 8 | N.A. | N.A. | |
| Family History of Lipedema | 20 | N.A. | N.A. | - |
| Concomitant Disease: | ||||
| Arterial Hypertension | 6 | 0 | 0 | - |
| Diabetes, Type 2 | 1 | 0 | 0 | |
| Hypothyroidism | 7 | 0 | 0 |
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempa, S.; Buechler, C.; Föh, B.; Felthaus, O.; Prantl, L.; Günther, U.L.; Müller, M.; Derer-Petersen, S.; Sina, C.; Schmelter, F.; et al. Serum Metabolomic Profiling of Patients with Lipedema. Int. J. Mol. Sci. 2023, 24, 17437. https://doi.org/10.3390/ijms242417437
Kempa S, Buechler C, Föh B, Felthaus O, Prantl L, Günther UL, Müller M, Derer-Petersen S, Sina C, Schmelter F, et al. Serum Metabolomic Profiling of Patients with Lipedema. International Journal of Molecular Sciences. 2023; 24(24):17437. https://doi.org/10.3390/ijms242417437
Chicago/Turabian StyleKempa, Sally, Christa Buechler, Bandik Föh, Oliver Felthaus, Lukas Prantl, Ulrich L. Günther, Martina Müller, Stefanie Derer-Petersen, Christian Sina, Franziska Schmelter, and et al. 2023. "Serum Metabolomic Profiling of Patients with Lipedema" International Journal of Molecular Sciences 24, no. 24: 17437. https://doi.org/10.3390/ijms242417437
APA StyleKempa, S., Buechler, C., Föh, B., Felthaus, O., Prantl, L., Günther, U. L., Müller, M., Derer-Petersen, S., Sina, C., Schmelter, F., & Tews, H. C. (2023). Serum Metabolomic Profiling of Patients with Lipedema. International Journal of Molecular Sciences, 24(24), 17437. https://doi.org/10.3390/ijms242417437










