Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner
Abstract
1. Introduction
2. Results
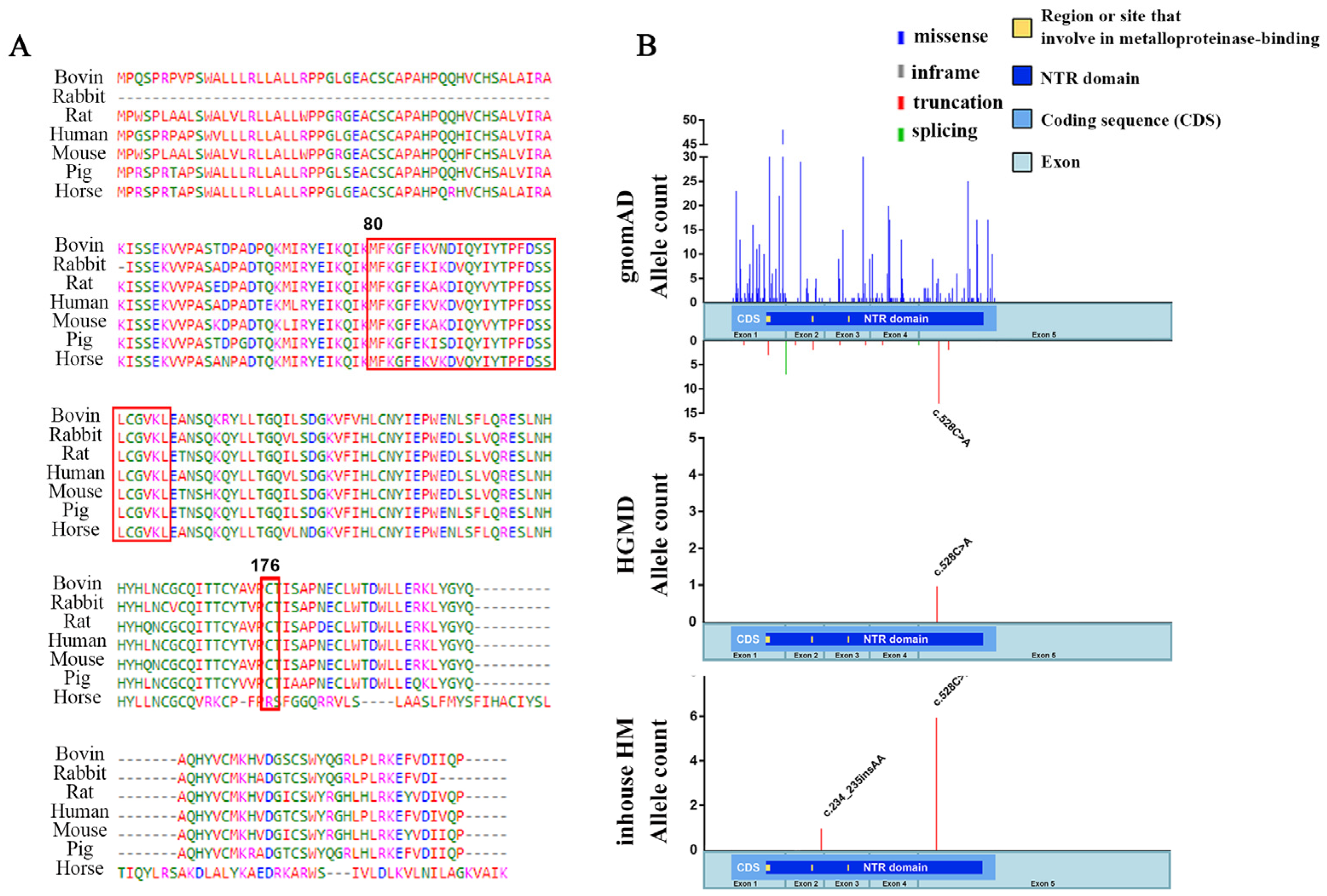
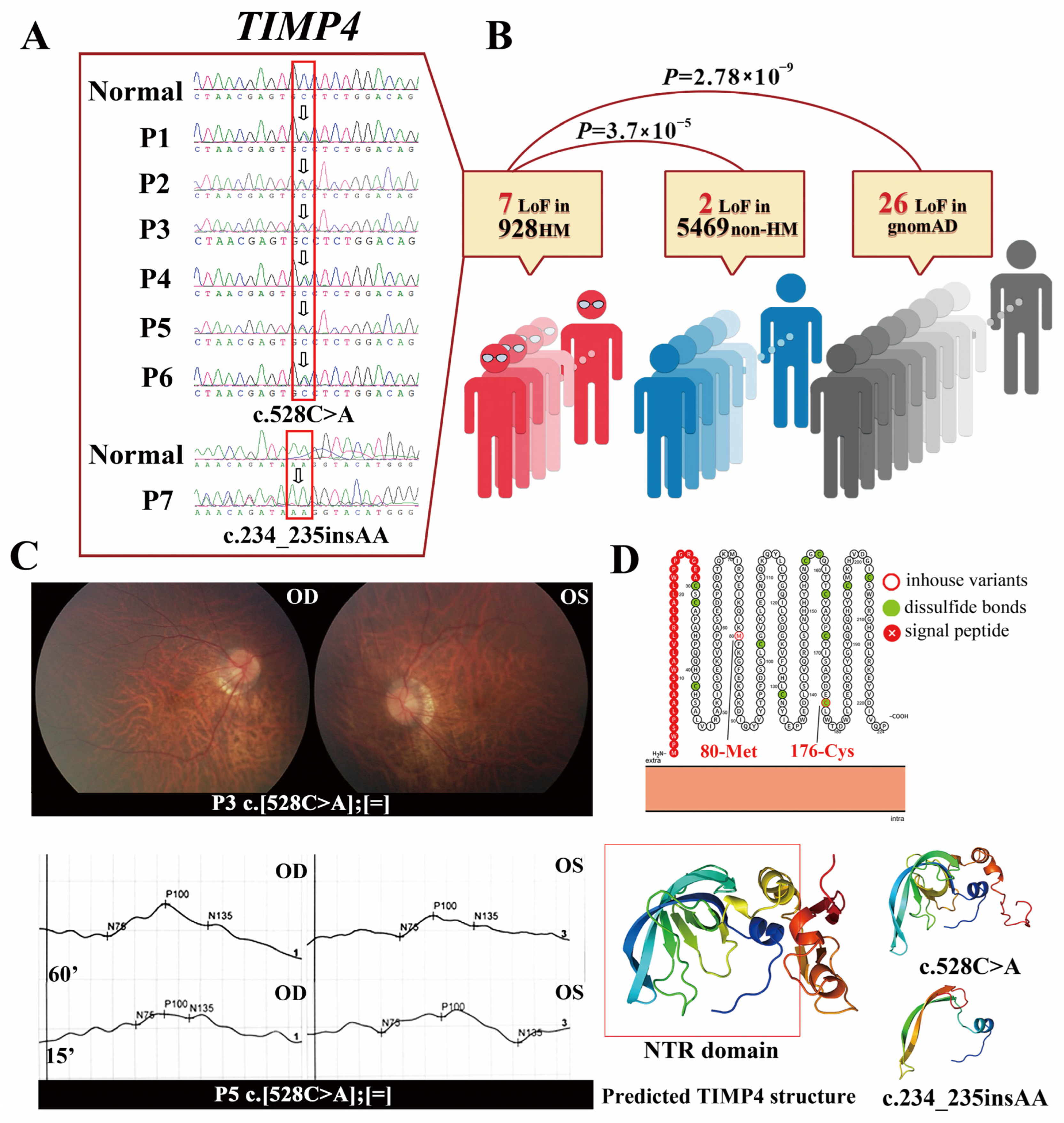
2.1. The TIMP4 Loss-of-Function Variants Clustered in Individuals with High Myopia
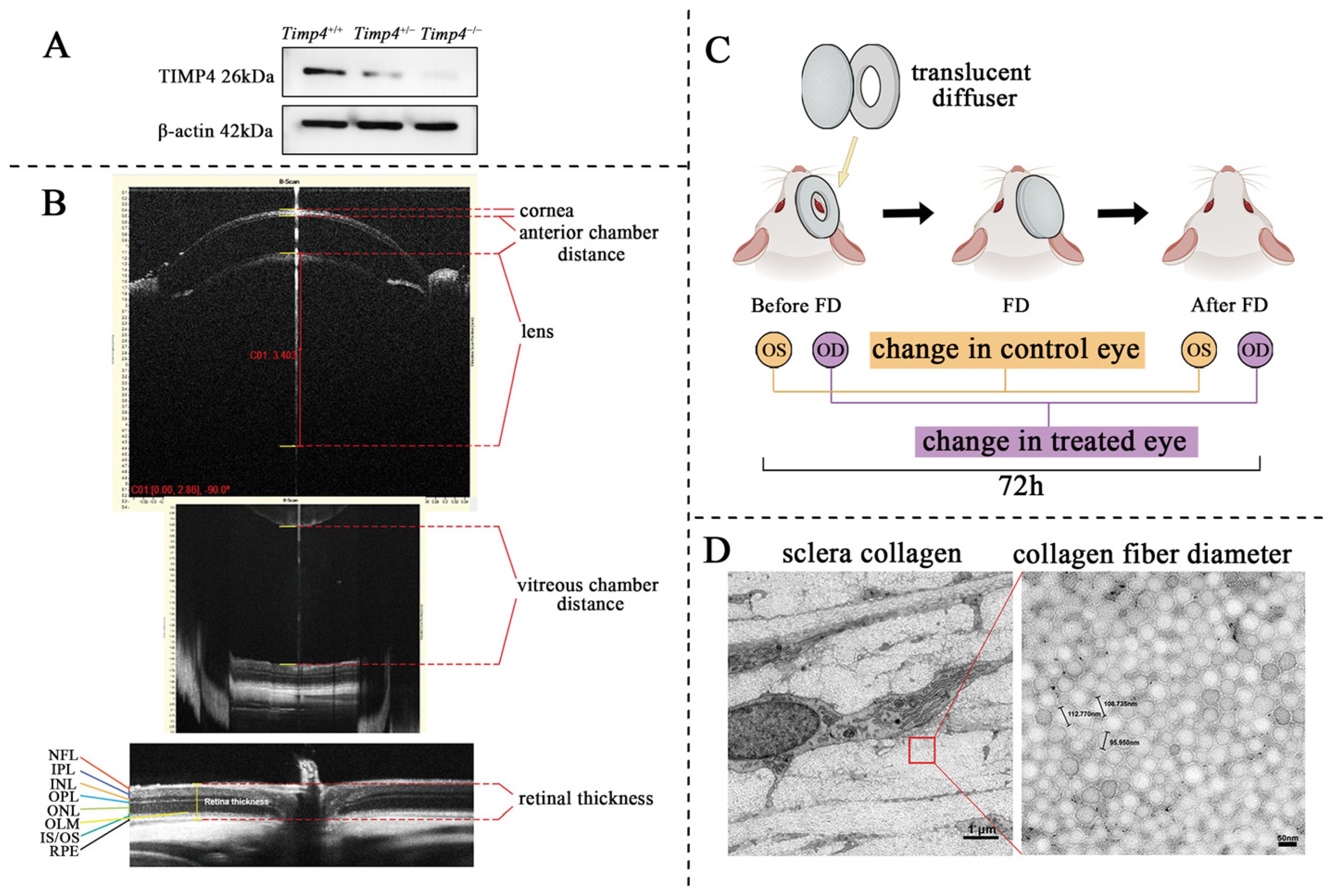
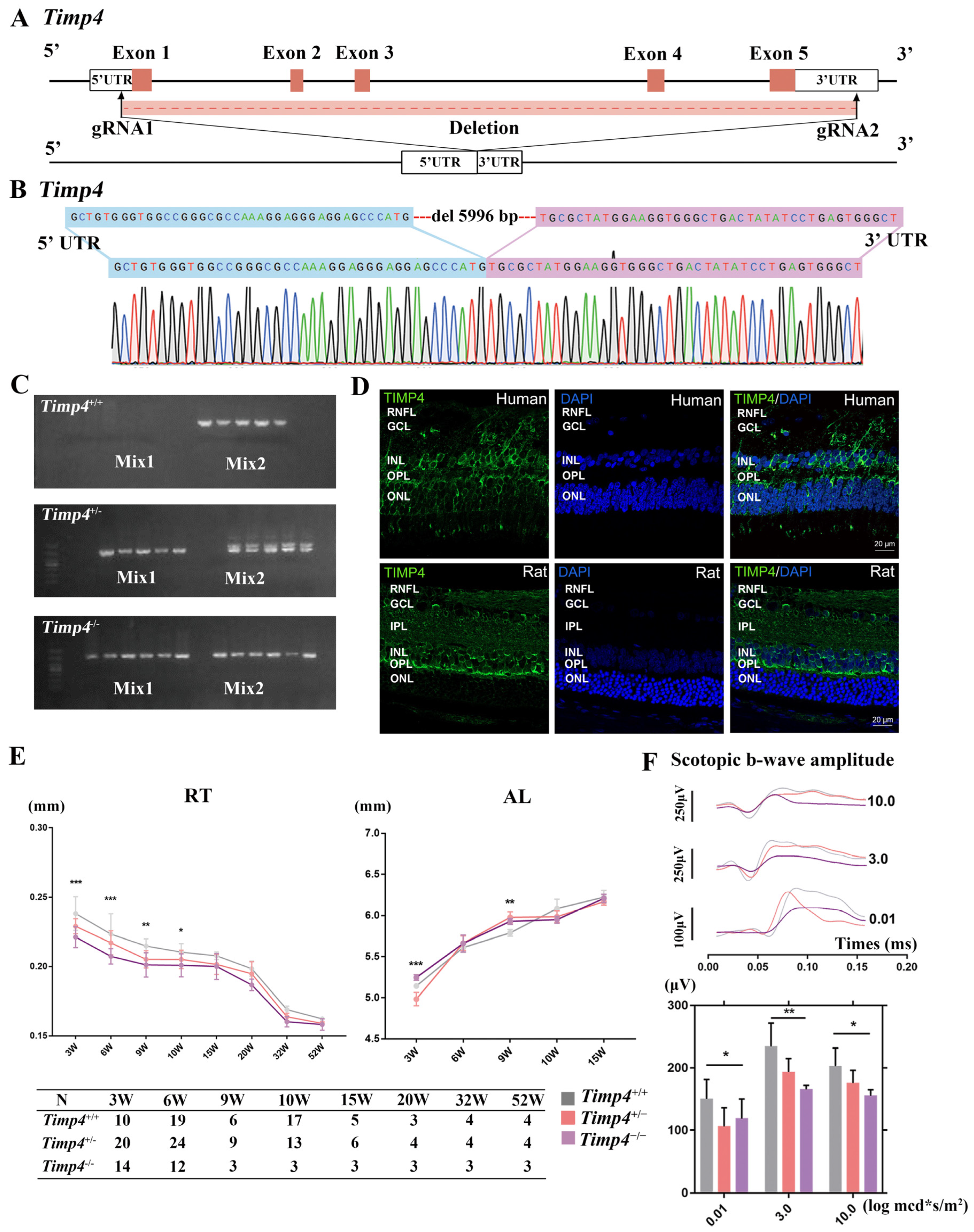
2.2. Timp4 Gene Editing Rat
2.3. Timp4 Expression in the Retina
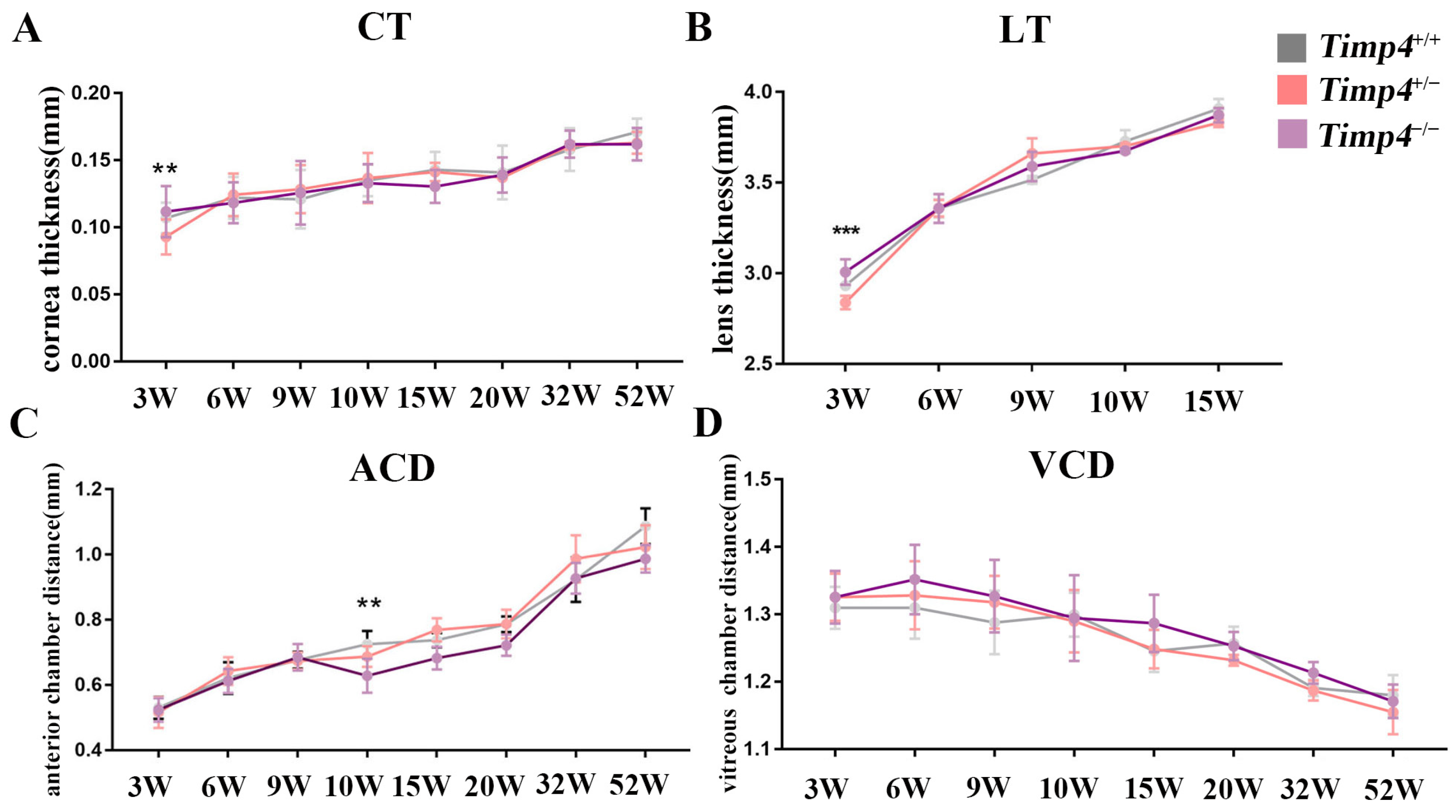
2.4. Ocular Morphology and Electroretinography Changes in Timp4−/− Rats in Normal Development
2.5. Ocular Morphology Changes of Timp4 Defect Rats under form Deprivation Pressure
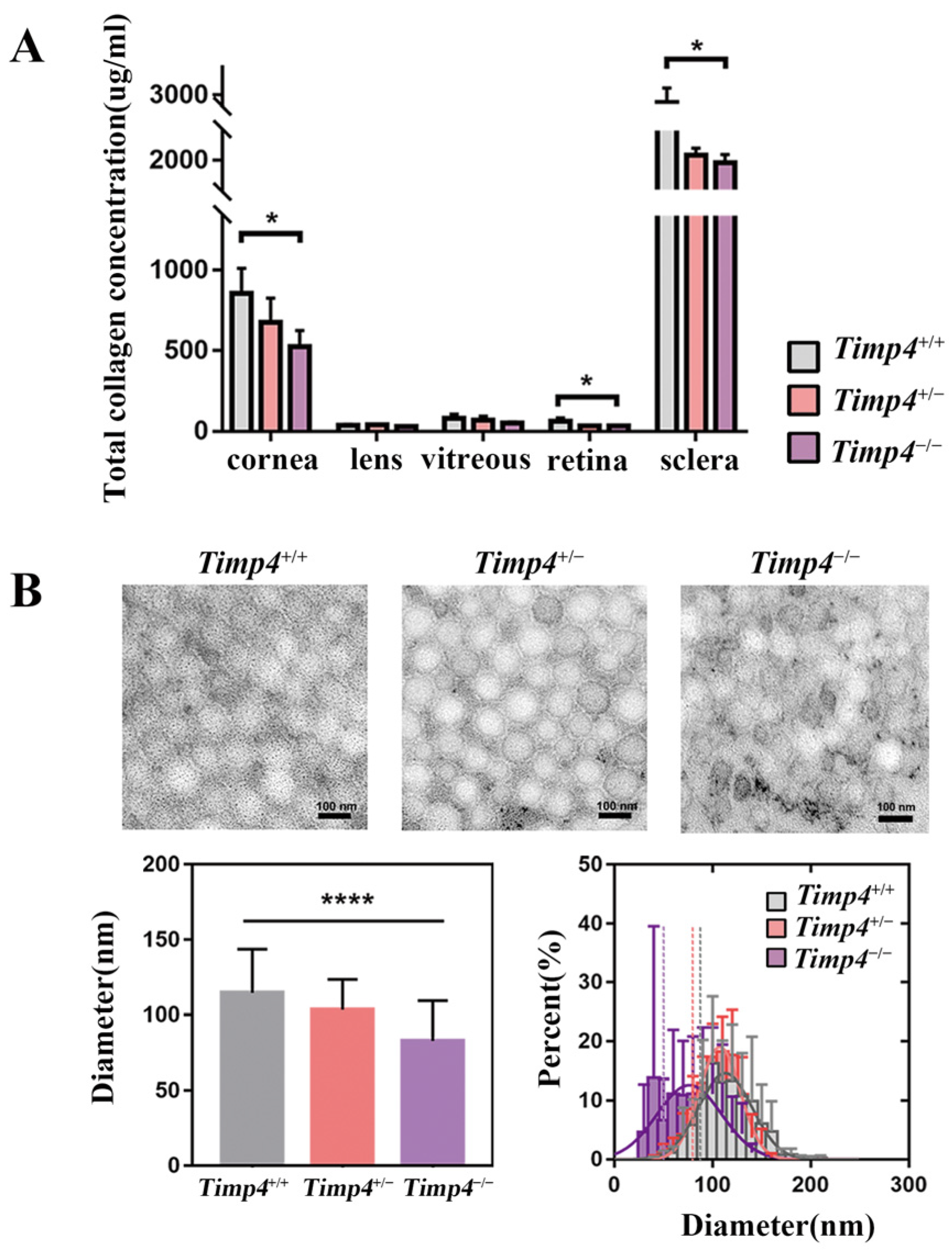
2.6. Content and Morphology Change in the Collagen of Timp4 Defect Rats
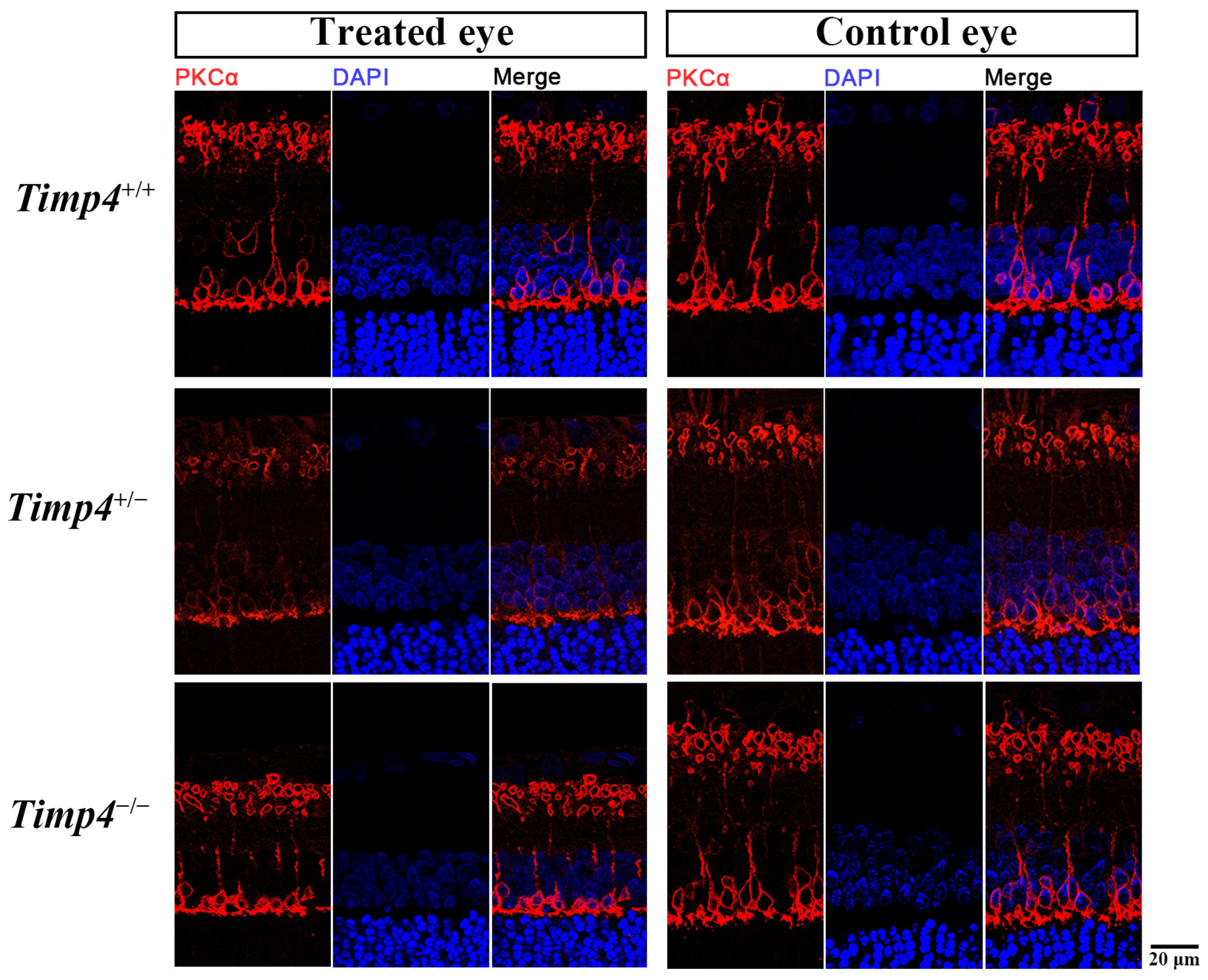
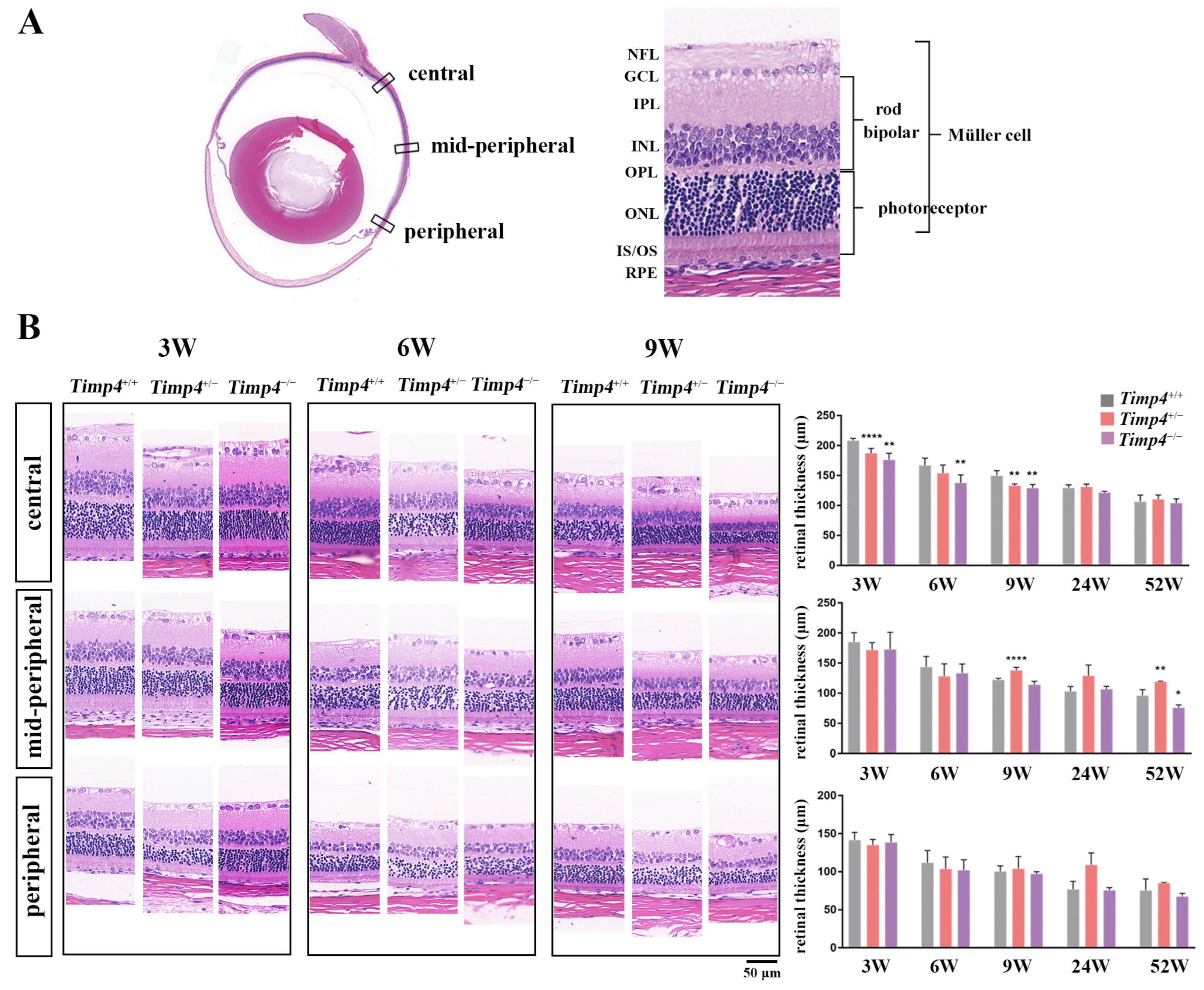
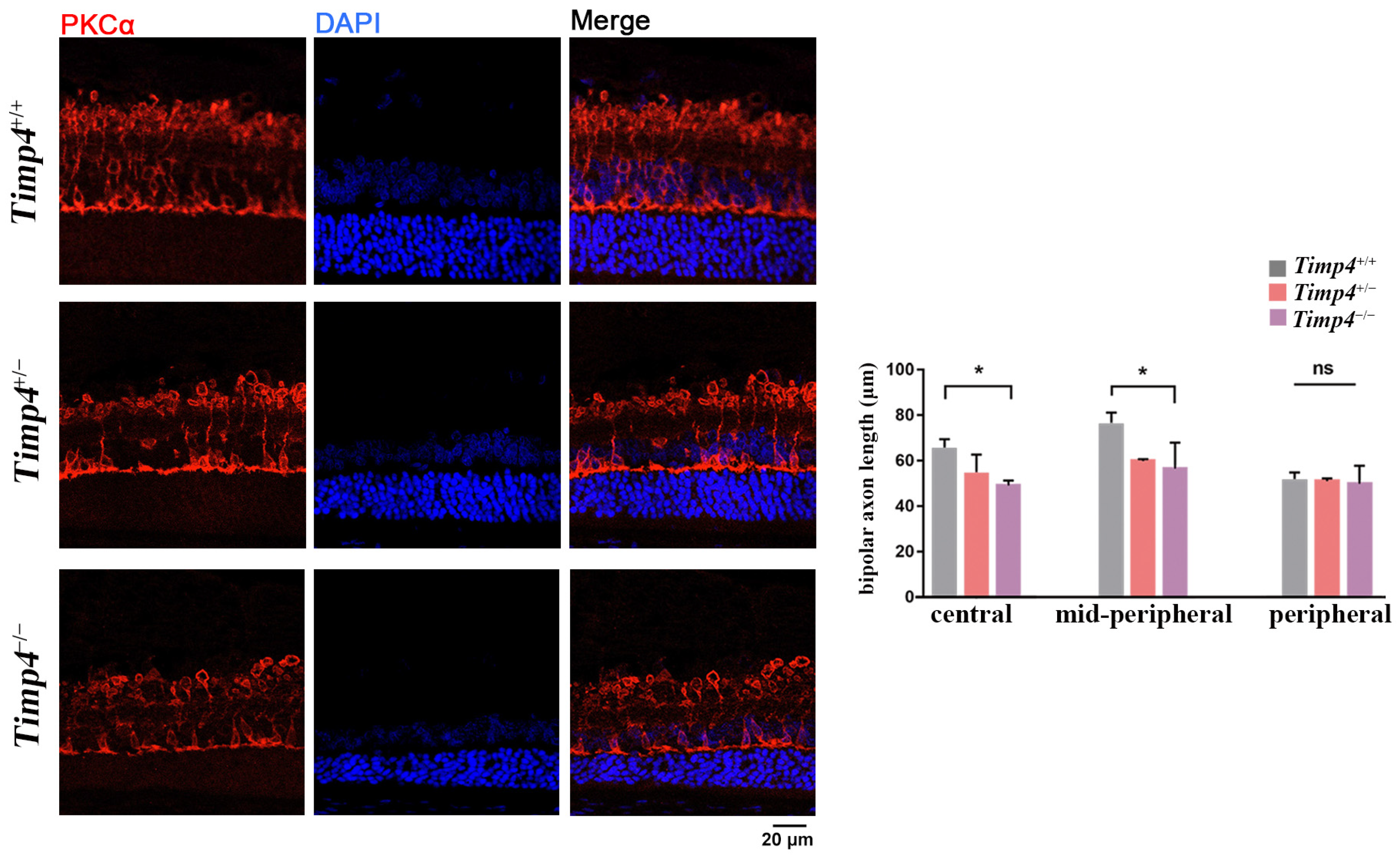
2.7. Morphology Changes in the Retina of Timp4−/− Rats
3. Discussion
4. Materials and Methods
4.1. Patients and Clinical Information
4.2. Candidate Gene Detection by WES and Multistep Bioanalysis
4.3. Knockdown of Timp4 Gene in Rats
4.4. Determination of Ocular Parameters
4.5. Determination of Ocular Components under Extra Pressure from Form Deprivation
4.6. Determination of Retina Function by Electroretinography
4.7. Hematoxylin-Eosin (HE) Staining and Immunofluorescence (IF)
4.8. Determination of Collagen Levels
4.9. Determination of Collagen Ultrastructure in the Sclera
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PPVs | Potential pathogenic variants |
| LoF | Loss of function |
| MMP | Matrix metalloproteinase |
| TIMP | Tissue inhibitor of metalloproteinase |
| ECM | Extracellular matrix |
| WES | Whole exome sequencing |
| HGMD | Human Gene Variant Database |
| OCT | Optical Coherence Tomography |
| ERG | Electroretinogram |
| RBC | Rod bipolar cell |
| FD | Form deprivation |
Appendix A
| Gene Name | Location | NCBI Gene ID | OMIM |
|---|---|---|---|
| MMP1 | 11q22.2 | 4312 | 120353 |
| MMP2 | 16q12.2 | 4313 | 120360 |
| MMP3 | 11q22.2 | 4314 | 185250 |
| MMP7 | 11q22.2 | 4316 | 178990 |
| MMP8 | 11q22.2 | 4317 | 120355 |
| MMP9 | 20q13.12 | 4318 | 120361 |
| MMP10 | 11q22.2 | 4319 | 185260 |
| MMP11 | 22q11.23 | 4320 | 185261 |
| MMP12 | 11q22.2 | 4321 | 601046 |
| MMP13 | 11q22.2 | 4322 | 600108 |
| MMP14 | 14q11.2 | 4323 | 600754 |
| MMP15 | 16q21 | 4324 | 602261 |
| MMP16 | 8q21.3 | 4325 | 602262 |
| MMP17 | 12q24.33 | 4326 | 602285 |
| MMP19 | 12q13.2 | 4327 | 601807 |
| MMP20 | 11q22.2 | 9313 | 604629 |
| MMP21 | 10q26.2 | 118856 | 608416 |
| MMP23A | 1p36.33 | 8511 | 603320 |
| MMP23B | 1p36.33 | 8510 | 603321 |
| MMP24 | 20q11.22 | 10893 | 604871 |
| MMP25 | 16p13.3 | 64386 | 608482 |
| MMP26 | 11p15.4 | 56547 | 605470 |
| MMP27 | 11q22.2 | 64066 | 618101 |
| MMP28 | 17q12 | 79148 | 608417 |
| TIMP1 | Xp11.3 | 7076 | 305370 |
| TIMP2 | 17q25.3 | 7077 | 188825 |
| TIMP3 | 22q12.3 | 7078 | 188826 |
| TIMP4 | 3p25.2 | 7079 | 601915 |
| Timp4+/+ | Timp4+/− | Timp4−/− | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p Value | |
| Axial length (mm) | |||||||
| 3W | 5.143 | 0.025 | 4.984 | 0.080 | 5.246 | 0.033 | 1.0 × 10−5 |
| 6W | 5.611 | 0.059 | 5.664 | 0.089 | 5.660 | 0.102 | 0.401 |
| 9W | 5.923 | 0.084 | 5.891 | 0.119 | 5.892 | 0.094 | 0.007 |
| 10W | 5.789 | 0.040 | 5.977 | 0.068 | 5.928 | 0.036 | 0.215 |
| 15W | 6.084 | 0.116 | 5.985 | 0.070 | 5.949 | 0.042 | 0.786 |
| Retina thickness (mm) | |||||||
| 3W | 0.2382 | 0.0121 | 0.2290 | 0.0054 | 0.2212 | 0.0075 | 3.6 × 10−5 |
| 6W | 0.2233 | 0.0147 | 0.2171 | 0.0086 | 0.2073 | 0.0055 | 4.4 × 10−4 |
| 9W | 0.2148 | 0.0051 | 0.2052 | 0.0062 | 0.2012 | 0.0088 | 0.013 |
| 10W | 0.2103 | 0.0061 | 0.2051 | 0.0066 | 0.2009 | 0.0083 | 0.022 |
| 15W | 0.2078 | 0.0027 | 0.2015 | 0.0072 | 0.2000 | 0.0094 | 0.213 |
| 20W | 0.1984 | 0.0055 | 0.1948 | 0.0086 | 0.1867 | 0.0042 | 0.163 |
| 32W | 0.1689 | 0.0026 | 0.1638 | 0.0027 | 0.1602 | 0.0037 | 0.055 |
| 52W | 0.1623 | 0.0017 | 0.1591 | 0.0012 | 0.1582 | 0.0041 | 0.088 |
| Vitreous chamber depth (mm) | |||||||
| 3W | 1.310 | 0.031 | 1.326 | 0.035 | 1.325 | 0.039 | 0.472 |
| 6W | 1.310 | 0.046 | 1.328 | 0.050 | 1.352 | 0.052 | 0.079 |
| 9W | 1.288 | 0.047 | 1.318 | 0.039 | 1.327 | 0.054 | 0.353 |
| 10W | 1.300 | 0.033 | 1.290 | 0.046 | 1.295 | 0.064 | 0.814 |
| 15W | 1.246 | 0.031 | 1.249 | 0.029 | 1.287 | 0.042 | 0.113 |
| 20W | 1.257 | 0.025 | 1.232 | 0.008 | 1.253 | 0.021 | 0.725 |
| 32W | 1.191 | 0.012 | 1.187 | 0.015 | 1.213 | 0.016 | 0.119 |
| 52W | 1.180 | 0.030 | 1.155 | 0.033 | 1.171 | 0.025 | 0.165 |
| Cornea thickness (mm) | |||||||
| 3W | 0.107 | 0.011 | 0.093 | 0.013 | 0.112 | 0.019 | 0.002 |
| 6W | 0.122 | 0.016 | 0.124 | 0.016 | 0.118 | 0.015 | 0.618 |
| 9W | 0.121 | 0.022 | 0.129 | 0.018 | 0.126 | 0.024 | 0.819 |
| 10W | 0.135 | 0.012 | 0.137 | 0.019 | 0.133 | 0.014 | 0.857 |
| 15W | 0.143 | 0.013 | 0.141 | 0.007 | 0.130 | 0.012 | 0.370 |
| 20W | 0.141 | 0.020 | 0.137 | 0.003 | 0.139 | 0.013 | 0.161 |
| 32W | 0.158 | 0.016 | 0.161 | 0.003 | 0.162 | 0.010 | 0.959 |
| 52W | 0.171 | 0.010 | 0.163 | 0.008 | 0.162 | 0.012 | 0.862 |
| Anterior chamber depth (mm) | |||||||
| 3W | 0.530 | 0.034 | 0.515 | 0.047 | 0.524 | 0.036 | 0.641 |
| 6W | 0.621 | 0.048 | 0.643 | 0.042 | 0.612 | 0.037 | 0.103 |
| 9W | 0.676 | 0.026 | 0.674 | 0.026 | 0.685 | 0.041 | 0.843 |
| 10W | 0.725 | 0.041 | 0.687 | 0.032 | 0.628 | 0.052 | 0.002 |
| 15W | 0.737 | 0.022 | 0.769 | 0.036 | 0.682 | 0.034 | 0.075 |
| 20W | 0.786 | 0.024 | 0.787 | 0.044 | 0.722 | 0.032 | 0.633 |
| 32W | 0.923 | 0.068 | 0.987 | 0.072 | 0.927 | 0.047 | 0.359 |
| 52W | 1.087 | 0.055 | 1.023 | 0.067 | 0.987 | 0.042 | 0.163 |
| Lens thickness (mm) | |||||||
| 3W | 2.932 | 0.024 | 2.839 | 0.037 | 3.007 | 0.070 | 1.3 × 10−5 |
| 6W | 3.359 | 0.050 | 3.359 | 0.045 | 3.358 | 0.079 | 0.079 |
| 9W | 3.515 | 0.021 | 3.661 | 0.083 | 3.589 | 0.081 | 0.295 |
| 10W | 3.728 | 0.060 | 3.702 | 0.015 | 3.676 | 0.013 | 0.751 |
| 15W | 3.908 | 0.053 | 3.830 | 0.024 | 3.873 | 0.039 | 0.135 |
| Timp4+/+ | Timp4+/− | Timp4−/− | ||||
|---|---|---|---|---|---|---|
| Total Collagen Concentration (μg/mL) | Tissue Weight (g) | Total Collagen Concentration (μg/mL) | Tissue Weight (g) | Total Collagen Concentration (μg/mL) | Tissue Weight (g) | |
| cornea | 854.2 ± 156.4 | 0.005 ± 0.001 | 676.3 ± 148.5 | 0.006 ± 0.002 | 522.4 ± 101.4 | 0.005 ± 0.001 |
| lens | 38.0 ± 5.4 | 0.022 ± 0.005 | 39.8 ± 3.1 | 0.023 ± 0.004 | 32.1 ± 3.2 | 0.019 ± 0.003 |
| vitreous | 80.4 ± 27.1 | 0.004 ± 0.001 | 68.5 ± 26.3 | 0.004 ± 0.001 | 51.0 ± 7.8 | 0.005 ± 0.002 |
| retina | 62.8 ± 21.5 | 0.013 ± 0.003 | 33.5 ± 5.5 | 0.010 ± 0.002 | 32.6 ± 5.8 | 0.010 ± 0.001 |
| sclera | 2719.9 ± 535.7 | 0.013 ± 0.002 | 2112.9 ± 167.4 | 0.015 ± 0.004 | 1941.2 ± 190.9 | 0.013 ± 0.002 |
| Timp4+/+ | Timp4+/− | Timp4−/− | |
|---|---|---|---|
| Average | 114.5 ± 29.22 | 103.4 ± 20.21 | 82.77 ± 26.68 |
| First quartile | 94.75 | 90.52 | 63.45 |
| Median | 113.1 | 103.7 | 84.7 |
| Third quartile | 135.0 | 117.3 | 102.3 |
| Central | Mid-Peripheral | Peripheral | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| 3W | Timp4+/+ | 208.22 | 3.76 | 184.83 | 15.67 | 141.55 | 10.2 |
| Timp4+/− | 187.44 | 7.97 | 171.84 | 12.25 | 135.22 | 7.2 | |
| Timp4−/− | 175.87 | 11.31 | 172.65 | 28.49 | 138.5 | 10.37 | |
| p | 3.41 × 10−6 | 0.349 | 0.359 | ||||
| 6W | Timp4+/+ | 166.67 | 12.29 | 143.53 | 17.65 | 111.8 | 16.18 |
| Timp4+/− | 153.95 | 13.73 | 128.06 | 20.86 | 103.61 | 15.73 | |
| Timp4−/− | 137.65 | 13.53 | 133.2 | 15.42 | 101.85 | 13.86 | |
| p | 0.006 | 0.296 | 0.481 | ||||
| 9W | Timp4+/+ | 149.47 | 8.8 | 122.23 | 2.4 | 100.33 | 7.46 |
| Timp4+/− | 133.23 | 2.73 | 138.03 | 4.91 | 103.95 | 16.01 | |
| Timp4−/− | 129.07 | 6.1 | 114.13 | 5.75 | 97.1 | 3.03 | |
| p | 4.27 × 10−4 | 4.22 × 10−5 | 0.591 | ||||
| NFL + GCL | IPL + INL | OPL + ONL + IS/OS | RPE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | ||
| 3W | Timp4+/+ | 36.42 | 7.26 | 85.00 | 7.81 | 89.67 | 4.55 | 6.67 | 0.81 |
| Timp4+/− | 28.66 | 8.69 | 71.74 | 8.12 | 75.48 | 8.72 | 5.58 | 0.72 | |
| Timp4−/− | 27.58 | 4.14 | 76.93 | 3.61 | 73.72 | 7.31 | 6.68 | 1.28 | |
| p | 0.0920 | 0.005 | 0.002 | 0.026 | |||||
| 6W | Timp4+/+ | 26.32 | 2.54 | 69.00 | 4.63 | 66.48 | 8.35 | 4.87 | 0.67 |
| Timp4+/− | 25.27 | 4.26 | 51.93 | 16.09 | 50.77 | 16.51 | 3.96 | 1.32 | |
| Timp4−/− | 25.52 | 1.28 | 53.00 | 2.91 | 52.90 | 3.95 | 5.03 | 0.98 | |
| p | 0.8220 | 0.021 | 0.059 | 0.138 | |||||
| 9W | Timp4+/+ | 37.53 | 2.32 | 57.20 | 2.79 | 52.27 | 1.39 | 3.73 | 1.00 |
| Timp4+/− | 35.90 | 7.55 | 50.58 | 4.80 | 44.17 | 2.55 | 4.80 | 0.69 | |
| Timp4−/− | 26.13 | 0.67 | 44.57 | 2.10 | 40.63 | 0.51 | 3.50 | 0.46 | |
| p | 0.0668 | 0.011 | 0.0002 | 0.057 | |||||
| Gene | MYP | OMIM | Inheritance Patterns | Animal Models | Phenotypes Supporting High Myopia | Reference (PMID) | ||
|---|---|---|---|---|---|---|---|---|
| Axial Length | Refractive Error | Others | ||||||
| SCO2 | 6 | 604272 | AD | mouse | / | / | / | [69] |
| ZNF644 | 21 | 614159 | AD | zebrafish | / | / | / | [70] |
| LRPAP1 | 23 | 104225 | AR | mouse | / | / | / | [23,71] |
| SLC39A5 | 24 | 608730 | AD | mouse | / | / | / | [72] |
| P4HA2 | 25 | 600608 | AD | mouse | / | / | / | [73] |
| ARR3 | 26 | 301770 | XL | mouse | / | / | / | [74,75] |
| CPSF1 | 27 | 606027 | AD | zebrafish | / | / | small eye size | [49] |
| LOXL3 | 28 | 607163 | AR | mouse | / | / | / | [54] |
| CTSH | / | 116820 | AR | mouse | / | / | abnormally gross eyeball | [22] |
| NDUFAF7 | / | 615898 | AD | mouse | / | / | / | [76] |
| BSG | / | 109480 | AD | mouse | AL elongation | / | / | [32] |
| OPN1LW | / | 300822 | XL | mouse | / | / | / | [77] |
| XYLT | / | 608125 | AD | mouse | / | / | / | [78] |
| DZIP1 | / | 608671 | AD | mouse | / | / | / | [79] |
| Organ/Tissue | Phenotype | Reference (PMID) |
|---|---|---|
| Heart | Timp4−/− mice were born and developed healthy, but their mortality increased significantly by TIMP2 expression increasing after cardiac pressure overload-induced by aortic banding, which could be rescued by exogenous MMPs inhibitors | [46] |
| Heart | after ischemia-reperfusion in vivo, more severe systolic and diastolic dysfunction, myocardial hypertrophy, and fibrosis appeared in Timp4−/− mice than in wild-type | [80] |
| Heart | left ventricular hypertrophy appeared, ventricular fibrosis increased, and long-term survival decreased in Timp4−/− mice under left ventricular pressure overload | [81] |
| Vascular | no difference in blood pressure hypertension induced by angiotensin II between Timp4−/− mice and wild-type mice | [82] |
| Fat | Timp4−/− mice showed higher food intake than the wild-type mice under a high-fat diet but less fat gain | [83] |
| Material | Volume/Well (μL) | Final Concentration | |
|---|---|---|---|
| Mix1 | 2x PCR buffer for KOD FX | 25 | 1× |
| 2 mM dNTPs | 10 | 0.4 mM each | |
| Primer-F | 1.5 | / | |
| Primer-R | 1.5 | / | |
| Template DNA | 2 | 200 ng/50 μL | |
| KOD FX (1.0 U/μL) | 1 | 1.0 U/50 μL | |
| Ultra-pure water | 9 | / | |
| Mix2 | 2x PCR buffer for KOD FX | 25 | 1× |
| 2mM dNTPs | 10 | 0.4 mM each | |
| Primer-F | 0.75 | / | |
| Primer-R | 1.5 | / | |
| Primer-WT-F | 0.75 | / | |
| Template DNA | 2 | 200 ng/50 μL | |
| KOD FX (1.0 U/μL) | 1 | 1.0 U/50 μL | |
| Ultra-pure water | 9 | / |
| Temperature | Time | Cycle |
|---|---|---|
| 94 °C | 2 min | |
| 98 °C | 10 s | 35x |
| 60 °C | 30 s | |
| 68 °C | 1 min | |
| 68 °C | 7 min |
| Age (Weeks) | 3 | 5 | 6 | 9 | 10 | 12 | 15 | 18 | 20 | 22 | 52 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timp4+/+ | Mean | 49.3 | 124.0 | 167.0 | 252.2 | 271.5 | 366.5 | 370.3 | 421.5 | 423.5 | 440.0 | 604.7 |
| SD | 8.1 | 13.7 | 9.9 | 10.1 | 16.3 | 14.8 | 8.7 | 9.2 | 29.0 | 5.7 | 13.7 | |
| Timp4+/− | Mean | 52.5 | 129.7 | 165.6 | 259.3 | 275.4 | 365.8 | 382.3 | 435.5 | 447.0 | 441.5 | 623.0 |
| SD | 6.4 | 10.8 | 10.4 | 20.8 | 12.6 | 13.3 | 16.7 | 16.7 | 15.3 | 24.3 | 28.3 | |
| Timp4−/− | Mean | 53.2 | 122.0 | 160.7 | 247.4 | 303.3 | 385.0 | 399.6 | 443.3 | 463.5 | 444.3 | 608.3 |
| SD | 7.9 | 25.5 | 26.1 | 18.7 | 10.6 | 14.3 | 7.7 | 13.3 | 7.1 | 17.5 | 11.6 |
| Antibody | Target | Immunogen | Host Species | Supplier | RRID/SKU | Concentration |
|---|---|---|---|---|---|---|
| PCKα | Protein Kinase C-α | amino acids residue 652–672 | Mouse | Santa Cruz, Dallas, USA | AB_628142 | 1:1000 |
| TIMP4 | Tissue inhibitor of matrix metalloproteinase4 | Amino acids residue 30–224 | Rabbit | Invitrogen, USA | PA5-92959 | 1:100 |
| DAPI | Nucleic acid | / | / | Roche, Basel, Swiss | 10236276001 | 1:3000 |
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Ikuno, Y. Overview of the Complications of high myopia. Retina 2017, 37, 2347–2351. [Google Scholar] [CrossRef] [PubMed]
- Enthoven, C.A.; Tideman, J.W.L.; Polling, J.R.; Tedja, M.S.; Raat, H.; Iglesias, A.I.; Verhoeven, V.J.M.; Klaver, C.C.W. Interaction between lifestyle and genetic susceptibility in myopia: The Generation R study. Eur. J. Epidemiol. 2019, 34, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Guo, Y.; Liao, C.; Chen, Y.; Su, G.; Zhang, G.; Zhang, L.; He, M. Incidence of and Factors Associated With Myopia and High Myopia in Chinese Children, Based on Refraction Without Cycloplegia. JAMA Ophthalmol. 2018, 136, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Baird, P.N.; Saw, S.-M.; Lanca, C.; Guggenheim, J.A.; Smith Iii, E.L.; Zhou, X.; Matsui, K.-O.; Wu, P.-C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, B.; Guan, L.; Xiao, X.; Zhang, J.; Li, S.; Jiang, H.; Jia, X.; Yang, J.; Guo, X.; et al. Unique Variants in OPN1LW Cause Both Syndromic and Nonsyndromic X-Linked High Myopia Mapped to MYP1. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4150–4155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, X.; Li, S.; Jia, X.; Zhang, Q. Frequent mutations of RetNet genes in eoHM: Further confirmation in 325 probands and comparison with late-onset high myopia based on exome sequencing. Exp. Eye Res. 2018, 171, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Huang, L.; Xu, Y.; Xiao, X.; Li, S.; Jia, X.; Gao, B.; Wang, P.; Guo, X.; Zhang, Q. Exome Sequencing on 298 Probands With Early-Onset High Myopia: Approximately One-Fourth Show Potential Pathogenic Mutations in RetNet Genes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8365–8372. [Google Scholar] [CrossRef]
- Hysi, P.G.; Choquet, H.; Khawaja, A.P.; Wojciechowski, R.; Tedja, M.S.; Yin, J.; Simcoe, M.J.; Patasova, K.; Mahroo, O.A.; Thai, K.K.; et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat. Genet. 2020, 52, 401–407. [Google Scholar] [CrossRef]
- Metlapally, R.; Wildsoet, C.F. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog. Progress. Mol. Biol. Transl. Sci. 2015, 134, 241–248. [Google Scholar] [CrossRef]
- Avetisov, E.S.; Savitskaya, N.F.; Vinetskaya, M.I.; Iomdina, E.N. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab. Pediatr. Syst. Ophthalmol. 1983, 7, 183–188. [Google Scholar] [PubMed]
- Stickler, G.B.; Belau, P.G.; Farrell, F.J.; Jones, J.D.; Pugh, D.G.; Steinberg, A.G.; Ward, L.E. Hereditary Progressive Arthro-Ophthalmopathy. Mayo Clin. Proc. 1965, 40, 433–455. [Google Scholar]
- Zeigler, S.M.; Sloan, B.; Jones, J.A. Pathophysiology and Pathogenesis of Marfan Syndrome. Adv. Exp. Med. Biol. 2021, 1348, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liu, Q.; Ma, X.; Bai, S.; Chen, P.; Zhao, Y.; Bai, C.; Liu, Y.; Liu, K.; Xin, M.; et al. MicroRNA-146b-5p promotes atrial fibrosis in atrial fibrillation by repressing TIMP4. J. Cell. Mol. Med. 2021, 25, 10543–10553. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Gentle, A. Role of the sclera in the development and pathological complications of myopia. Prog. Prog. Retin. Eye Res. 2003, 22, 307–338. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Liu, H.-H.; Kenning, M.S.; Jobling, A.I.; McBrien, N.A.; Gentle, A. Reduced Scleral TIMP-2 Expression Is Associated With Myopia Development: TIMP-2 Supplementation Stabilizes Scleral Biomarkers of Myopia and Limits Myopia Development. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1971–1981. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, D.-N.; Sun, J.; Zhou, J. Correlations Between MMPs and TIMPs Levels in Aqueous Humor from High Myopia and Cataract Patients. Curr. Eye Res. 2017, 42, 600–603. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, Q.; Reinach, P.S.; Yang, J.; Ma, L.; Wang, X.; Wen, Y.; Srinivasalu, N.; Qu, J.; Zhou, X. Cause and Effect Relationship between Changes in Scleral Matrix Metallopeptidase-2 Expression and Myopia Development in Mice. Am. J. Pathol. 2018, 188, 1754–1767. [Google Scholar] [CrossRef]
- Siegwart, J.T., Jr.; Norton, T.T. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3484–3492. [Google Scholar] [CrossRef]
- Rada, J.A.; Brenza, H.L. Increased latent gelatinase activity in the sclera of visually deprived chicks. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1555–1565. [Google Scholar]
- Aldahmesh, M.A.; Khan, A.O.; Alkuraya, H.; Adly, N.; Anazi, S.; Al-Saleh, A.A.; Mohamed, J.Y.; Hijazi, H.; Prabakaran, S.; Tacke, M.; et al. Mutations in LRPAP1 are associated with severe myopia in humans. Am. J. Hum. Genet. 2013, 93, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.W.; Wang, P.F.; Cheung, J.K.W.; Yu, F.; Zheng, H.; Luo, S.; Yip, S.P.; To, C.H.; Lam, T.C. Transcriptional profiling of the chick retina identifies down-regulation of VIP and UTS2B genes during early lens-induced myopia. Mol. Omics 2022, 18, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Zajgla, J.; Del Pozo, L.; Ceballos, G.; Maldonado, V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol. Cancer 2008, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Willenbrock, F.; Crabbe, T.; Slocombe, P.M.; Sutton, C.W.; Docherty, A.J.; Cockett, M.I.; O’Shea, M.; Brocklehurst, K.; Phillips, I.R.; Murphy, G. The activity of the tissue inhibitors of metalloproteinases is regulated by C-terminal domain interactions: A kinetic analysis of the inhibition of gelatinase A. Biochemistry 1993, 32, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Suo, N.-C.; Lei, C.-L.; Zhang, Y.-C.; Li, X.-Z.; Li, F.-Z.; Gong, K. Effects of latanoprost on the expression of TGF-β1 and Wnt/β-catenin signaling pathway in the choroid of form-deprivation myopia rats. Cell Mol. Biol. 2020, 66, 71–75. [Google Scholar] [CrossRef]
- John, J.; Sharma, A.; Kukshal, P.; Bhatia, T.; Nimgaonkar, V.L.; Deshpande, S.N.; Thelma, B.K. Rare Variants in Tissue Inhibitor of Metalloproteinase 2 as a Risk Factor for Schizophrenia: Evidence From Familial and Cohort Analysis. Schizophr. Bull. 2019, 45, 256–263. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Wallman, J.; Gottlieb, M.D.; Rajaram, V.; Fugate-Wentzek, L.A. Local retinal regions control local eye growth and myopia. Science 1987, 237, 73–77. [Google Scholar] [CrossRef]
- Jin, Z.-B.; Wu, J.; Huang, X.-F.; Feng, C.-Y.; Cai, X.-B.; Mao, J.-Y.; Xiang, L.; Wu, K.-C.; Xiao, X.; Kloss, B.A.; et al. Trio-based exome sequencing arrests de novo mutations in early-onset high myopia. Proc. Natl. Acad. Sci. USA 2017, 114, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Omi, N. Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Front. Neuroanat. 2017, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Teves, M.; Díez-Hermano, S.; Porrero, C.; Sánchez-Jiménez, A.; Prensa, L.; Clascá, F.; García-Amado, M.; Villacorta-Atienza, J.A. Benchmarking of tools for axon length measurement in individually-labeled projection neurons. PLoS Comput. Biol. 2021, 17, e1009051. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.L.; Dhingra, A.; Vardi, N.; Wong, R.O.L. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat. Neurosci. 2006, 9, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Haverkamp, S.; Schubert, T.; Baden, T. Retinal bipolar cells: Elementary building blocks of vision. Nat. Rev. Neurosci. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Chakraborty, R.; Park, H.N.; Hanif, A.M.; Sidhu, C.S.; Iuvone, P.M.; Pardue, M.T. ON pathway mutations increase susceptibility to form-deprivation myopia. Exp. Eye Res. 2015, 137, 79–83. [Google Scholar] [CrossRef]
- Snellman, J.; Kaur, T.; Shen, Y.; Nawy, S. Regulation of ON bipolar cell activity. Prog. Progress. Retin. Eye Res. 2008, 27, 450–463. [Google Scholar] [CrossRef]
- Martemyanov, K.A.; Sampath, A.P. The Transduction Cascade in Retinal ON-Bipolar Cells: Signal Processing and Disease. Annu. Rev. Vis. Sci. 2017, 3, 25–51. [Google Scholar] [CrossRef]
- Xiong, W.-H.; Pang, J.-J.; Pennesi, M.E.; Duvoisin, R.M.; Wu, S.M.; Morgans, C.W. The Effect of PKCα on the Light Response of Rod Bipolar Cells in the Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4961–4974. [Google Scholar] [CrossRef]
- Varshney, S.; Hunter, D.D.; Brunken, W.J. Extracellular Matrix Components Regulate Cellular Polarity and Tissue Structure in the Developing and Mature Retina. J. Ophthalmic Vis. Res. 2015, 10, 329–339. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef]
- Sood, D.; Chwalek, K.; Stuntz, E.; Pouli, D.; Du, C.; Tang-Schomer, M.; Georgakoudi, I.; Black, L.D.; Kaplan, D.L. Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Yoshida, T.; Liu, H.; Ichinose, S.; Ishida, T.; Nakahama, K.I.; Nagaoka, N.; Moriyama, M.; Morita, I.; Ohno-Matsui, K. Establishment of novel therapy to reduce progression of myopia in rats with experimental myopia by fibroblast transplantation on sclera. J. Tissue Eng. Regen. Med. 2018, 12, e451–e461. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, L.; Zhang, J.; Cai, X.; Yang, Y.; Yu, J.; Qiu, J.; Ge, J.; Yu, K.; Zhuang, J. Up-Regulation of SorCS1, an Important Sorting Receptor, in the Retina of a Form-Deprivation Rat Model. Cell Mol. Neurobiol. 2020, 40, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Koskivirta, I.; Kassiri, Z.; Rahkonen, O.; Kiviranta, R.; Oudit, G.Y.; McKee, T.D.; Kytö, V.; Saraste, A.; Jokinen, E.; Liu, P.P.; et al. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J. Biol. Chem. 2010, 285, 24487–24493. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, D.; Xiao, X.; Li, S.; Jia, X.; Sun, W.; Guo, X.; Zhang, Q. Evaluation of 12 myopia-associated genes in Chinese patients with high myopia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Li, S.; Sun, W.; Xiao, X.; Wang, Y.; Jiang, Y.; Zhang, Q. Variants in HNRNPH1 are associated with high myopia in humans and ocular coloboma in zebrafish. Clin. Genet. 2022, 102, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Sun, W.; Xiao, X.; Li, S.; Jia, X.; Zhou, L.; Wang, P.; Zhang, Q. CPSF1 mutations are associated with early-onset high myopia and involved in retinal ganglion cell axon projection. Hum. Mol. Genet. 2019, 28, 1959–1970. [Google Scholar] [CrossRef]
- Wildeman, M.; van Ophuizen, E.; den Dunnen, J.T.; Taschner, P.E.M. Improving sequence variant descriptions in mutation databases and literature using the Mutalyzer sequence variation nomenclature checker. Hum. Mutat. 2008, 29, 6–13. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lee, D.; Nakai, A.; Miwa, Y.; Tomita, Y.; Serizawa, N.; Katada, Y.; Hatanaka, Y.; Tsubota, K.; Negishi, K.; Kurihara, T. Retinal Degeneration in a Murine Model of Retinal Ischemia by Unilateral Common Carotid Artery Occlusion. BioMed Res. Int. 2021, 2021, 7727648. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kurihara, T.; Miyauchi, M.; Ishida, A.; Jiang, X.; Ikeda, S.-I.; Torii, H.; Tsubota, K. Oral crocetin administration suppressed refractive shift and axial elongation in a murine model of lens-induced myopia. Sci. Rep. 2019, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Zhang, T.; Lin, Z.; Li, Z.; Zhang, A.; Sun, X.; Gao, J. Loss of Lysyl Oxidase-like 3 Attenuates Embryonic Lung Development in Mice. Sci. Rep. 2016, 6, 33856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Lan, C.; Yang, Q.; Zhong, W.; Gu, Z.; Xiang, X.; Mao, Y.; Liao, X. Expression SCO1 of and SCO2 after form-deprivation myopia in Guinea pigs. Eur. J. Ophthalmol. 2021, 32, 3050–3057. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Raviola, E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature 1977, 266, 66–68. [Google Scholar] [CrossRef]
- Tejedor, J.; de la Villa, P. Refractive changes induced by form deprivation in the mouse eye. Investig. Ophthalmol. Vis. Sci. 2003, 44, 32–36. [Google Scholar] [CrossRef]
- Sherman, S.M.; Norton, T.T.; Casagrande, V.A. Myopia in the lid-sutured tree shrew (Tupaia glis). Brain Res. 1977, 124, 154–157. [Google Scholar] [CrossRef]
- Fujikado, T.; Kawasaki, Y.; Suzuki, A.; Ohmi, G.; Tano, Y. Retinal function with lens-induced myopia compared with form-deprivation myopia in chicks. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von. Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 1997, 235, 320–324. [Google Scholar] [CrossRef]
- Guggenheim, J.A.; McBrien, N.A. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1380–1395. [Google Scholar]
- Thomson, K.; Karouta, C.; Ashby, R. Topical application of dopaminergic compounds can inhibit deprivation myopia in chicks. Exp. Eye Res. 2020, 200, 108233. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, X.; Chen, Z.; Zhang, L.; Wang, L.; Xu, J.; Wu, M.J.C. High-energy short-wave blue light conversion films via carbon quantum dots for preventing retinal photochemical damage. Carbon 2022, 199, 431–438. [Google Scholar] [CrossRef]
- Chen, L.; Ouyang, J.; Li, X.; Xiao, X.; Sun, W.; Li, S.; Zhou, L.; Liao, Y.; Zhang, Q. DNAH17 is essential for rat spermatogenesis and fertility. J. Genet. 2021, 100, 14. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Hare, W.A. Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vis. Vision. Res. 2000, 40, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, X.; Huang, K.; Luo, M.; Wang, L. Morphological and distributional properties of SMI-32 immunoreactive ganglion cells in the rat retina. J. Comp. Neurol. 2022, 530, 1276–1287. [Google Scholar] [CrossRef]
- Samuel, C.S. Determination of collagen content, concentration, and sub-types in kidney tissue. Methods Mol. Biol. 2009, 466, 223–235. [Google Scholar] [CrossRef]
- Ouyang, X.; Han, Y.; Xie, Y.; Wu, Y.; Guo, S.; Cheng, M.; Wang, G. The collagen metabolism affects the scleral mechanical properties in the different processes of scleral remodeling. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 118, 109294. [Google Scholar] [CrossRef]
- Mu, N.; Wang, H.; Chen, D.; Wang, F.; Ji, L.; Zhang, C.; Li, M.; Lu, P. A Novel Rat Model of Dry Eye Induced by Aerosol Exposure of Particulate Matter. Investig. Ophthalmol. Vis. Sci. 2022, 63, 39. [Google Scholar] [CrossRef]
- Hill, S.; Deepa, S.S.; Sataranatarajan, K.; Premkumar, P.; Pulliam, D.; Liu, Y.; Soto, V.Y.; Fischer, K.E.; Van Remmen, H. Sco2 deficient mice develop increased adiposity and insulin resistance. Mol. Cell. Endocrinol. 2017, 455, 103–114. [Google Scholar] [CrossRef]
- Olsen, J.B.; Wong, L.; Deimling, S.; Miles, A.; Guo, H.; Li, Y.; Zhang, Z.; Greenblatt, J.F.; Emili, A.; Tropepe, V. G9a and ZNF644 Physically Associate to Suppress Progenitor Gene Expression during Neurogenesis. Stem Cell Rep. 2016, 7, 454–470. [Google Scholar] [CrossRef][Green Version]
- Komoriya, K.; Kato, Y.; Hayashi, Y.; Ohsuye, K.; Nishigaki, R.; Sugiyama, Y. Characterization of the hepatic disposition of lanoteplase, a rationally designed variant of tissue plasminogen activator in rodents. Drug Metab Dispos 2007, 35, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, H.; Wu, W.; Xie, E.; Yu, Y.; He, X.; Li, J.; Zheng, W.; Wang, X.; Cao, X.; et al. The zinc transporter Slc39a5 controls glucose sensing and insulin secretion in pancreatic β-cells via Sirt1- and Pgc-1α-mediated regulation of Glut2. Protein Cell 2019, 10, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lyu, Z.; Li, B.; You, Z.; Cui, N.; Li, Y.; Li, Y.; Huang, B.; Chen, R.; Chen, Y.; et al. P4HA2 induces hepatic ductular reaction and biliary fibrosis in chronic cholestatic liver diseases. Hepatology (Baltim. Md.) 2023, 78, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Skinbjerg, M.; Liow, J.-S.; Seneca, N.; Hong, J.; Lu, S.; Thorsell, A.; Heilig, M.; Pike, V.W.; Halldin, C.; Sibley, D.R.; et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: A PET study in a receptor internalization-deficient mouse model. Neuroimage 2010, 50, 1402–1407. [Google Scholar] [CrossRef]
- Hutchinson, J.L.; Zhao, X.; Hill, R.; Mundell, S.J. Arrestin-3 differentially regulates platelet GPCR subsets. Platelets 2020, 31, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Zurita Rendón, O.; Silva Neiva, L.; Sasarman, F.; Shoubridge, E.A. The arginine methyltransferase NDUFAF7 is essential for complex I assembly and early vertebrate embryogenesis. Hum. Mol. Genet. 2014, 23, 5159–5170. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-T.; Li, J.; Zhu, P.; Chiodo, V.A.; Smith, W.C.; Freedman, B.; Baehr, W.; Pang, J.; Hauswirth, W.W. Human L- and M-opsins restore M-cone function in a mouse model for human blue cone monochromacy. Mol. Vis. 2018, 24, 17–28. [Google Scholar]
- Ferencz, B.; Condac, E.; Poudel, N.; Munteanu, M.C.; Sivasami, P.; Choudhury, B.; Naidu, N.N.; Zhang, F.; Breshears, M.; Linhardt, R.J.; et al. Xylosyltransferase 2 deficiency and organ homeostasis. Glycoconj. J. 2020, 37, 755–765. [Google Scholar] [CrossRef]
- Morningstar, J.E.; Gensemer, C.; Moore, R.; Fulmer, D.; Beck, T.C.; Wang, C.; Moore, K.; Guo, L.; Sieg, F.; Nagata, Y.; et al. Mitral Valve Prolapse Induces Regionalized Myocardial Fibrosis. J. Am. Heart Assoc. 2021, 10, e022332. [Google Scholar] [CrossRef]
- Takawale, A.; Fan, D.; Basu, R.; Shen, M.; Parajuli, N.; Wang, W.; Wang, X.; Oudit, G.Y.; Kassiri, Z. Myocardial recovery from ischemia-reperfusion is compromised in the absence of tissue inhibitor of metalloproteinase 4. Circulation Heart Fail. 2014, 7, 652–662. [Google Scholar] [CrossRef]
- Yarbrough, W.M.; Baicu, C.; Mukherjee, R.; Van Laer, A.; Rivers, W.T.; McKinney, R.A.; Prescott, C.B.; Stroud, R.E.; Freels, P.D.; Zellars, K.N.; et al. Cardiac-restricted overexpression or deletion of tissue inhibitor of matrix metalloproteinase-4: Differential effects on left ventricular structure and function following pressure overload-induced hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H752–H761. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Lee, J.; Morton, J.S.; Takawale, A.; Fan, D.; Kandalam, V.; Wang, X.; Davidge, S.T.; Kassiri, Z. TIMP3 is the primary TIMP to regulate agonist-induced vascular remodelling and hypertension. Cardiovasc. Res. 2013, 98, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Sakamuri, S.S.V.P.; Watts, R.; Takawale, A.; Wang, X.; Hernandez-Anzaldo, S.; Bahitham, W.; Fernandez-Patron, C.; Lehner, R.; Kassiri, Z. Absence of Tissue Inhibitor of Metalloproteinase-4 (TIMP4) ameliorates high fat diet-induced obesity in mice due to defective lipid absorption. Sci. Rep. 2017, 7, 6210. [Google Scholar] [CrossRef] [PubMed]

| Nucleotide Change | BCVA | RE | AL (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | (NM_003256.4) | Onset Age | OD | OS | OD | OS | OD | OS | Fundus | VEP |
| P1 | c.528C>A | 12 | 1.0 | 1.0 | −8.00 | −8.50 | / | / | the bilateral temporal lens with cloudy anterior and posterior cortex, large optic disc with conus | / |
| P2 | c.528C>A | 2 | 0.5 | 1.0 | −9.00 | −6.00 | / | / | large optic papilla, light temporal retina, and peripheral tigroid fundus, fovea reflex present | / |
| P3 | c.528C>A | 5 | 0.9- | 0.9− | −6.25 | −5.25 | 25.18 | 24.89 | / | + |
| P4 | c.528C>A | 4 | 0.8- | 0.8− | −10.25 | −11.25 | / | / | tigroid fundus, boundary line on the mid-peripheral retina | / |
| P5 | c.528C>A | 3 | 0.1 | 0.1 | −11.25 | −11.00 | 26.85 | 26.98 | light nipple color, thin arteries, tigroid fundus, arc spots, poor retinal luster, peripheral retina degeneration, boundary line on the peripheral retina | + |
| P6 | c.528C>A | 12 | 1.0 | 1.0 | −6.25 | −7.25 | 26.37 | 26.83 | conus and tigroid fundus | / |
| P7 | c.234_235insAA | 4 | 0.7 | 0.7 | −10.75 | −11.50 | / | / | tigroid fundus with fovea reflex present | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Jiang, Z.; Yi, Z.; Ouyang, J.; Li, X.; Zhang, Q.; Wang, P. Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 16928. https://doi.org/10.3390/ijms242316928
Zhou W, Jiang Z, Yi Z, Ouyang J, Li X, Zhang Q, Wang P. Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner. International Journal of Molecular Sciences. 2023; 24(23):16928. https://doi.org/10.3390/ijms242316928
Chicago/Turabian StyleZhou, Wenhui, Zixuan Jiang, Zhen Yi, Jiamin Ouyang, Xueqing Li, Qingjiong Zhang, and Panfeng Wang. 2023. "Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner" International Journal of Molecular Sciences 24, no. 23: 16928. https://doi.org/10.3390/ijms242316928
APA StyleZhou, W., Jiang, Z., Yi, Z., Ouyang, J., Li, X., Zhang, Q., & Wang, P. (2023). Defect of TIMP4 Is Associated with High Myopia and Participates in Rat Ocular Development in a Dose-Dependent Manner. International Journal of Molecular Sciences, 24(23), 16928. https://doi.org/10.3390/ijms242316928






