2.1. Obtaining and Characterization of Gold Nanoparticles Preparations
Five preparations of GNPs were obtained via the citrate reduction of chloroauric acid under various ratios of reactants. Their micrographs obtained via transmission electron microscopy (TEM) are given in
Appendix A (
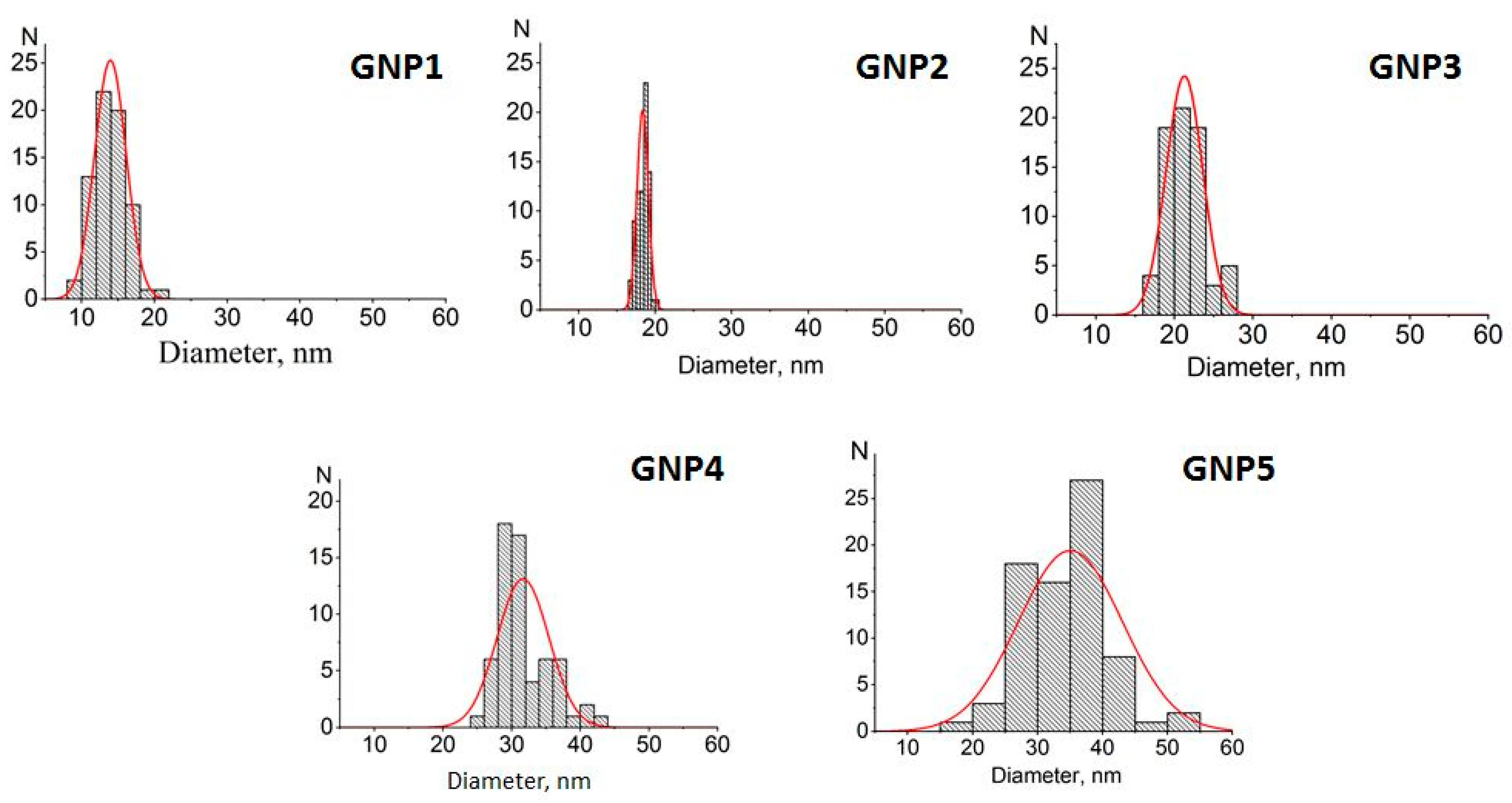
Figure A1), and histograms for the distribution of GNP diameters are depicted in
Figure 1. In accordance with the calculated size and shape characteristics (see
Table 1), the preparations were further named GNPs with average diameters of 14 nm, 18.5 nm, 21 nm, 31.5 nm, and 35.5 nm. The ellipticity coefficients varied from 1.08 to 1.28, which indicated that the shape of the nanoparticles was close to spherical, especially for the four preparations with smaller diameters.
Based on the earlier established dependence between the average diameter and the peak of the absorption spectrum of spherical gold nanoparticles [
16], the GNP preparations were additionally characterized using spectrophotometric data. The found diameters (see
Table 1) imperceptibly differed from the microscopic results, from 0.5% (18.5 nm vs. 18.4 nm) to 6.5% (13 nm vs. 13.9 nm), thus confirming the effectiveness of the simple and rapid spectrophotometric characterization of GNPs.
2.2. Preparation of Antibody–GNP Conjugates and Determination of Their Composition
Varying the ratio of GNPs and antibodies in the course of the adsorption leads to obtaining products with different compositions and structures. An important difference between these products is whether the antibodies form only one layer or multiple layers on the surface of the particles. Complexes with polylayer immobilization of antibodies on the GNP surfaces have been considered in several studies [
18,
19,
20]. The binding affinity upon the formation of the outer layers is much is lower than the affinity for the first layer, which is why these layers are called soft and hard protein coronas, respectively [
21,
22,
23]. The soft corona can dissociate during the storage and use of conjugates, thereby reducing the yield of detectable complexes, the accuracy, and the reliability of assay results. In addition, the outer layers limit the access of antigen molecules to the first layer of the immobilized antibodies. Therefore, the choice of a monolayer coating of antibodies must be reasonable for analytical purposes [
24].
Based on the arguments presented above, the GNP:antibody ratio for their conjugation was chosen in such a way as to be limited to the first layer of immobilized antibodies. For this purpose, we were guided by the fact that the densest arrangement accounts for the occupation of a 25 nm
2 area on the surface by one IgG molecule. Across the different studies, the measured amounts of IgG adsorbed on GNPs have significantly varied depending on the sorption conditions and the used method. Thus, Driskel’s group, using different methods for determining the concentration of antibodies, studied IgG–GNP conjugates with a diameter of 60 nm [
15,
25]. They found that 227 to 660 IgG molecules (443.5 on average value) can bind to one particle via physical adsorption. This value corresponds to approximately 25 nm
2 of GNP surface per one bound IgG molecule; this value was used in our following calculations. To provide the corresponding proportions of reactants for monolayer saturation, their required concentrations were calculated; see
Table 2.
The results of the immobilization experiments were characterized through measurements of the composition of the obtained conjugates. As we proposed earlier [
16], the fluorescence of tryptophan residues was registered for added IgG solutions and for supernatants with IgG that did not bind to GNPs. The calculated content of the bound antibodies, the percentage of their binding, and the composition of the resulting conjugates are summarized in
Table 3.
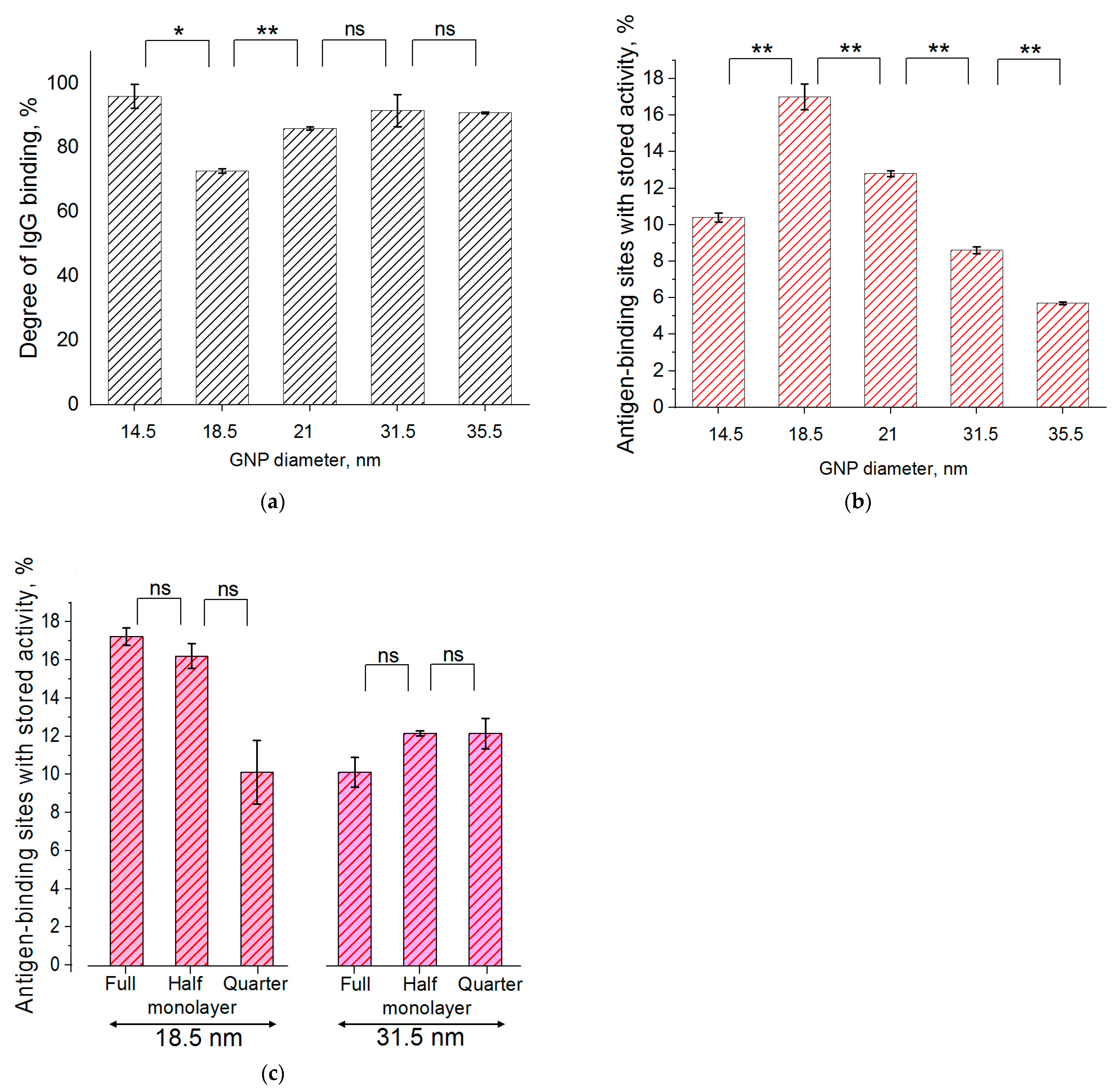
As can be seen, the majority of the added antibodies bound to the GNPs in all preparations. The percentage of IgG binding varied from 73 to 96%, thus confirming the similarity of the conditions chosen for IgG immobilization on the surface of all five GNP preparations. Statistical analysis showed that, in this row, the neighboring preparations were significantly different only for the conjugate with an average GNP diameter equal to 18.5 nm. See
Appendix B,
Figure A2, for the estimation of
p values using Student’s
t-test, which confirms the statistical significance of the differences between pairwise-compared values (GNP1 vs. GNP2 and GNP2 vs. GNP3).
2.3. Determination of Active Centers of the Immobilized Antibodies (Reaction with Carboxyfluorescein)
To correctly assess the changes in the antigen-binding properties of the antibodies caused by their immobilization, we took into account the results of our earlier characterization of homogeneous interactions for the chosen fluorescein–(antibody against the fluorescein) pair [
16]. The experiments conducted with the registration of fluorescence quenching in immune complexes showed that in the antibody preparation, 60% of the antibody valences were able to bind to fluorescein, and 40% of them were inactive. This value is comparable to the results of other studies for monoclonal antibodies (for example, a 1.31–1.71 stoichiometry for antigen complexes with bivalent antibodies at a theoretical 2:1 stoichiometry in [
26]) and may be associated with deviations from the native structure during both the biosynthesis and storage of the antibodies. So, IgG molecules that were initially inactive (not bound to fluorescein in solution) were excluded from the consideration of immobilization-caused inactivation.
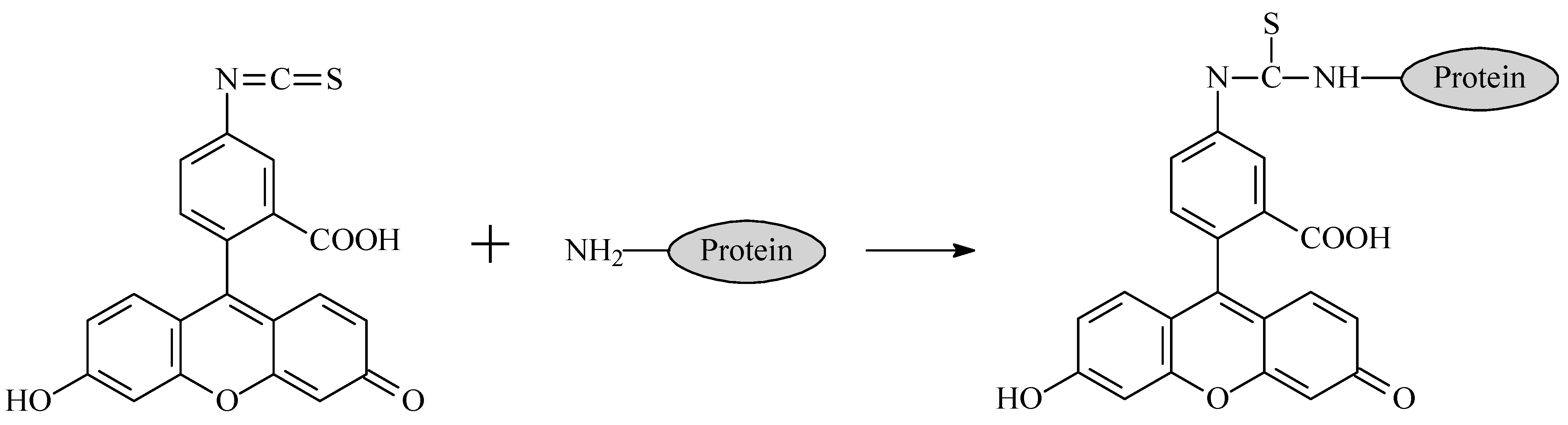
The measurement of the binding of a carboxylated fluorescein derivative to immobilized antibodies was based on the detection of the fluorescence of (i) solutions of fluorescein with known concentrations that were added for binding and (ii) a supernatant in which unbound antigen was separated from the formed immune complexes. To unify the conditions used for the measurements, the supernatants of the same precipitated GNPs were used to prepare calibration solutions of fluorescein [
24].
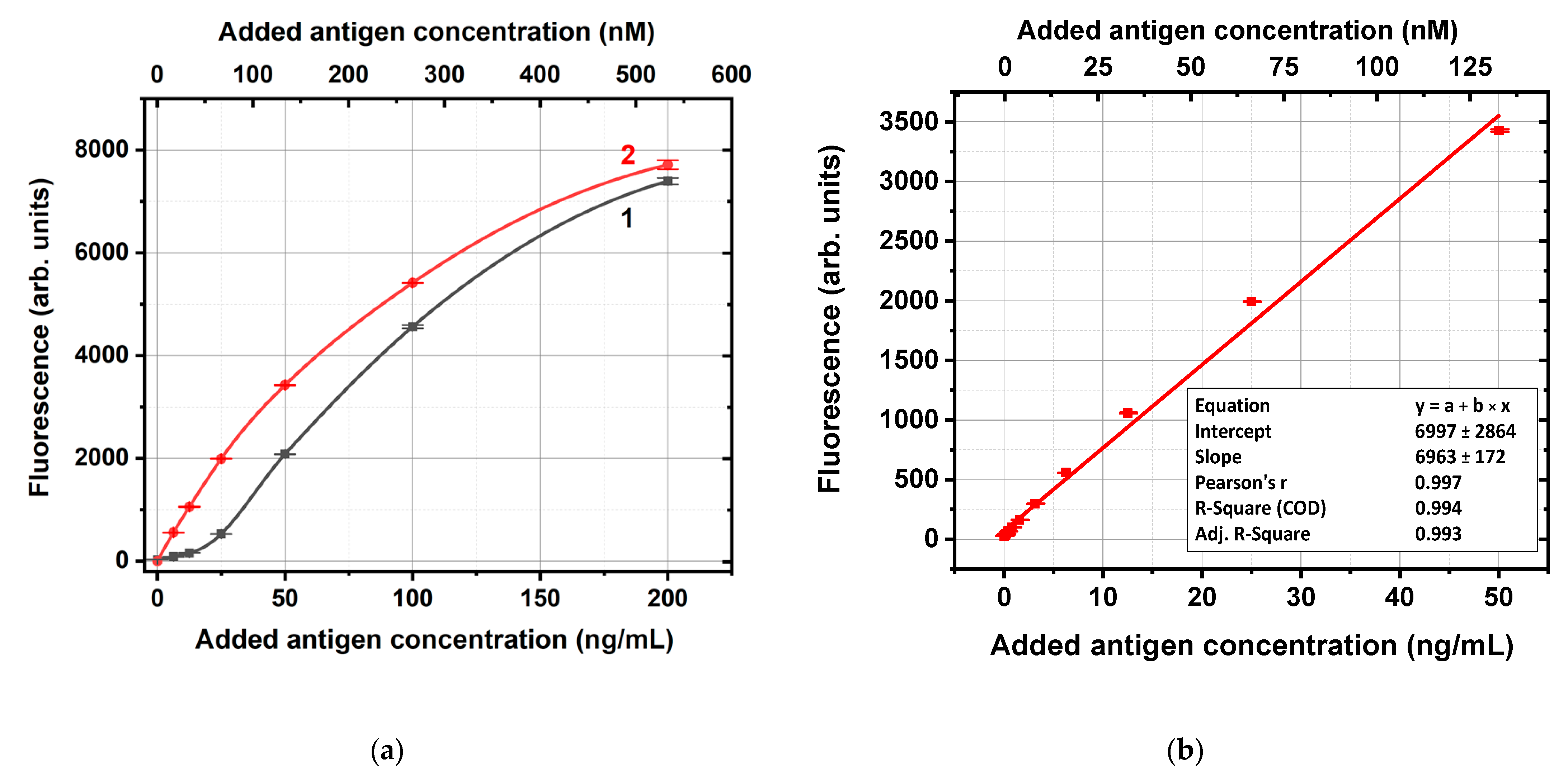
Figure 2a shows, as an example, the fluorescence levels obtained using the conjugate of antifluorescein IgG and GNPs with an average diameter of 14 nm. The concentration dependence for solutions of carboxyfluorescein in supernatants in the range of concentrations from 0 to 50 ng/mL was almost linear (R
2 > 0.99, see
Figure 2b), whereas for higher concentrations, it deviated from linearity. So, the fluorescence values for supernatants with unbound carboxyfluorescein molecules (
Figure 2a, curve 1) and the calibration linear approximation from
Figure 2b were used to calculate the carboxyfluorescein content in these supernatants as well as the bound antigen concentrations. If some supernatants samples had fluorescence outside the calibration curve, they were additionally diluted for the following calculations.
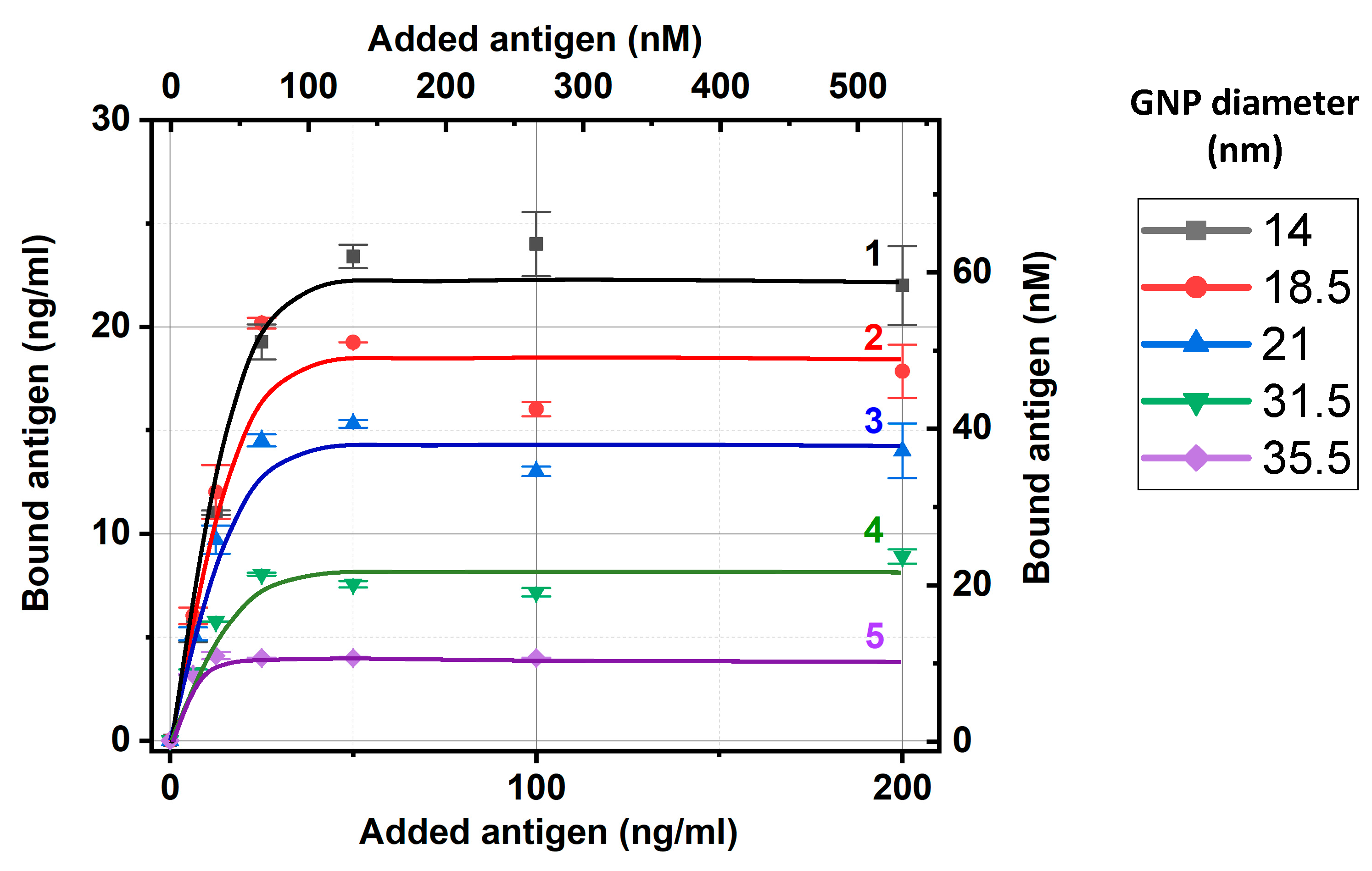
Considering the same experiments with all GNP preparations, we also used fluorescence for the added and nonbound carboxyfluorescein and calculated the bound carboxyfluorescein concentrations, as described above. The obtained dependences of carboxyfluorescein binding with the five tested preparations of IgG–GNP conjugates are presented in
Figure 3. As can be seen, all dependencies reached saturation, which allowed the estimation of the maximal capacities of the IgG–GNP conjugates to bound antigen molecules. To unify these estimations, we used an added antigen concentration of 50 ng/mL, which aligns with the saturation regions and enabled the linear approximation of the calibration curves in the range of 0–50 ng/mL without additional dilution of the tested samples.
The relative positions of the dependences in
Figure 3 reflect the differences in the surface capacity of the antibody conjugates for GNPs with different diameters. The larger the nanoparticles, the smaller their total surface area in the colloidal solution at a fixed optical density and the lower the concentration of bound antibodies per unit volume of solution. Therefore, it was logical to expect that a smaller number of antibodies would bind less carboxyfluorescein, i.e., the saturation levels in
Figure 3 decrease with increasing diameter of the GNPs.
The obtained data about antigen binding at 50 ng/mL (given at
Figure 3) and the conjugates’ composition (
Section 2.2) were used to estimate the percentage of active antigen-binding sites in the conjugates. The results of these calculations are summarized in
Table 4.
For the different-sized GNPs, the percentage of active antigen-binding sites varied more than three times, from 6% to 17%. The maximal value was reached for the GNPs with an average diameter equal to 18.5 nm; its decrease at smaller and larger diameters was statistically significant. See
Appendix B,
Figure A2b, for the estimation of
p values using Student’s
t-test for sequential pairwise comparisons. The factors that could be considered in the relationship with recorded differences included the electrostatic properties of GNPs and the differences in surface curvatures. These could influence the orientation of the antibodies, including their structural rearrangements during immobilization. Additionally, the negative surface charge of the GNPs [
27], reversibly interacting with solved charged molecules, and the local charges at the surface of immobilized immunoglobulin molecules could have influenced the movement of charged antigens at this level and at the time of its stay near the binding sites of the immobilized antibodies. Of course, these interactions depend on many factors that can only be listed as subjects for further detailed studies.
The above-presented levels of the retention of antibody activity after immobilization on the surface of nanoparticles are rather low and inferior even to those of systems in which larger antigens are bound [
16,
25,
28]. This situation requires an explanation and a search for additional factors that prevent effective binding in this case. To test the observed regularities in more detail, we performed additional experiments using another derivative of fluorescein (
Section 2.4) and varying coverages of the GNP surfaces with IgG (
Section 2.5).
2.4. Determination of Active Centers of the Immobilized Antibodies (Reaction with Aminofluorescein)
To check the impact of the electrostatic properties of the antigen derivative on the efficiency of its binding, a positively charged derivative of fluorescein containing an amino group instead of a carboxyl group was selected. Using an IgG conjugate with GNPs having an average diameter of 21 nm as an example, the antigen-binding ability of the conjugate with respect to this derivative was characterized.
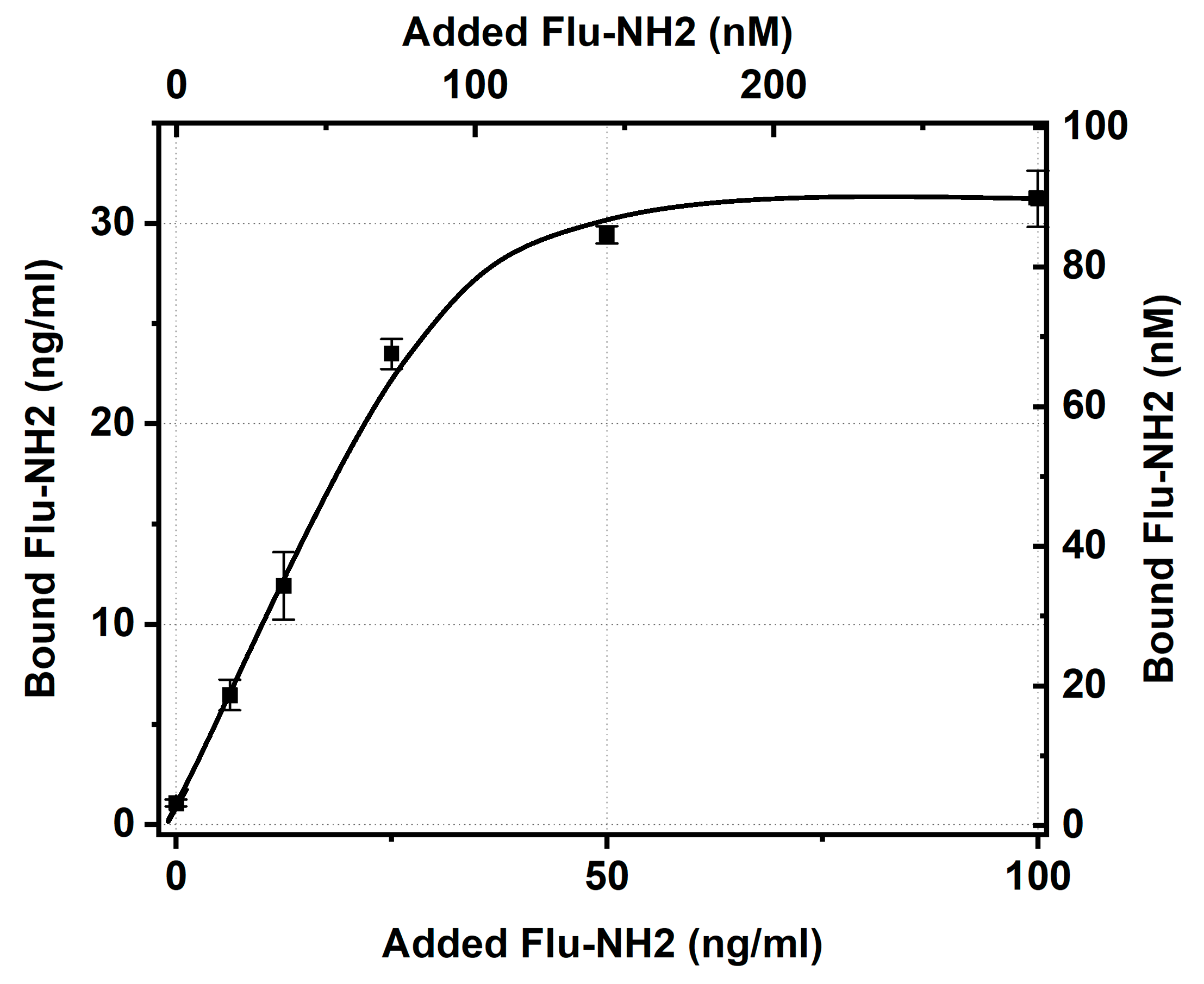
The testing of aminofluorescein binding and the IgG–GNP conjugate reached saturation with concentration dependence (
Figure 4), which allowed taking its value for an added antigen concentration of 50 ng/mL (from the plateau region, similar to the data processing for
Figure 3) and calculating the percentage of antigen-binding sites of immobilized IgG with stored activity (
Table 5).
As can be seen, the amino derivative of fluorescein bound to conjugated antibodies 2.1 times better than the carboxyl derivative. However, even in the case of aminofluorescein, the majority of the antibodies’ sites after immobilization lost their ability to bind this antigen. The inability to bind fluorescein, regardless of its variant, may be explained by either the complete blockage of the antigen-binding site as a result of immobilization or the structural disruption of this site caused by the interaction of IgG with the surface of the GNPs. A more complex question is why amino and carboxy derivatives, which both come into contact with the antigen-binding sites of the immobilized antibodies, differ in the limits of their binding. Probably, the antigen derivative and GNPs having the same charge limits the time required for the antigen to migrate at the GNP surface or the time needed for the transformation of the initial contact with IgG to a strong immune complex. However, the assessment of these factors’ contribution requires additional investigation.
The study results prove that the degree of antigen-binding ability stored by antibodies after their immobilization on the GNP surface is not a universal parameter for all antibodies: it depends on which antigen these antibodies bind.
2.5. Study of Antigen (Carboxyfluorescein)-Binding Properties of the Immobilized Antibodies with Varying Contents on the Nanoparticle Surfaces
Earlier studies of the interactions between IgG–GNP conjugates and protein antigens (peroxidase, C-reactive protein) [
16,
25,
28] showed that the antigen-binding efficiency is sensitive to the degree of GNP coverage by the antibodies. With a decrease in the surface density of immobilization, on the one hand, the accessibility of differently located antigen-binding sites increases, and the steric hindrances for nearly located antibodies decrease. On the other hand, different degrees of coverage can affect the contacts of antibodies with the GNP surface, their orientation, and structural changes. Therefore, it was of interest to evaluate how the degree of coverage and antigen-binding properties are related in the case of antibodies and low-molecular-weight antigens.
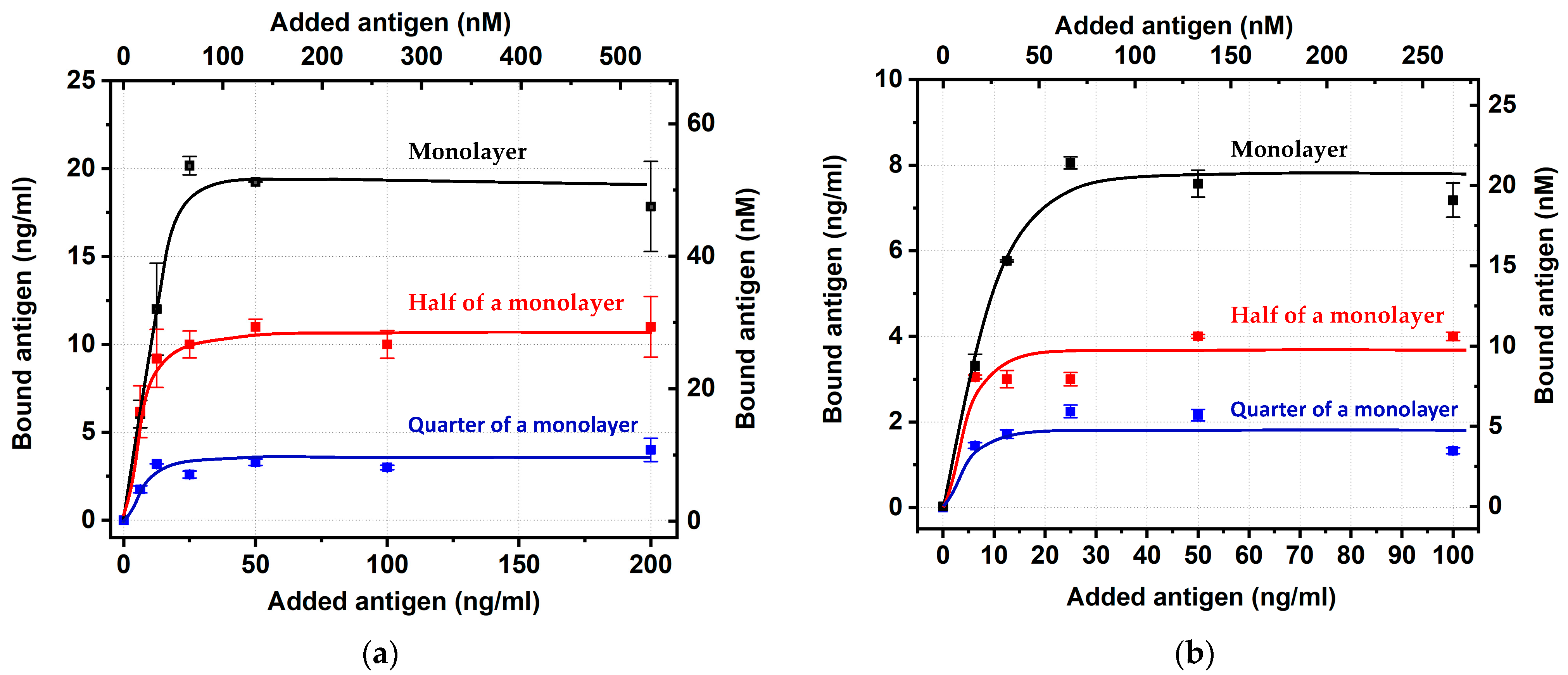
Using GNPs with average diameters of 18.5 and 31.5 nm, conjugates were synthesized containing different amounts of antibodies on the surface, namely, (i) with a completely filled monolayer of antibodies, (ii) containing two times fewer antibodies, and (iii) containing four times fewer antibodies. Firstly, they were characterized in terms of IgG binding. As can be seen from
Table 6, all preparations demonstrated high percentages of binding that were comparable with the earlier considered (
Table 3) monolayer preparations.
Next, the conjugates were tested for binding with carboxyfluorescein. The obtained concentration dependences (
Figure 5) reached saturation in all six cases, thus allowing the estimation of the percentage of the antigen-binding sites with stored activity. The calculations were implemented basing on the binding values for an added antigen concentration equal to 50 ng/mL (similar to the data processing for
Figure 3 and
Figure 4), which, in all cases, agreed with the plateau regions.
The calculated percentage values did not reveal any patterns regarding the degree of coverage (see
Table 7 and
Figure A2c in
Appendix B). Thus, in the situation with the absence of the above-listed factors that influence the interactions with protein antigens, the preparations of IgG–GNP conjugates with different IgG contents were comparable in their reactivity for the binding of low-molecular-weight antigens. Additionally, the IgG:GNP ratio is a useful tool for modulating the analytical parameters of immunosensors [
29,
30,
31,
32]; the obtained data confirmed the possibility of applying these tools without risks caused by antibody immobilization.