Hen Egg White Lysozyme (HEWL) Confers Resistance to Verticillium Wilt in Cotton by Inhibiting the Spread of Fungus and Generating ROS Burst
Abstract
:1. Introduction
2. Results
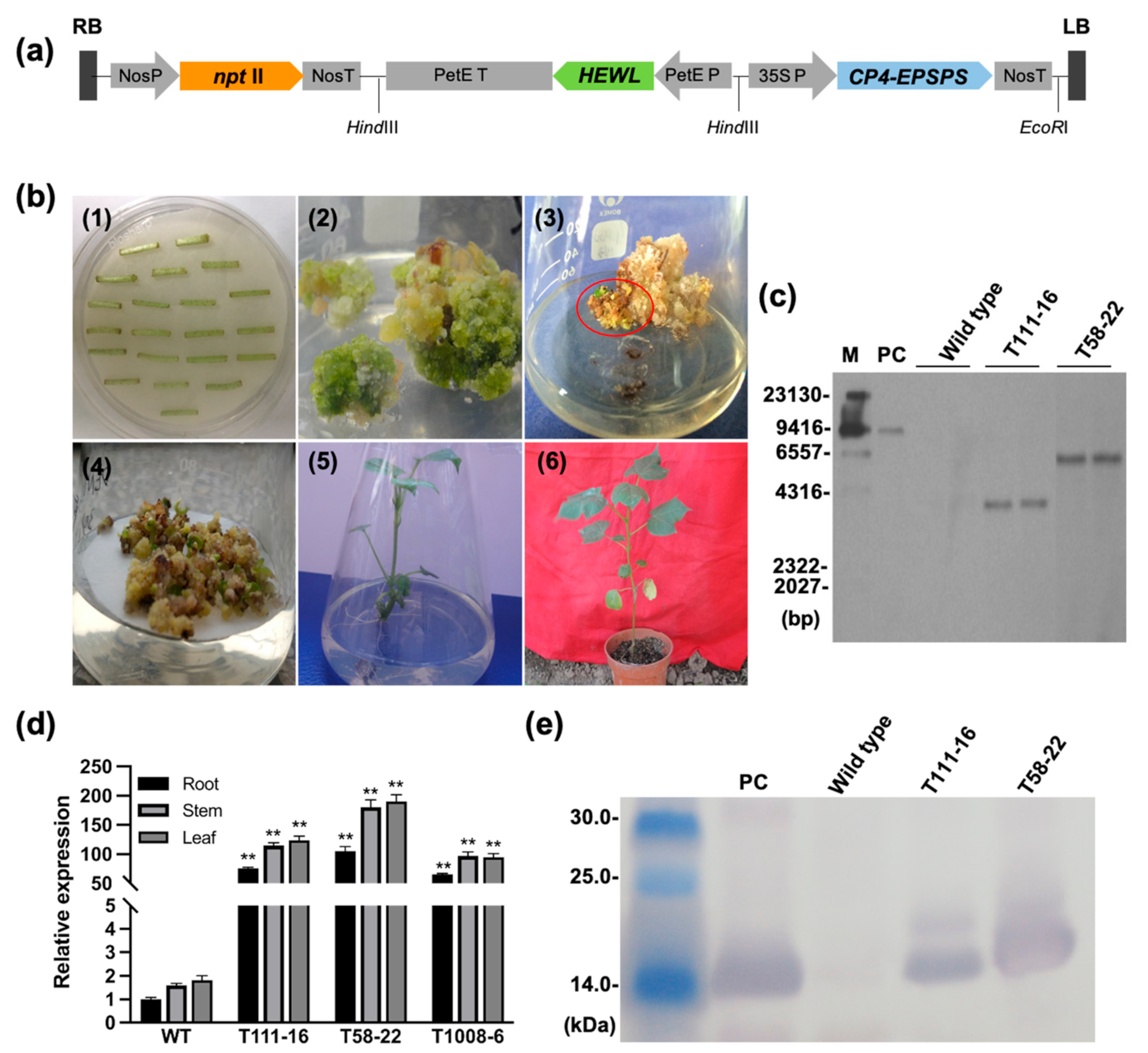
2.1. Generation of HEWL Transgenic Cotton Plants
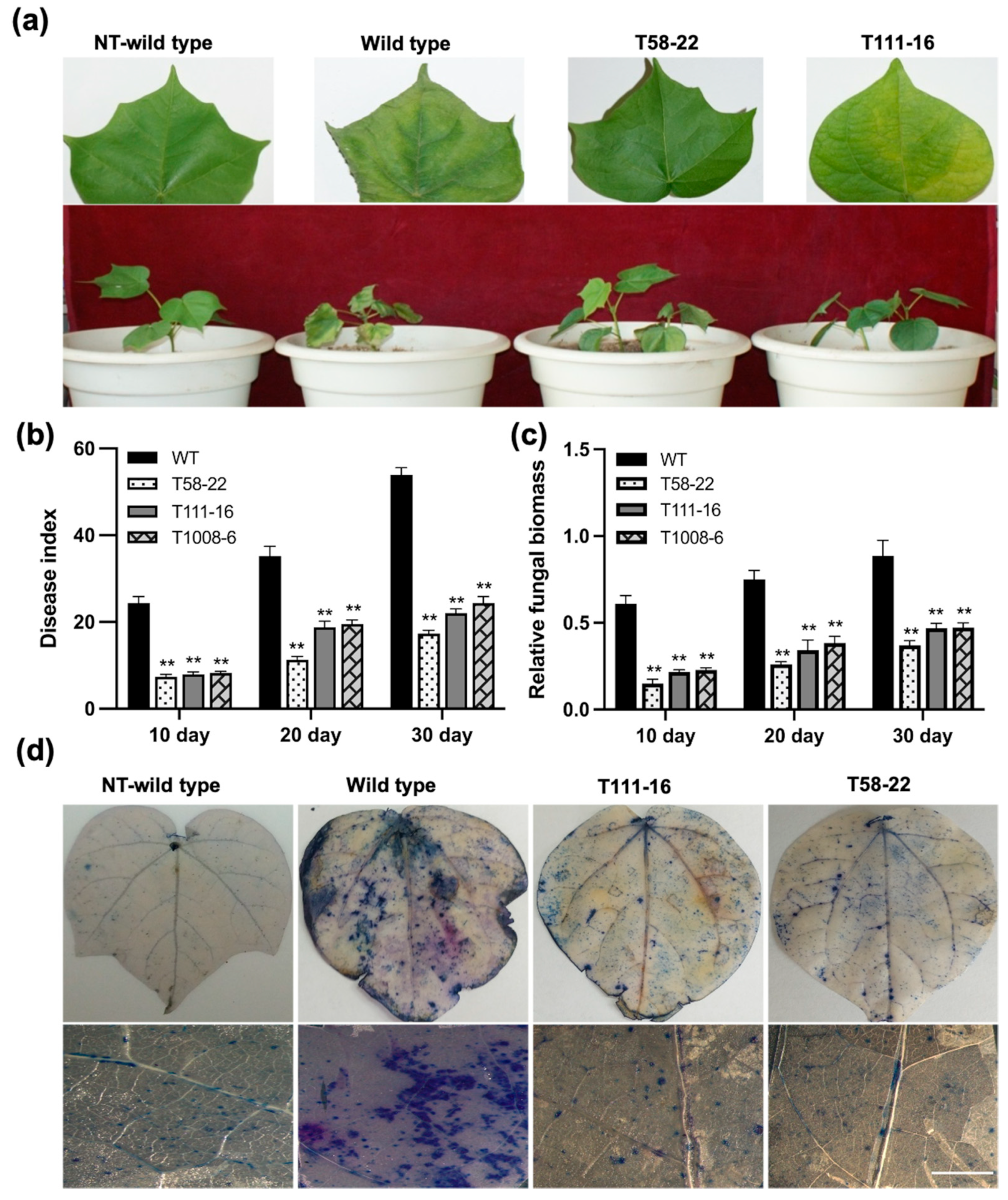
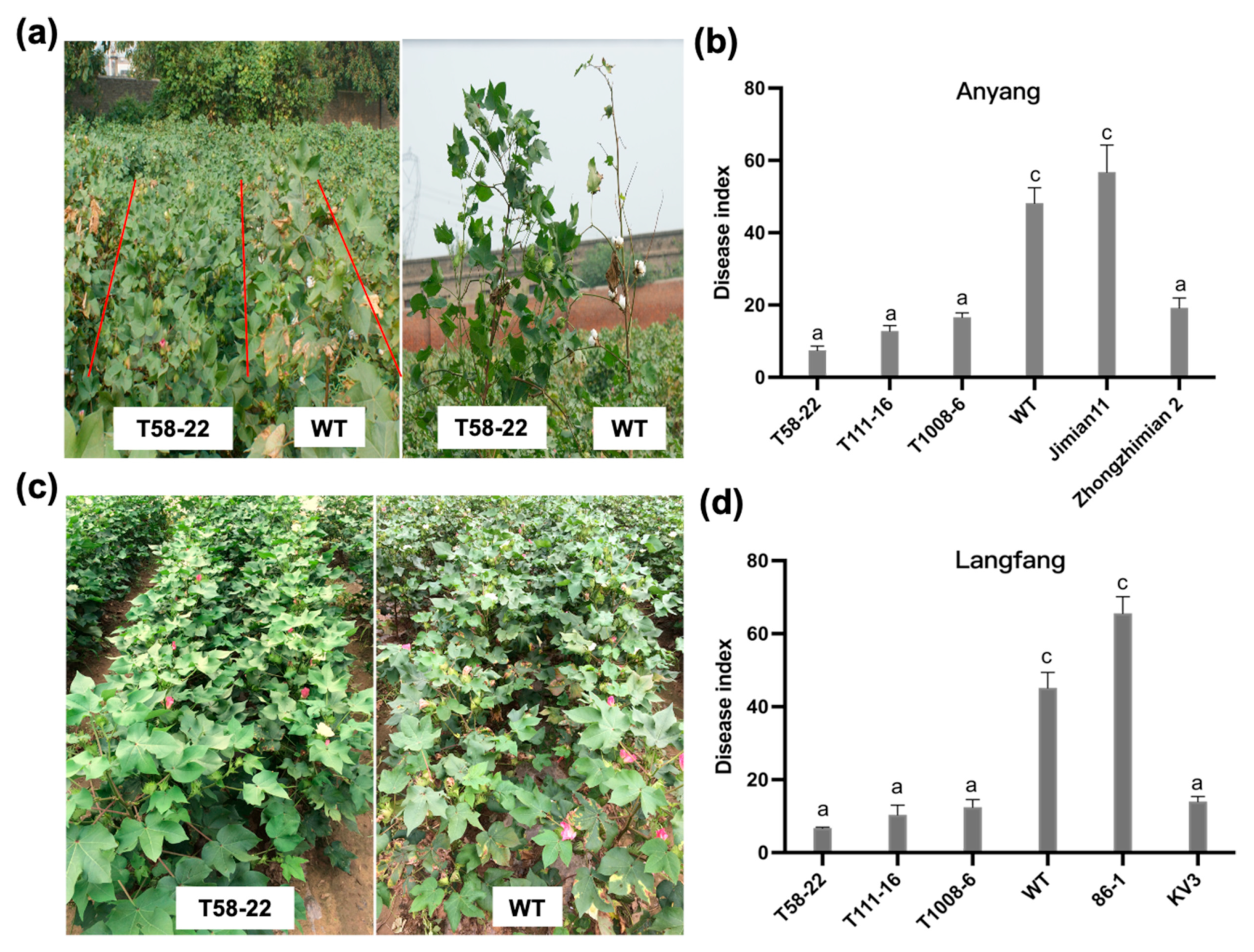
2.2. HEWL Expression Improved Resistance to Verticillium Wilt in Transgenic Cotton
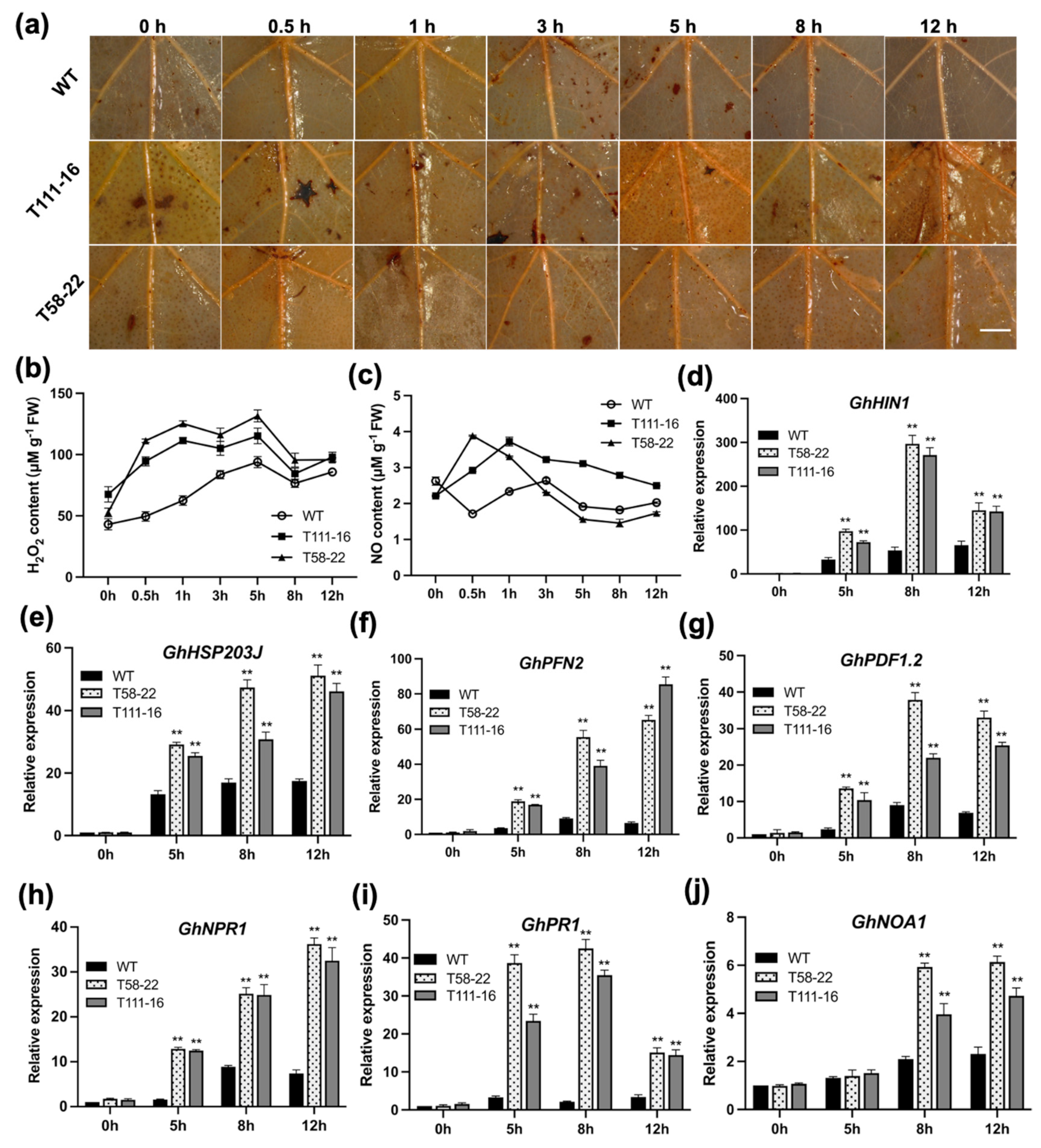
2.3. HEWL Transgenic Cotton Enhances Disease Resistance by Generating ROS Burst and Activating PR Genes
2.4. HEWL Inhibits the Growth of V. dahliae in Cotton
3. Discussion
3.1. Fungal Inhibitory Activity of HEWL
3.2. HEWL Produces a ROS Burst in Transgenic Cotton
3.3. HEWL Elicits Defense Responses against Pathogens in Cotton Plants
3.4. The Strategies and Future Prospects of Controlling Cotton Diseases
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. V. dahliae Strain and Culture Conditions
4.3. Cotton Transformation and Transgenic Plant Selection
4.4. PCR and qPCR
4.5. Southern Blot and Western Blot
4.6. Evaluation of Cotton Resistance to Verticillium Wilt
4.7. Trypan Blue and DAB Staining
4.8. Determination of H2O2, NO Content
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deketelaere, S.; Tyvaert, L.; Franca, S.C.; Hofte, M. Desirable traits of a good biocontrol agent against Verticillium Wilt. Front. Microbiol. 2017, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, H.L.; Wang, X.N.; Yang, Y.H.; Zhang, C.J.; Zhu, H.Q.; Shi, L.; Tang, C.M.; Zhao, M.W. Dynamic infection of Verticillium dahliae in upland cotton. Plant Biol. 2020, 22, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Xu, Z.P.; Sun, H.; Sun, L.Q.; Shaban, M.; Yang, X.Y.; Zhu, L.F. Genome-wide identification of papain-like cysteine proteases in Gossypium hirsutum and functional characterization in response to Verticillium dahliae. Front. Plant Sci. 2019, 10, 134. [Google Scholar] [CrossRef]
- Shaban, M.; Miao, Y.; Ullah, A.; Khan, A.Q.; Menghwar, H.; Khan, A.H.; Ahmed, M.M.; Tabassum, M.A.; Zhu, L. Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Biochem. 2018, 125, 193–204. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, Y.M.; McKenna, J.A.; McGinness, B.S.; Hinch, J.; Poon, S.; Connelly, A.A.; Anderson, M.A.; Heath, R.L. Field resistance to Fusarium oxysporum and Verticillium dahliae in transgenic cotton expressing the plant defensin NaD1. J. Exp. Bot. 2014, 65, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Wang, J. Genetic Transformation of cotton with the Harpin-encoding gene hpa(Xoo) of Xanthomonas oryzae pv. oryzae and evaluation of resistance against Verticillium Wilt. Methods Mol. Biol. 2019, 1902, 257–280. [Google Scholar]
- Song, Y.; Liu, L.; Wang, Y.; Valkenburg, D.J.; Zhang, X.; Zhu, L.; Thomma, B. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 2018, 16, 638–648. [Google Scholar] [CrossRef]
- Tong, S.; Yuan, M.; Liu, Y.; Li, X.; Jin, D.; Cheng, X.; Lin, D.; Ling, H.; Yang, D.; Wang, Y.; et al. Ergosterol-targeting fusion antifungal peptide significantly increases the Verticillium wilt resistance of cotton. Plant Biotechnol. J. 2021, 19, 926–936. [Google Scholar] [CrossRef]
- Xiao, S.; Hu, Q.; Shen, J.; Liu, S.; Yang, Z.; Chen, K.; Klosterman, S.J.; Javornik, B.; Zhang, X.; Zhu, L. GhMYB4 downregulates lignin biosynthesis and enhances cotton resistance to Verticillium dahliae. Plant Cell Rep. 2021, 40, 735–751. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.Y.; Zhao, P.M.; Han, L.B.; Jiao, G.L.; Zheng, Y.Y.; Huang, S.J.; Xia, G.X. Overexpression of a profilin (GhPFN2) promotes the progression of developmental phases in cotton fibers. Plant Cell Physiol. 2010, 51, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, Z.; Zhu, L.; Zhang, C.; Chen, Y.; Zhou, Y.; Li, F.; Li, X. Overexpression of cotton (Gossypium hirsutum) dirigent1 gene enhances lignification that blocks the spread of Verticillium dahliae. Acta Biochim. Biophys. Sin. 2012, 44, 555–564. [Google Scholar] [CrossRef]
- Pitsili, E.; Phukan, U.J.; Coll, N.S. Cell death in plant immunity. Cold Spring Harb. Perspect. Biol. 2020, 12, a036483. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Jones, J.D.G.; Ding, P. Plant immune networks. Trends Plant Sci. 2022, 27, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wu, Z.; Chen, S.; Ao, K.; Huang, W.; Yaghmaiean, H.; Sun, T.; Xu, F.; Zhang, Y.; Wang, S.; et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature 2021, 598, 500–503. [Google Scholar] [CrossRef]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Ling, X.; Ma, Z. Gbvdr6, a gene encoding a receptor-like protein of cotton (Gossypium barbadense), confers resistance to Verticillium Wilt in Arabidopsis and upland cotton. Front. Plant Sci. 2018, 8, 2272. [Google Scholar] [CrossRef]
- Parkhi, V.; Kumar, V.; Campbell, L.A.M.; Bell, A.A.; Rathore, K.S. Expression of Arabidopsis NPR1 in transgenic cotton confers resistance to non-defoliating isolates of Verticillium dahliae but not the defoliating isolates. J. Phytopathol. 2010, 158, 822–825. [Google Scholar] [CrossRef]
- Feng, H.J.; Li, C.; Zhou, J.L.; Yuan, Y.; Feng, Z.L.; Shi, Y.Q.; Zhao, L.H.; Zhang, Y.L.; Wei, F.; Zhu, H.Q. A cotton WAKL protein interacted with a DnaJ protein and was involved in defense against Verticillium dahliae. Int. J. Biol. Macromol. 2021, 167, 633–643. [Google Scholar] [CrossRef]
- Liu, N.N.; Sun, Y.; Pei, Y.K.; Zhang, X.Y.; Wang, P.; Li, X.C.; Li, F.G.; Hou, Y.X. A pectin methylesterase inhibitor enhances resistance to Verticillium Wilt. Plant Physiol. 2018, 176, 2202–2220. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, X.; Zhou, J.; Wu, Y.; Zhang, X.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Shi, Y.; et al. Genome-wide analysis of ribosomal protein GhRPS6 and its role in cotton Verticillium Wilt resistance. Int. J. Mol. Sci. 2021, 22, 1795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Wang, X.; Chen, B.; Zhao, J.; Cui, J.; Li, Z.; Yang, J.; Wu, L.; Wu, J.; et al. The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 2019, 20, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, N.; Ma, L.; Lu, J.; Wang, H.; Wang, C.; Yu, S.; Wei, H. The cotton BEL1-Like transcription Factor GhBLH7-D06 negatively regulates the defense response against Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 7126. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Zhu, W.; Wang, Y.; Zhu, L. GhHB12, a HD-ZIP I transcription factor, negatively regulates the cotton resistance to Verticillium dahliae. Int. J. Mol. Sci. 2018, 19, 3997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hu, X.; Wang, P.; Wang, H.; Ge, X.; Li, F.; Hou, Y. GhODO1, an R2R3-type MYB transcription factor, positively regulates cotton resistance to Verticillium dahliae via the lignin biosynthesis and jasmonic acid signaling pathway. Int. J. Biol. Macromol. 2022, 201, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xiao, S.; Wang, X.; Ao, C.; Zhang, X.; Zhu, L. GhWRKY1-like enhances cotton resistance to Verticillium dahliae via an increase in defense-induced lignification and S monolignol content. Plant Sci. 2021, 305, 110833. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Hu, F.; Wang, Y. Online preconcentration of lysozyme in hen egg white using responsive polymer coating in CE. J. Sep. Sci. 2021, 44, 3477–3488. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, K.; Wang, N.; Li, G.Q.; Sun, N.; Liu, D.H. Codon optimization, constitutive expression and antimicrobial characterization of hen egg white lysozyme (HEWL) in Pichia pastoris. Afr. J. Biotechnol. 2012, 11, 11887–11893. [Google Scholar]
- Takahashi, Y.; Berberich, T.; Yamashita, K.; Uehara, Y.; Miyazaki, A.; Kusano, T. Identification of tobacco HIN1 and two closely related genes as spermine-responsive genes and their differential expression during the Tobacco mosaic virus -induced hypersensitive response and during leaf- and flower-senescence. Plant Mol. Biol. 2004, 54, 613–622. [Google Scholar] [CrossRef]
- Takagi, K.; Tasaki, K.; Komori, H.; Katou, S. Hypersensitivity-related genes HSR201 and HSR203J are regulated by calmodulin-binding protein 60-type transcription factors and required for pathogen signal-induced salicylic acid synthesis. Plant Cell Physiol. 2022, 63, 1008–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Y.; Han, L.; Su, L.; Xia, G.; Wang, H. Overexpression of GhPFN2 enhances protection against Verticillium dahliae invasion in cotton. Sci. China Life Sci. 2017, 60, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Eggermont, K.; Schenk, P.M.; Van den Ackerveken, G.; Cammue, B.P.; Thomma, B.P. The Arabidopsis mutant iop1 exhibits induced over-expression of the plant defensin gene PDF1.2 and enhanced pathogen resistance. Mol. Plant Pathol. 2003, 4, 479–486. [Google Scholar] [CrossRef]
- Zhong, X.; Xi, L.; Lian, Q.; Luo, X.; Wu, Z.; Seng, S.; Yuan, X.; Yi, M. The NPR1 homolog GhNPR1 plays an important role in the defense response of Gladiolus hybridus. Plant Cell Rep. 2015, 34, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Wunsche, H.; Baldwin, I.T.; Wu, J. Silencing NOA1 elevates herbivory-induced jasmonic acid accumulation and compromises most of the carbon-based defense metabolites in Nicotiana attenuata(F). J. Integr. Plant Biol. 2011, 53, 619–631. [Google Scholar] [CrossRef]
- During, K.; Porsch, P.; Mahn, A.; Brinkmann, O.; Gieffers, W. The non-enzymatic microbicidal activity of lysozymes. FEBS Lett. 1999, 449, 93–100. [Google Scholar] [CrossRef]
- Kurdyumov, A.S.; Manuvera, V.A.; Baskova, I.P.; Lazarev, V.N. A comparison of the enzymatic properties of three recombinant isoforms of thrombolytic and antibacterial protein—Destabilase-Lysozyme from medicinal leech. BMC Biochem. 2015, 16, 27. [Google Scholar] [CrossRef]
- Zavalova, L.L.; Yudina, T.G.; Artamonova, I.I.; Baskova, I.P. Antibacterial non-glycosidase activity of invertebrate destabilase-lysozyme and of its helical amphipathic peptides. Chemotherapy 2006, 52, 158–160. [Google Scholar] [CrossRef]
- Averyanov, A. Oxidative burst and plant disease resistance. Front. Biosci. 2009, 1, 142–152. [Google Scholar]
- Khan, A.U.; Wilson, T. Reactive Oxygen Species as Cellular Messengers. Chem. Biol. 1995, 2, 437–445. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, Z.W.; Liu, P.F.; Liang, Y.P.; Zhao, Z.Z.; Li, W.H.; Liu, X.L.; Xia, Y. Oxathiapiprolin, a novel chemical inducer activates the plant disease resistance. Int. J. Mol. Sci. 2020, 21, 1223. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Y.; Lu, Y.; Liu, G.; Liu, J.; Shi, H. The co-modulation of RAV transcription factors in ROS burst and extensive transcriptional reprogramming underlies disease resistance in cassava. Plant Cell Rep. 2022, 41, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Zhao, J.; Ding, L.Y.; Zou, L.F.; Li, Y.R.; Chen, G.Y.; Zhang, T.Z. Constitutive expression of a novel antimicrobial protein, Hcm1, confers resistance to both Verticillium and Fusarium wilts in cotton. Sci. Rep. 2016, 6, 20773. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Sakaguchi, A.; Nishizawa, Y.; Kouzai, Y.; Minami, E.; Yano, S.; Koga, H.; Meshi, T.; Nishimura, M. Surface alpha-1,3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 2012, 8, e1002882. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Shi, L.; Han, Q.; Hu, H.L.; Zhao, M.W.; Tang, C.M.; Li, S.P. Biocontrol of Verticillium wilt and colonization of cotton plants by an endophytic bacterial isolate. J. Appl. Microbiol. 2012, 113, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, H.; Wang, L.; Li, Z.; Shi, Y.; Zhao, L.; Feng, Z.; Zhu, H. Potential of endophytic fungi isolated from cotton roots for biological control against Verticillium Wilt disease. PLoS ONE 2017, 12, e0170557. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, X.; Luo, X.; Wang, P.; Fan, Q.; Hu, G.; Xiao, J.; Li, F.; Wu, J. Simultaneous editing of two copies of Gh14-3-3d confers enhanced transgene-clean plant defense against Verticillium dahliae in Allotetraploid upland cotton. Front. Plant Sci. 2018, 9, 842. [Google Scholar] [CrossRef]
- Wu, S.J.; Wang, H.H.; Li, F.F.; Chen, T.Z.; Zhang, J.; Jiang, Y.J.; Ding, Y.Z.; Guo, W.Z.; Zhang, T.Z. Enhanced Agrobacterium-mediated transformation of embryogenic calli of upland cotton via efficient selection and timely subculture of somatic embryos. Plant Mol. Biol. Rep. 2008, 26, 174–185. [Google Scholar] [CrossRef]
- Song, Y.; Zhai, Y.; Li, L.; Yang, Z.; Ge, X.; Yang, Z.; Zhang, C.; Li, F.; Ren, M. BIN2 negatively regulates plant defence against Verticillium dahliae in Arabidopsis and cotton. Plant Biotechnol. J. 2021, 19, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Xu, F.C.; Zhao, J.R.; Li, B.; Xu, L.; Gao, W. GbMPK3 overexpression increases cotton sensitivity to Verticillium dahliae by regulating salicylic acid signaling. Plant Sci. 2020, 292, 110374. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Brubaker, C.L.; Wendel, J.F. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol. Biol. Rep. 1993, 11, 6. [Google Scholar]
- Guo, W.; Li, G.; Wang, N.; Yang, C.; Zhao, Y.; Peng, H.; Liu, D.; Chen, S. A Na(+)/H(+) antiporter, K2-NhaD, improves salt and drought tolerance in cotton (Gossypium hirsutum L.). Plant Mol. Biol. 2020, 102, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.E.; Nour-Eldin, H.H.; Halkier, B.A. A Western blot protocol for detection of proteins heterologously expressed in Xenopus laevis Oocytes. Methods Mol. Biol. 2016, 1405, 99–107. [Google Scholar] [PubMed]
- Conlon, H.E.; Salter, M.G. Plant protein extraction. Methods Mol. Biol. 2007, 362, 379–383. [Google Scholar]
- Zhang, Y.; Shi, Y.; Zhao, L.; Wei, F.; Feng, Z.; Feng, H. Phosphoproteomics profiling of cotton (Gossypium hirsutum L.) roots in response to Verticillium dahliae inoculation. ACS Omega 2019, 4, 18434–18443. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Long, L.; Zhu, L.F.; Xu, L.; Gao, W.H.; Sun, L.Q.; Liu, L.L.; Zhang, X.L. Proteomic and virus-induced gene silencing (VIGS) Analyses reveal that gossypol, brassinosteroids, and jasmonic acid contribute to the resistance of cotton to Verticillium dahliae. Mol. Cell Proteom. 2013, 12, 3690–3703. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol. Biol. 2010, 639, 292–298. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Li, G.; Wang, N.; Yang, C.; Peng, H.; Wang, M.; Liu, D. Hen Egg White Lysozyme (HEWL) Confers Resistance to Verticillium Wilt in Cotton by Inhibiting the Spread of Fungus and Generating ROS Burst. Int. J. Mol. Sci. 2023, 24, 17164. https://doi.org/10.3390/ijms242417164
Guo W, Li G, Wang N, Yang C, Peng H, Wang M, Liu D. Hen Egg White Lysozyme (HEWL) Confers Resistance to Verticillium Wilt in Cotton by Inhibiting the Spread of Fungus and Generating ROS Burst. International Journal of Molecular Sciences. 2023; 24(24):17164. https://doi.org/10.3390/ijms242417164
Chicago/Turabian StyleGuo, Wenfang, Gangqiang Li, Nan Wang, Caifeng Yang, Huakang Peng, Mengqi Wang, and Dehu Liu. 2023. "Hen Egg White Lysozyme (HEWL) Confers Resistance to Verticillium Wilt in Cotton by Inhibiting the Spread of Fungus and Generating ROS Burst" International Journal of Molecular Sciences 24, no. 24: 17164. https://doi.org/10.3390/ijms242417164
APA StyleGuo, W., Li, G., Wang, N., Yang, C., Peng, H., Wang, M., & Liu, D. (2023). Hen Egg White Lysozyme (HEWL) Confers Resistance to Verticillium Wilt in Cotton by Inhibiting the Spread of Fungus and Generating ROS Burst. International Journal of Molecular Sciences, 24(24), 17164. https://doi.org/10.3390/ijms242417164





