Albumin-Based Liver Reserve Models vs. MELD 3.0 in Prognostic Prediction for Hepatocellular Carcinoma Patients with Renal Insufficiency
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
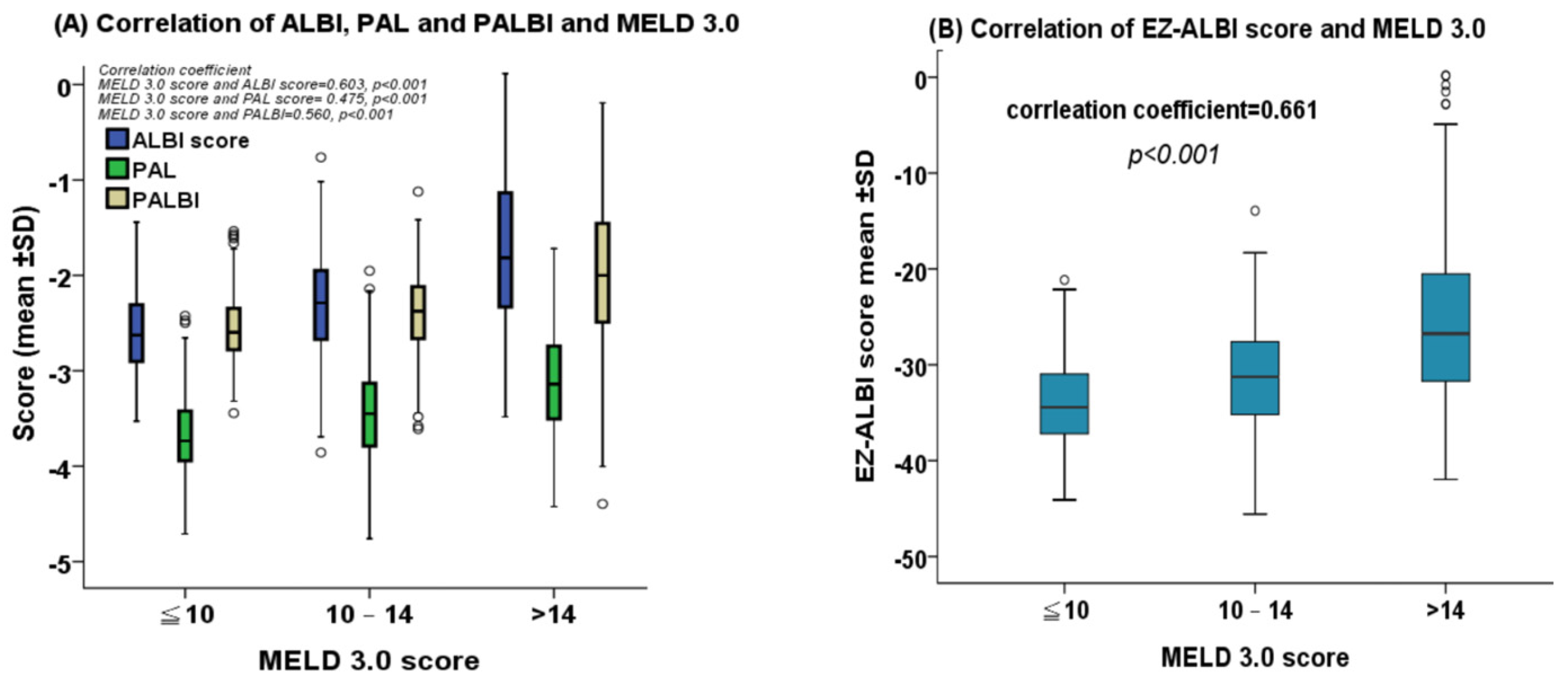
2.2. Correlation between MELD 3.0 Score and ALBI, PALBI, PAL, and EZ-ALBI Scores
2.3. Kaplan–Meier Survival Analysis
2.4. Multivariate Cox Proportional Hazards Model
2.5. Comparison of Prognostic Performance
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Diagnosis and Definition
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kulik, L.; El-Serag, H.B. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019, 156, 477–491.e471. [Google Scholar] [CrossRef]
- Zhang, C.H.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Masuzaki, R.; Moriyama, M.; Omata, M. Molecular mechanisms: Connections between nonalcoholic fatty liver disease, steatohepatitis and hepatocellular carcinoma. Int. J. Mol. Sci. 2020, 21, 1525. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Schirmacher, P.; Vilgrain, V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment strategies for hepatocellular carcinoma—A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Chang, Y.; Jeong, S.W.; Young Jang, J.; Jae Kim, Y. Recent Updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int. J. Mol. Sci. 2020, 21, 8165. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Dimitroulis, D.; Garmpi, A.; Psilopatis, I.; Sarantis, P.; Koustas, E.; Kanavidis, P.; Prevezanos, D.; Kouraklis, G.; et al. Targeted therapies for hepatocellular carcinoma treatment: A new era ahead—A systematic review. Int. J. Mol. Sci. 2022, 23, 14117. [Google Scholar] [CrossRef]
- Ginès, P.; Schrier, R.W. Renal failure in cirrhosis. N. Engl. J. Med. 2009, 361, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014, 85, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Montalti, R.; Giglio, M.C.; Rompianesi, G.; Tomassini, F.; Scarpellini, E.; De Simone, G.; De Palma, G.D.; Troisi, R.I. Hepatocellular carcinoma in patients with chronic renal disease: Challenges of interventional treatment. Surg. Oncol. 2021, 36, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fragaki, M.; Sifaki-Pistolla, D.; Orfanoudaki, E.; Kouroumalis, E. Comparative evaluation of ALBI, MELD, and Child-Pugh scores in prognosis of cirrhosis: Is ALBI the new alternative? Ann. Gastroenterol. 2019, 32, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, J.; Evers, M.J.; Bui, J.T.; Lokken, R.P.; Lipnik, A.J.; Gaba, R.C.; Ray, C.E., Jr. Albumin-bilirubin and platelet-albumin-bilirubin Grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J. Vasc. Interv. Radiol. 2017, 28, 1224–1231. [Google Scholar] [CrossRef]
- Chong, C.C.; Chan, A.W.; Wong, J.; Chu, C.M.; Chan, S.L.; Lee, K.F.; Yu, S.C.; To, K.F.; Johnson, P.; Lai, P.B. Albumin-bilirubin grade predicts the outcomes of liver resection versus radiofrequency ablation for very early/early stage of hepatocellular carcinoma. Surgeon 2017, 16, 163–170. [Google Scholar] [CrossRef]
- Pinato, D.J.; Sharma, R.; Allara, E.; Yen, C.; Arizumi, T.; Kubota, K.; Bettinger, D.; Jang, J.W.; Smirne, C.; Kim, Y.W.; et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatol. 2017, 66, 338–346. [Google Scholar] [CrossRef]
- Kariyama, K.; Nouso, K.; Hiraoka, A.; Wakuta, A.; Oonishi, A.; Kuzuya, T.; Toyoda, H.; Tada, T.; Tsuji, K.; Itobayashi, E.; et al. EZ-ALBI score for predicting hepatocellular carcinoma prognosis. Liver Cancer 2020, 9, 734–743. [Google Scholar] [CrossRef]
- Ananchuensook, P.; Sriphoosanaphan, S.; Suksawatamnauy, S.; Siripon, N.; Pinjaroen, N.; Geratikornsupuk, N.; Kerr, S.J.; Thanapirom, K.; Komolmit, P. Validation and prognostic value of EZ-ALBI score in patients with intermediate-stage hepatocellular carcinoma treated with trans-arterial chemoembolization. BMC Gastroenterol. 2022, 22, 295. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Liu, P.; Hsu, C.; Ko, C.; Huang, Y.; Su, C.; Hsia, C.; Tsai, P.; Chou, S.; Lee, R.; et al. Easy albumin–bilirubin score as a new prognostic predictor in hepatocellular carcinoma. Hepatol. Res. 2021, 51, 1129–1138. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Su, C.-W.; Chiou, Y.-Y.; Chiu, N.-C.; Liu, C.-A.; Fang, K.-C.; Huo, T.-I.; Huang, Y.-H.; Chang, C.-C.; Hou, M.-C.; et al. Hepatocellular carcinoma: Nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology 2017, 285, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, T.; Goulis, L.; Doumtsis, P.; Tzoumari, T.; Akriviadis, E.; Cholongitas, E. ALBI and PALBI Grades Are Associated with the Outcome of Patients with Stable Decompensated Cirrhosis. Ann. Hepatol. 2018, 18, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yuan, J.; Tang, B.; Zhang, F.; Lu, S.; Chen, R.; Zhang, L.; Ren, Z.; Yin, X. Albumin-bilirubin index and platelet-albumin-bilirubin index contribute to identifying survival benefit candidates in patients with hepatocellular carcinoma and Child-Pugh grade A undergoing transcatheter arterial chemoembolization with sorafenib treatment. Ann. Transl. Med. 2021, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Meira Júnior, J.D.; Fonseca, G.M.; Carvalho Neto, F.N.; Jeismann, V.B.; Kruger, J.A.P.; Silva, J.P.M.; Coelho, F.F.; Herman, P. Platelet-albumin (PAL) score as a predictor of perioperative outcomes and survival in patients with hepatocellular carcinoma undergoing liver resection in a Western center. Surg. Oncol. 2022, 42, 101752. [Google Scholar] [CrossRef]
- Shindoh, J.; Kawamura, Y.; Kobayashi, Y.; Kiya, Y.; Sugawara, T.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Ikeda, K.; Hashimoto, M. Platelet-albumin score as a sensitive measure for surgical risk prediction and survival outcomes of patients with hepatocellular carcinoma. J. Gastrointest. Surg. 2019, 23, 76–83. [Google Scholar] [CrossRef]
- Ho, S.-Y.; Liu, P.-H.; Hsu, C.-Y.; Huang, Y.-H.; Liao, J.-I.; Su, C.-W.; Hou, M.-C.; Huo, T.-I. Comparison of four albumin-based liver reserve models (ALBI/EZ-ALBI/PALBI/PAL) against MELD for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. Cancers 2023, 15, 1925. [Google Scholar] [CrossRef]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for end-stage liver disease updated for the modern era. Gastroenterology 2021, 161, 1887–1895.e4. [Google Scholar] [CrossRef]
- Asrani, S.K.; Jennings, L.W.; Kim, W.R.; Kamath, P.S.; Levitsky, J.; Nadim, M.K.; Testa, G.; Leise, M.D.; Trotter, J.F.; Klintmalm, G. MELD-GRAIL-Na: Glomerular filtration rate and mortality on liver-transplant waiting list. Hepatology 2020, 71, 1766–1774. [Google Scholar] [CrossRef]
- Takahashi, H.; Kawanaka, M.; Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Morishita, A.; Munekage, K.; et al. Association of serum albumin levels and long-term prognosis in patients with biopsy-confirmed nonalcoholic fatty liver disease. Nutrients 2023, 15, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Song, M.J.; Kim, S.H.; Park, M. Comparing various scoring system for predicting overall survival according to treatment modalities in hepatocellular carcinoma focused on Platelet-albumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grade: A nationwide cohort study. PLoS ONE 2019, 14, e0216173. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Lei, Z.; Tang, X.; Ma, W.; Si, A.; Yang, P.; Li, Q.; Geng, Z.; Zhou, J.; Cheng, Z. External validation of six liver functional reserve models to predict posthepatectomy liver failure after major resection for hepatocellular carcinoma. J. Cancer 2021, 12, 5260–5267. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, R.; Zhang, M.; Liu, W.; Li, H.; Li, D. Prognostic value of platelet-albumin-bilirubin grade in Child-Pugh A and B patients with hepatocellular carcinoma: A meta-analysis. Front. Oncol. 2022, 12, 914997. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef]
- I Tsilimigras, D.; Moris, D.; Hyer, J.M.; Bagante, F.; Sahara, K.; Moro, A.; Paredes, A.Z.; Mehta, R.; Ratti, F.; Marques, H.P.; et al. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br. J. Surg. 2020, 107, 854–864. [Google Scholar] [CrossRef]
- Wallace, D.; Cowling, T.; McPhail, M.J.; Brown, S.E.; Aluvihare, V.; Suddle, A.; Auzinger, G.; Heneghan, M.A.; Rowe, I.A.; Walker, K.; et al. Assessing the time-dependent impact of performance status on outcomes after liver transplantation. Hepatology 2020, 72, 1341–1352. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hsu, C.Y.; Huang, Y.H.; Hsia, C.Y.; Chiou, Y.Y.; Su, C.W.; Lin, H.C.; Huo, T.I. Vascular invasion in hepatocellular carcinoma: Prevalence, determinants and prognostic impact. J. Clin. Gastroenterol. 2014, 48, 734–741. [Google Scholar] [CrossRef]

| Variable | n = 1120 |
|---|---|
| Age (years, mean ± SD) | 72 ± 11 |
| Male/female, n (%) | 816/304 (73/27) |
| Etiologies of liver disease | |
| HBV, n (%) | 403 (36) |
| HCV, n (%) | 310 (27) |
| HBV + HCV, (%) | 49 (4) |
| Others, (%) | 358 (32) |
| Laboratory values (mean ± SD) | |
| Albumin (g/L) | 3.5 ± 0.6 |
| Bilirubin (mg/dL) | 1.7 ± 3.9 |
| Creatinine (mg/dL) | 2.0 ± 1.7 |
| Sodium (mmol/L) | 138 ± 5 |
| INR of PT | 1.1 ± 0.5 |
| Platelet (1000 ul/L) | 174 ± 95 |
| GFR (mL/min/1.73 m2) | 43 ± 15 |
| Diabetes mellitus, n (%) | 430 (38) |
| AFP (ng/mL), median [IQR] | 30 [5–861] |
| Tumor nodules (single/multiple), n (%) | 712/408 (64/36) |
| Tumor size, mean ± SD | 6.2 ± 4.5 |
| Tumor size > 3 cm, n (%) | 681 (68) |
| Tumor burden score, median, [IQR] | 5.3 [3.2–9.7] |
| Tumor burden score (low/medium/high), n (%) | 310/718/92 (28/64/8) |
| Vascular invasion, n (%) | 255 (23) |
| Ascites, n (%) | 313 (28) |
| CTP score (mean ± SD) | 6.4 ± 1.7 |
| CTP class (A/B/C), n (%) | 749/288/83 (67/26/8) |
| ALBI score(mean ± SD) | −2.21 ± 0.71 |
| ALBI grade (1/2/3) | 372/601/147 (33/54/13) |
| EZ-ALBI score (mean ± SD) | −30.05 ± 8.06 |
| EZ-ALBI grade (1/2/3) | 346/639/135 (31/57/12) |
| PALBI score (mean ± SD) | −2.30 ± 0.56 |
| PALBI grade (1/2/3) | 452/323/345 (40/29/31) |
| PAL score (mean ± SD) | −3.41 ± 0.55 |
| PAL grade (1/2/3) | 304/560/256 (27/50/23) |
| MELD 3.0 score (mean ± SD) | 14.64 ± 6.2 |
| MELD 3.0 (≤10/10–14/>14) | 393/318/409 (35/28/37) |
| Performance status (0/1/2/3–4) | 566/174/203/177 (51/15/18/10/16) |
| BCLC (0/A/B/C/D) n (%) | 66/241/177/436/200 (6/22/15/38/19) |
| Treatment | |
| Surgical resection | 209 (19) |
| Liver transplantation | 6 (1) |
| Percutaneous ablation | 226 (20) |
| TACE | 329 (29) |
| Others | 350 (31) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Overall Survival | HR | CI | p | HR | CI | p |
| Age (≤72/>72 years) | 1.269 | 1.112–1.449 | <0.001 | 1.264 | 1.104–1.446 | <0.001 |
| Sex (male/female) | 0.997 | 0.908–1.052 | 0.543 | |||
| HBsAg (negative/positive) | 1.222 | 1.069–1.396 | 0.003 | |||
| Anti-HCV (negative/positive) | 0.945 | 0.822–1.085 | 0.421 | |||
| Albumin level (≥3.5/<3.5 g/dL) | 2.221 | 1.947–2.535 | <0.001 | |||
| Bilirubin level (≤1.7/>1.7/mg/dL) | 2.275 | 1.925–2.690 | <0.001 | |||
| Platelet (≥150,000/<150,000/μL) | 1.195 | 1.104–1.295 | <0.001 | |||
| INR of PT (<1.1/≥1.1) | 1.143 | 1.005–1.301 | 0.042 | |||
| AFP level (<20/≥20 ng/mL) | 1.919 | 1.680–2.191 | <0.001 | 1.856 | 1.702–2.025 | <0.001 |
| Multiple tumors (no/yes) | 1.456 | 1.275–1.662 | <0.001 | |||
| Tumor size (≤3 cm/>3 cm) | 2.161 | 1.867–2.500 | <0.001 | |||
| Vascular invasion (no/yes) | 3.481 | 2.983–4.063 | <0.001 | 1.957 | 1.643–2.332 | <0.001 |
| Ascites (no/yes) | 2.664 | 2.231–3.072 | <0.001 | 1.650 | 1.406–1.936 | <0.001 |
| Tumor burden score | ||||||
| Low | 1 | 1 | ||||
| Medium | 1.997 | 1.710–2.333 | <0.001 | 1.576 | 1.341–1.852 | <0.001 |
| High | 5.683 | 4.391–7.355 | <0.001 | 2.076 | 1.558–2.767 | <0.001 |
| Performance status | ||||||
| 0 | 1 | 1 | ||||
| 1–2 | 1.800 | 1.496–2.166 | <0.001 | 1.304 | 1.077–1.578 | <0.001 |
| 3–4 | 2.709 | 2.337–3.139 | <0.001 | 1.541 | 1.221–1.726 | <0.001 |
| Model 1 | ||||||
| ALBI | ||||||
| grade 1 | 1 | 1 | ||||
| grade 2 | 2.051 | 1.766–2.382 | <0.001 | 1.432 | 1.221–1.680 | <0.001 |
| grade 3 | 5.057 | 4.082–6.265 | <0.001 | 2.362 | 1.852–3.013 | <0.001 |
| Model 2 | ||||||
| EZ-ALBI | ||||||
| grade 1 | 1 | 1 | ||||
| grade 2 | 2.010 | 1.727–2.339 | <0.001 | 1.491 | 1.273–1.747 | <0.001 |
| grade 3 | 4.805 | 3.846–6.002 | <0.001 | 2.255 | 1.758–2.894 | <0.001 |
| Model 3 | ||||||
| PALBI | ||||||
| grade 1 | 1 | 1 | ||||
| grade 2 | 1.755 | 1.495–2.061 | <0.001 | 1.427 | 1.211–1.682 | <0.001 |
| grade 3 | 3.465 | 2.947–4.051 | <0.001 | 1.812 | 1.503–2.184 | <0.001 |
| Model 4 | ||||||
| PAL | ||||||
| grade 1 | 1 | 1 | ||||
| grade 2 | 1.681 | 1.430–1.975 | <0.001 | 1.381 | 1.170–1.629 | <0.001 |
| grade 3 | 3.092 | 2.564–3.730 | <0.001 | 1.827 | 1.490–2.422 | <0.001 |
| Model 5 | ||||||
| MELD 3.0 score | ||||||
| ≤10 | 1 | 1 | ||||
| 10–14 | 1.473 | 1.246–1.742 | <0.001 | 1.220 | 1.029–1.446 | 0.022 |
| >14 | 2.692 | 2.300–3.150 | <0.001 | 1.851 | 1.560–2.197 | <0.001 |
| Liver Reserve Models | Homogeneity (Wald χ2) | AICc |
|---|---|---|
| ALBI | 239.031 | 11,281.79 |
| EZ-ALBI | 203.652 | 11,316.459 |
| PALBI | 221.884 | 11,298.227 |
| PAL | 136.085 | 11,384.026 |
| MELD 3.0 | 156.026 | 11,364.088 |
| Non-Invasive Liver Reserve Models | Formula |
|---|---|
| ALBI, grade 1/2/3 (≤−2.6/>−2.6 and ≤−1.39/>−1.39) | (log(Bilirubin [μmol/L]) × 0.66) + (Albumin [g/L] × −0.085) |
| EZ-ALBI, grade 1/2/3 (≤−34.4, >−34.4 and ≤−22.2, >−22.2) | T.Bil (mg/dL) − (9 × Alb (g/dL)) |
| PALBI grade 1/2/3/(≤−2.53), score>−2.53 and ≤−2.09)/(score>−2.09). | 2.02 × log10bilirubin [μmol/L] level − 0.37 × (log10bilirubin level)2 − 0.04 × albumin level − 3.48 × log10platelet count [1000/μL] (PLT) + 1.01 × (log10PLT)2 |
| PAL grade 1/2/3 ≤−3.77, >−3.77 and ≤−3.04, >−3.04 | 0.777 × albumin [g/dL] − 0.575 × log10 (platelet count) [104/μL]. |
| MELD 3.0 * (≤10, score 10, between and l4, >14) | 1.33 (if female) + [4.56 × loge(bilirubin (mg/dl))] + [0.82 × (137 − Na (mEq/L))] − [0.24 × (137 − Na) × loge(bilirubin)] + [9.09 × loge(INR)] + [11.14 × loge(creatinine)] − [1.85 × (3.5 − albumin(g/dL))] − [1.83 × (3.5 −albumin) × loge(creatinine)] + 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, S.-Y.; Liu, P.-H.; Hsu, C.-Y.; Tseng, H.-T.; Huang, Y.-H.; Su, C.-W.; Hou, M.-C.; Huo, T.-I. Albumin-Based Liver Reserve Models vs. MELD 3.0 in Prognostic Prediction for Hepatocellular Carcinoma Patients with Renal Insufficiency. Int. J. Mol. Sci. 2023, 24, 16987. https://doi.org/10.3390/ijms242316987
Ho S-Y, Liu P-H, Hsu C-Y, Tseng H-T, Huang Y-H, Su C-W, Hou M-C, Huo T-I. Albumin-Based Liver Reserve Models vs. MELD 3.0 in Prognostic Prediction for Hepatocellular Carcinoma Patients with Renal Insufficiency. International Journal of Molecular Sciences. 2023; 24(23):16987. https://doi.org/10.3390/ijms242316987
Chicago/Turabian StyleHo, Shu-Yein, Po-Hong Liu, Chia-Yang Hsu, Hung-Ting Tseng, Yi-Hsiang Huang, Chien-Wei Su, Ming-Chih Hou, and Teh-Ia Huo. 2023. "Albumin-Based Liver Reserve Models vs. MELD 3.0 in Prognostic Prediction for Hepatocellular Carcinoma Patients with Renal Insufficiency" International Journal of Molecular Sciences 24, no. 23: 16987. https://doi.org/10.3390/ijms242316987
APA StyleHo, S.-Y., Liu, P.-H., Hsu, C.-Y., Tseng, H.-T., Huang, Y.-H., Su, C.-W., Hou, M.-C., & Huo, T.-I. (2023). Albumin-Based Liver Reserve Models vs. MELD 3.0 in Prognostic Prediction for Hepatocellular Carcinoma Patients with Renal Insufficiency. International Journal of Molecular Sciences, 24(23), 16987. https://doi.org/10.3390/ijms242316987






