Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology
Abstract
:1. Introduction
2. Results
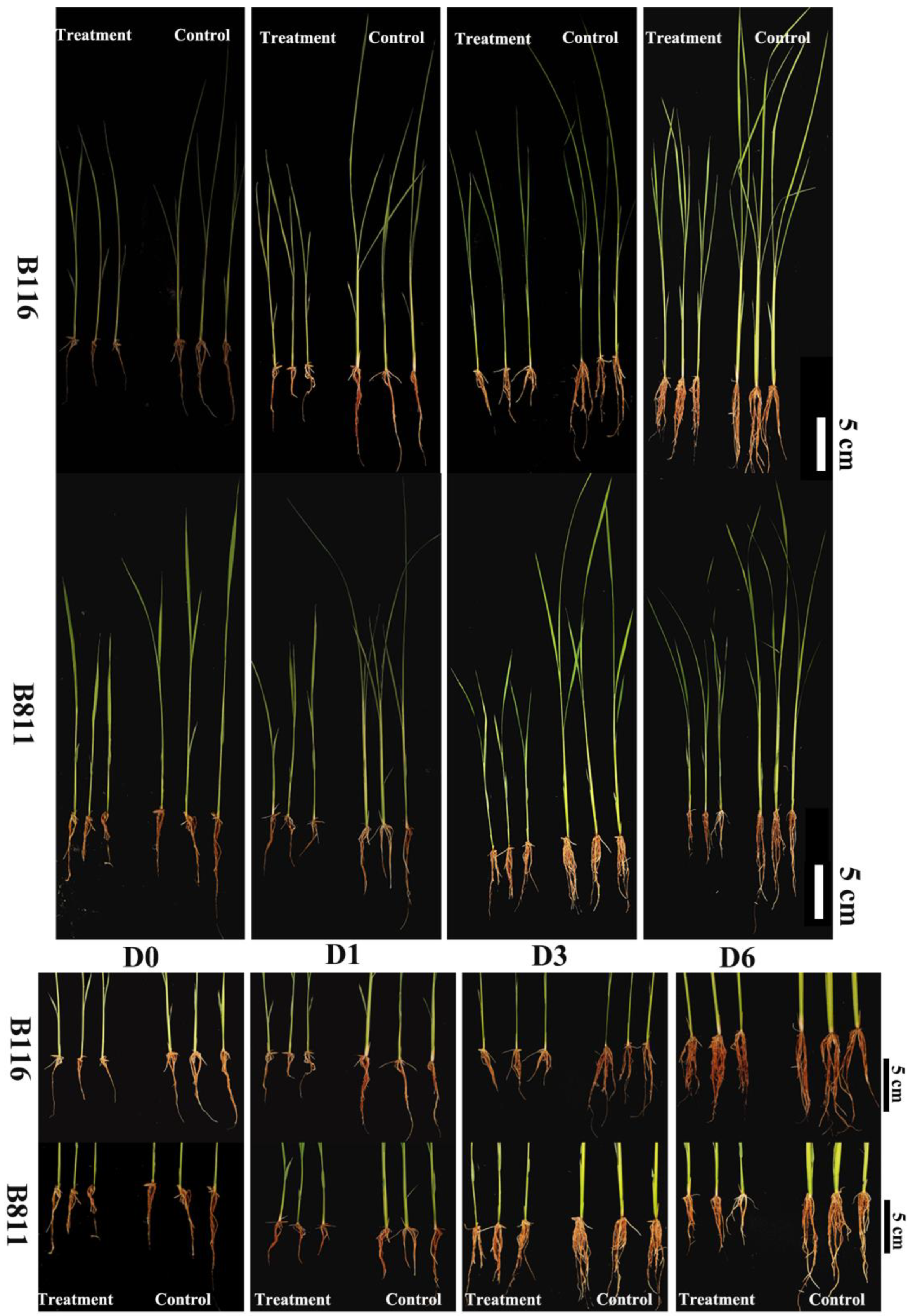
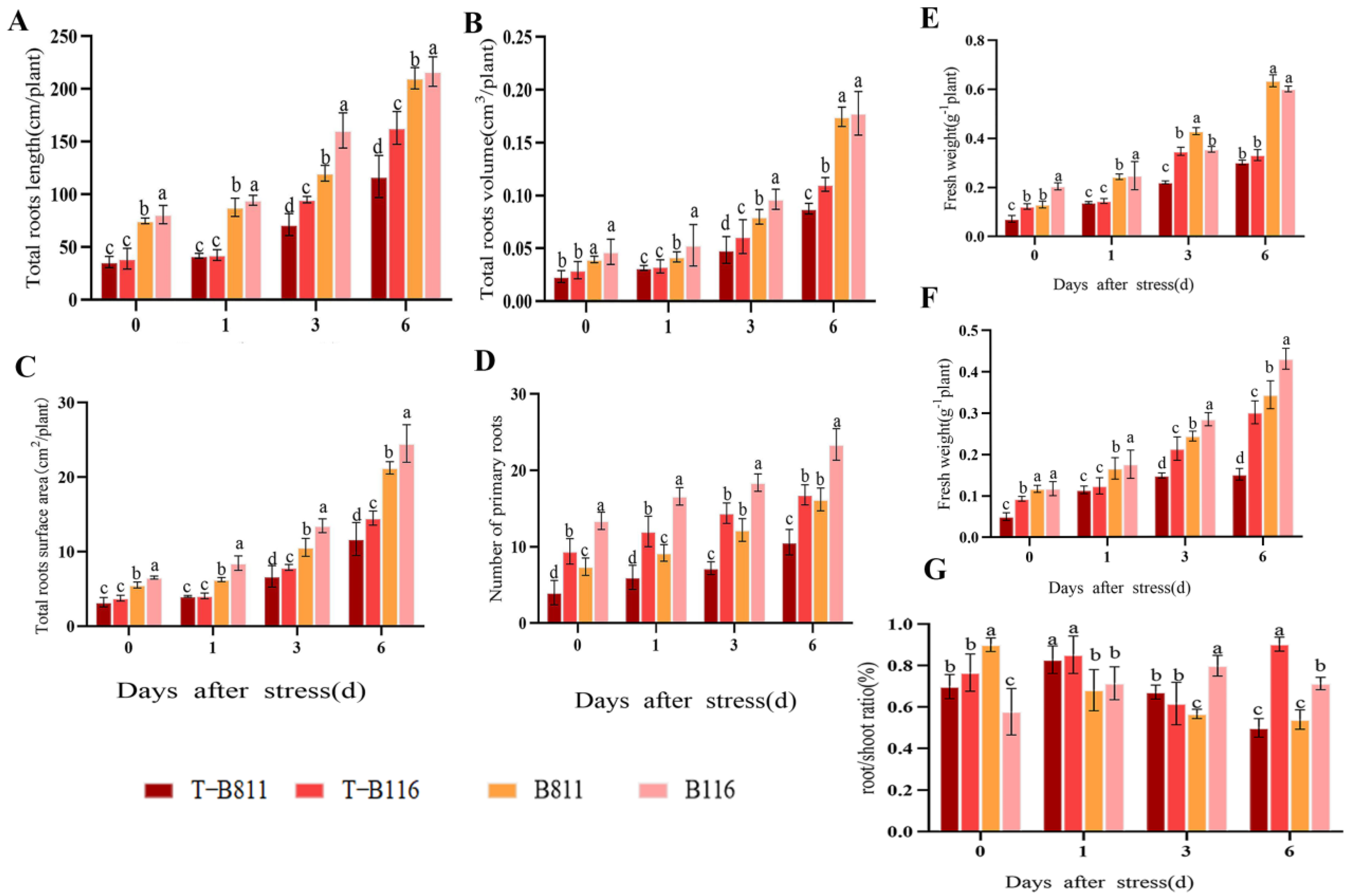
2.1. Root Growth Status of Rice Seedlings during Growth Recovery after Combined Stress from Low Temperatures and Low Light Intensities
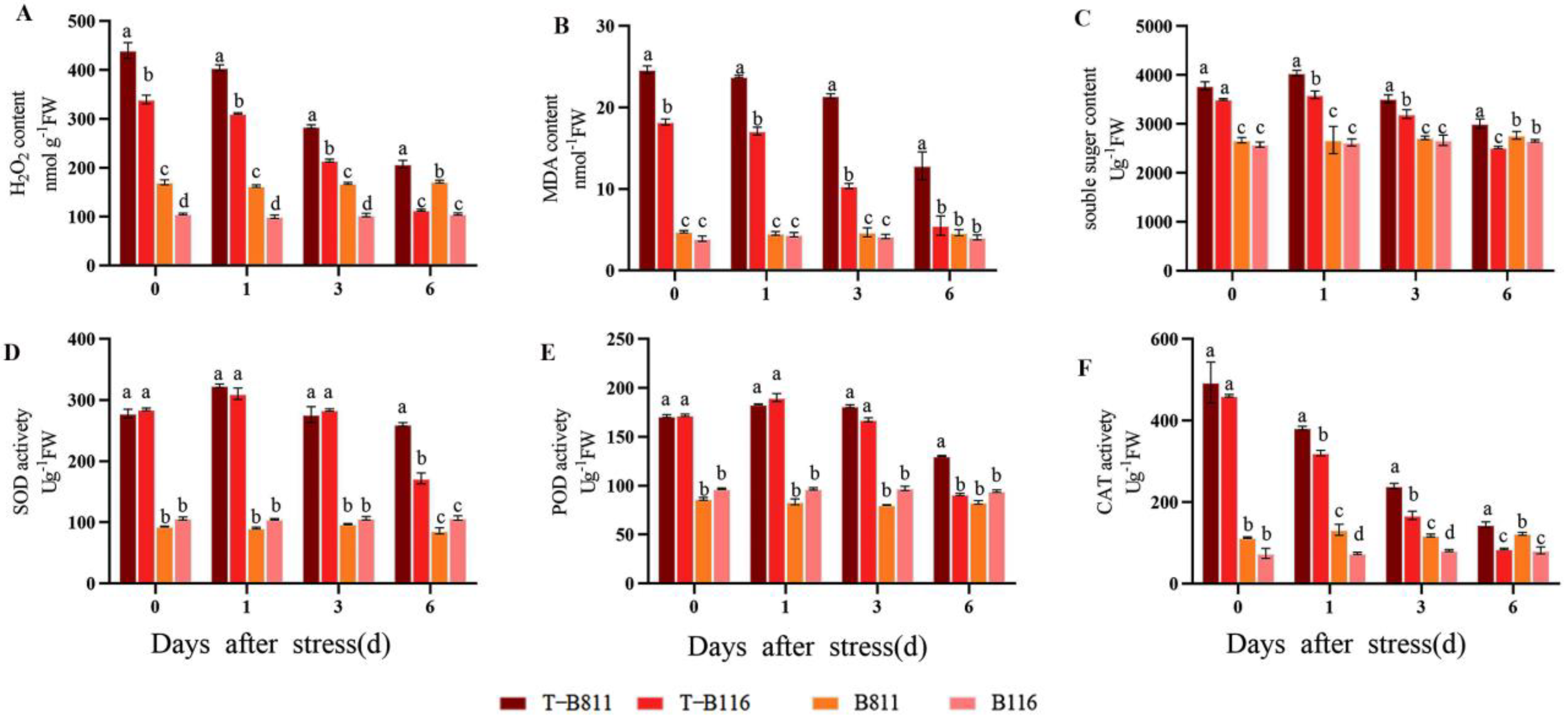
2.2. Reactive Oxygen Species Metabolism in Rice Seedling Roots during Growth Recovery after Combined Stress from Low Temperatures and Low Light Intensities
2.3. Differential Analysis of Gene Expression in Rice Seedling Roots during Growth Recovery after Combined Stress from Low Temperature and Low Light Intensity
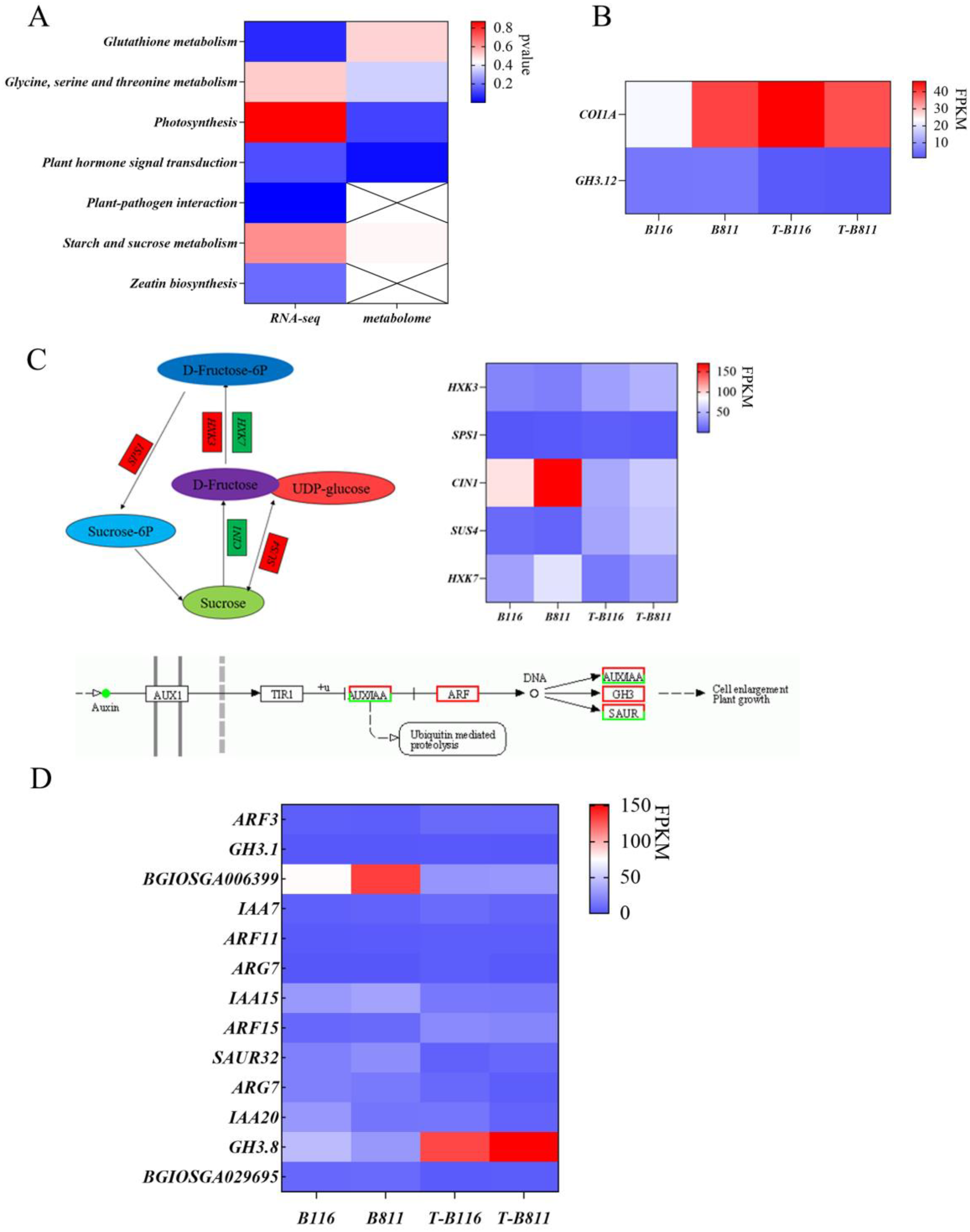
2.4. Functional Enrichment Analysis of Differentially Expressed Genes in Rice Seedling Roots during Growth Recovery after Combined Stress from Low Temperatures and Low Light Intensities
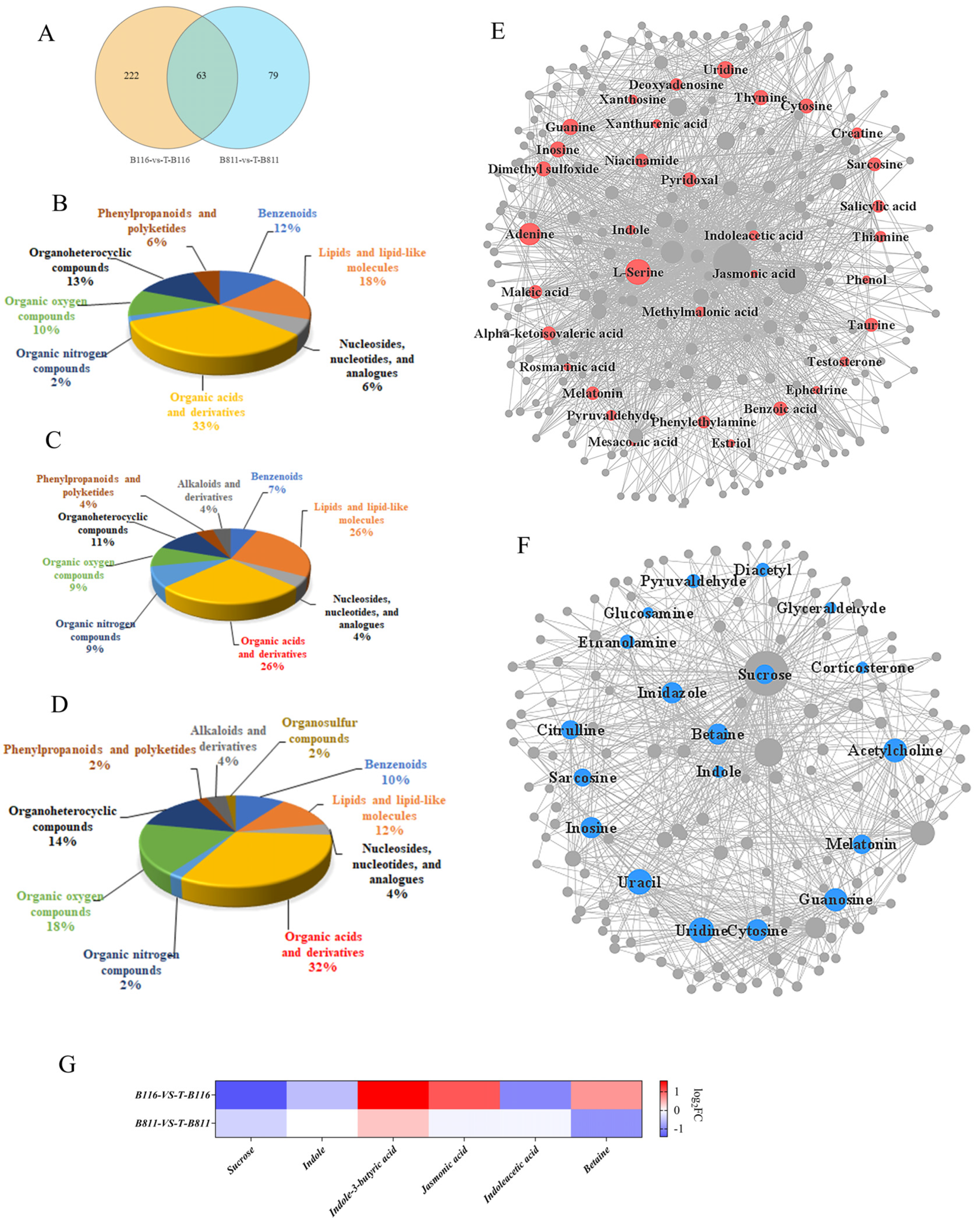
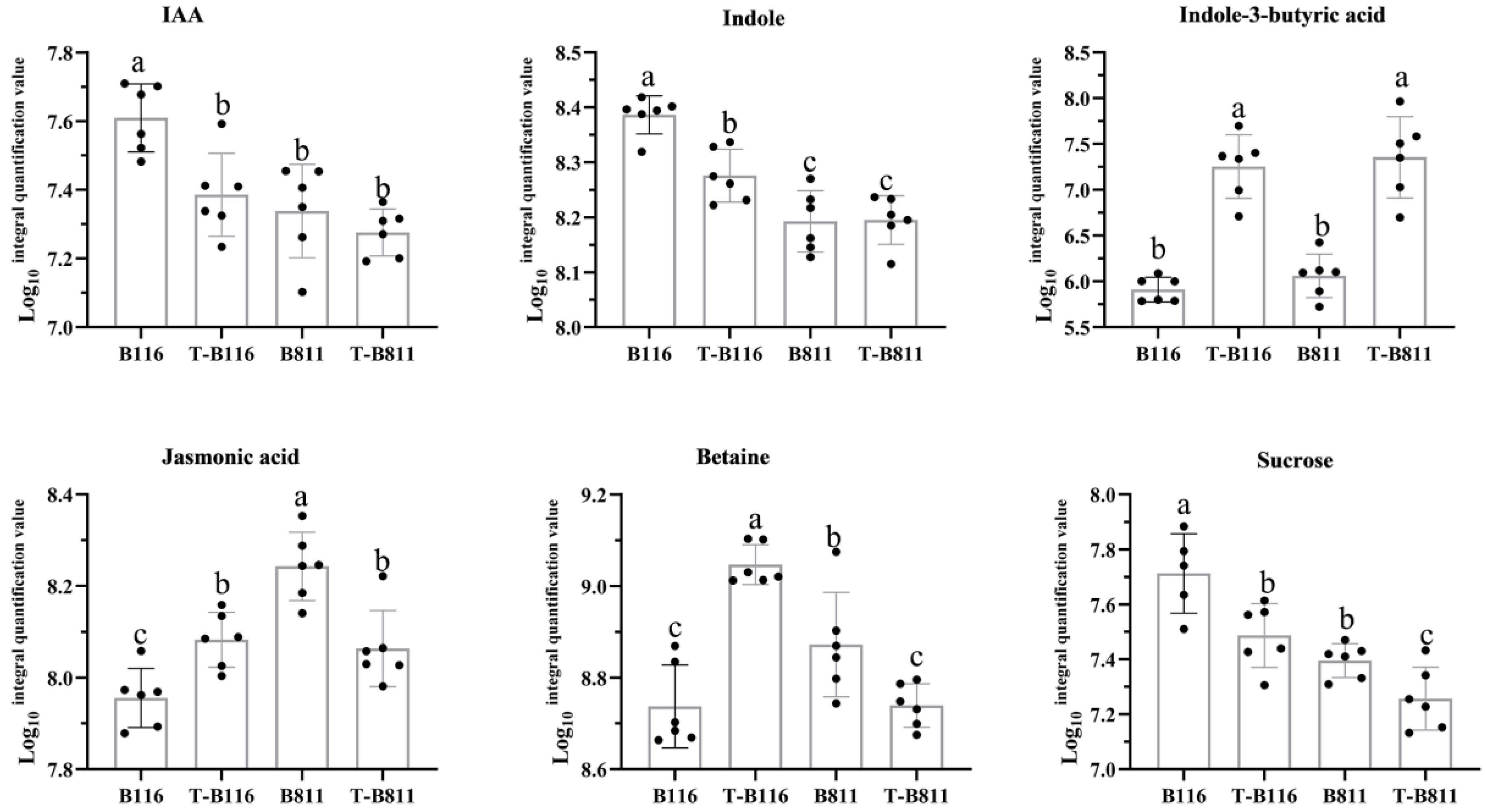
2.5. Differential Analysis of Root Metabolites of Rice Seedlings during Growth Recovery after Exposure to Combined Stress from Low Temperatures and Low Light Intensities
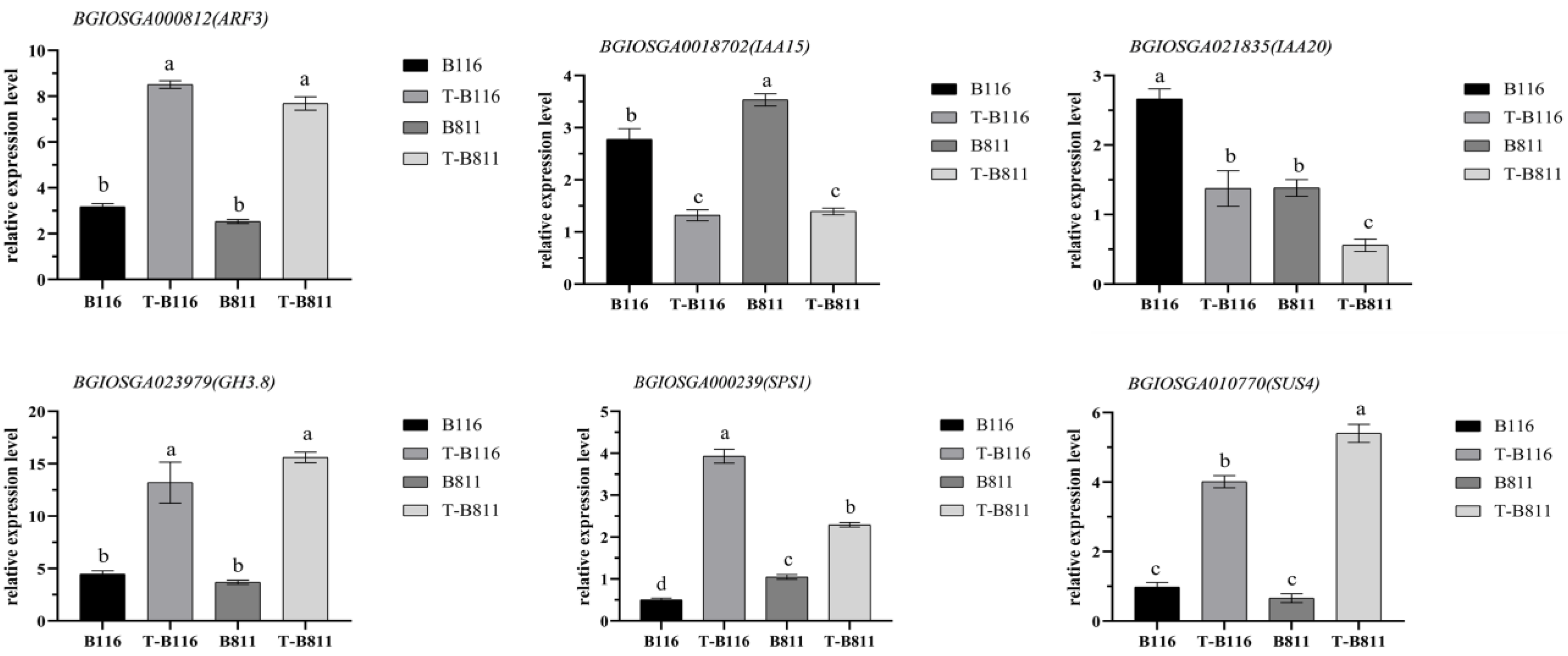
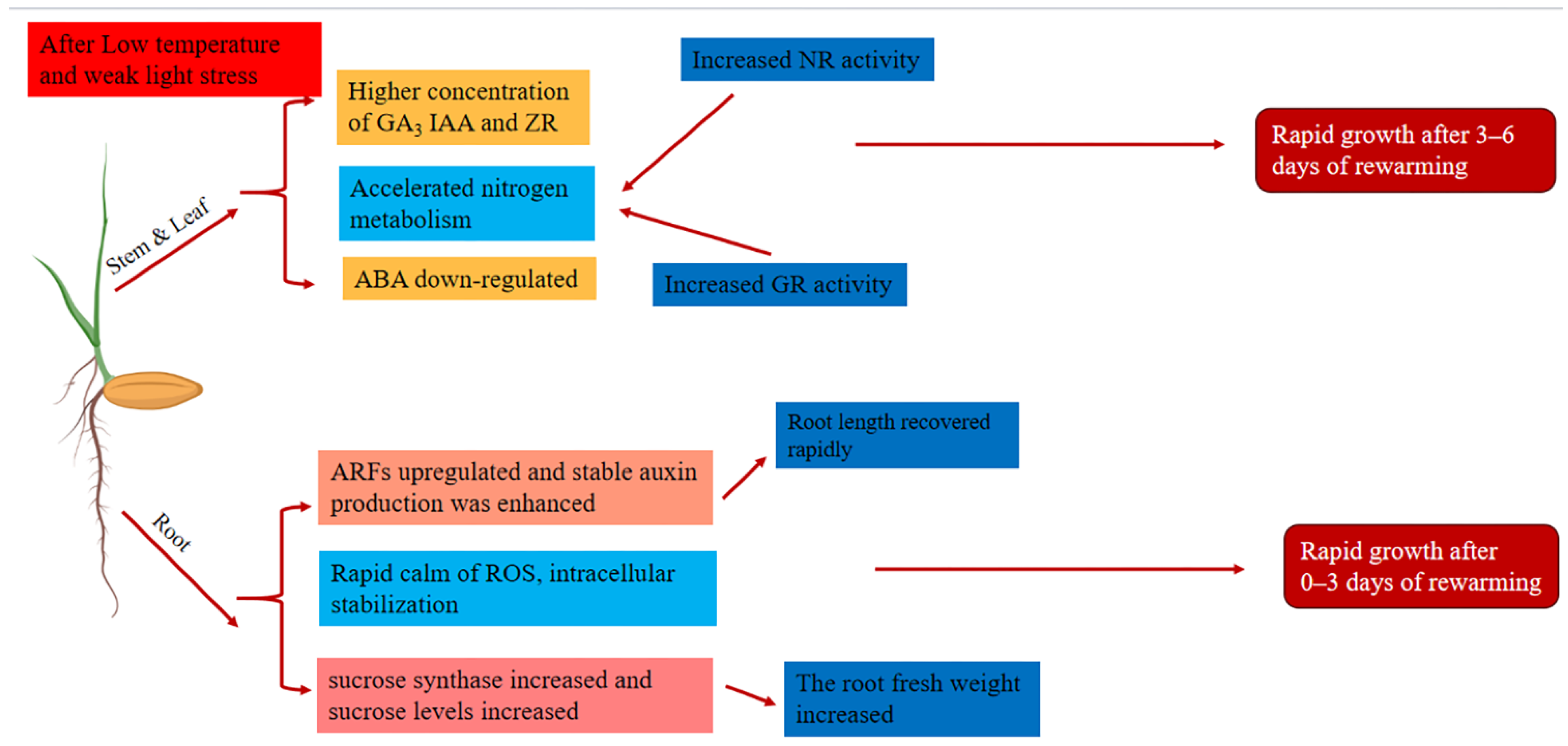
2.6. Analysis of Key Genes and Metabolites for the Rapid Growth and Recovery of Rice Seedlings after Exposure to Combined Stress from Low Temperatures and Low Light Intensities
3. Discussion
4. Materials and Methods
4.1. Planting and Experimental Design
4.2. Investigation of Root Growth Status
4.3. Root Reactive Oxygen Metabolism
4.4. RNA-seq Assay Analysis
4.5. Extraction of Metabolites
4.6. Identification and Differential Analysis of Metabolites
4.7. RT-qPCR
4.8. Data Analysis and Chart Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Lu, J.F. Research progress on the physiological and ecological characteristics and cultivation techniques of direct seeding rice in the middle and lower reaches of the Yangtze River. Acta Agric. Nucleatae Sin. 2013, 27, 487–494. [Google Scholar]
- Huang, M.; Zhang, W.; Jiang, L.; Zou, Y. Impact of temperature changes on early-rice productivity in a subtropical environment of China. Field Crop. Res. 2013, 146, 10–15. [Google Scholar] [CrossRef]
- Zhu, D.F.; Zhang, Y.P. Analysis on the Causes of Large Area Rotten Seeds and Dead Seedlings of Early Rice in Zhejiang Province Caused by “Late Spring Cold” and Its Prevention and Control and Enlightenment. China Rice 2020, 26, 10–12. [Google Scholar]
- Guo, R.G.; Duan, L.C.; Wu, Z.M.; Li, H.J. Analysis on the Characteristics of Cold and Dew Wind Disasters in Late Spring of Double Season Direct Seeded Rice in Jiangxi Province. J. Jiangxi Agric. 2021, 33, 65–71. [Google Scholar]
- Li, M.Y.; Shi, Q.H.; Pan, X.H. The development trend, existing problems, and countermeasures of direct seeding rice in Jiangxi Province. Mod. Agric. Sci. Technol. 2008, 21, 236–238. [Google Scholar]
- Wu, L.Q.; Cai, Z.H.; Zhang, G.L.; Liu, Y.T.; Zhao, R. The effect of low temperature on the physiological characteristics and root tip anatomical structure of rice seedlings with different cold tolerance. Chin. J. Agrometeorol. 2018, 39, 805–813. [Google Scholar]
- Tjia, T.O.S.; Meitha, K.; Septiani, P.; Awaludin, R.; Sumardi, D. Extracellular self-DNA induces local inhibition of growth, regulates production of reactive oxygen species, and gene expression in rice roots. Biol. Plant. 2023, 67, 9–18. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, J.; Sun, Z.; Su, J.; Luo, X.; Song, J.; Li, P.; Sun, Y.; Yu, C.; Peng, X. Zinc application after low temperature stress promoted rice tillers recovery: Aspects of nutrient absorption and plant hormone regulation. Plant Sci. 2022, 314, 111104. [Google Scholar] [CrossRef]
- Akhtamov, M.; Adeva, C.; Shim, K.C.; Lee, H.S.; Kim, S.H.; Jeon, Y.A.; Luong, N.H.; Kang, J.W.; Lee, J.Y.; Ahn, S.N. Characterization of Quantitative Trait Loci for Germination and Coleoptile Length under Low-Temperature Condition Using Introgression Lines Derived from an Interspecific Cross in Rice. Genes 2020, 11, 1200. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.C.; Liu, X.; Fu, L.; Hou, Y.J.; Du, Y.; Xie, S.; Zhang, C.; et al. Reciprocal Regulation of the TOR Kinase and ABA Receptor Balances Plant Growth and Stress Response. Mol. Cell 2018, 69, 100–112. [Google Scholar] [CrossRef]
- Wang, X.L.; Qin, R.R.; Sun, R.H.; Hou, X.G.; Qi, L.; Shi, J. Effects of plant population density and root-induced cytokinin on the corn compensatory growth during post-drought rewatering. PLoS ONE 2018, 13, e0198878. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, J.; Li, B.Y.; Zhou, X.Y.; Gu, F.L.; Xiang, R.Y.; Lei, L.X.; Tang, R.Y.; Zhao, Z.W. Effects of low temperature stress on cold tolerance and physiological and biochemical indexes of different Hybrid rice combinations at seedling stage. Jiangsu Agric. Sci. 2020, 48, 59–63. [Google Scholar]
- Cai, J.; Zeng, Z.; Connor, J.N.; Huang, C.Y.; Melino, V.; Kumar, P.; Miklavcic, S.J. RootGraph: A graphic optimization tool for automated image analysis of plant roots. J. Exp. Bot. 2015, 66, 6551–6562. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of Low Temperature Stress on Spikelet-Related Parameters during Anthesis in Indica-Japonica Hybrid Rice. Front. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Huang, W.N.; Zhang, X.X.; Gao, Y.; Li, A.H.; Dai, Y.; Yu, L.; Liu, G.Q.; Pan, C.H.; Li, Y.H.; et al. Fine mapping of qRC10-2, a quantitative trait locus for cold tolerance of rice roots at seedling and mature stages. PLoS ONE 2014, 9, e96046. [Google Scholar] [CrossRef] [PubMed]
- Shimono, H.; Okada, M. Plasticity of rice tiller production is related to genotypic variation in the biomass response to elevated atmospheric CO2 concentration and low temperatures during vegetative growth. Environ. Exp. Bot. 2013, 87, 227–234. [Google Scholar] [CrossRef]
- Liu, C.T.; Wang, W.; Mao, B.G.; Chu, C.C. Cold stress tolerance in rice: Physiological changes, molecular mechanism, and future prospects. Yi Chuan 2018, 40, 171–185. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, L.; Liu, C.; Chen, Z.; Guan, S.; Ma, W.; Pan, G. Low-temperature stress affects reactive oxygen species, osmotic adjustment substances, and antioxidants in rice (Oryza sativa L.) at the reproductive stage. Sci. Rep. 2022, 12, 6224. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z.; Feng, K.; Ji, Y.; Xu, Y.; Tu, D.; Teng, B.; Liu, Q.; Liu, J.; Zhou, Y.; et al. Strategies for indica rice adapted to high-temperature stress in the middle and lower reaches of the Yangtze River. Front. Plant Sci. 2022, 13, 1081807. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Zhao, Y.; Xu, Y.; Song, S.; Chong, K. Comparative metabolomic analysis reveals a reactive oxygen species-dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol. 2016, 211, 1295–1310. [Google Scholar] [CrossRef]
- Zou, J.; Yang, L.; Li, Y.; Piao, M.; Li, Y.; Yao, N.; Zhang, X.; Zhang, Q.; Hu, G.; Yang, D.; et al. Comparative Proteomics Combined with Morphophysiological Analysis Revealed Chilling Response Patterns in Two Contrasting Maize Genotypes. Cells 2022, 11, 1321. [Google Scholar] [CrossRef]
- Wang, X.; Shan, X.; Wu, Y.; Su, S.; Li, S.; Liu, H.; Han, J.; Xue, C.; Yuan, Y. iTRAQ-based quantitative proteomic analysis reveals new metabolic pathways responding to chilling stress in maize seedlings. J. Proteom. 2016, 146, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, L.; Fu, X.; Huang, S.; Fu, H.; Shi, X.; Hu, L.; Cai, Y.; He, H.; Chen, X. Physiological and Transcriptomic Analyses Reveal the Mechanisms of Compensatory Growth Ability for Early Rice after Low Temperature and Weak Light Stress. Plants 2022, 11, 2523. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z.; Guo, T. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seqapproaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Muhlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature responses of roots: Impact on growth, root system architecture and implications for phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Seghal, A.; Jumaa, S.; Bheemanahalli, R.; Kakar, N.; Redoña, E.D.; Wijewardana, C.; Alsajri, F.A.; Chastain, D.; Gao, W.; et al. Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance. Agronomy 2021, 11, 112. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Papasotiropoulos, V.; Katsileros, A.; Zrenner, R.M.; Hincha, D.K.; Zuther, E.; Schwarz, D. Rootstock Sub-Optimal Temperature Tolerance Determines Transcriptomic Responses after Long-Term Root Cooling in Rootstocks and Scions of Grafted Tomato Plants. Front. Plant Sci. 2017, 8, 911. [Google Scholar] [CrossRef]
- Nawaz, G.; Han, Y.; Usman, B.; Liu, F.; Qin, B.; Li, R. Knockout of OsPRP1, a gene encoding proline-rich protein, confers enhanced cold sensitivity in rice (Oryza sativa L.) at the seedling stage. 3 Biotech 2019, 9, 254. [Google Scholar] [CrossRef]
- Longkumer, T.; Chen, C.; Biancucci, M.; Bhaskara, G.B.; Verslues, P.E. Spatial differences in stoichiometry of EGR phosphatase and Microtubule-associated Stress Protein 1 control root meristem activity during drought stress. Plant Cell 2022, 34, 742–758. [Google Scholar] [CrossRef]
- Li, W.Y.; Chen, B.X.; Chen, Z.J.; Gao, Y.T.; Chen, Z.; Liu, J. Reactive Oxygen Species Generated by NADPH Oxidases Promote Radicle Protrusion and Root Elongation during Rice Seed Germination. Int. J. Mol. Sci. 2017, 18, 110. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, Q.; Wu, Z.; Jiang, W.; Wang, L.; Zhang, Q.; Liu, J.; Guo, D.; Hu, X.; Zhang, Z.; et al. Functional characterization of OsCAT2 gene in rice that regulates ROS scavenging and plant growth and development. Plant Growth Regul. 2023. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, L.; Fu, X.; Huang, S.; Zhao, D.; He, H.; Chen, X. Physiological analysis reveals the mechanism of accelerated growth recovery for rice seedlings by nitrogen application after low temperature stress. Front. Plant Sci. 2023, 14, 1133592. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Sola-Landa, A.; Barreiro, C. RNA-Seq-Based Comparative Transcriptomics: RNA Preparation and Bioinformatics. In Microbial Steroids: Methods and Protocols; Humana: New York, NY, USA, 2017; Volume 1645, pp. 59–72. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Pandit, E.; Nayak, D.K.; Behera, L.; Mohapatra, T. Genes, pathways and transcription factors involved in seedling stage chilling stress tolerance in indica rice through RNA-Seq analysis. BMC Plant Biol. 2019, 19, 352. [Google Scholar] [CrossRef] [PubMed]
- Jawahir, V.; Zolman, B.K. Long chain acyl CoA synthetase 4 catalyzes the first step in peroxisomal indole-3-butyric acid to IAA conversion. Plant Physiol. 2021, 185, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Manzano, C.; Pallero-Baena, M.; Casimiro, I.; De Rybel, B.; Orman-Ligeza, B.; Van Isterdael, G.; Beeckman, T.; Draye, X.; Casero, P.; Del, P.J. The Emerging Role of Reactive Oxygen Species Signaling during Lateral Root Development. Plant Physiol. 2014, 165, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; He, C.; Ma, Y.; Herde, M.; Ding, Z. Jasmonic Acid Enhances Al-Induced Root Growth Inhibition. Plant Physiol. 2017, 173, 1420–1433. [Google Scholar] [CrossRef]
- Wang, R.; Estelle, M. Diversity and specificity: Auxin perception and signaling through the TIR1/AFB pathway. Curr. Opin. Plant Biol. 2014, 21, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 14817. [Google Scholar] [CrossRef] [PubMed]
- Naser, V.; Shani, E. Auxin response under osmotic stress. Plant Mol. Biol. 2016, 91, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of Low Temperature at Booting Stage on Sucrose Metabolism and Endogenous Hormone Contents in Winter Wheat Spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, G.M.; Thomas, J.; Liyanage, R.; Lay, J.O.; Basu, S.; Ramegowda, V.; Do, A.M.; Benitez, L.C.; Bolacel, B.E.; Pereira, A. Cold tolerance response mechanisms revealed through comparative analysis of gene and protein expression in multiple rice genotypes. PLoS ONE 2019, 14, e0218019. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shen, Y.; Yang, Q.Q.; Jiang, L. Study on meteorological indicators of late spring cold in Jiangsu province from 1981 to 2020. Henan J. Sci. 2021, 39, 1333–1339. [Google Scholar]
- Khasanova, A.; Lovell, J.T.; Bonnette, J.; Weng, X.; Jenkins, J.; Yoshinaga, Y.; Schmutz, J.; Juenger, T.E. The Genetic Architecture of Shoot and Root Trait Divergence Between Mesic and Xeric Ecotypes of a Perennial Grass. Front. Plant Sci. 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, E.A. Ropls: PCA, PLS(-DA) and OPLS(-DA) for Multivariate Analysis and Feature Selection of Omics Data. R Package Version1.0. 2016. Available online: https://bioconductor.statistik.tu-dortmund.de/packages/3.6/bioc/vignettes/ropls/inst/doc/ropls-vignette (accessed on 25 October 2023).
- Ivanisevic, J.; Want, E.J. From Samples to Insights into Metabolism: Uncovering Biologically Relevant Information in LC-HRMS Metabolomics Data. Metabolites 2019, 9, 308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Zhong, L.; Wang, H.; He, H.; Chen, X. Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology. Int. J. Mol. Sci. 2023, 24, 17359. https://doi.org/10.3390/ijms242417359
Fu X, Zhong L, Wang H, He H, Chen X. Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology. International Journal of Molecular Sciences. 2023; 24(24):17359. https://doi.org/10.3390/ijms242417359
Chicago/Turabian StyleFu, Xiaoquan, Lei Zhong, Hui Wang, Haohua He, and Xiaorong Chen. 2023. "Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology" International Journal of Molecular Sciences 24, no. 24: 17359. https://doi.org/10.3390/ijms242417359
APA StyleFu, X., Zhong, L., Wang, H., He, H., & Chen, X. (2023). Elucidation of the Mechanism of Rapid Growth Recovery in Rice Seedlings after Exposure to Low-Temperature Low-Light Stress: Analysis of Rice Root Transcriptome, Metabolome, and Physiology. International Journal of Molecular Sciences, 24(24), 17359. https://doi.org/10.3390/ijms242417359





