High-Sensitivity Troponin I and Cardiovascular Events in Stable Coronary Artery Disease: Insights from a Longitudinal Outpatient Study
Abstract
:1. Introduction
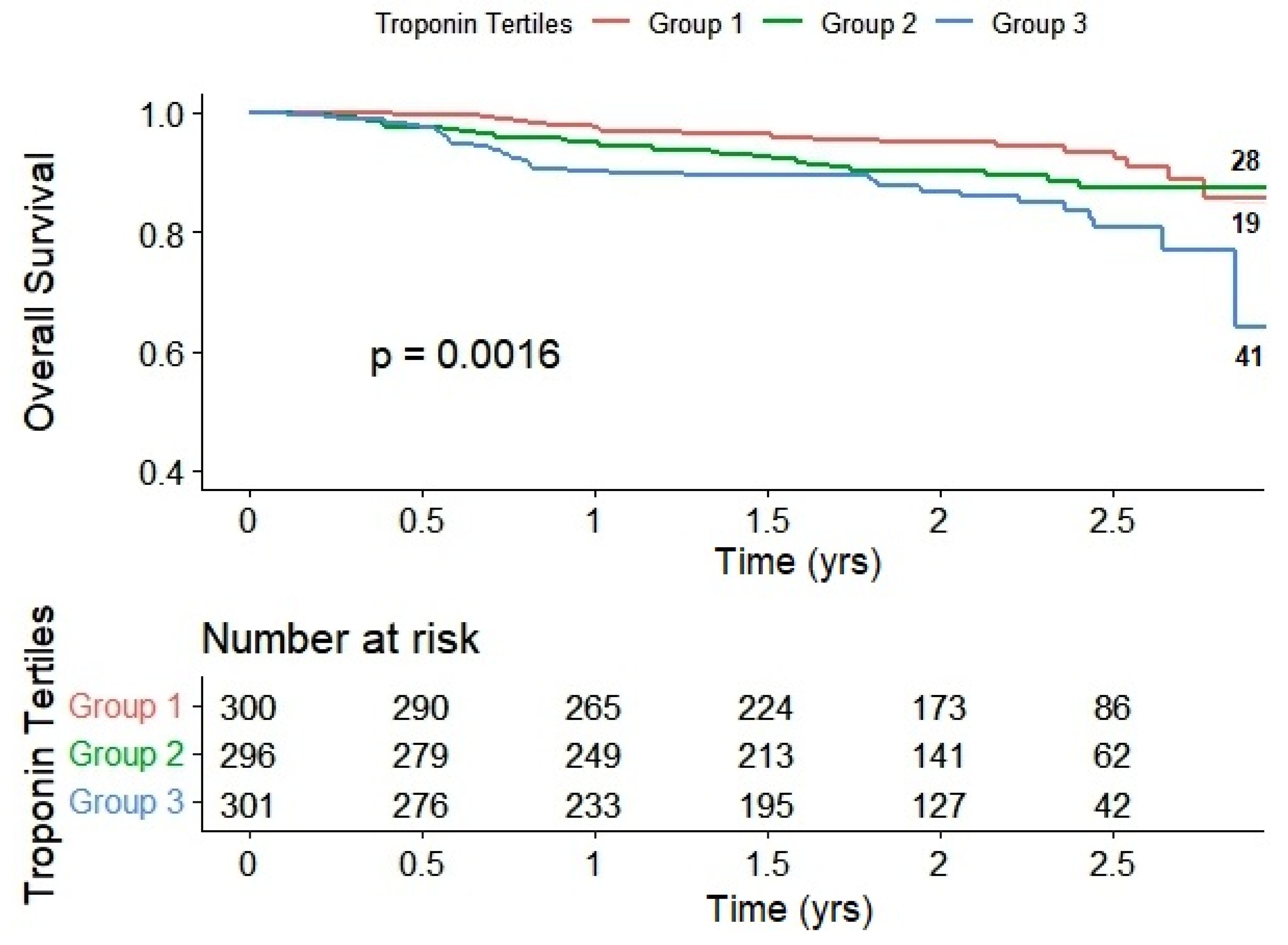
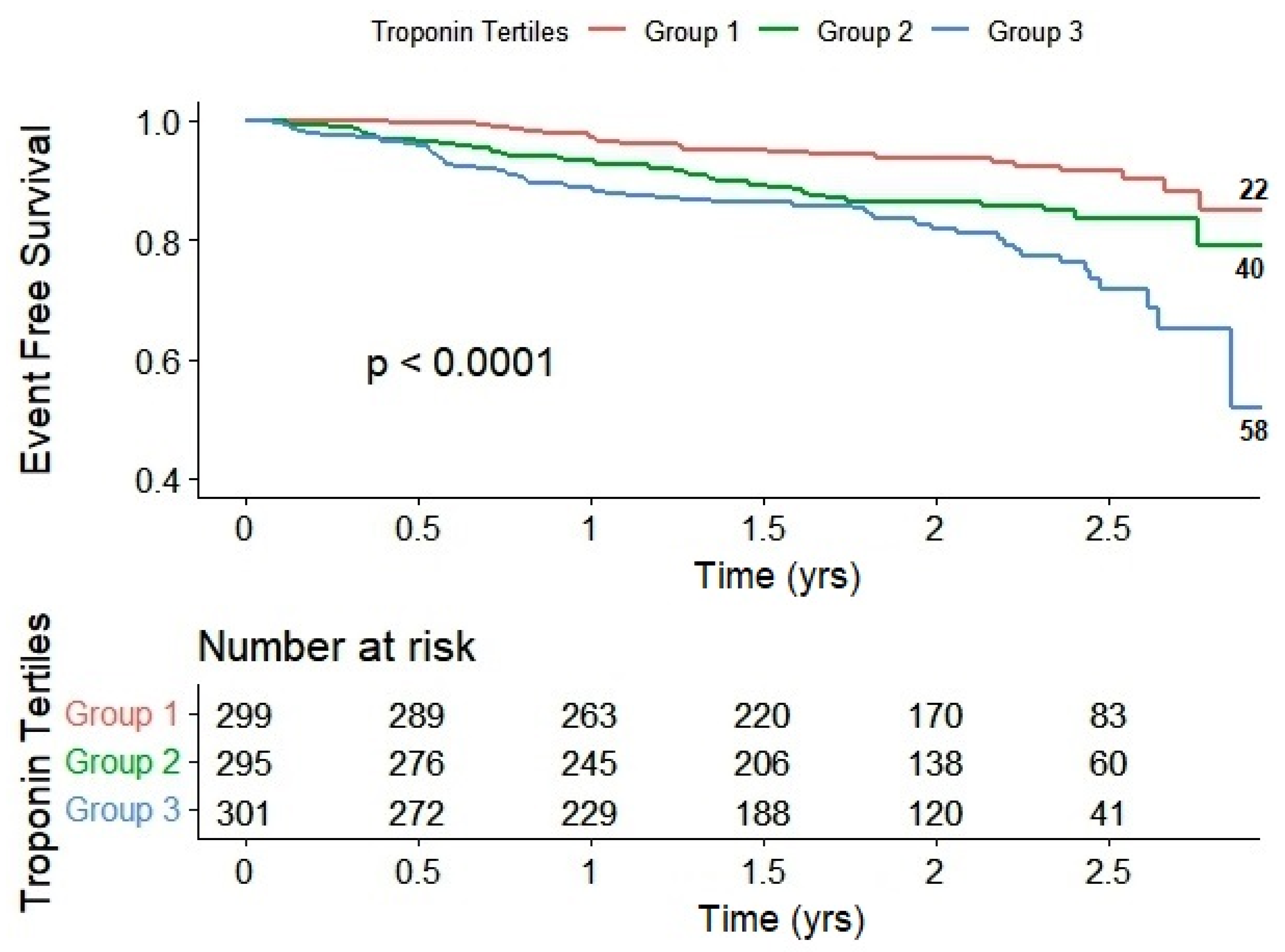
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Laboratory Analyses
4.3. Clinical Endpoints
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF). Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction. Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- DE Gennaro, L.; Brunetti, N.D.; Cuculo, A.; Pellegrino, P.L.; Izzo, P.; Roma, F.; DI Biase, M. Increased Troponin Levels in Nonischemic Cardiac Conditions and Noncardiac Diseases. J. Interv. Cardiol. 2008, 21, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Rahayu, S.R.; Fibriana, A.I.; Susanto, H.; Kartasurya, M.I.; Bahrudin, U. Cardiac Troponin Elevation After Long-Distance Cycling is Associated with Oxidative Stress and Exercise Intensity: An Observational Study. Asian J. Sports Med. 2020, 11, e107053. [Google Scholar] [CrossRef]
- Aldous, S.J.; Florkowski, C.M.; Crozier, I.G.; Elliott, J.; George, P.; Lainchbury, J.G.; Mackay, R.J.; Than, M.; Flaws, D.F.; Borowsky, J. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2011, 48 Pt 3, 241–248, Erratum in Ann. Clin. Biochem. 2012, 49 Pt 2, 208. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S. A New Season for Cardiac Troponin Assays: It’s Time to Keep a Scorecard. Clin. Chem. 2009, 55, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Everett, B.M.; Brooks, M.M.; Vlachos, H.E.; Chaitman, B.R.; Frye, R.L.; Bhatt, D.L.; BARI 2D Study Group. Troponin and Cardiac Events in Stable Ischemic Heart Disease and Diabetes. N. Engl. J. Med. 2015, 373, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Kontos, M.C.; Shah, R.; Fritz, L.M.; Anderson, F.; Tatum, J.L.; Ornato, J.P.; Jesse, R.L. Implication of different cardiac troponin I levels for clinical outcomes and prognosis of acute chest pain patients. J. Am. Coll. Cardiol. 2004, 43, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S. High-Sensitivity Cardiac Troponin for Screening Large Populations of Healthy People: Is There Risk? Clin. Chem. 2011, 57, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Segre, C.A.W.; Hueb, W.; Garcia, R.M.R.; Rezende, P.C.; Favarato, D.; Strunz, C.M.C.; Sprandel, M.d.C.O.; Roggério, A.; Carvalho, A.L.; Maranhão, R.C.; et al. Troponin in diabetic patients with and without chronic coronary artery disease. BMC Cardiovasc. Disord. 2015, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Welsh, P.; Evans, J.D.; Tschiderer, L.; Boachie, C.; Jukema, J.W.; Ford, I.; Trompet, S.; Stott, D.J.; Kearney, P.M.; et al. High-Sensitivity Cardiac Troponin Concentration and Risk of First-Ever Cardiovascular Outcomes in 154,052 Participants. J. Am. Coll. Cardiol. 2017, 70, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Mariathas, M.; Allan, R.; Ramamoorthy, S.; Olechowski, B.; Hinton, J.; Azor, M.; Nicholas, Z.; Calver, A.; Corbett, S.; Mahmoudi, M.; et al. True 99th centile of high sensitivity cardiac troponin for hospital patients: Prospective, observational cohort study. BMJ 2019, 364, l729. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Collinson, P.O.; IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2012, 58, 54–61, Erratum in Clin. Chem. 2012, 58, 796. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Jernberg, T.; Lindahl, B. Cardiac Troponin Elevation in Patients without a Specific Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Jaffe, A.S. The Evolving Role of Cardiac Troponin: From Acute to Chronic Coronary Syndromes. J. Am. Coll. Cardiol. 2023, 82, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Hinton, J.; Mariathas, M.N.; Gabara, L.; Allan, R.; Nicholas, Z.; Kwok, C.S.; Ramamoorthy, S.; Calver, A.; Corbett, S.; Jabbour, R.J.; et al. Association between troponin level and medium-term mortality in 20 000 hospital patients. Heart 2023, 109, 1772–1777. [Google Scholar] [CrossRef]
- Clarke, M.S.F.; Caldwell, R.W.; Chiao, H.; Miyake, K.; McNeil, P.L. Contraction-Induced Cell Wounding and Release of Fibroblast Growth Factor in Heart. Circ. Res. 1995, 76, 927–934. [Google Scholar] [CrossRef]
- Takashio, S.; Yamamuro, M.; Izumiya, Y.; Sugiyama, S.; Kojima, S.; Yamamoto, E.; Tsujita, K.; Tanaka, T.; Tayama, S.; Kaikita, K.; et al. Coronary Microvascular Dysfunction and Diastolic Load Correlate with Cardiac Troponin T Release Measured by a Highly Sensitive Assay in Patients with Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2013, 62, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H. Release of cardiac troponin from healthy and damaged myocardium. Front. Lab. Med. 2017, 1, 144–150. [Google Scholar] [CrossRef]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 949) | Group 1 (0.09–0.1) (n = 316) | Group 2 (0.14–0.2) (n = 316) | Group 3 (0.34–1.12) (n = 317) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 67.5 ± 9.5 | 67.1 ± 9.5 | 68.0 ± 9.5 | 67.3 ± 9.5 | 0.07 |
| Male Sex | 660 (69.5%) | 249 (78.8%) | 215 (68.0%) | 196 (61.8%) | <0.001 |
| Hypertension | 682 (71.9%) | 235 (74.4%) | 231 (73.1%) | 216 (68.1%) | 0.18 |
| Diabetes | 494 (52.1%) | 163 (51.6%) | 167 (52.8%) | 164 (51.7%) | 0.94 |
| Dyslipidemia | 716 (75.4%) | 239 (75.6%) | 244 (77.2%) | 233 (73.5%) | 0.55 |
| Previous MI | 486 (51.6%) | 138 (43.8%) | 161 (51.4%) | 187 (59.2%) | 0.004 |
| Previous Angina | 199 (21%) | 51 (16.1%) | 72 (22.8%) | 76 (24.0%) | 0.03 |
| Smoking | 503 (53.2%) | 165 (52.4%) | 164 (52.2%) | 174 (54.9%) | 0.75 |
| Previous PCI | 383 (40.5%) | 134 (42.4%) | 136 (43.3%) | 113 (35.6%) | 0.16 |
| Previous CABG | 332 (35.1%) | 115 (36.4%) | 116 (36.9%) | 101 (31.9%) | 0.34 |
| Previous HF | 340 (35.9%) | 82 (25.9%) | 110 (34.9%) | 148 (46.7%) | <0.001 |
| LVEF % | 55 (40–63) | 60 (51–65) | 55 (40–61.5) | 43 (35–60) | 0.49 |
| CAD Single-Vessel Double-Vessel Triple-Vessel | 96 (10.6%) 286 (31.5%) 526 (57.9%) | 42 (13.7%) 102 (33.2%) 163 (53.1%) | 26 (8.7%) 87 (29.1%) 186 (62.2%) | 28 (9.3%) 97 (32.1%) 177 (58.6%) | 0.12 |
| Creatinine (mg/dL) | 1.11 (0.95–1.30) | 1.06 (0.9–1.22) | 1.12 (0.94–1.31) | 1.17 (0.98–1.39) | 0.83 |
| Total Cholesterol (mg/dL) | 153 (129–188) | 149 (126–185) | 156 (130–185) | 155 (133–192) | 0.68 |
| LDL-Cholesterol (mg/dL) | 82 (64–109) | 77 (62–105) | 83 (65–108) | 86 (66–113) | 0.58 |
| HDL-Cholesterol (mg/dL) | 42 (36–50) | 42 (35–49) | 42 (36–51) | 42 (36–49) | 0.99 |
| Triglycerides (mg/dL) | 120 (87–181) | 120 (85–176) | 118 (85–179) | 126 (90–185) | 0.36 |
| Fasting Glucose (mg/dL) | 118 (103–149) | 119 (105–150) | 115 (102–143) | 121 (103–152) | 0.54 |
| Glycated Hemoglobin (%) | 6.5 (5.8–7.7) | 6.3 (5.8–7.6) | 6.5 (5.9–7.7) | 6.6 (5.9–7.9) | 0.85 |
| Hemoglobin (g/dL) | 14.0 (12.9–15.1) | 14.1 (12.9–15.2) | 14.0 (13.0–15.1) | 13.8 (12.6–14.9) | 0.45 |
| Troponin (ng/L) | 9.0 (5.0–17.0) | 4.0 (3.0–6.0) | 9.0 (8.0–11.0) | 26.0 (17.0–57.0) | <0.001 |
| Group 1 (n = 316) | Group 2 (n = 316) | Group 3 (n = 317) | p-Value * | |
|---|---|---|---|---|
| HF Hospitalization | 4 (1.3%) | 13 (4.2%) | 25 (8.0%) | <0.001 |
| MI | 12 (3.9%) | 5 (1.6%) | 7 (2.3%) | 0.13 |
| PCI or CABG | 22 (7.1%) | 18 (5.8%) | 22 (7.1%) | 0.52 |
| Death | 19 (6.0%) | 28 (8.9%) | 41 (12.9%) | 0.002 |
| Combined Events (Death + Hospitalization) | 22 (7.0%) | 40 (12.8%) | 58 (18.5%) | <0.001 |
| Group 1 (Reference) | Group 2 | Group 3 | ||
|---|---|---|---|---|
| Overall Death | HR (95% CI), p-value | 1.0 | 1.12 (0.60–2.10), p = 0.71 | 2.02 (1.13–3.60), p = 0.017 |
| Combined Events | HR (95% CI), p-value | 1.0 | 1.33 (0.77–2.32), p = 0.31 | 2.30 (1.37–3.88), p = 0.002 |
| AUC (95% CI) | Sensibility (%) | Specificity (%) | Cut Off (ng/L) | p-Value | |
|---|---|---|---|---|---|
| Death | 0.619 (0.587–0.650) | 48.9 | 69.7 | >13 | <0.0001 |
| Combined Events | 0.645 (0.613–0.675) | 50.8 | 70.8 | >13 | <0.0001 |
| AUC (95% CI) | Sensibility (%) | Specificity (%) | Cut Off (Ratio) | p-Value | |
|---|---|---|---|---|---|
| Death | 0.611 (0.579–0.642) | 53.4 | 66.0 | 0.24 | <0.0001 |
| Combined Events | 0.639 (0.607–0.669) | 53.3 | 67.7 | 0.24 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strunz, C.M.C.; Hueb, W.; Rezende, P.C.; Vendramini, S.P.d.A.; Assis, A.C.R.d.; Roggerio, A.; Tairova, M.S.; Silva, M.F.; Oliveira, S.A.; Kisser, G.d.C.A.; et al. High-Sensitivity Troponin I and Cardiovascular Events in Stable Coronary Artery Disease: Insights from a Longitudinal Outpatient Study. Int. J. Mol. Sci. 2023, 24, 17286. https://doi.org/10.3390/ijms242417286
Strunz CMC, Hueb W, Rezende PC, Vendramini SPdA, Assis ACRd, Roggerio A, Tairova MS, Silva MF, Oliveira SA, Kisser GdCA, et al. High-Sensitivity Troponin I and Cardiovascular Events in Stable Coronary Artery Disease: Insights from a Longitudinal Outpatient Study. International Journal of Molecular Sciences. 2023; 24(24):17286. https://doi.org/10.3390/ijms242417286
Chicago/Turabian StyleStrunz, Celia Maria Cassaro, Whady Hueb, Paulo Cury Rezende, Sabrina Pacheco do Amaral Vendramini, Arthur Cicupira Rodrigues de Assis, Alessandra Roggerio, Maria Stanislavovna Tairova, Marcela Francisca Silva, Senili Avila Oliveira, Gyovanna de Cassia Agreste Kisser, and et al. 2023. "High-Sensitivity Troponin I and Cardiovascular Events in Stable Coronary Artery Disease: Insights from a Longitudinal Outpatient Study" International Journal of Molecular Sciences 24, no. 24: 17286. https://doi.org/10.3390/ijms242417286
APA StyleStrunz, C. M. C., Hueb, W., Rezende, P. C., Vendramini, S. P. d. A., Assis, A. C. R. d., Roggerio, A., Tairova, M. S., Silva, M. F., Oliveira, S. A., Kisser, G. d. C. A., & Kalil Filho, R. (2023). High-Sensitivity Troponin I and Cardiovascular Events in Stable Coronary Artery Disease: Insights from a Longitudinal Outpatient Study. International Journal of Molecular Sciences, 24(24), 17286. https://doi.org/10.3390/ijms242417286






