Quantification of Antiphospholipid Antibodies: The Importance of Using an Appropriate Methodology for Each Clinical Profile
Abstract
:1. Introduction
2. Results
2.1. Study Population
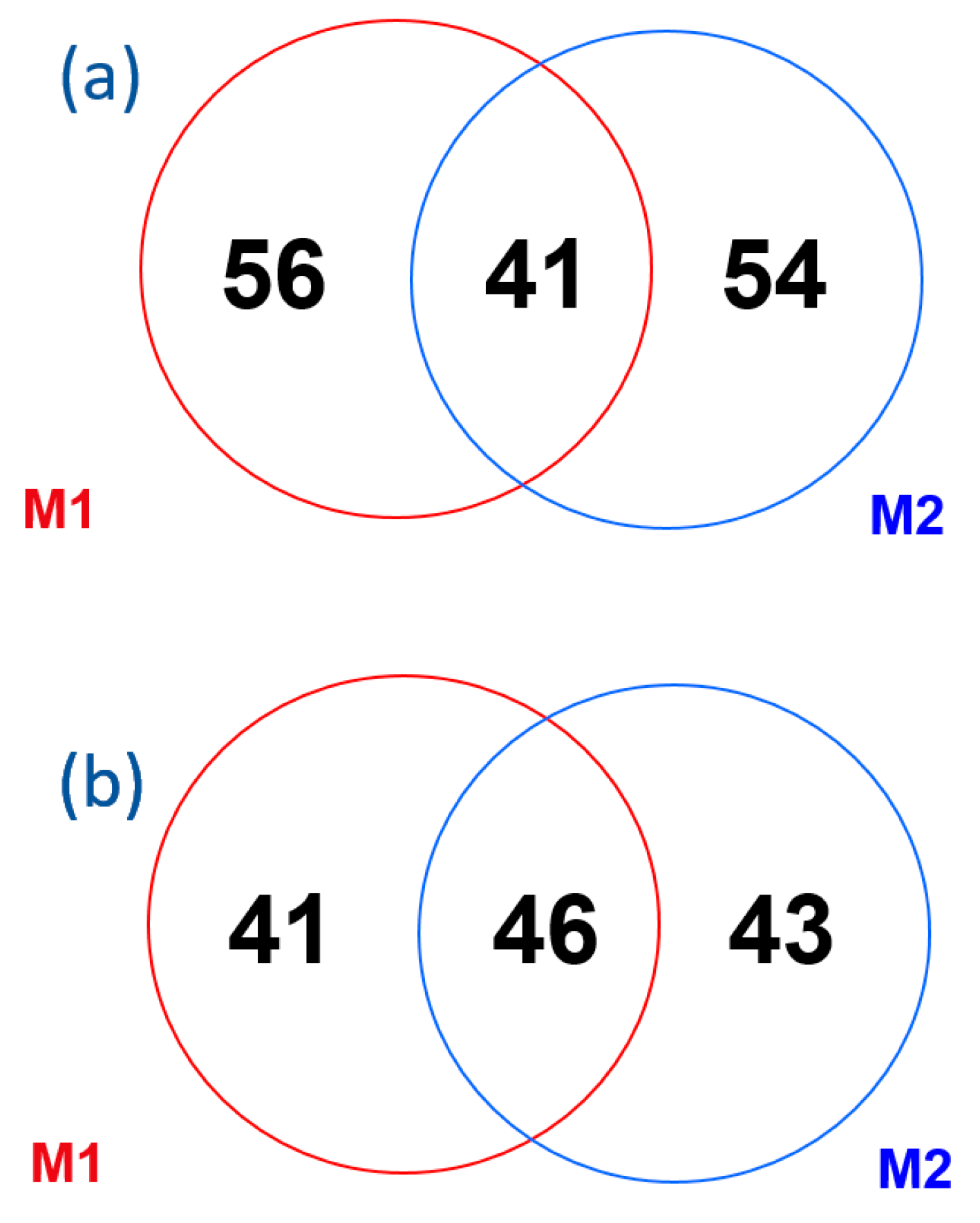
2.2. Method Comparison
| aPL | M1 and M2 Positives | M1 Positives | M2 Positives | Kappa Index | 95% CI | Level of Agreement |
|---|---|---|---|---|---|---|
| aCL IgG | 24 | 32 | 31 | 0.40 | 0.28–0.52 | Fair |
| aCL IgM | 18 | 35 | 40 | 0.28 | 0.17–0.40 | Fair |
| aβ2GPI IgG | 28 | 25 | 29 | 0.48 | 0.36–0.60 | Moderate |
| aβ2GPI IgM | 23 | 18 | 36 | 0.43 | 0.31–0.56 | Moderate |
2.3. Lupus Anticoagulant
2.4. Patients with APS Associated Clinical Events
2.5. Correlation between Methodologies According to Clinical Events
| Clinical Manifestation | Number of Patients | M1 and M2 Positives | M1 Positives | M2 Positives | Kappa Index | 95% CI | Level of Agreement |
|---|---|---|---|---|---|---|---|
| Any Sidney APS clinical criteria | 403 | 27 | 42 | 24 | 0.36 | 0.23–0.48 | Fair |
| Gestational only | 74 | 7 | 11 | 1 | 0.46 | 0.21–0.70 | Moderate |
| Gestational | 80 | 8 | 11 | 2 | 0.45 | 0.21–0.69 | Moderate |
| Thrombotic and gestational | 6 | 1 | 0 | 1 | 0.57 | −0.12–1.00 | No agreement |
| Thrombotic only | 323 | 19 | 31 | 22 | 0.32 | 0.18–0.46 | Fair |
| Thrombotic | 329 | 20 | 31 | 23 | 0.33 | 0.19–0.47 | Fair |
2.6. Patients with Other Autoimmune Disease
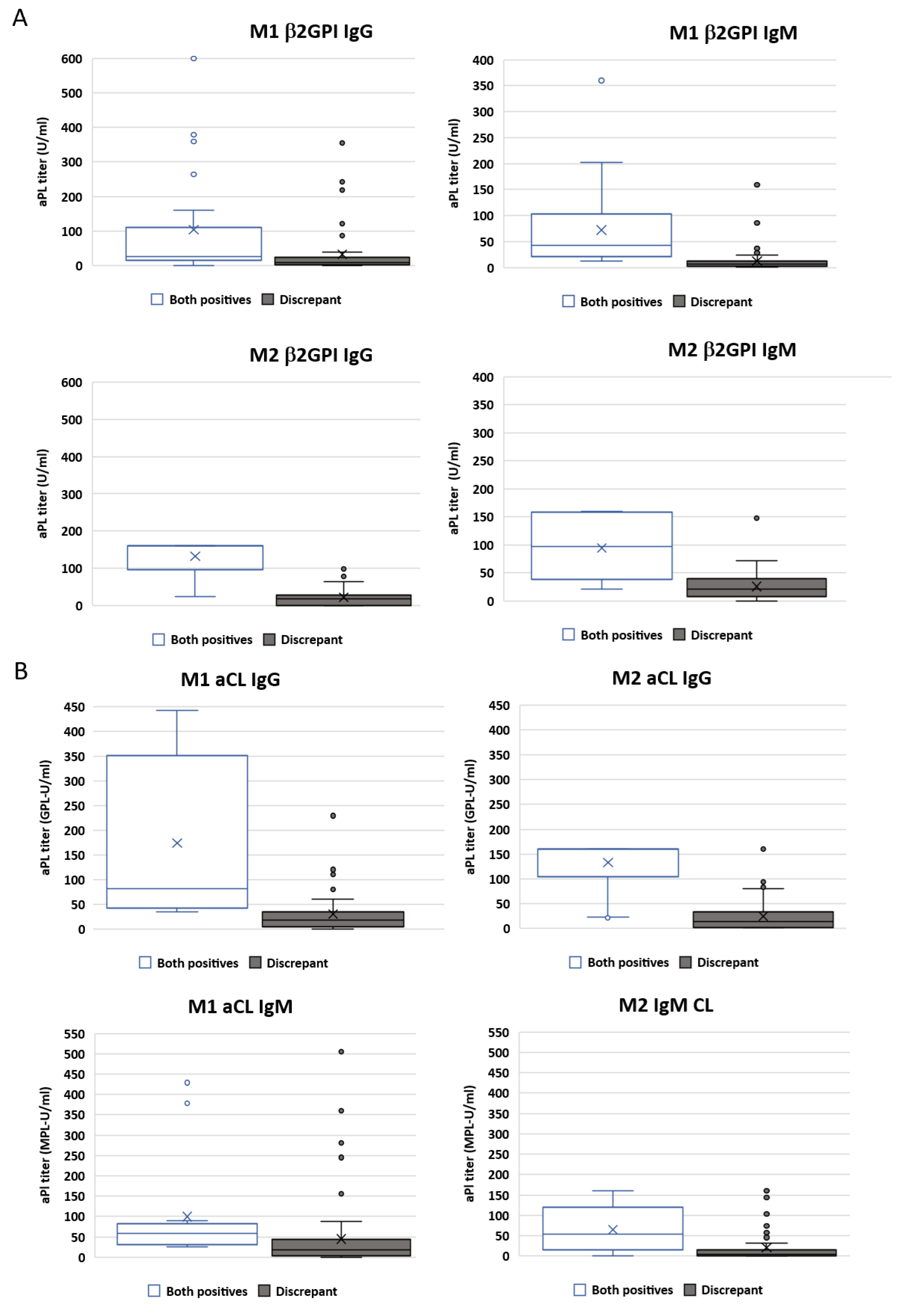
2.7. Importance of Antibody Titres
2.8. Diagnostic Performance
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Cut-Off Established Using Healthy Donors
4.3. Assays
4.4. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaneko, K.; Ozawa, N.; Murashima, A. Obstetric anti-phospholipid syndrome: From pathogenesis to treatment. Immunol. Med. 2022, 45, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Zuily, S.; Ahmadzadeh, Y.; Amigo, M.C.; Avcin, T.; Bertolaccini, M.L.; Branch, D.W.; de Jesus, G.; Devreese, K.M.J.; Frances, C.; et al. Development of a New International Antiphospholipid Syndrome Classification Criteria Phase I/II Report: Generation and Reduction of Candidate Criteria. Arthritis Care Res. 2021, 73, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Marante, O.; Rodriguez de Frias, E.; Serrano, M.; Lozano Morillo, F.; Naranjo, L.; Gil-Etayo, F.J.; Paz-Artal, E.; Pleguezuelo, D.E.; Serrano, A. The Weight of IgA Anti-beta2glycoprotein I in the Antiphospholipid Syndrome Pathogenesis: Closing the Gap of Seronegative Antiphospholipid Syndrome. Int. J. Mol. Sci. 2020, 21, 8972. [Google Scholar] [CrossRef] [PubMed]
- Pregnolato, F.; Chighizola, C.B.; Encabo, S.; Shums, Z.; Norman, G.L.; Tripodi, A.; Chantarangkul, V.; Bertero, T.; De Micheli, V.; Borghi, M.O.; et al. Anti-phosphatidylserine/prothrombin antibodies: An additional diagnostic marker for APS? Immunol. Res. 2013, 56, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, C.B.; Sciascia, S.; Andreoli, L.; Meroni, P.L. Beyond current concepts in anti-phospholipid syndrome: The 16th International Congress on Anti-phospholipid Antibodies (ICAPA) in Manchester. Autoimmun. Rev. 2020, 19, 102615. [Google Scholar] [CrossRef]
- Radin, M.; Foddai, S.G.; Cecchi, I.; Rubini, E.; Schreiber, K.; Roccatello, D.; Bertolaccini, M.L.; Sciascia, S. Antiphosphatidylserine/Prothrombin Antibodies: An Update on Their Association with Clinical Manifestations of Antiphospholipid Syndrome. Thromb. Haemost 2020, 120, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.N.; Gharavi, A.E.; Boey, M.L.; Patel, B.M.; Mackworth-Young, C.G.; Loizou, S.; Hughes, G.R. Anticardiolipin antibodies: Detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet 1983, 2, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Villalta, D.; Alessio, M.G.; Tampoia, M.; Da Re, A.; Stella, S.; Da Re, M.; Tozzoli, R.; Bizzaro, N. Accuracy of the first fully automated method for anti-cardiolipin and anti-beta2 glycoprotein I antibody detection for the diagnosis of antiphospholipid syndrome. Ann. N. Y. Acad. Sci. 2009, 1173, 21–27. [Google Scholar] [CrossRef]
- Coulam, C.B.; McIntyre, J.A.; Wagenknecht, D.; Rote, N. Interlaboratory inconsistencies in detection of anticardiolipin antibodies. Lancet 1990, 335, 865. [Google Scholar] [CrossRef]
- Devreese, K.M.J. Testing for antiphospholipid antibodies: Advances and best practices. Int. J. Lab. Hematol. 2020, 42 (Suppl. S1), 49–58. [Google Scholar] [CrossRef]
- Reber, G.; Schousboe, I.; Tincani, A.; Sanmarco, M.; Kveder, T.; de Moerloose, P.; Boffa, M.C.; Arvieux, J. Inter-laboratory variability of anti-beta2-glycoprotein I measurement. A collaborative study in the frame of the European Forum on Antiphospholipid Antibodies Standardization Group. Thromb. Haemost 2002, 88, 66–73. [Google Scholar] [PubMed]
- Chayoua, W.; Kelchtermans, H.; Moore, G.W.; Gris, J.C.; Musial, J.; Wahl, D.; Zuily, S.; Gianniello, F.; Fontana, P.; Remijn, J.; et al. Detection of Anti-Cardiolipin and Anti-beta2glycoprotein I Antibodies Differs between Platforms without Influence on Association with Clinical Symptoms. Thromb. Haemost 2019, 119, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Kaneshige, R.; Motoki, Y.; Yoshida, M.; Oku, K.; Morishita, E.; Ieko, M.; Ichihara, K.; Nojima, J. Determination of diagnostic threshold in harmonization and comparison of clinical utility for five major antiphospholipid antibody assays used in Japan. J. Clin. Lab. Anal. 2022, 36, e24340. [Google Scholar] [CrossRef]
- de Laat, B.; van Berkel, M.; Urbanus, R.T.; Siregar, B.; de Groot, P.G.; Gebbink, M.F.; Maas, C. Immune responses against domain I of beta(2)-glycoprotein I are driven by conformational changes: Domain I of beta(2)-glycoprotein I harbors a cryptic immunogenic epitope. Arthritis Rheum. 2011, 63, 3960–3968. [Google Scholar] [CrossRef]
- Ioannou, Y.; Rahman, A. Domain I of beta2-glycoprotein I: Its role as an epitope and the potential to be developed as a specific target for the treatment of the antiphospholipid syndrome. Lupus 2010, 19, 400–405. [Google Scholar] [CrossRef]
- Carrasco, A.; Cabrera-Marante, O.; Serrano, A. Taller de autoinmunidad 2019: Estado actual del diagnóstico del síndrome antifosfolipídico. Inmunología 2019, 38, 44–46. [Google Scholar]
- Martinez-Flores, J.A.; Serrano, M.; Alfaro, J.; Mora, S.; Paz-Artal, E.; Morales, J.M.; Serrano, A. Heterogeneity between diagnostic tests for IgA anti-beta2 glycoprotein I: Explaining the controversy in studies of association with vascular pathology. Anal. Chem. 2013, 85, 12093–12098. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.; Martinez-Flores, J.A.; Serrano, M.; Lora, D.; Paz-Artal, E.; Morales, J.M.; Serrano, A. Evaluation of three fully automated immunoassay systems for detection of IgA anti-beta 2-glycoprotein I antibodies. Int. J. Lab. Hematol. 2016, 38, 560–568. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Meroni, P.L.; Borghi, M.O. Antiphospholipid Antibody Assays in 2021: Looking for a Predictive Value in Addition to a Diagnostic One. Front. Immunol. 2021, 12, 726820. [Google Scholar] [CrossRef]
- de Laat, B.; Derksen, R.H.; van Lummel, M.; Pennings, M.T.; de Groot, P.G. Pathogenic anti-beta2-glycoprotein I antibodies recognize domain I of beta2-glycoprotein I only after a conformational change. Blood 2006, 107, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Devreese, K.M. Antiphospholipid antibody testing and standardization. Int. J. Lab. Hematol. 2014, 36, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Chighizola, C.B.; Rovelli, F.; Gerosa, M. Antiphospholipid syndrome in 2014: More clinical manifestations, novel pathogenic players and emerging biomarkers. Arthritis Res. Ther. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Rodriguez-Pinto, I.; Cervera, R. Catastrophic antiphospholipid syndrome: An update. Panminerva Med. 2017, 59, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Sevim, E.; Zisa, D.; Andrade, D.; Sciascia, S.; Pengo, V.; Tektonidou, M.G.; Ugarte, A.; Gerosa, M.; Belmont, H.M.; Zamorano, M.A.A.; et al. Characteristics of Patients with Antiphospholipid Antibody Positivity in the APS ACTION International Clinical Database and Repository. Arthritis Care Res. 2022, 74, 324–335. [Google Scholar] [CrossRef]
- Cervera, R.; Piette, J.C.; Font, J.; Khamashta, M.A.; Shoenfeld, Y.; Camps, M.T.; Jacobsen, S.; Lakos, G.; Tincani, A.; Kontopoulou-Griva, I.; et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef]
- Sciascia, S.; Radin, M.; Cecchi, I.; Levy, R.A.; Erkan, D. 16th International congress on antiphospholipid antibodies task force report on clinical manifestations of antiphospholipid syndrome. Lupus 2021, 30, 1314–1326. [Google Scholar] [CrossRef]
- Chayoua, W.; Kelchtermans, H.; Gris, J.C.; Moore, G.W.; Musial, J.; Wahl, D.; de Groot, P.G.; de Laat, B.; Devreese, K.M.J. The (non-)sense of detecting anti-cardiolipin and anti-beta2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J. Thromb. Haemost 2020, 18, 169–179. [Google Scholar] [CrossRef]
- Ruffatti, A.; Olivieri, S.; Tonello, M.; Bortolati, M.; Bison, E.; Salvan, E.; Facchinetti, M.; Pengo, V. Influence of different IgG anticardiolipin antibody cut-off values on antiphospholipid syndrome classification. J. Thromb. Haemost 2008, 6, 1693–1696. [Google Scholar] [CrossRef]
- Boffa, M.C.; Boinot, C.; De Carolis, S.; Rovere-Querini, P.; Aurousseau, M.H.; Allegri, F.; Nicaise-Roland, P.; Barra, A.; Botta, A.; Ambrozic, A.; et al. Laboratory criteria of the obstetrical antiphospholipid syndrome. Data from a multicentric prospective European women cohort. Thromb. Haemost 2009, 102, 25–28. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. The 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Arthritis Rheumatol. 2023, 75, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Marziale, A.; Bettacchioli, E.; Picart, G.; Nafai, S.; Galinat, H.; Meroni, P.L.; Frostegard, J.; Alarcon-Riquelme, M.E.; Renaudineau, Y. Antiphospholipid autoantibody detection is important in all patients with systemic autoimmune diseases. J. Autoimmun. 2020, 115, 102524. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Foddai, S.G.; Alessandri, C.; Alunno, A.; Andreoli, L.; Barinotti, A.; Calligaro, A.; Canti, V.; Carubbi, F.; Cecchi, I.; et al. Clinical Delphi on aPL Negativization: Report from the APS Study Group of the Italian Society for Rheumatology (SIR-APS). Thromb. Haemost 2022, 122, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Marante, O.; Lora, D.; Serrano, M.; Rodriguez de Frias, E.A.; Naranjo, L.; Perez, D.; Paz-Artal, E.; Pleguezuelo, D.E.; Serrano, A. Antiphospholipid antibodies quantification using ALBIA technology: How to define an optimal cutoff? Clin. Chem. Lab. Med. 2021, 59, e454–e457. [Google Scholar] [CrossRef]
- Devreese, K.M.J. Solid Phase Assays for Antiphospholipid Antibodies. Semin. Thromb. Hemost 2022, 48, 661–671. [Google Scholar] [CrossRef]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for lupus anticoagulant/antiphospholipid antibodies of the International Society on Thrombosis and Haemostasis: Update of the guidelines for lupus anticoagulant detection and interpretation. J. Thromb. Haemost 2020, 18, 2828–2839. [Google Scholar] [CrossRef]
- Forastiero, R.; Papalardo, E.; Watkins, M.; Nguyen, H.; Quirbach, C.; Jaskal, K.; Kast, M.; Teodorescu, M.; Lakos, G.; Binder, W.; et al. Evaluation of different immunoassays for the detection of antiphospholipid antibodies: Report of a wet workshop during the 13th International Congress on Antiphospholipid Antibodies. Clin. Chim. Acta 2014, 428, 99–105. [Google Scholar] [CrossRef]
- Sciascia, S.; Radin, M.; Ramirez, C.; Seaman, A.; Bentow, C.; Casas, S.; Cecchi, I.; Rubini, E.; Foddai, S.G.; Baldovino, S.; et al. Evaluation of novel assays for the detection of autoantibodies in antiphospholipid syndrome. Autoimmun. Rev. 2020, 19, 102641. [Google Scholar] [CrossRef]
- Wan, L.Y.; Gu, J.Y.; Liu, T.T.; Hu, Q.Y.; Jia, J.C.; Teng, J.L.; Sun, Y.; Liu, H.L.; Cheng, X.B.; Ye, J.N.; et al. Clinical performance of automated chemiluminescent methods for anticardiolipin and anti-beta2-glycoprotein I antibodies detection in a large cohort of Chinese patients with antiphospholipid syndrome. Int. J. Lab. Hematol. 2020, 42, 206–213. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derkesen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Vandevelde, A.; Chayoua, W.; de Laat, B.; Moore, G.W.; Musial, J.; Zuily, S.; Wahl, D.; Devreese, K.M.J. Added value of antiphosphatidylserine/prothrombin antibodies in the workup of thrombotic antiphospholipid syndrome: Communication from the ISTH SSC Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibodies. J. Thromb. Haemost 2022, 20, 2136–2150. [Google Scholar] [CrossRef] [PubMed]

| Study Population (n = 1020) | ||
|---|---|---|
| Variables | Median (SD)/n | IQR/% |
| Age | 56 (19.13) | 41–70 |
| Sex (Male) | 398 | 39.0 |
| Dyslipidemia | 247 | 24.2 |
| Diabetes mellitus | 151 | 14.8 |
| Smoking | 239 | 23.4 |
| High blood pressure | 324 | 31.8 |
| Clinical manifestations included in APS classification criteria | 403 | 39.5 |
| Thrombotic events | 329 | 81.64 |
| Obstetric events | 80 | 19.85 |
| Autoimmune diseases | 172 | 16.9 |
| SLE | 64 | 37.2 |
| RA | 36 | 20.9 |
| SS | 23 | 13.4 |
| SScl | 20 | 11.6 |
| APS Events (n = 403) | No APS Events (n = 617) | |||
|---|---|---|---|---|
| aPL | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value |
| aβ2GPI IgM | 0.80 | <0.0001 | 0.64 | <0.0001 |
| aCL IgM | 0.46 | <0.0001 | 0.18 | <0.0001 |
| aβ2GPI IgG | 0.61 | <0.0001 | 0.37 | <0.0001 |
| aCL IgG | 0.71 | <0.0001 | 0.49 | <0.0001 |
| Thrombotic Events (n = 329) | Gestational Events (n = 80) | |||
|---|---|---|---|---|
| aPL | Correlation Coefficient | p-Value | Correlation Coefficient | p-Value |
| aβ2GPI IgM | 0.84 | <0.0001 | 0.81 | <0.0001 |
| aCL IgM | 0.50 | <0.0001 | 0.48 | <0.0001 |
| aβ2GPI IgG | 0.60 | <0.0001 | 0.66 | <0.0001 |
| aCL IgG | 0.70 | <0.0001 | 0.66 | <0.0001 |
| Autoimmune Disease | Non-Autoimmune Disease | p-Value | |
|---|---|---|---|
| APS related event | 0.55 (0.28–0.83) | 0.30 (0.17–0.44) | 0.110 |
| Non-APS related event | 0.55 (0.32–0.77) | 0.37 (0.23–0.20) | 0.182 |
| AUC | |
|---|---|
| M1 and thrombotic events | Non-significant (p = 0.16) |
| M2 and thrombotic events | 0.52 (0.5–0.54) |
| M1 and obstetric morbidity considering only females | 0.57 (0.52–0.62) |
| M2 and obstetric morbidity considering only females | Non-significant (p = 0.49) |
| M1 | M2 | ||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| All patients | APS event | 17.12 (13.57–21.16) | 89.30 (86.59–91.63) | 12.66 (9.57–16.30) | 91.41 (88.91–93.50) |
| Thrombotic event | 15.50 (11.76–19.87) | 87.84 (85.17–90.19) | 13.07 (9.62–17.20) | 91.17 (88.80–93.18) | |
| Obstetric event | 23.75 (14.95–34.58) | 87.66 (85.39–89.69) | 12.50 (6.16–21.79) | 90.00 (87.90–91.84) | |
| Autoimmune disease | APS event | 27.66 (15.62–42.64) | 88.00 (80.98–93.13) | 23.40 (12.30–38.03) | 88.80 (81.98–93.73) |
| Thrombotic event | 22.50 (10.84–38.45) | 85.61 (78.44–91.11) | 22.50 (10.84–38.45) | 87.88 (81.14–92.87) | |
| Obstetric event | 50.00 (17.70–84.30) | 85.37 (79.01–90.39) | 25.00 (3.19–65.09) | 85.98 (79.70–90.90) | |
| No autoimmune disease | APS event | 15.73 (12.11–19.94) | 89.63 (86.60–92.18) | 11.24 (8.15–14.98) | 92.07 (89.32–94.30 |
| Thrombotic event | 14.53 (10.68–19.13) | 88.37 (85.42–90.91) | 11.76 (8.29–16.05) | 91.95 (89.38–94.07) | |
| Obstetric event | 20.83 (12.16–32.02) | 88.14 (85.66–90.33) | 11.11 (4.92–20.72) | 90.85 (88.60–92.79) | |
| M1 | M2 | ||||
|---|---|---|---|---|---|
| Positive Likelihood Ratio | Negative Likelihood Ratio | Positive Likelihood Ratio | Negative Likelihood Ratio | ||
| All patients | APS event | 1.6 (1.17–2.19) | 0.93 (0.88–0.98) | 1.47 (1.02–2.12) | 0.96 (0.91–1) |
| Thrombotic event | 1.28 (0.92–1.76) | 0.96 (0.91–1.02) | 1.48 (1.03–2.14) | 0.95 (0.91–1) | |
| Obstetric event | 1.92 (1.25–2.95) | 0.87 (0.77–0.99) | 1.25 (0.68–2.30) | 0.97 (0.89–1.06) | |
| Autoimmune disease | APS event | 2.3 (1.19–4.47) | 0.82 (0.68–0.99) | 1.95 (0.97–3.94) | 0.87 (0.73–1.03) |
| Thrombotic event | 1.56 (0.77–3.18) | 0.91 (0.76–1.08) | 1.75 (0.84–3.61) | 0.89 (0.74–1.06) | |
| Obstetric event | 3.42 (1.56–7.49) | 0.59 (0.29–1.17) | 1.71 (0.49–6) | 0.88 (0.59–1.32) | |
| No autoimmune disease | APS event | 1.52 (1.07–2.16) | 0.94 (0.89–0.99) | 1.42 (0.93–2.16) | 0.96 (0.92.1.01) |
| Thrombotic event | 1.25 (0.87–1.79) | 0.97 (0.91–1.02) | 1.46 (0.96–2.23) | 0.96 (0.91–1.01) | |
| Obstetric event | 1.76 (1.08–2.87) | 0.9 (0.8–1.01) | 1.21 (0.61–2.42) | 0.98 (0.9–1.06) | |
| Sensitivity | Specificity | Positive LR | Negative LR | ||
|---|---|---|---|---|---|
| aCL M1 | IgM | 5.46 (3.5–8.2) | 94.9 (92.9–96.6) | 1.09 (0.6–1.9) | 1.00 (0.97–1.03) |
| IgG | 7.94 (5.5–11.0) | 96.11 (94.3–97.5) | 2.04 (1.2–3.4) | 0.96 (0.93–0.99) | |
| aβ2GPI M1 | IgM | 5.71 (3.7–8.4) | 97.08 (95.4–98.3) | 1.96 (1.1–3.6) | 0.97 (0.94–1.00) |
| IgG | 8.44 (5.9–11.6) | 96.92 (95.2–98.1) | 2.74 (1.6–4.7) | 0.94 (0.91–0.98) | |
| aCL M2 | IgM | 7.44 (5.1–10.5) | 95.5 (93.5–96.9) | 1.64 (1.0–2.7) | 0.97 (0.94–1.00) |
| IgG | 7.69 (5.3–10.7) | 96.11 (94.3–97.5) | 1.98 (1.2–3.3) | 0.96 (0.93–0.99) | |
| aβ2GPI M2 | IgM | 6.70 (4.5–9.6) | 94.81 (92.8–96.4) | 1.29 (0.8–2.1) | 0.98 (0.95–1.02) |
| IgG | 6.95 (4.7–9.9) | 95.3 (93.3–96.8) | 1.48 (0.9–2.5) | 0.98 (0.95–1.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Marante, O.; Garcinuño, S.; Pleguezuelo, D.E.; Gil-Etayo, F.J.; Tenica, I.; Rodríguez de Frías, E.; Zafra, D.; Castro, N.; Paz-Artal, E.; Serrano, A.; et al. Quantification of Antiphospholipid Antibodies: The Importance of Using an Appropriate Methodology for Each Clinical Profile. Int. J. Mol. Sci. 2023, 24, 17373. https://doi.org/10.3390/ijms242417373
Cabrera-Marante O, Garcinuño S, Pleguezuelo DE, Gil-Etayo FJ, Tenica I, Rodríguez de Frías E, Zafra D, Castro N, Paz-Artal E, Serrano A, et al. Quantification of Antiphospholipid Antibodies: The Importance of Using an Appropriate Methodology for Each Clinical Profile. International Journal of Molecular Sciences. 2023; 24(24):17373. https://doi.org/10.3390/ijms242417373
Chicago/Turabian StyleCabrera-Marante, Oscar, Sara Garcinuño, Daniel Enrique Pleguezuelo, Francisco J. Gil-Etayo, Iulian Tenica, Edgard Rodríguez de Frías, Denis Zafra, Nerea Castro, Estela Paz-Artal, Antonio Serrano, and et al. 2023. "Quantification of Antiphospholipid Antibodies: The Importance of Using an Appropriate Methodology for Each Clinical Profile" International Journal of Molecular Sciences 24, no. 24: 17373. https://doi.org/10.3390/ijms242417373
APA StyleCabrera-Marante, O., Garcinuño, S., Pleguezuelo, D. E., Gil-Etayo, F. J., Tenica, I., Rodríguez de Frías, E., Zafra, D., Castro, N., Paz-Artal, E., Serrano, A., & Serrano, M. (2023). Quantification of Antiphospholipid Antibodies: The Importance of Using an Appropriate Methodology for Each Clinical Profile. International Journal of Molecular Sciences, 24(24), 17373. https://doi.org/10.3390/ijms242417373






