Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury
Abstract
:1. Introduction
2. Results
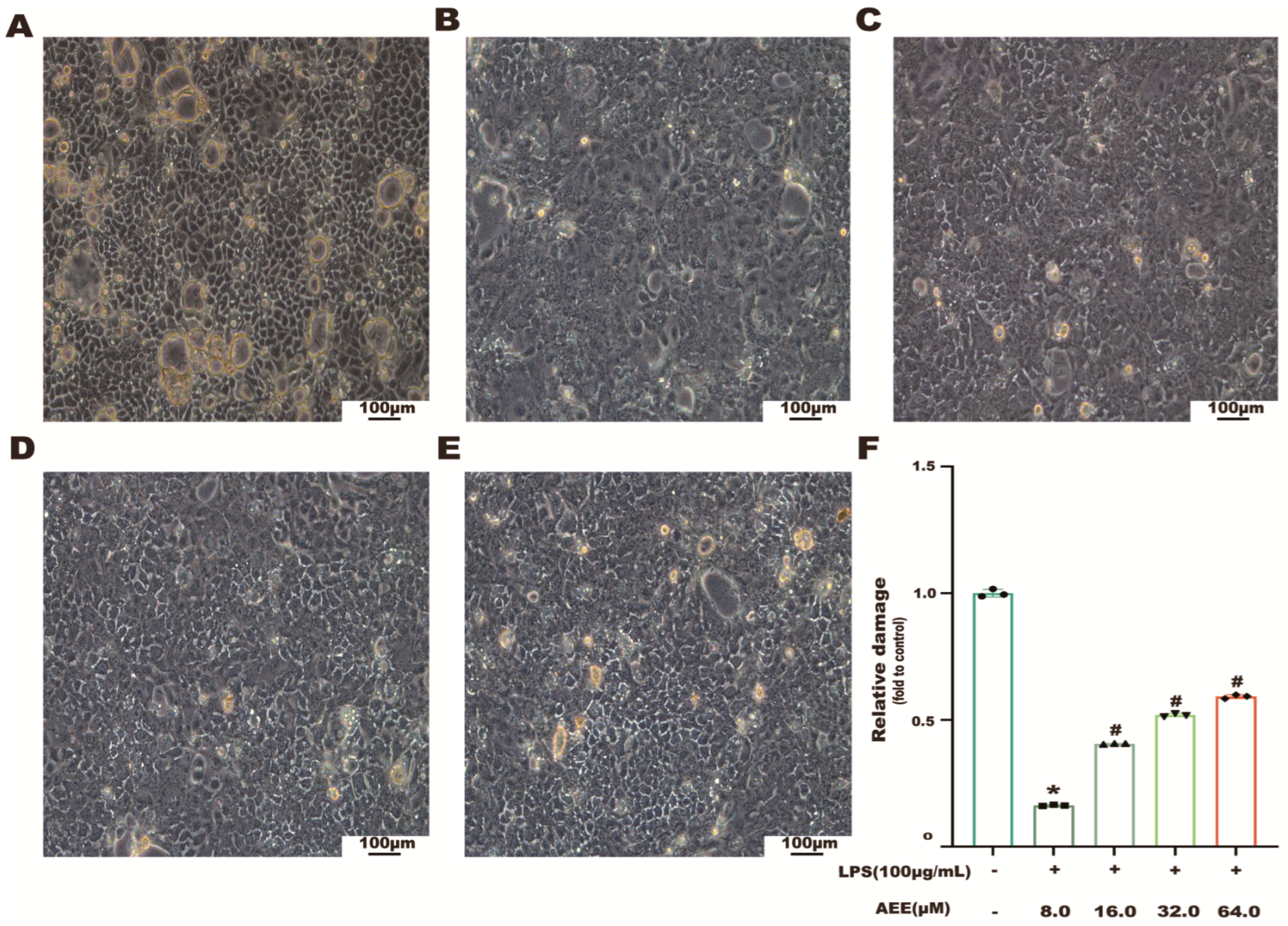
2.1. AEE Alleviated LPS-Induced Cytotoxicity
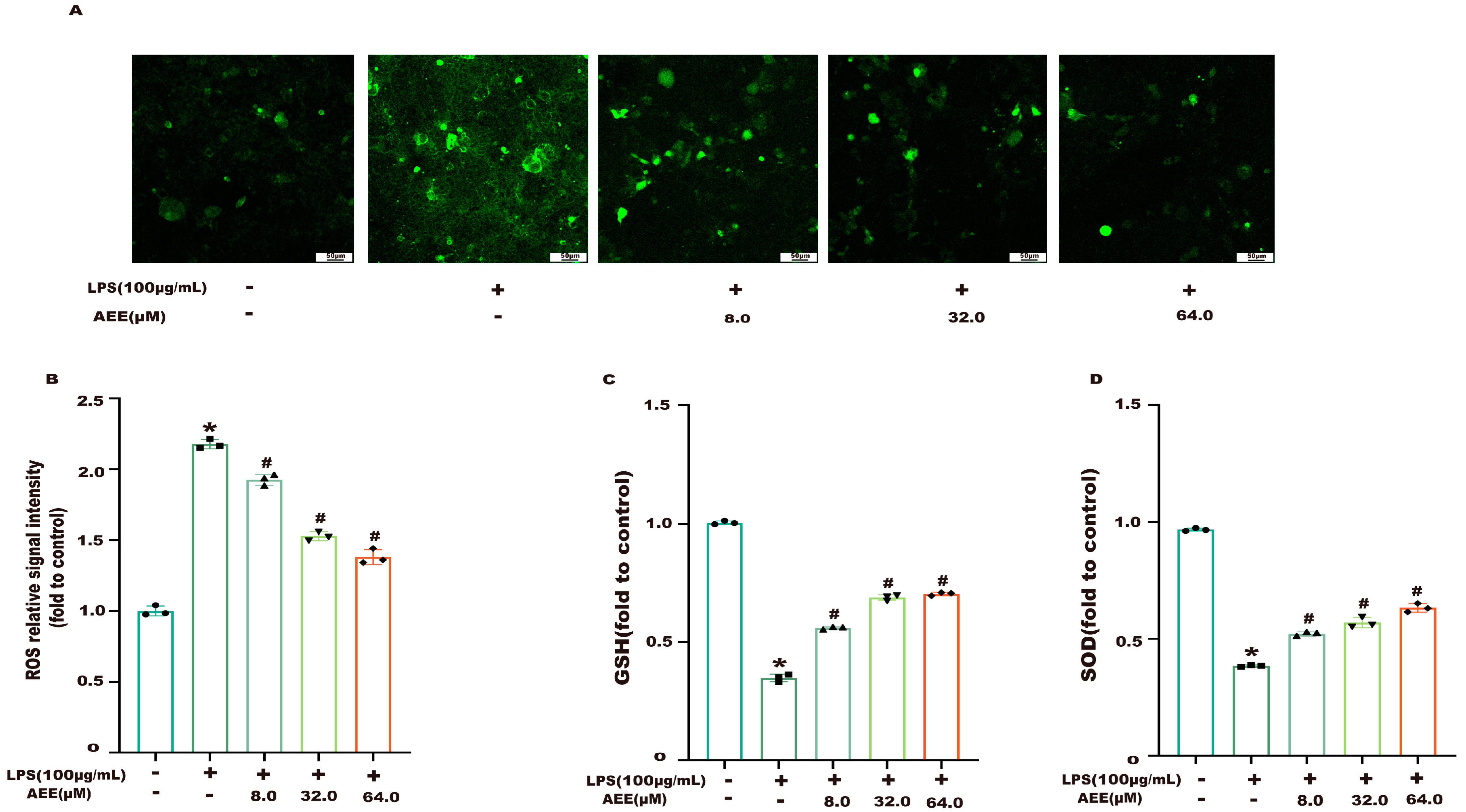
2.2. AEE Alleviated LPS-Induced Oxidative Stress
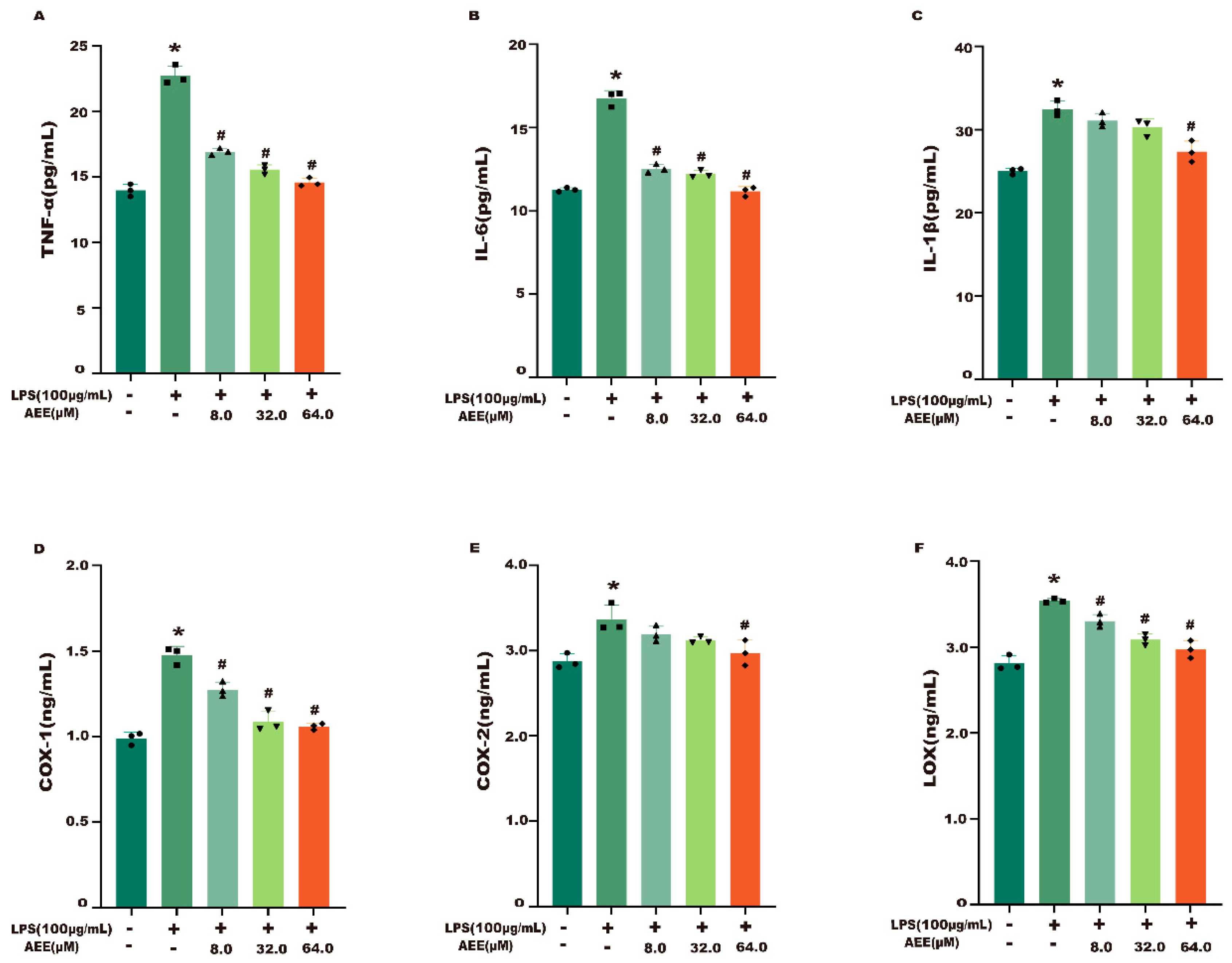
2.3. AEE Alleviated LPS-Induced Inflammatory Responses
2.4. Effects of AEE on the Morphology of LPS-Induced Caco-2 Cells
2.5. AEE Alleviated LPS-Induced Caco-2 Cell Monolayer Damage
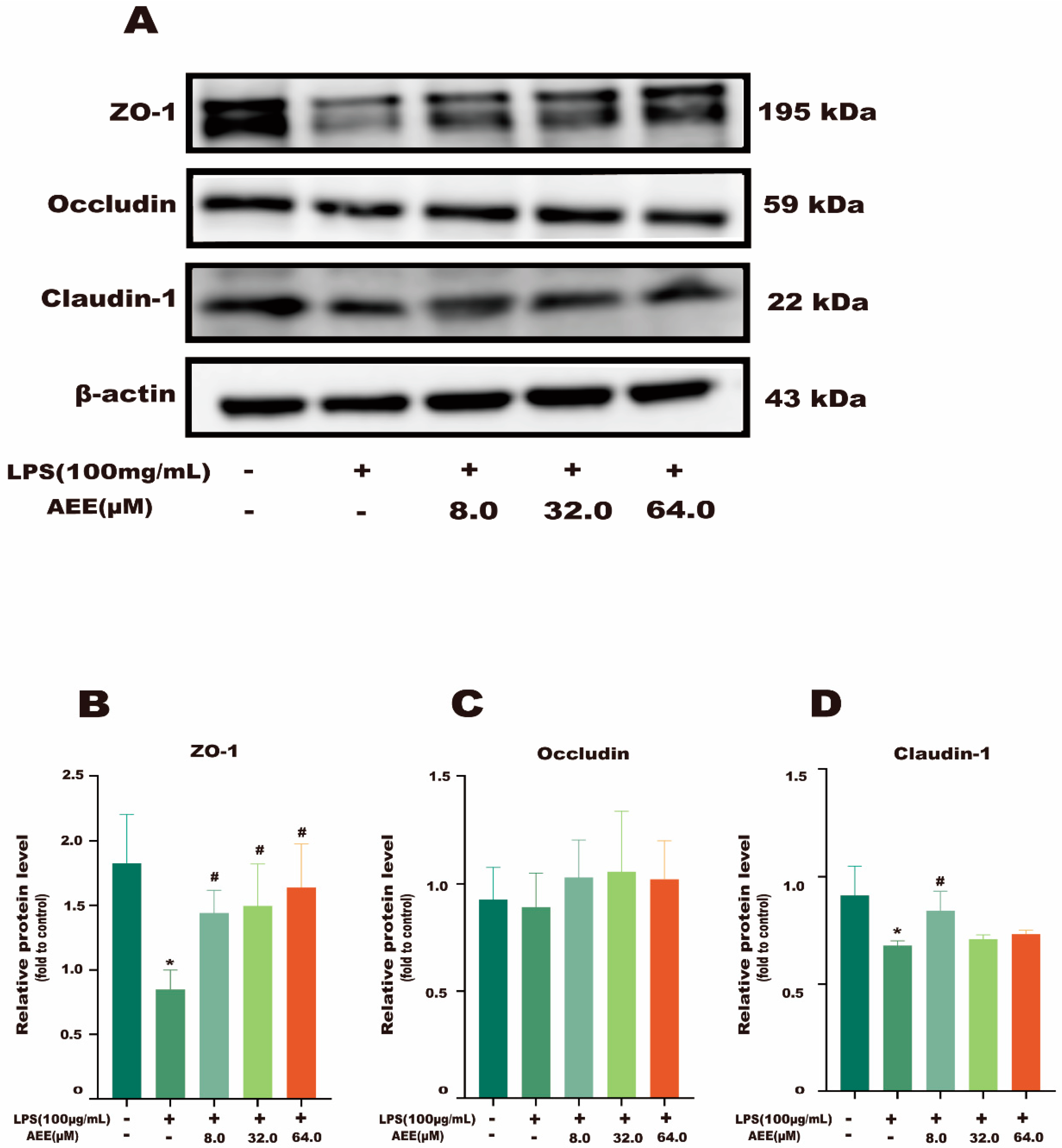
2.6. AEE Inhibited LPS-Induced Downregulation of TJ Proteins
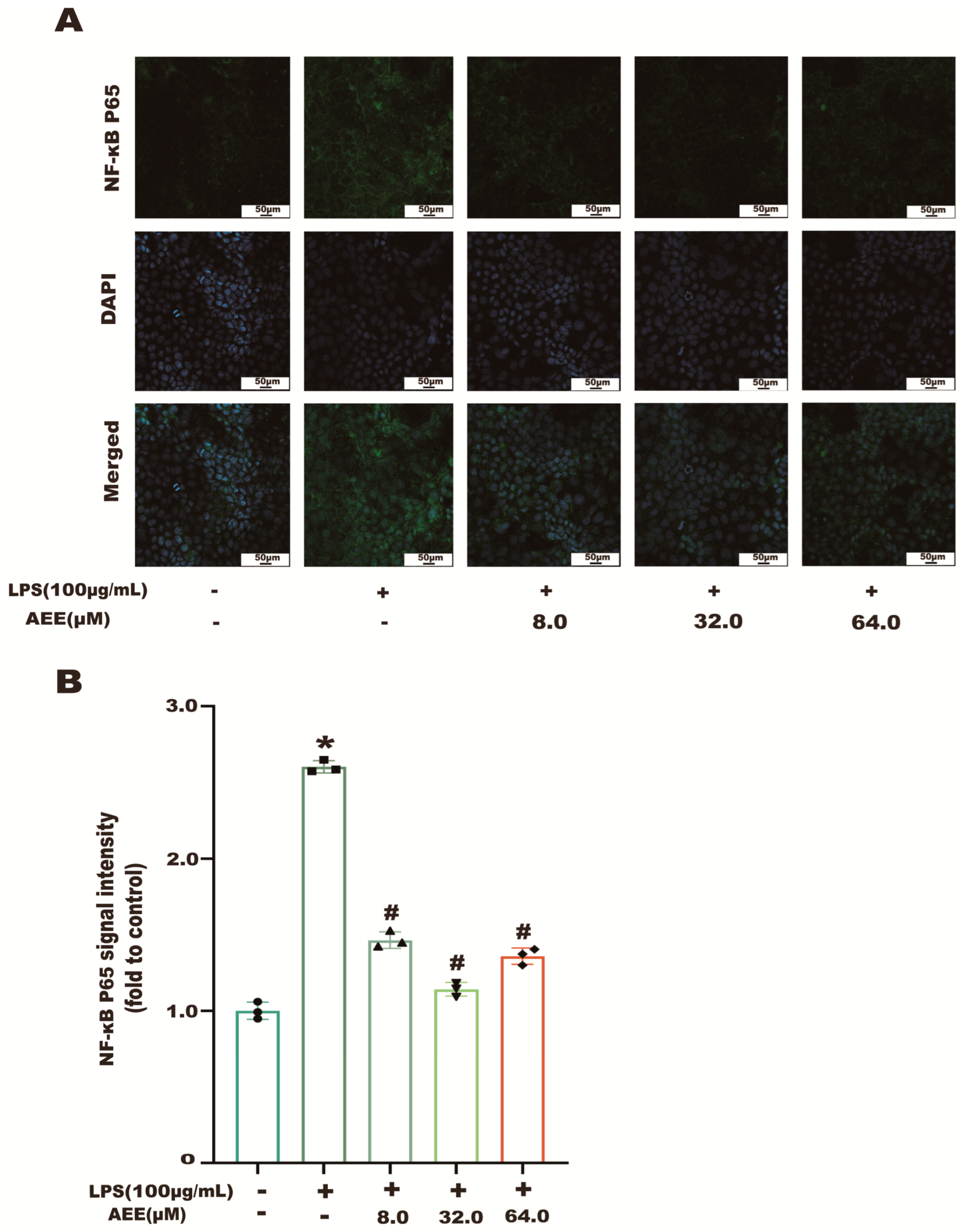
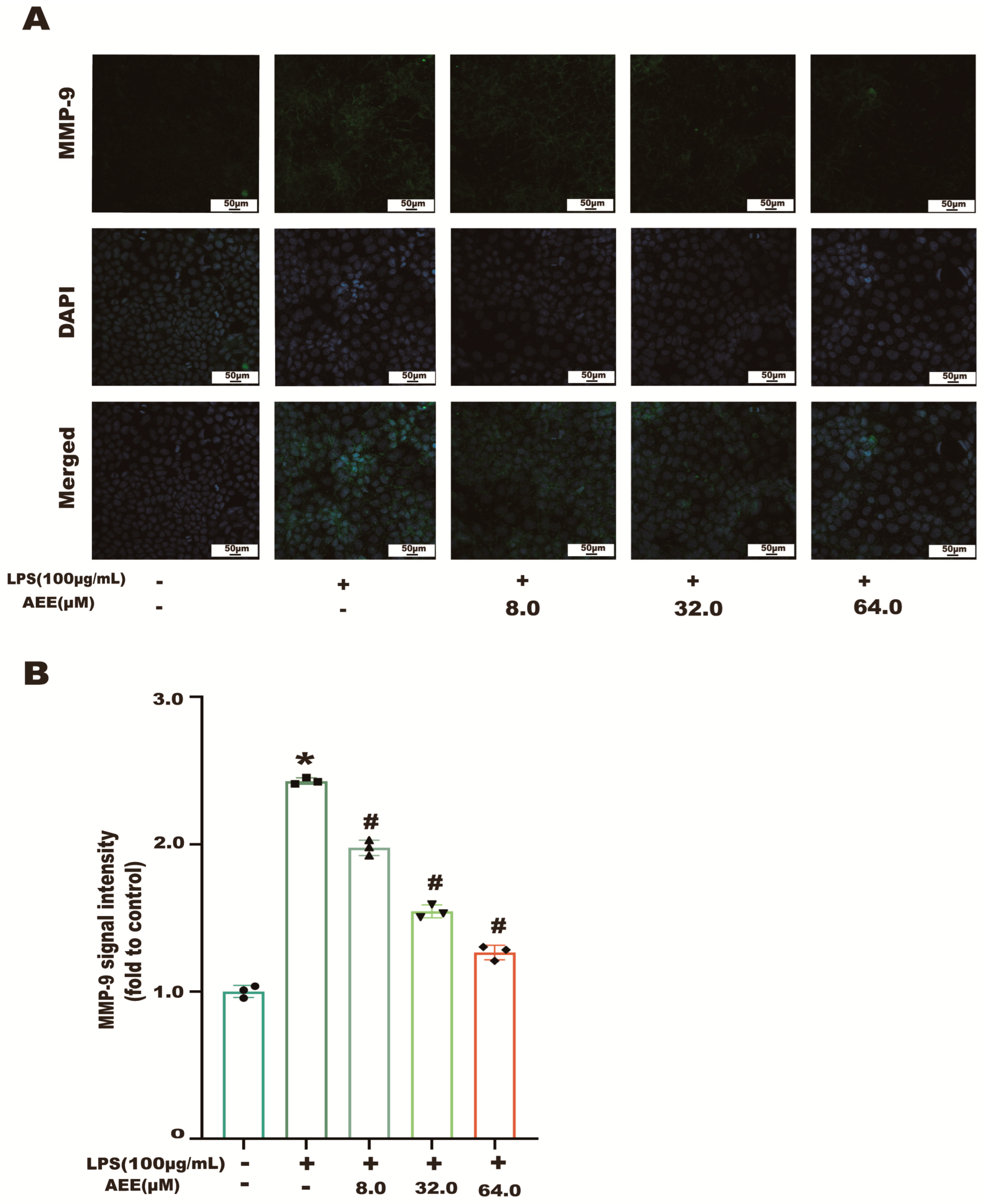
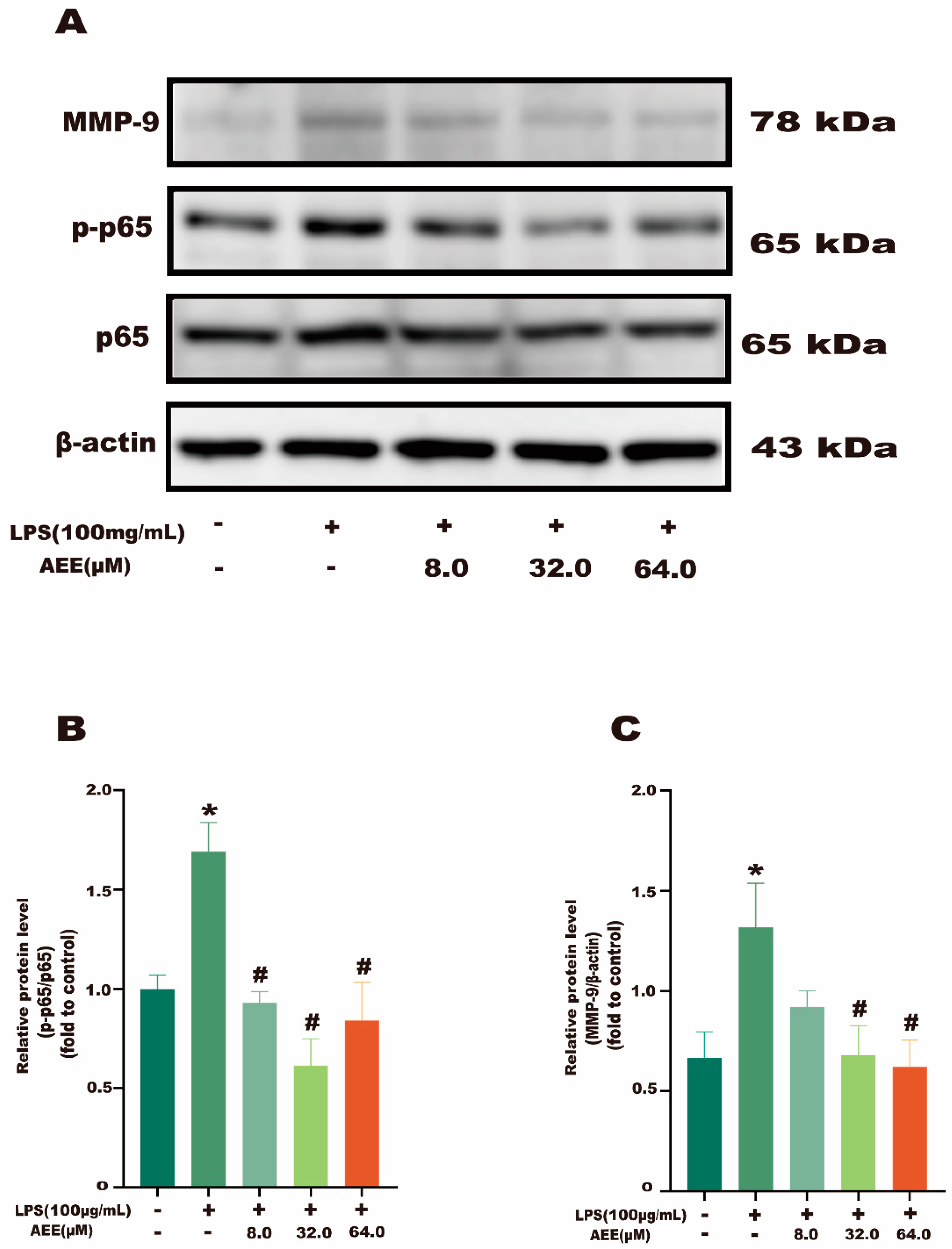
2.7. AEE Inhibited LPS-Induced NF-κB and MMP-9 Activation
2.8. Metabolomic Analysis of LPS-Induced Caco-2 Cells
2.8.1. Metabolite Analysis of Cell Lysates
2.8.2. Metabolic Pathway Analysis
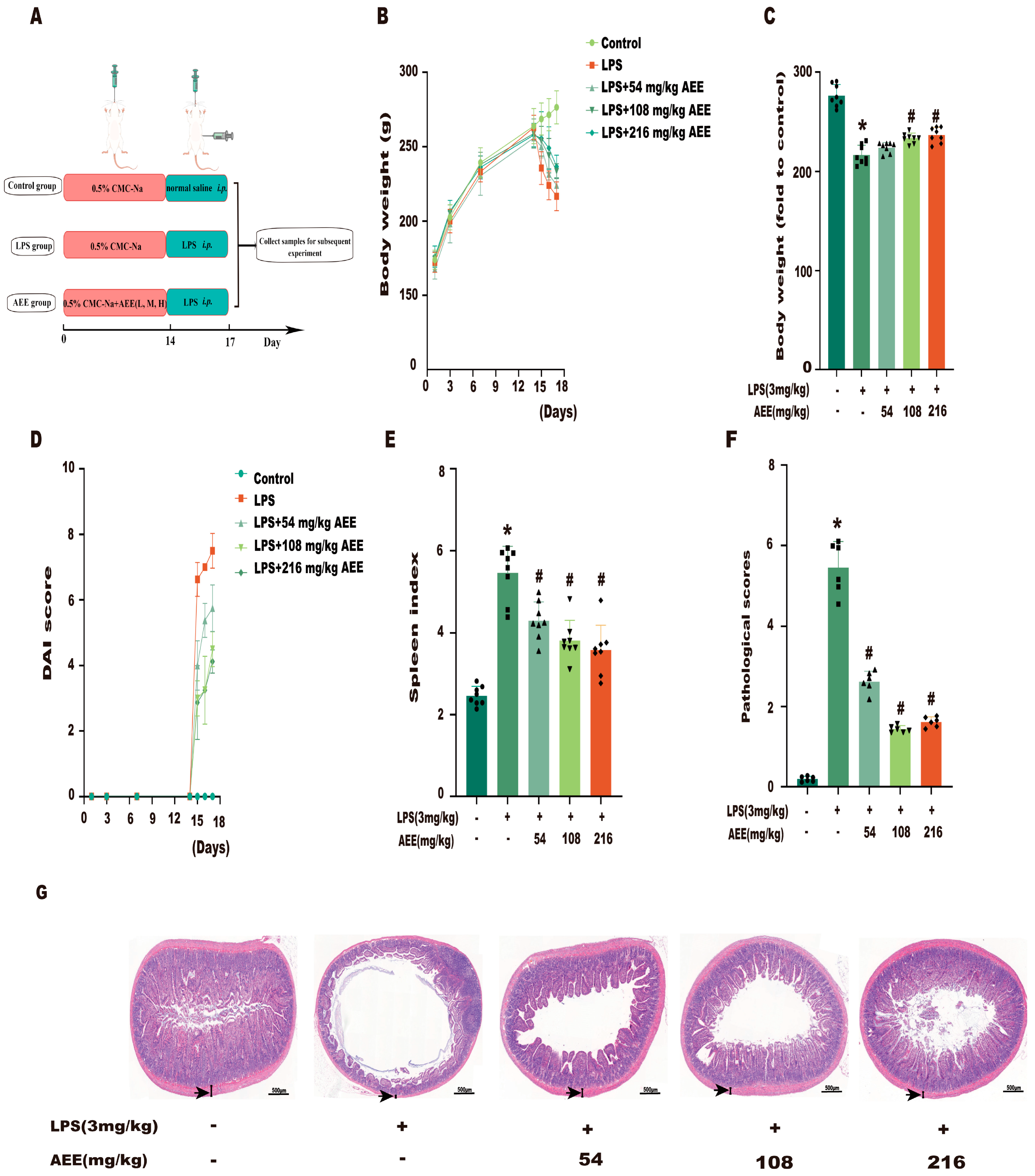
2.9. AEE Pretreatment Alleviated LPS-Induced Colitis in Rats
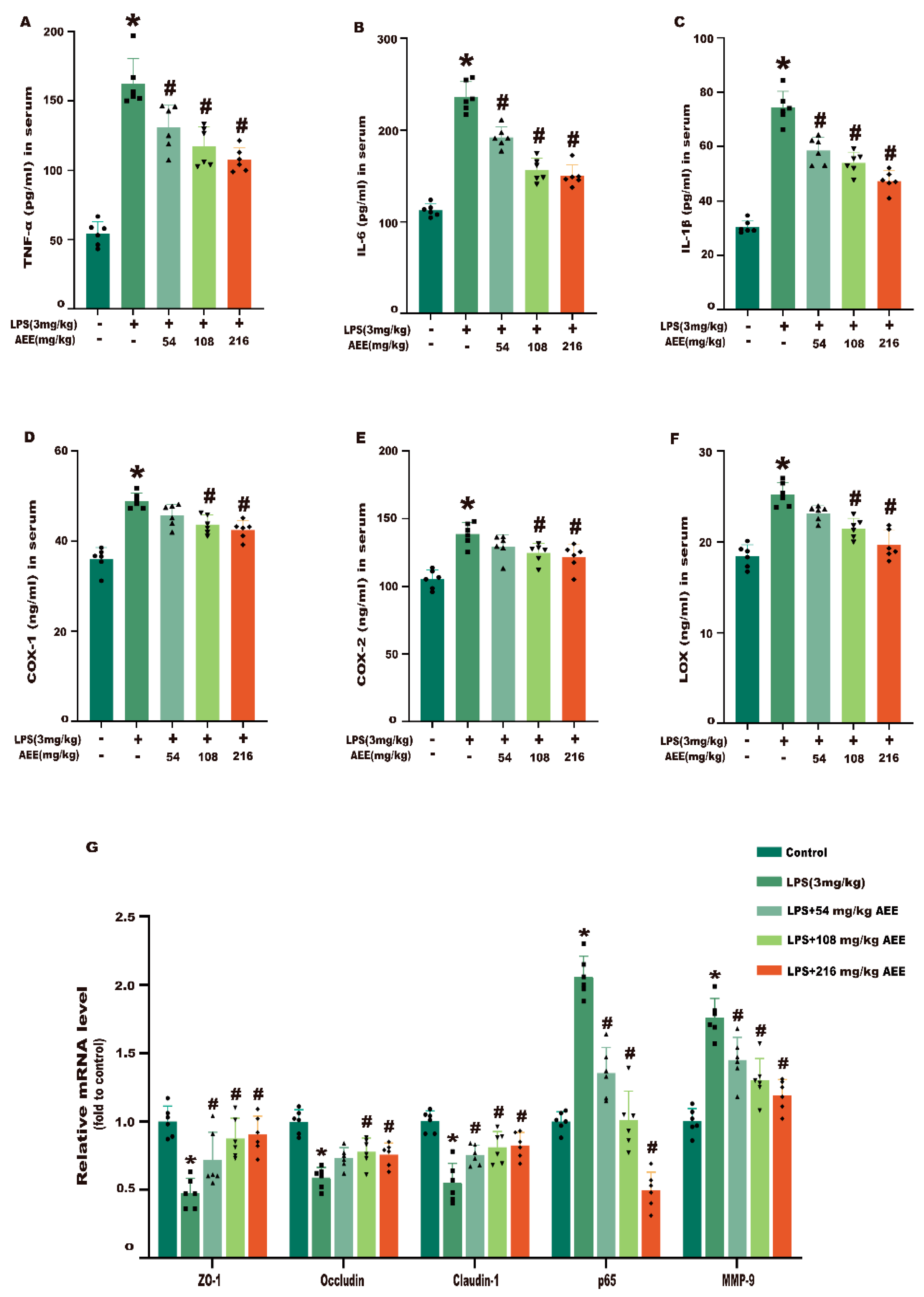
2.10. AEE Improved Inflammation and Intestinal Barrier Damage in LPS-Induced Rats
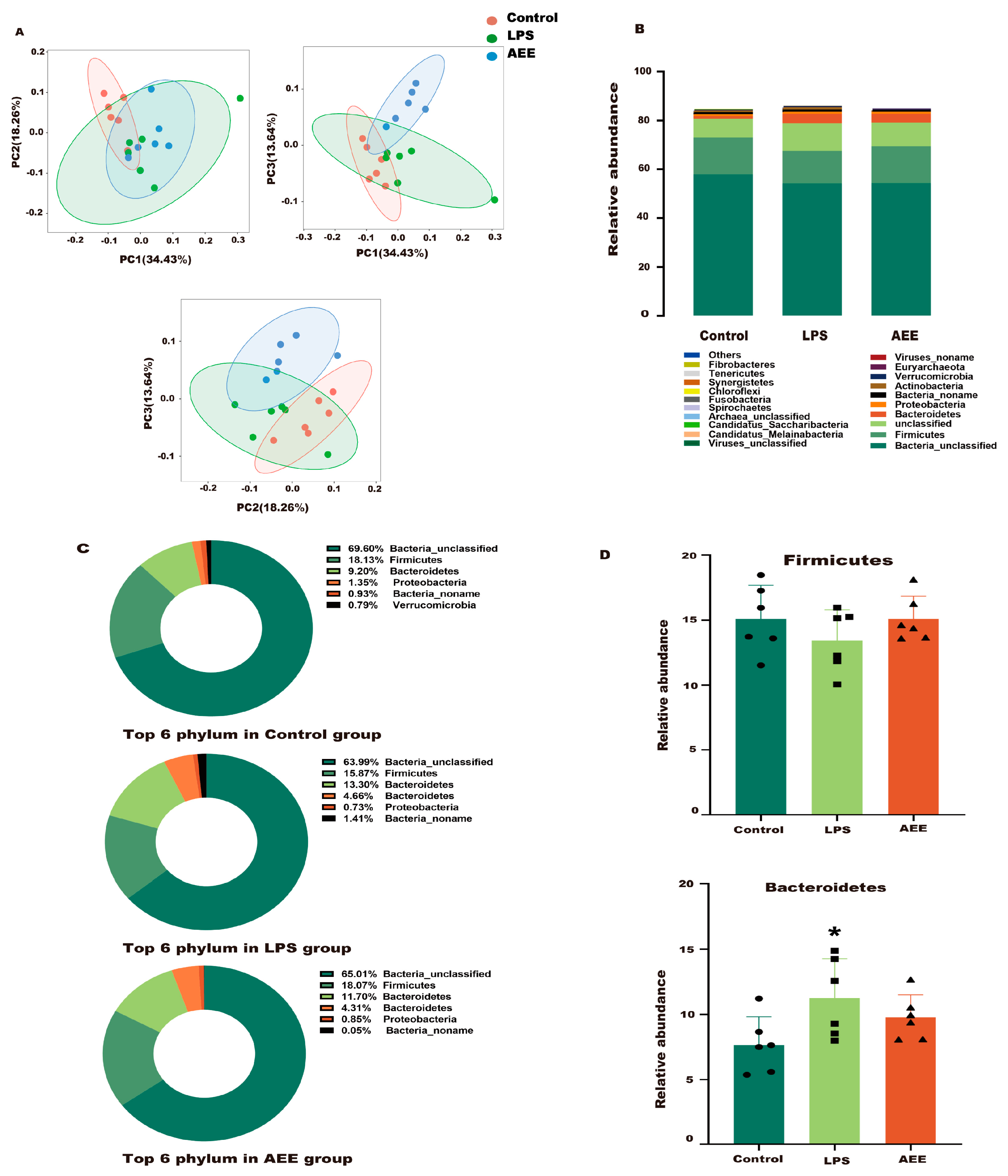
2.11. AEE Improved the Gut Microbiota Structure in LPS-Treated Rats
2.11.1. Phylum Level
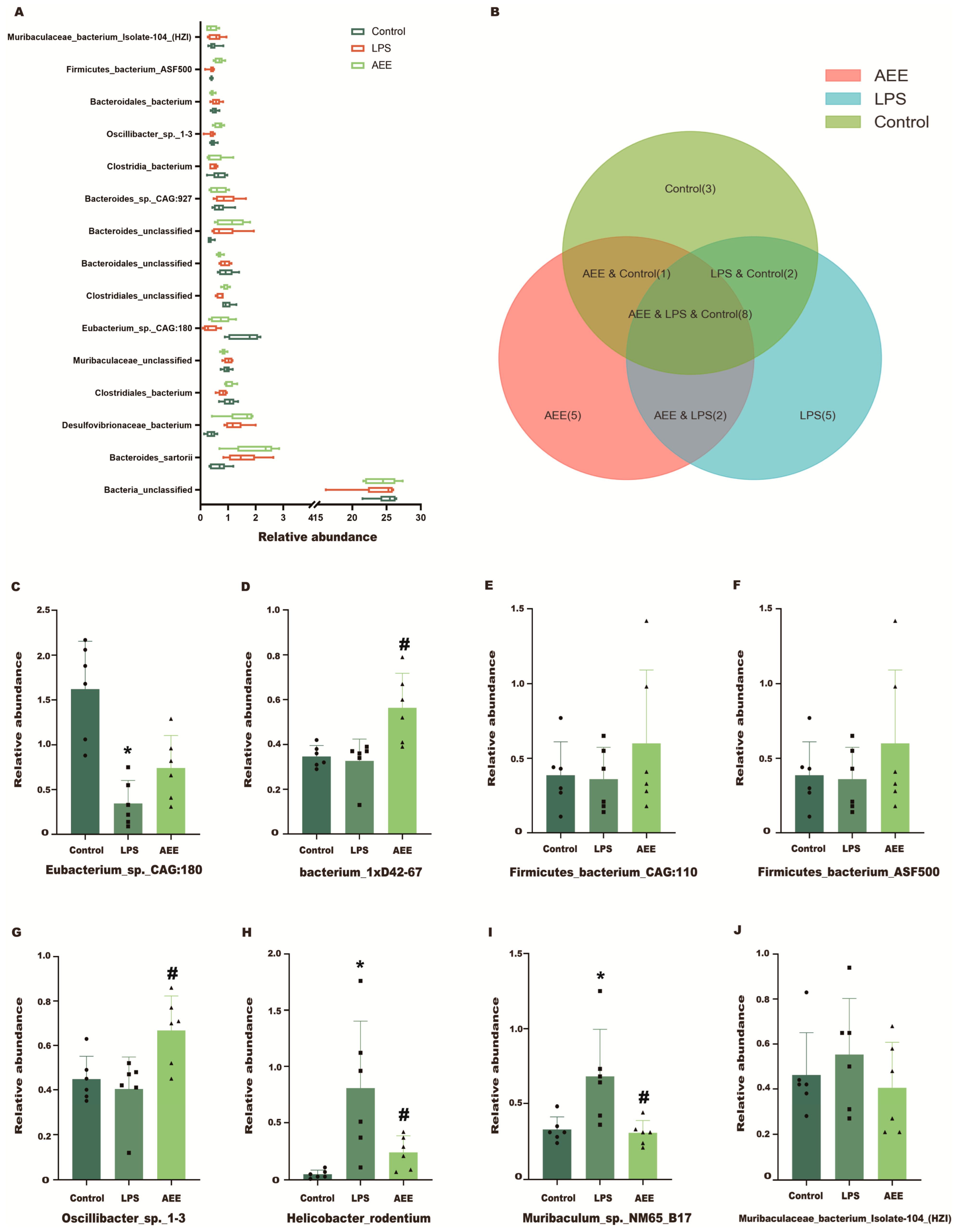
2.11.2. Genus Level
2.11.3. Species Level
2.11.4. Differential Species Analysis
2.11.5. Functional Level Analysis of Cecum Microbes by Metagenome
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Cell Viability
4.4. AEE Treatment and LPS Stimulation
4.5. Measurement of Trans-Epithelial Monolayer Resistance
4.6. Measurement of Paracellular Permeability
4.7. Measurement of LDH, ROS, SOD, and GSH in Cell Supernatant
4.8. Protein Extraction and Western Blotting
4.9. Immunofluorescence
4.10. Metabolomics Analysis
4.10.1. Cell Collection and Processing
4.10.2. UPLC-QTOF-MS/MS Conditions
4.10.3. Metabolomics Data Analysis
4.11. Drug Preparation in the Animal Study
4.12. Animal Experiment
4.13. Measurement of Disease Activity Index (DAI) Scores and Immune Organ Index
4.14. Measurement of Inflammatory Factors in Serum and Cell Supernatant
4.15. Histopathological Analysis
4.16. RNA Extraction and RT-qPCR
4.17. Metagenome Sequencing for the Contents of the Cecum
4.17.1. DNA Extractions
4.17.2. DNA Library Construction
4.17.3. Data Analysis
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEE | Aspirin eugenol ester |
| LPS | Lipopolysaccharide |
| AP side | Side of the intestine |
| BL side | Lower chamber is the basal side |
| Papp | Apparent permeability coefficient |
| LY | Lucifer yellow |
| TEER | Transepithelial electrical resistance |
| TJ | Tight junction |
| DAI | Disease Activity Index |
| ELISA | Enzyme-linked immunosorbent assay |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| LDH | Lactate dehydrogenase |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| NF-κB | Nuclear factor-kappa B |
| MMP-9 | Matrix metalloproteinase-9 |
| ROS | Reactive oxygen species |
| COX | Cyclooxygenase |
| LOX | Lipoxygenase |
| LTs | Leukotrienes |
| AA | Arachidonic acid |
| HE | Hematoxylin–Eosin |
| CCK-8 | Cell counting kit-8 |
| BCA | Bicinchoninic acid |
| CMC-Na | Carboxymethylcellulose |
References
- Zmora, N.; Levy, M.; Pevsner-Fishcer, M.; Elinav, E. Inflammasomes and intestinal inflammation. Mucosal Immunol. 2017, 10, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Ash, C. Protection and resolvin gut inflammation. Science 2017, 356, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, Y.; Shi, Y.; Liu, T.; Cao, Y.; Tang, Y.; Ge, X.; Nie, H.; Zheng, C.; Li, Y.C. 1,25-Dihydroxyvitamin D Protects Intestinal Epithelial Barrier by Regulating the Myosin Light Chain Kinase Signaling Pathway. Inflamm. Bowel Dis. 2015, 21, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.-J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef]
- Liu, H.; Patel, N.R.; Walter, L.; Ingersoll, S.; Sitaraman, S.V.; Garg, P. Constitutive expression of MMP9 in intestinal epithelium worsens murine acute colitis and is associated with increased levels of proinflammatory cytokine Kc. Am. J. Physiol. Liver Physiol. 2013, 304, G793–G803. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Huang, X.L.; Chen, R.R.; Li, T.; Ye, H.J.; Xie, W.; Huang, Z.M.; Cao, G.Z. Paeoniflorin Prevents Intestinal Barrier Dis-ruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and Inflammation in the Intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Kagan, J.C. Lipopolysaccharide Detection across the Kingdoms of Life. Trends Immunol. 2017, 38, 696–704. [Google Scholar] [CrossRef]
- Han, F.; Lu, Z.; Liu, Y.; Xia, X.; Zhang, H.; Wang, X.; Wang, Y. Cathelicidin-BF ameliorates lipopolysaccharide-induced intestinal epithelial barrier disruption in rat. Life Sci. 2016, 152, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-D.; Huang, M.-Z.; Yang, Y.-J.; Liu, X.-W.; Qin, Z.; Li, S.-H.; Li, J.-Y. Aspirin Eugenol Ester Attenuates Paraquat-Induced Hepatotoxicity by Inhibiting Oxidative Stress. Front. Physiol. 2020, 11, 582801. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Z.; Zhang, Z.-D.; Yang, Y.-J.; Liu, X.-W.; Qin, Z.; Li, J.-Y. Aspirin Eugenol Ester Protects Vascular Endothelium from Oxidative Injury by the Apoptosis Signal Regulating Kinase-1 Pathway. Front. Pharmacol. 2020, 11, 588755. [Google Scholar] [CrossRef]
- Huang, M.Z.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin Eugenol Ester Reduces H(2)O(2)-Induced Oxidative Stress of HU-VECs via Mitochondria-Lysosome Axis. Oxid. Med. Cell Longev. 2019, 2019, 8098135. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Z.; Lu, X.R.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Cellular Metabolomics Reveal the Mechanism Underlying the Anti-Atherosclerotic Effects of Aspirin Eugenol Ester on Vascular Endothelial Dysfunction. Int. J. Mol. Sci. 2019, 20, 3165. [Google Scholar] [CrossRef]
- Huang, M.; Yang, Y.; Liu, X.; Qin, Z.; Li, J. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Br. J. Pharmacol. 2019, 176, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, J.; Sun, G.; Yang, H.; Sun, X.; Yao, X.; Lin, A.; Liu, H. 5-Aminosalicylic acid ameliorates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota and bile acid metabolism. Cell. Mol. Life Sci. CMLS 2022, 79, 460. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nature clinical practice. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar]
- de Barboza, G.D.; Guizzardi, S.; Moine, L.; Tolosa de Talamoni, N. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, Z.D.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Ge, W.B.; Li, J.Y.; Yang, Y.J. Aspirin eugenol ester alleviates lipopolysaccharide-induced acute lung injury in rats while stabilizing serum metabolites levels. Front. Immunol. 2022, 13, 939106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Yang, Y.J.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Li, J.Y. Protective Activity of Aspirin Eugenol Ester on Paraquat-Induced Cell Damage in SH-SY5Y Cells. Oxid. Med. Cell Longev. 2021, 2021, 6697872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Isolation and identification of peptides from simulated gastrointestinal digestion of preserved egg white and their anti-inflammatory activity in TNF-α-induced Caco-2 cells. J. Nutr. Biochem. 2019, 63, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bodega, G.; Alique, M.; Puebla, L.; Carracedo, J.; Ramírez, R.M. Microvesicles: ROS scavengers and ROS producers. J. Extracell. Vesicles 2019, 8, 1626654. [Google Scholar] [CrossRef]
- Coskun, M.; Vermeire, S.; Nielsen, O.H. Novel Targeted Therapies for Inflammatory Bowel Disease. Trends Pharmacol. Sci. 2017, 38, 127–142. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Vu, T.-Y.; Chandi, V.; Polimati, H.; Tatipamula, V.B. Dual COX and 5-LOX inhibition by clerodane diterpenes from seeds of Polyalthia longifolia (Sonn.) Thwaites. Sci. Rep. 2020, 10, 15965. [Google Scholar] [CrossRef]
- Tanaka, K.; Suemasu, S.; Ishihara, T.; Tasaka, Y.; Arai, Y.; Mizushima, T. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID)-dependent exacerbation of colitis. Eur. J. Pharmacol. 2009, 603, 120–132. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Márton, M.-R.; Herz, C.; Maul, R.; Baldermann, S.; Schreiner, M.; Lamy, E. Nasturtium (Indian cress, Tropaeolum majus nanum) dually blocks the COX and LOX pathway in primary human immune cells. Phytomedicine 2016, 23, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; Sorgi, C.A.; Hori, J.I.; Paula-Silva, F.W.G.; Arantes, E.C.; Serezani, C.H.; Zamboni, D.S.; Faccioli, L.H. Opposing roles of LTB4 and PGE2 in regulating the inflammasome-dependent scorpion venom-induced mortality. Nat. Commun. 2016, 7, 10760. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Xu, Y.; Xu, J.; Ling, Y.; Cai, Y. Chitosan nanoparticles reduce LPS-induced inflammatory reaction via inhibition of NF-κB pathway in Caco-2 cells. Int. J. Biol. Macromol. 2016, 86, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Che, S.; Ruan, Z.; Song, L.; Tang, R.; Zhang, L. Regulatory effects of flavonoids luteolin on BDE-209-induced intestinal epithelial barrier damage in Caco-2 cell monolayer model. Food Chem. Toxicol. 2021, 150, 112098. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Chen, Y.; Du, J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Eri, R.; Simms, L.A.; Florin, T.H.; Radford-Smith, G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm. Bowel Dis. 2009, 15, 100–113. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, Y.; Ye, L.; Wang, Y.; Zhang, K.; Wang, L.; Huang, Y.; Wang, L.; Xian, S.; Zhang, Y.; et al. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm. Sin. B 2019, 9, 711–723. [Google Scholar] [CrossRef]
- Hui, Q.; Ammeter, E.; Liu, S.; Yang, R.; Lu, P.; Lahaye, L.; Yang, C. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide-induced inflammation in the porcine intestinal epithelial cells. J. Anim. Sci. 2020, 98, skaa245. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, X.; Liang, X.; Hong, D.; Guan, Z.; Guan, Y.; Zhu, W. Mechanistic studies of the transport of peimine in the Caco-2 cell model. Acta Pharm. Sin. B 2016, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Wu, Z.; Ji, Y.; Wang, B.; Dai, Z.; Wu, G. L-Glutamate Enhances Barrier and Antioxidative Functions in Intestinal Porcine Epithelial Cells. J. Nutr. 2015, 145, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Guo, Y.; Yang, Y. Effects of dietary l-tryptophan supplementation on intestinal response to chronic unpredictable stress in broilers. Amino Acids 2017, 49, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, O.; Martins, I.M.; Hou, H.; Zhao, X.; Blumberg, J.B.; Li, B. Collagen peptides ameliorate intestinal epithelial barrier dysfunction in immunostimulatory Caco-2 cell monolayers via enhancing tight junctions. Food Funct. 2017, 8, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y.; Xiong, S.; Wu, M.; Li, B.; Ruan, Z.; Hu, X. Dietary l-tryptophan alleviated LPS-induced intestinal barrier injury by regulating tight junctions in a Caco-2 cell monolayer model. Food Funct. 2019, 10, 2390–2398. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Wei, X.; Li, N.; Wu, X.; Cao, G.; Qiao, H.; Wang, J.; Hao, R. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef]
- Jobin, C.; Sartor, R.B. The I kappa B/NF-kappa B system: A key determinant of mucosalinflammation and protection. Am. J. Physiol. Cell Physiol. 2000, 278, C451–C462. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Aljenoobi, F.I.; Jan, B.L.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Momordica charantia polysaccharides ameliorate oxidative stress, inflammation, and apoptosis in ethanol-induced gastritis in mucosa through NF-kB signaling pathway inhibition. Int. J. Biol. Macromol. 2018, 111, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- de Bruyn, M.; Vandooren, J.; Ugarte-Berzal, E.; Arijs, I.; Vermeire, S.; Opdenakker, G. The molecular biology of matrix metalloproteinases and tissue inhibitors of metalloproteinases in inflammatory bowel diseases. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 295–358. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, M.; Han, T. Lycium barbarum polysaccharides ameliorate intestinal barrier dysfunction and inflammation through the MLCK-MLC signaling pathway in Caco-2 cells. Food Funct. 2020, 11, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zeng, M.; Zhu, S.; Li, S.; Qian, Y.; Wu, H. Phycocyanin ameliorates mouse colitis via phycocyanobilin-dependent antioxidant and anti-inflammatory protection of the intestinal epithelial barrier. Food Funct. 2022, 13, 3294–3307. [Google Scholar] [CrossRef]
- de Bruyn, M.; Sabino, J.; Vandeputte, D.; Vermeire, S.; Raes, J.; Opdenakker, G. Comparisons of gut microbiota profiles in wild-type and gelatinase B/matrix metalloproteinase-9-deficient mice in acute DSS-induced colitis. Npj Biofilms Microbiomes 2018, 4, 18. [Google Scholar] [CrossRef]
- de Bruyn, M.; Breynaert, C.; Arijs, I.; De Hertogh, G.; Geboes, K.; Thijs, G.; Matteoli, G.; Hu, J.; Van Damme, J.; Arnold, B.; et al. Inhibition of gelatinase B/MMP-9 does not attenuate colitis in murine models of inflammatory bowel disease. Nat. Commun. 2017, 8, 15384. [Google Scholar] [CrossRef]
- Bouchet, S.; Bauvois, B. Neutrophil Gelatinase-Associated Lipocalin (NGAL), Pro-Matrix Metalloproteinase-9 (pro-MMP-9) and Their Complex Pro-MMP-9/NGAL in Leukaemias. Cancers 2014, 6, 796–812. [Google Scholar] [CrossRef]
- Bchir, S.; Nasr, H.B.; Bouchet, S.; Benzarti, M.; Garrouch, A.; Tabka, Z.; Susin, S.; Chahed, K.; Bauvois, B. Concomitant elevations of MMP-9, NGAL, proMMP-9/NGAL and neutrophil elastase in serum of smokers with chronic obstructive pulmonary disease. J. Cell. Mol. Med. 2017, 21, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, D.; Sun, H. Herba Origani alleviated DSS-induced ulcerative colitis in mice through remolding gut microbiota to regulate bile acid and short-chain fatty acid metabolisms. Biomed. Pharmacother. 2023, 161, 114409. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Teng, Y.; Liu, H.; Li, J.; Zhu, B.; Xia, X. Rhopilema esculentum polysaccharides enhance epithelial cell barrier in vitro and alleviate chronic colitis in mice. Int. J. Biol. Macromol. 2023, 241, 124560. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, W.; Lan, P.; Mou, X. The microbiome in inflammatory bowel diseases: From pathogenesis to therapy. Protein Cell 2020, 12, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Xu, D.; Wu, Q.; Liu, W.; Hu, G.; Meng, H.; Wang, J. Therapeutic efficacy and underlying mechanisms of Gastrodia elata polysaccharides on dextran sulfate sodium-induced inflammatory bowel disease in mice: Modulation of the gut microbiota and improvement of metabolic disorders. Int. J. Biol. Macromol. 2023, 248, 125919. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Li, L.; Liu, T.; Song, X.; Sun, Y.; Cao, X.; Wang, B.; Jiang, K.; Cao, H. Bile Acid-Gut Microbiota Axis in Inflammatory Bowel Disease: From Bench to Bedside. Nutrients 2021, 13, 3143. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Xiao-Rong, L.; Ning, M.; Xi-Wang, L.; Shi-Hong, L.; Zhe, Q.; Li-Xia, B.; Ya-Jun, Y.; Jian-Yong, L. Untargeted and Targeted Metabolomics Reveal the Underlying Mechanism of Aspirin Eugenol Ester Ameliorating Rat Hyperlipidemia via Inhibiting FXR to Induce CYP7A1. Front. Pharmacol. 2021, 12, 733789. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, Y.; Xie, Y.; Sun, Q.; Li, Y. Clostridium butyricum protects the epithelial barrier by maintaining tight junction protein expression and regulating microflora in a murine model of dextran sodium sulfate-induced colitis. Scand. J. Gastroenterol. 2018, 53, 1031–1042. [Google Scholar] [CrossRef]
- Huang, B.; Wang, L.; Liu, M.; Wu, X.; Lu, Q.; Liu, R. The underlying mechanism of A-type procyanidins from peanut skin on DSS -induced ulcerative colitis mice by regulating gut microbiota and metabolism. J. Food Biochem. 2022, 46, e14103. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Q.; Liu, X.-W.; Zhang, Z.-D.; Ma, N.; Lu, X.-R.; Ge, W.-B.; Li, J.-Y.; Yang, Y.-J. Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury. Int. J. Mol. Sci. 2023, 24, 17434. https://doi.org/10.3390/ijms242417434
Tao Q, Liu X-W, Zhang Z-D, Ma N, Lu X-R, Ge W-B, Li J-Y, Yang Y-J. Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury. International Journal of Molecular Sciences. 2023; 24(24):17434. https://doi.org/10.3390/ijms242417434
Chicago/Turabian StyleTao, Qi, Xi-Wang Liu, Zhen-Dong Zhang, Ning Ma, Xiao-Rong Lu, Wen-Bo Ge, Jian-Yong Li, and Ya-Jun Yang. 2023. "Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury" International Journal of Molecular Sciences 24, no. 24: 17434. https://doi.org/10.3390/ijms242417434
APA StyleTao, Q., Liu, X.-W., Zhang, Z.-D., Ma, N., Lu, X.-R., Ge, W.-B., Li, J.-Y., & Yang, Y.-J. (2023). Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury. International Journal of Molecular Sciences, 24(24), 17434. https://doi.org/10.3390/ijms242417434






