The Plasma Membrane H+ ATPase CsPMA2 Regulates Lipid Droplet Formation, Appressorial Development and Virulence in Colletotrichum siamense
Abstract
:1. Introduction
2. Results
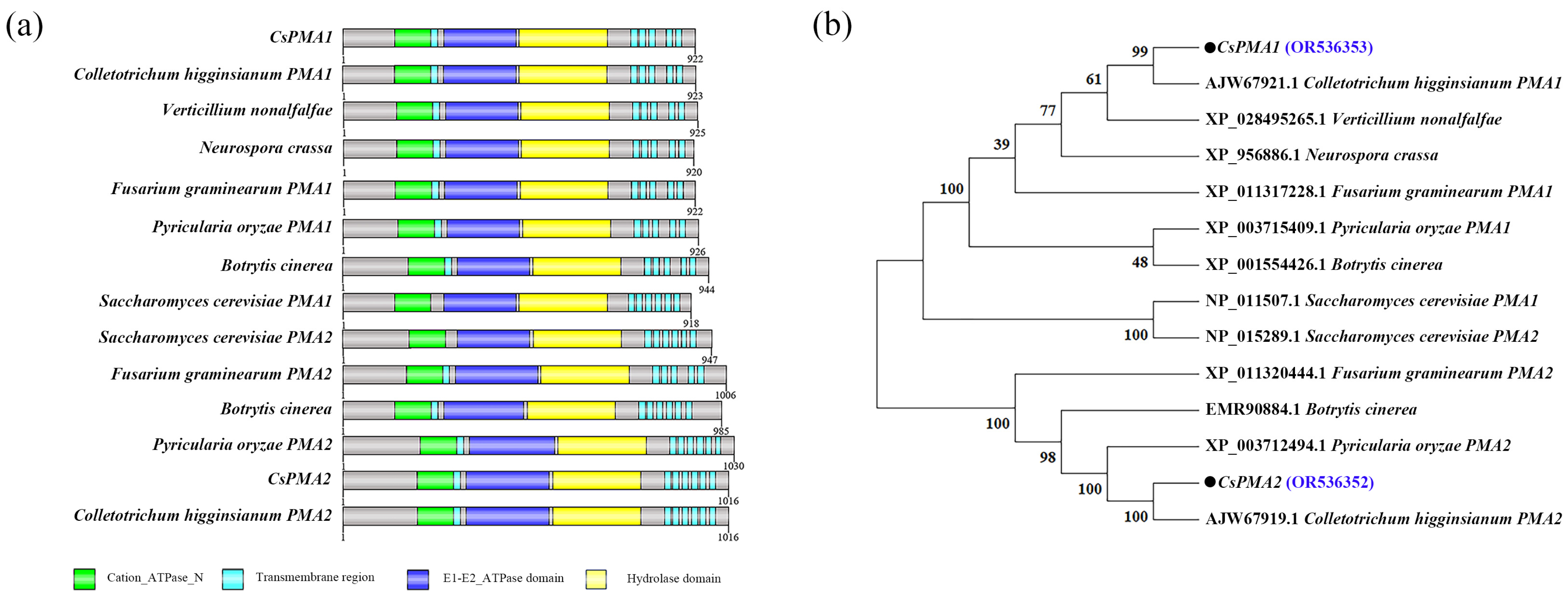
2.1. Identification and Deletion of the CsPMA2 Gene in C. siamense
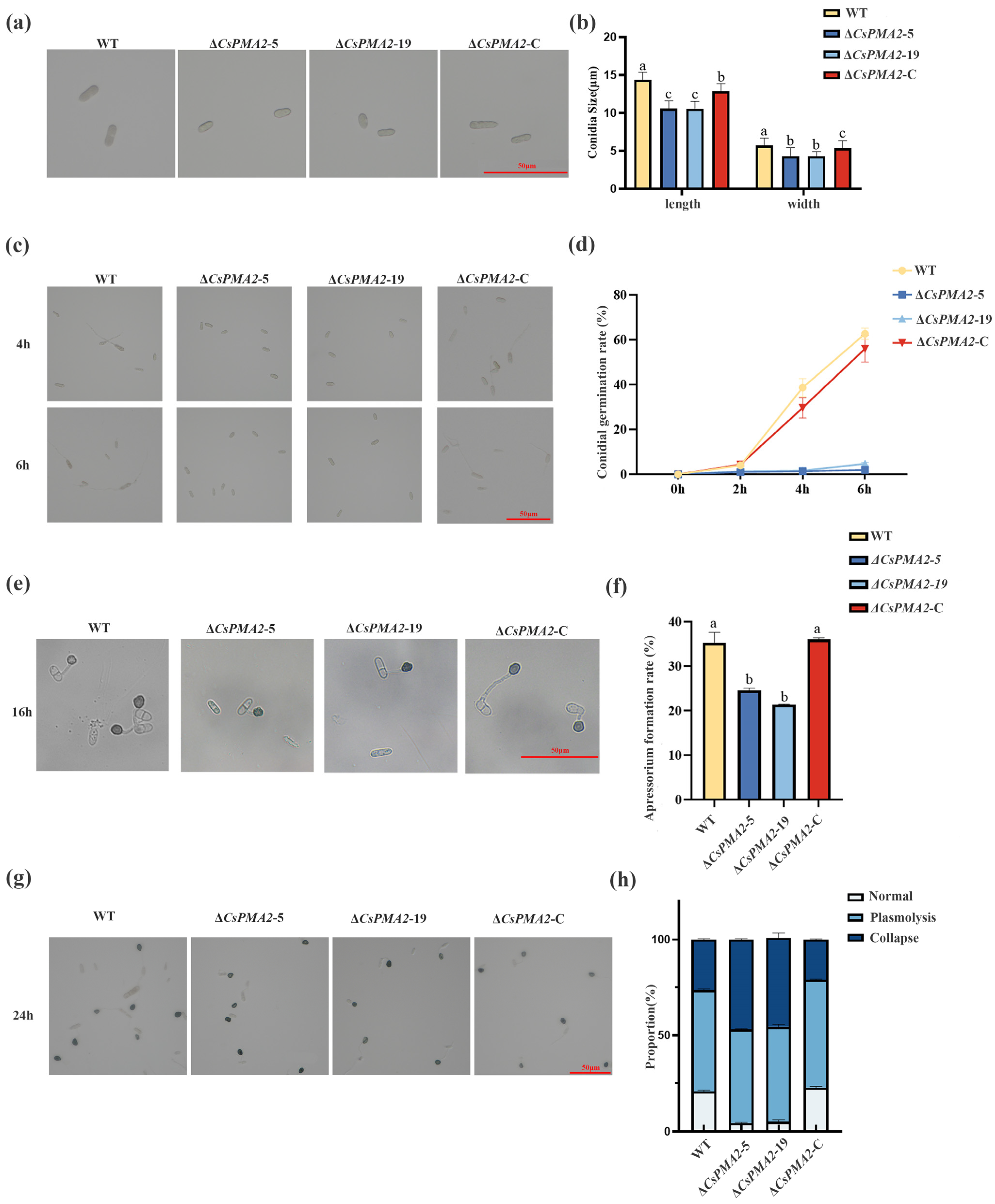
2.2. CsPMA2 Deletion Mutants Showed Small Conidia, Decreased Conidial Germination and Appressorium Formation Rates, and Retarded Appressoria Development in C. siamense
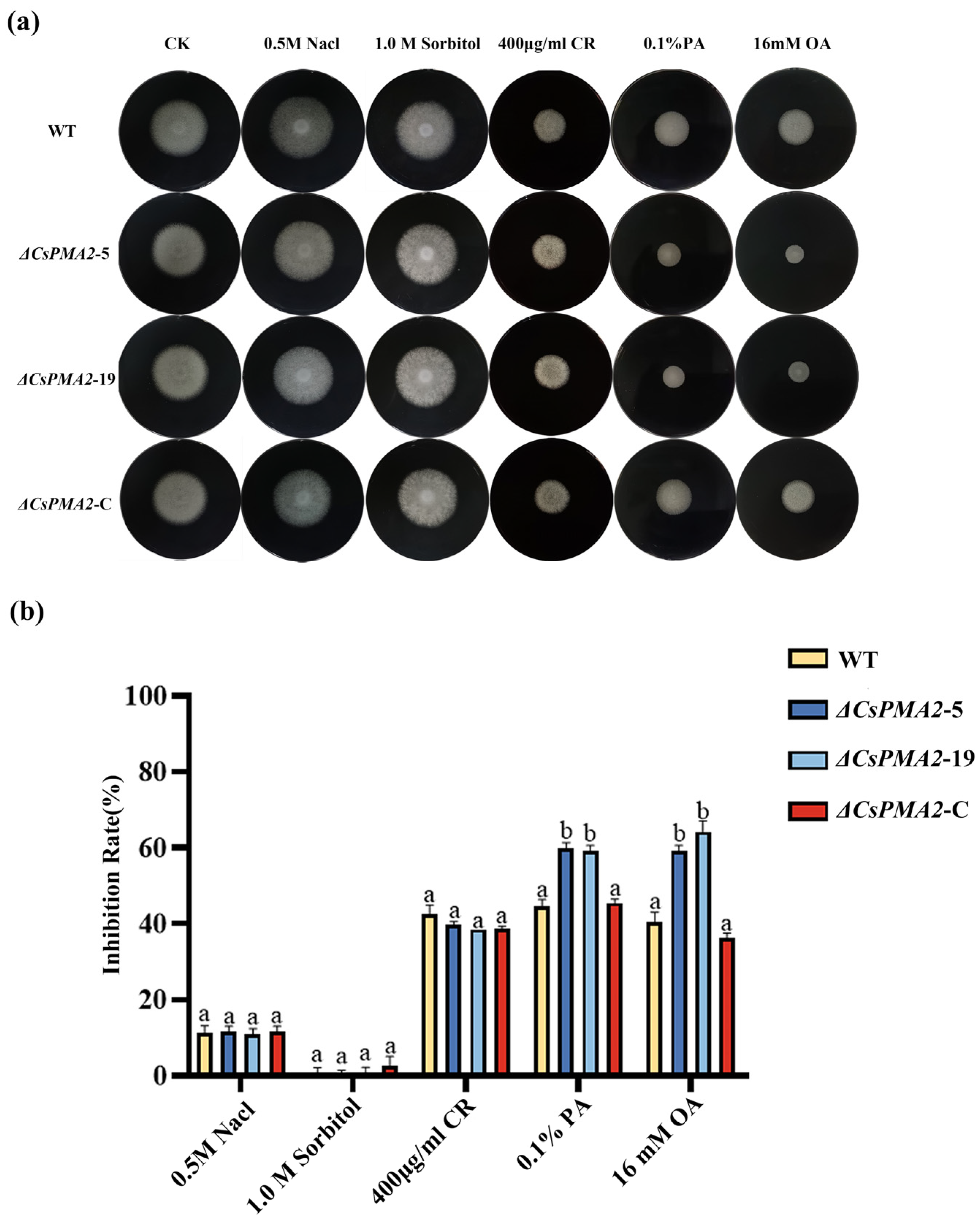
2.3. CsPMA2 Gene Is Involved in the Response to Acid Stress
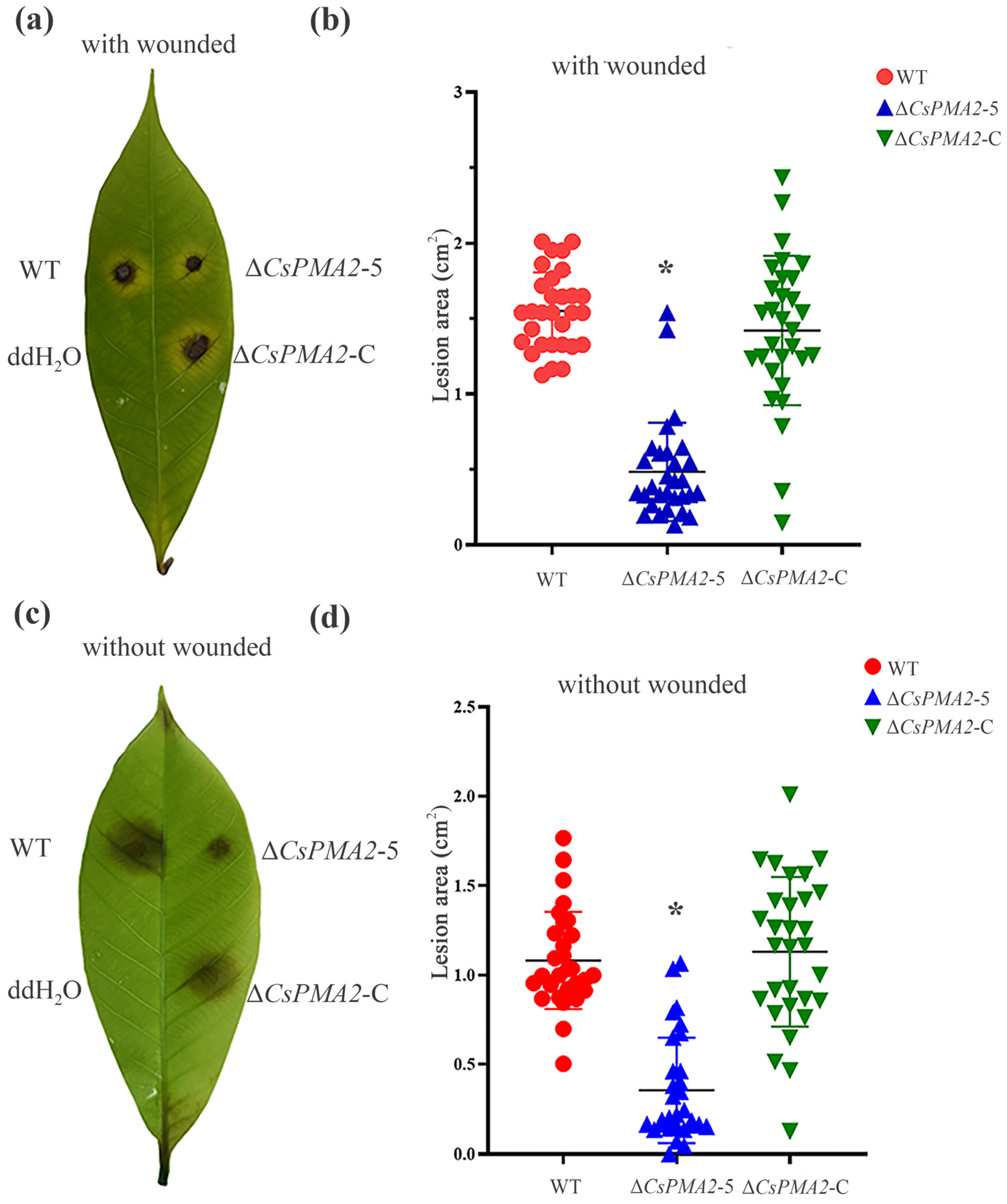
2.4. CsPMA2 Deletion Attenuates the Virulence of C. siamense
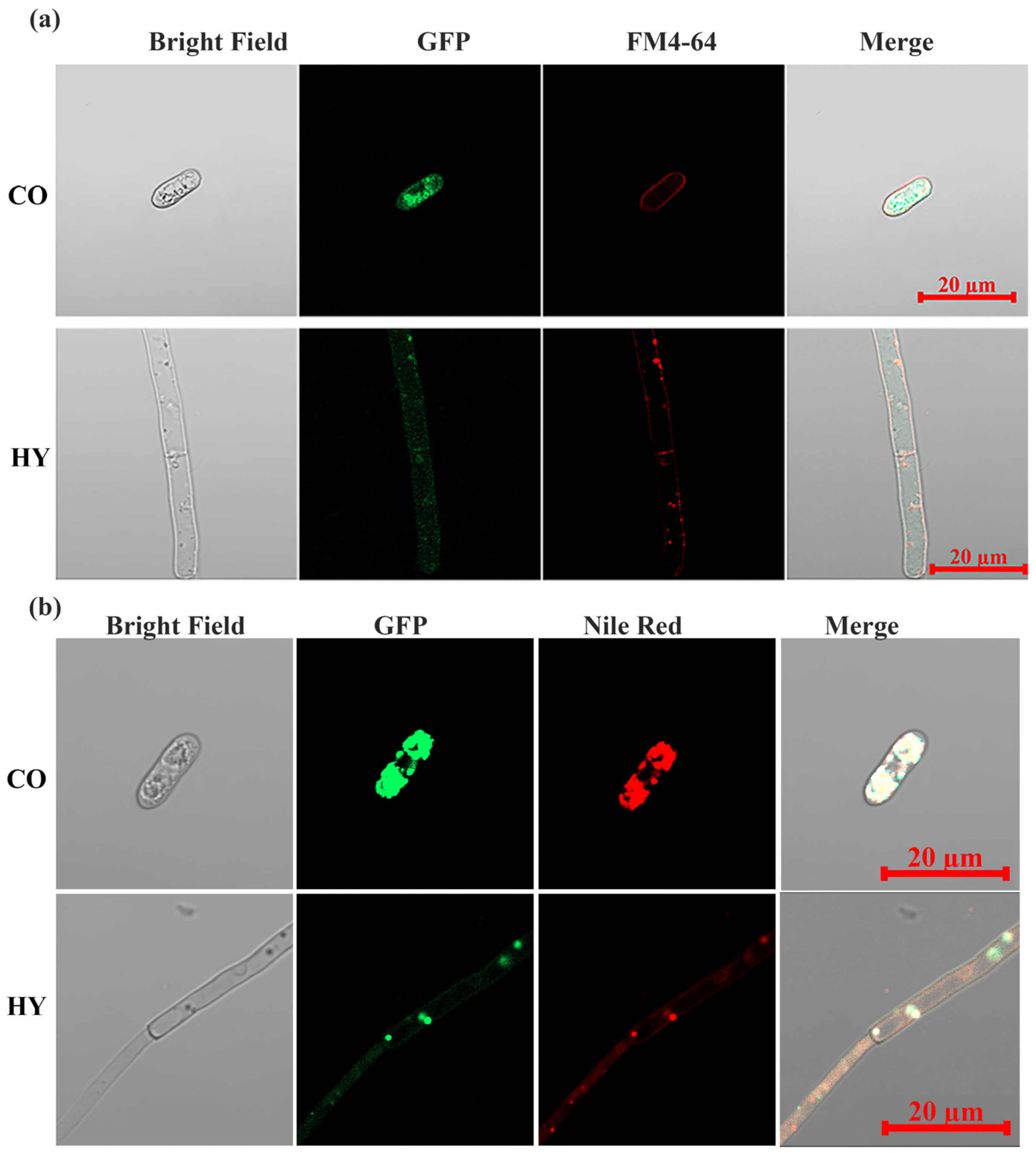
2.5. CsPMA2 Is Localized to Lipids on the Plasma Membrane and Intracellular
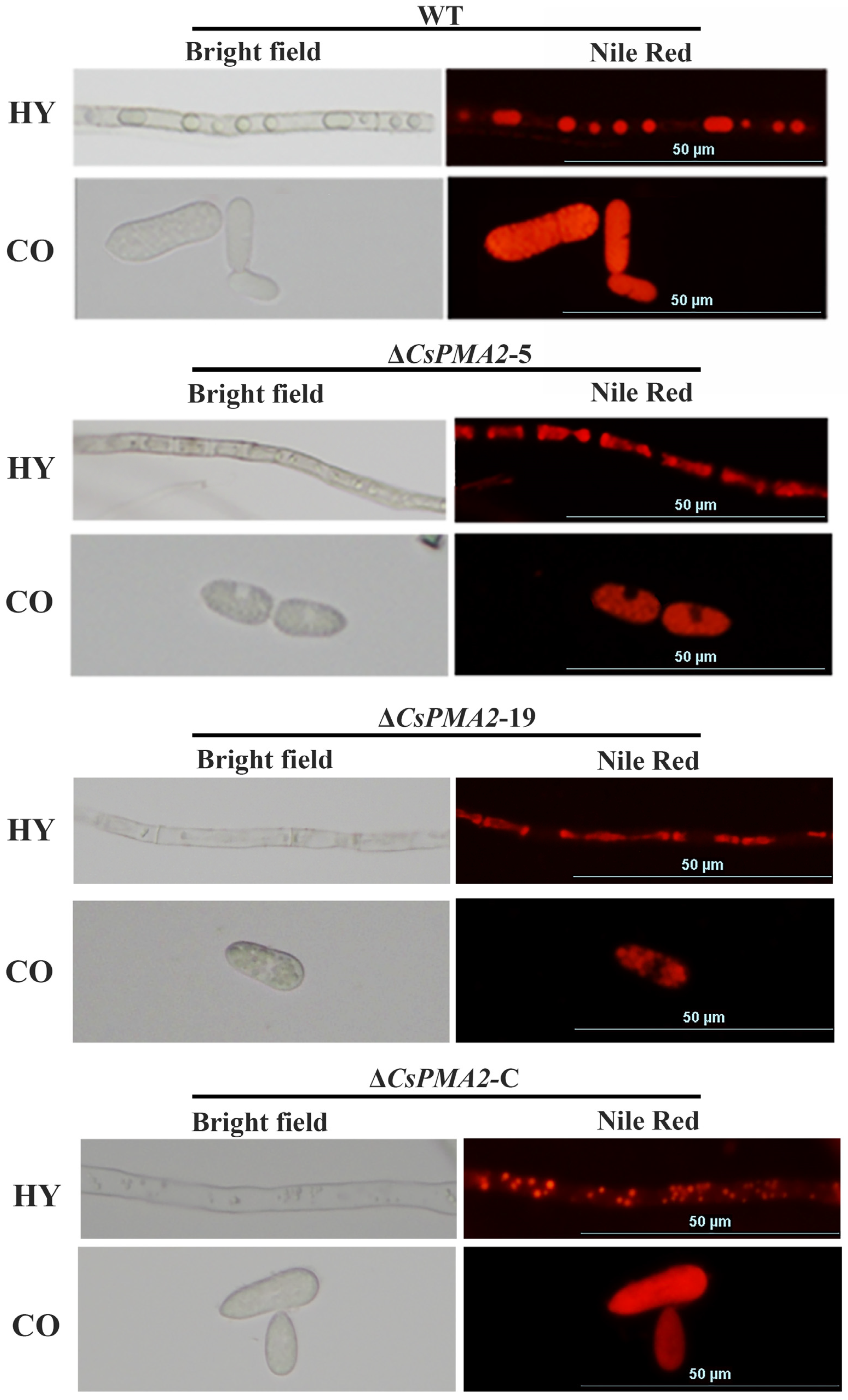
2.6. CsPMA2 Is Needed for Lipid Droplet Stability and the Content Balance of Some Lipid Species in C. siamense
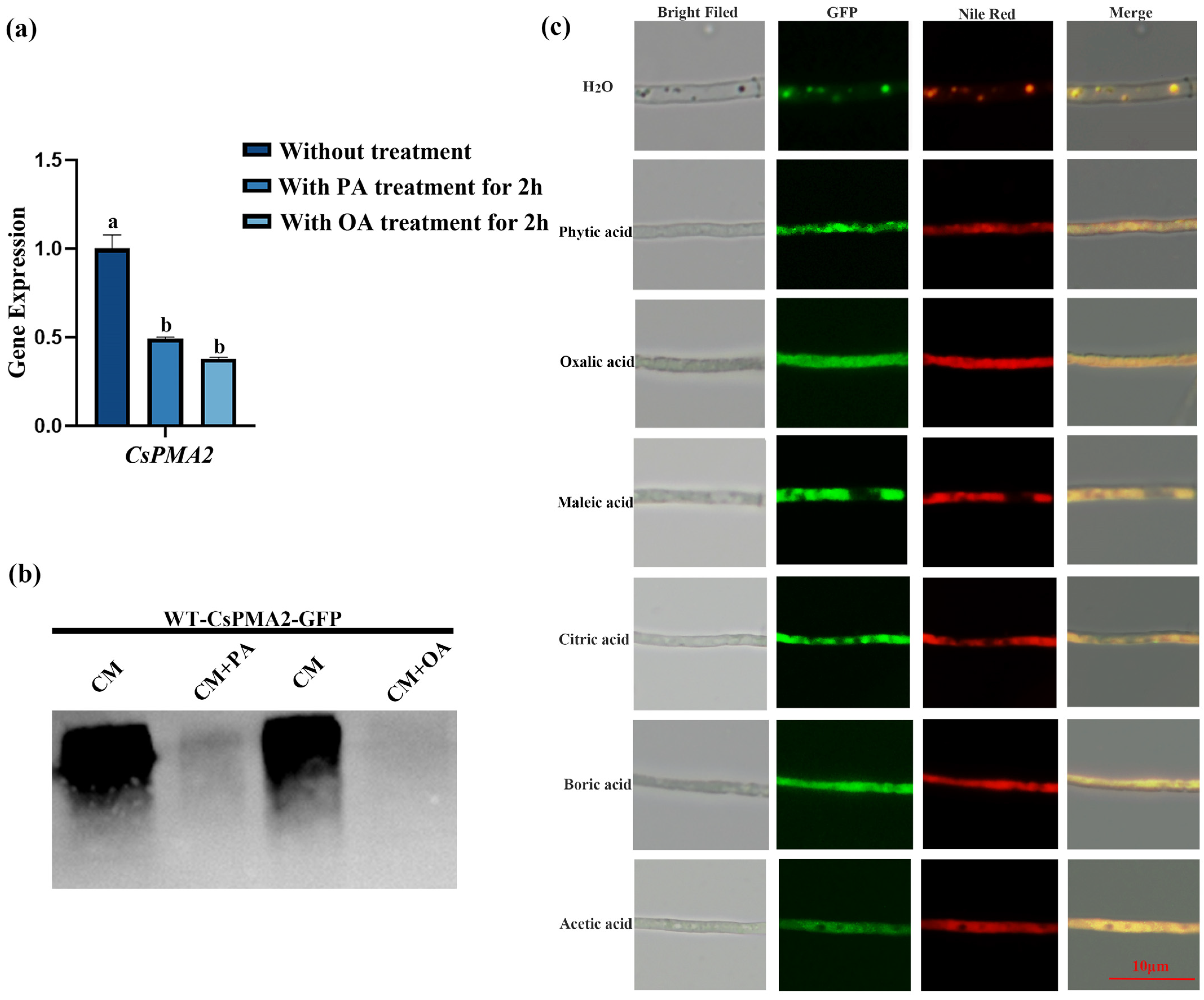
2.7. Acid Stress Affects the Expression of CsPMA2 and the Accumulation of Lipid Droplets
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. CsPMA2 Gene Cloning and Sequence Analysis
4.3. Targeted Disruption of CsPMA2, Complementation and Protein Subcellular Localization
4.4. Phenotypic Characterization
4.5. Fluorescence Microscopy Observation
4.6. Lipid Content Determination
4.7. Quantitative Real-Time PCR (qRT–PCR) Analysis and Western Blot Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano, R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim. Biophys. Acta 1988, 947, 1–28. [Google Scholar] [CrossRef]
- Dyla, M.; Basse Hansen, S.; Nissen, P.; Kjaergaard, M. Structural dynamics of P-type ATPase ion pumps. Biochem. Soc. Trans. 2019, 47, 1247–1257. [Google Scholar] [CrossRef]
- Portillo, F. Regulation of plasma membrane H(+)-ATPase in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Palmgren, M. Proton and calcium pumping P-type ATPases and their regulation of plant responses to the environment. Plant Physiol. 2021, 187, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R.; Kielland-Brandt, M.C.; Fink, G.R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 1986, 319, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Ambesi, A.; Miranda, M.; Petrov, V.V.; Slayman, C.W. Biogenesis and function of the yeast plasma-membrane H(+)-ATPase. J. Exp. Biol. 2000, 203, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Requena, N.; Breuninger, M.; Franken, P.; Ocon, A. Symbiotic status, phosphate, and sucrose regulate the expression of two plasma membrane H+-ATPase genes from the mycorrhizal fungus Glomus mosseae. Plant Physiol. 2003, 132, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Yu, N.; Bano, S.A.; Liu, C.; Miller, A.J.; Cousins, D.; Zhang, X.; Ratet, P.; Tadege, M.; Mysore, K.S.; et al. A H+-ATPase That energizes nutrient uptake during mycorrhizal symbioses in rice and Medicago truncatula. Plant Cell 2014, 26, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Lavy, K.; Kupiec, M. Glucose inhibits yeast AMPK (Snf1) by three independent mechanisms. Biology 2023, 12, 1007. [Google Scholar] [CrossRef]
- Ghislain, M.; Goffeau, A. The pma1 and pma2 H(+)-ATPases from Schizosaccharomyces pombe are functionally interchangeable. J. Biol. Chem. 1991, 266, 18276–18279. [Google Scholar] [CrossRef]
- Zhgun, A.; Dumina, M.; Valiakhmetov, A.; Eldarov, M. The critical role of plasma membrane H+-ATPase activity in cephalosporin C biosynthesis of Acremonium chrysogenum. PLoS ONE 2020, 15, e0238452. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.R.; Mariscal, M.; Serrano, A.; Segorbe, D.; Fernandez-Acero, T.; Martin, H.; Turra, D.; Di Pietro, A. Cytosolic pH controls fungal MAPK signaling and pathogenicity. mBio 2023, 14, e0028523. [Google Scholar] [CrossRef] [PubMed]
- Schlesser, A.; Ulaszewski, S.; Ghislain, M.; Goffeau, A. A second transport ATPase gene in Saccharomyces cerevisiae. J. Biol. Chem. 1988, 263, 19480–19487. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; Garciadeblas, B.; Perez-Martin, J.; Rodriguez-Navarro, A. Growth at high pH and sodium and potassium tolerance in media above the cytoplasmic pH depend on ENA ATPases in Ustilago maydis. Eukaryot. Cell 2009, 8, 821–829. [Google Scholar] [CrossRef]
- Young, M.R.; Heit, S.; Bublitz, M. Structure, function and biogenesis of the fungal proton pump Pma1. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1871, 119600. [Google Scholar] [CrossRef]
- Holsbeeks, I.; Lagatie, O.; Van Nuland, A.; Van de Velde, S.; Thevelein, J.M. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem. Sci. 2004, 29, 556–564. [Google Scholar] [CrossRef]
- Vazquez-Carrada, M.; Feldbrugge, M.; Olicon-Hernandez, D.R.; Guerra-Sanchez, G.; Pardo, J.P. Functional analysis of the plasma membrane H(+)-ATPases of Ustilago maydis. J. Fungi 2022, 8, 550. [Google Scholar] [CrossRef]
- Bowman, B.J.; Bowman, E.J. H+-ATPases from mitochondria, plasma membranes, and vacuoles of fungal cells. J. Membr. Biol. 1986, 94, 83–97. [Google Scholar] [CrossRef]
- Wu, L.; Yuan, Z.; Wang, P.; Mao, X.; Zhou, M.; Hou, Y. The plasma membrane H(+) -ATPase FgPMA1 regulates the development, pathogenicity, and phenamacril sensitivity of Fusarium graminearum by interacting with FgMyo-5 and FgBmh2. Mol. Plant Pathol. 2022, 23, 489–502. [Google Scholar] [CrossRef]
- Balhadere, P.V.; Talbot, N.J. PDE1 encodes a P-type ATPase involved in appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 2001, 13, 1987–2004. [Google Scholar] [CrossRef]
- Remy, E.; Meyer, M.; Blaise, F.; Chabirand, M.; Wolff, N.; Balesdent, M.H.; Rouxel, T. The Lmpma1 gene of Leptosphaeria maculans encodes a plasma membrane H+-ATPase isoform essential for pathogenicity towards oilseed rape. Fungal Genet. Biol. 2008, 45, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Kane, P.M. Proton transport and pH control in Fungi. Adv. Exp. Med. Biol. 2016, 892, 33–68. [Google Scholar] [CrossRef] [PubMed]
- Korn, M.; Schmidpeter, J.; Dahl, M.; Muller, S.; Voll, L.M.; Koch, C. A genetic screen for pathogenicity genes in the hemibiotrophic fungus Colletotrichum higginsianum identifies the plasma membrane proton pump Pma2 required for host penetration. PLoS ONE 2015, 10, e0125960. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.G.; Kim, Y.Y.; Lee, G.; Kim, J.B. Physiological and pathological roles of lipogenesis. Nat. Metab. 2023, 5, 735–759. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, T.; Lesmes, U.; Weiss, J.; McClements, D.J. Modulation of physicochemical properties of lipid droplets using β-lactoglobulin and/or lactoferrin interfacial coatings. Food Hydrocoll. 2011, 25, 1181–1189. [Google Scholar] [CrossRef]
- Teixeira, V.; Martins, T.S.; Prinz, W.A.; Costa, V. Target of rapamycin complex 1 (TORC1), protein kinase A (PKA) and cytosolic pH regulate a transcriptional circuit for lipid droplet formation. Int. J. Mol. Sci. 2021, 22, 9017. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.; Jeon, J.; Kim, K.S.; Park, J.; Park, S.Y.; Lee, Y.H. The PEX7-mediated peroxisomal import system is required for fungal development and pathogenicity in Magnaporthe oryzae. PLoS ONE 2011, 6, e28220. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Sangappillai, V.; Nadarajah, K. Fatty acid synthase beta dehydratase in the lipid biosynthesis pathway is required for conidiogenesis, pigmentation and appressorium formation in Magnaporthe oryzae S6. Int. J. Mol. Sci. 2020, 21, 7224. [Google Scholar] [CrossRef]
- Chen, D.; Cai, X.; Xing, J.; Chen, S.; Zhao, J.; Qu, Z.; Li, G.; Liu, H.; Zheng, L.; Huang, J.; et al. A lipid droplet-associated protein Nem1 regulates appressorium function for infection of Magnaporthe oryzae. aBIOTECH 2023, 4, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Cannon, P.F.; Simmons, C.M. Diversity and host preference of leaf endophytic fungi in the Iwokrama Forest Reserve, Guyana. Mycologia 2002, 94, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Petan, T. Lipid droplets and the management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452. [Google Scholar] [PubMed]
- Wang, C.; St Leger, R.J. The Metarhizium anisopliae perilipin homolog MPL1 regulates lipid metabolism, appressorial turgor pressure, and virulence. J. Biol. Chem. 2007, 282, 21110–21115. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, J.; Lu, J.; Liu, Y.; Xi, Y.; Song, M.; Guan, X.; Li, Z.; Li, X.; Zhang, Y.; et al. The Colletotrichum siamense hydrophobin CsHydr1 interacts with the lipid droplet-coating protein CsCap20 and regulates lipid metabolism and virulence. J. Fungi 2022, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhao, C.; Chen, D.; Yun, C.; Li, H.; Bai, L. Structure and activation mechanism of the hexameric plasma membrane H(+)-ATPase. Nat. Commun. 2021, 12, 6439. [Google Scholar] [CrossRef]
- Kono, Y.; Ishibashi, Y.; Fukuda, S.; Higuchi, T.; Tani, M. Simultaneous structural replacement of the sphingoid long-chain base and sterol in budding yeast. FEBS J. 2023, 290, 5605–5627. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef]
- Liu, X.H.; Gao, H.M.; Xu, F.; Lu, J.P.; Devenish, R.J.; Lin, F.C. Autophagy vitalizes the pathogenicity of pathogenic fungi. Autophagy 2012, 8, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, B.; Cai, J.; Zheng, X.; Feng, Y.; Huang, G. Colletotrichum species causing anthracnose of rubber trees in China. Sci. Rep. 2018, 8, 10435. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Li, G.H.; Zhao, P.J. Appressoria-small but incredibly powerful structures in plant-pathogen interactions. Int. J. Mol. Sci. 2023, 24, 2141. [Google Scholar] [CrossRef]
- Kim, J.W.; Shim, S.H. The fungus Colletotrichum as a source for bioactive secondary metabolites. Arch. Pharm. Res. 2019, 42, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Chung, J.; Farese, R.V., Jr. Lipid droplet biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yan, J.; Liu, C.; Xing, J.; Ren, Z.; Hendy, A.; Zheng, L.; Huang, J.; Chen, X.L. Perilipin LDP1 coordinates lipid droplets formation and utilization for appressorium-mediated infection in Magnaporthe oryzae. Environ. Microbiol. 2020, 22, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, D.; Jiang, K.; Zhang, K.Q.; Yang, J. AoPEX1 and AoPEX6 are required for mycelial growth, conidiation, stress response, fatty acid utilization, and trap formation in Arthrobotrys oligospora. Microbiol. Spectr. 2022, 10, e0027522. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Li, X.; Li, Y.N.; Li, Y.; Wu, S.; Xu, L.; Zhou, R.; Yang, J.; Li, G.; et al. MoLrp1-mediated signaling induces nuclear accumulation of MoMsn2 to facilitate fatty acid oxidation for infectious growth of the rice blast fungus. Plant Commun. 2023, 4, 100561. [Google Scholar] [CrossRef]
- Gaspar, M.L.; Aregullin, M.A.; Chang, Y.F.; Jesch, S.A.; Henry, S.A. Phosphatidic acid species 34:1 mediates expression of the myo-inositol 3-phosphate synthase gene INO1 for lipid synthesis in yeast. J. Biol. Chem. 2022, 298, 102148. [Google Scholar] [CrossRef]
- Romanauska, A.; Kohler, A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell 2018, 174, 700–715.e18. [Google Scholar] [CrossRef]
- Fernandez-Murray, J.P.; Tavasoli, M.; Williams, J.; McMaster, C.R. The leucine zipper domain of the transcriptional repressor Opi1 underlies a signal transduction mechanism regulating lipid synthesis. J. Biol. Chem. 2023, 299, 105417. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fang, S.; Li, Z.; Wang, N.; Li, X.; Liu, W.; Zhang, Y.; Lin, C.; Miao, W. CsAtf1, a bZIP transcription factor, is involved in fludioxonil sensitivity and virulence in the rubber tree anthracnose fungus Colletotrichum siamense. Fungal Genet. Biol. 2022, 158, 103649. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.O.; Elowsky, C.; Pham, N.T.T.; Wilso, R.A. Spermine-mediated tight sealing of the Magnaporthe oryzae appressorial pore–rice leaf surface interface. Nat. Microbiol. 2020, 5, 1472–1480. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xi, Y.; Lv, Y.; Yan, J.; Song, M.; Yang, H.; Zhang, Y.; Miao, W.; Lin, C. The Plasma Membrane H+ ATPase CsPMA2 Regulates Lipid Droplet Formation, Appressorial Development and Virulence in Colletotrichum siamense. Int. J. Mol. Sci. 2023, 24, 17337. https://doi.org/10.3390/ijms242417337
Liu Y, Xi Y, Lv Y, Yan J, Song M, Yang H, Zhang Y, Miao W, Lin C. The Plasma Membrane H+ ATPase CsPMA2 Regulates Lipid Droplet Formation, Appressorial Development and Virulence in Colletotrichum siamense. International Journal of Molecular Sciences. 2023; 24(24):17337. https://doi.org/10.3390/ijms242417337
Chicago/Turabian StyleLiu, Yu, Yitao Xi, Yanyu Lv, Jingting Yan, Miao Song, Hong Yang, Yu Zhang, Weiguo Miao, and Chunhua Lin. 2023. "The Plasma Membrane H+ ATPase CsPMA2 Regulates Lipid Droplet Formation, Appressorial Development and Virulence in Colletotrichum siamense" International Journal of Molecular Sciences 24, no. 24: 17337. https://doi.org/10.3390/ijms242417337
APA StyleLiu, Y., Xi, Y., Lv, Y., Yan, J., Song, M., Yang, H., Zhang, Y., Miao, W., & Lin, C. (2023). The Plasma Membrane H+ ATPase CsPMA2 Regulates Lipid Droplet Formation, Appressorial Development and Virulence in Colletotrichum siamense. International Journal of Molecular Sciences, 24(24), 17337. https://doi.org/10.3390/ijms242417337




