Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise
Abstract
1. Introduction
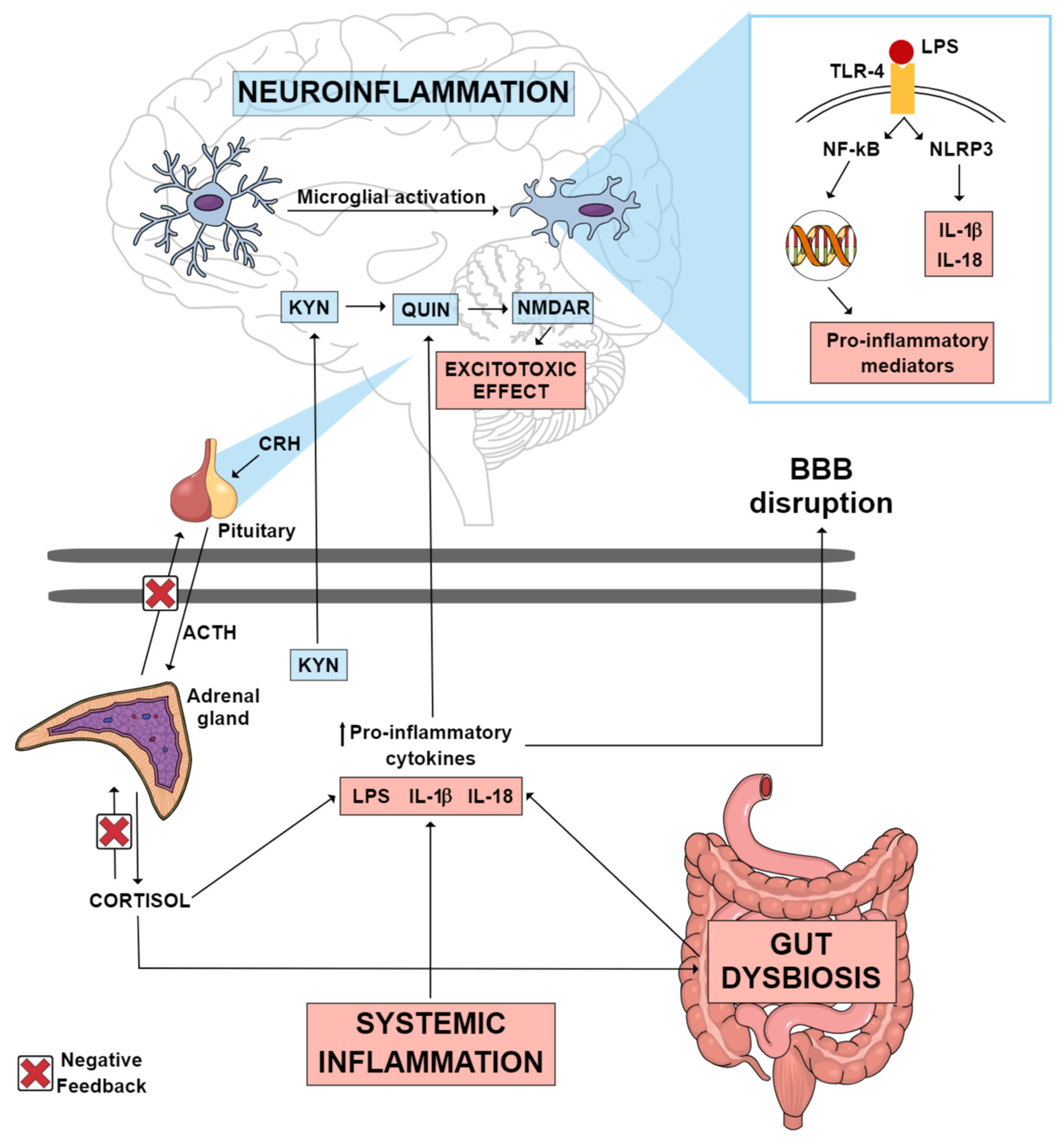
2. Gut Microbiota and MDD
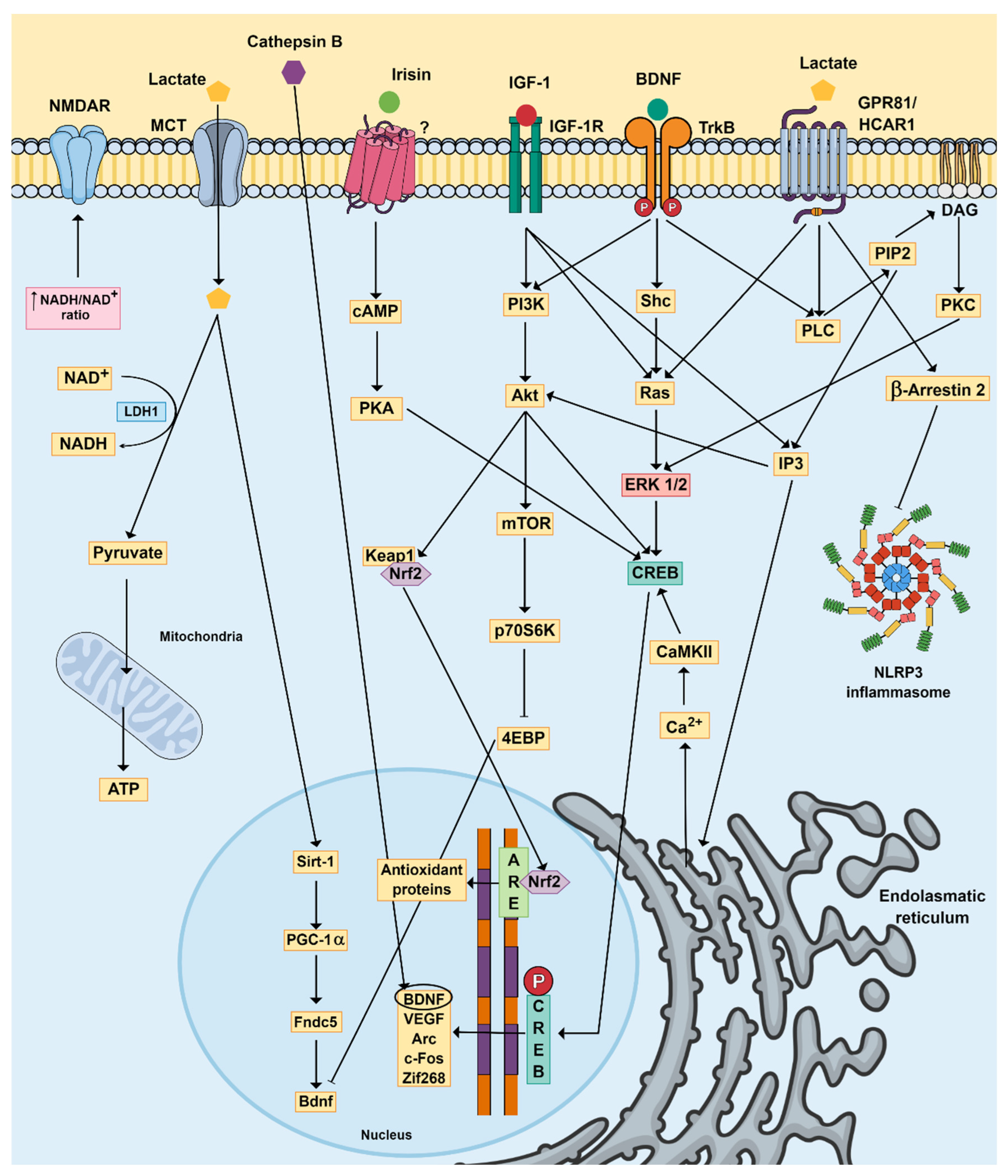
3. Physical Exercise, Brain Function and MDD
4. Physical Exercise and Gut Microbiota
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef]
- Cai, H.; Xie, X.M.; Zhang, Q.; Cui, X.; Lin, J.X.; Sim, K.; Ungvari, G.S.; Zhang, L.; Xiang, Y.T. Prevalence of Suicidality in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Comparative Studies. Front. Psychiatry 2021, 12, 690130. [Google Scholar] [CrossRef]
- Penninx, B.W.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Green, M.J.; Davies, N.; Demou, E.; Howe, L.D.; Harrison, S.; Smith, D.J.; Howard, D.M.; McIntosh, A.M.; Munafò, M.; et al. Effects of depression on employment and social outcomes: A Mendelian randomisation study. J. Epidemiol. Community Health 2022, 76, 563–571. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-induced depression-like model in mice: Meta-analysis and systematic evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.M.; Zanotti-Fregonara, P.; Fujita, M.; Newman, L.; Farmer, C.; Ballard, E.D.; Machado-Vieira, R.; Yuan, P.; Niciu, M.J.; Lyoo, C.H.; et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Liang, S.; Zhao, L.; Ni, P.; Wang, Q.; Guo, W.; Xu, Y.; Cai, J.; Tao, S.; Li, X.; Deng, W.; et al. Frontostriatal circuitry and the tryptophan kynurenine pathway in major psychiatric disorders. Psychopharmacology 2023. [Google Scholar] [CrossRef]
- Chen, X.; Beltran, D.J.; Tsygankova, V.D.; Woolwine, B.J.; Patel, T.; Baer, W.; Felger, J.C.; Miller, A.H.; Haroon, E. Kynurenines increase MRS metabolites in basal ganglia and decrease resting-state connectivity in frontostriatal reward circuitry in depression. Transl. Psychiatry 2021, 11, 456. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology from the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef]
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef] [PubMed]
- Aubé, A.C.; Blottière, H.M.; Scarpignato, C.; Cherbut, C.; Rozé, C.; Galmiche, J.P. Inhibition of acetylcholine induced intestinal motility by interleukin 1 beta in the rat. Gut 1996, 39, 470–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, K.; Ganguly, A.K. The effect of subdiaphragmatic vagotomy on the gastric mucus barrier in rats. Clin. Exp. Pharmacol. Physiol. 1987, 14, 735–741. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ishima, T.; Zhang, J.; Qu, Y.; Chang, L.; Pu, Y.; Fujita, Y.; Tan, Y.; Wang, X.; Hashimoto, K. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J. Neuroinflamm. 2020, 17, 241. [Google Scholar] [CrossRef]
- Wang, S.; Ishima, T.; Qu, Y.; Shan, J.; Chang, L.; Wei, Y.; Zhang, J.; Pu, Y.; Fujita, Y.; Tan, Y.; et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J. Affect. Disord. 2021, 292, 565–573. [Google Scholar] [CrossRef]
- Konsman, J.P.; Luheshi, G.N.; Bluthé, R.M.; Dantzer, R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000, 12, 4434–4446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564. [Google Scholar] [CrossRef]
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Kamohara, M.; Takasaki, J.; Matsumoto, S.; Saito, T.; Ohishi, T.; Hiyama, H.; Matsuo, A.; Matsushime, H.; Furuichi, K. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003, 303, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Y.; Luan, Y.; Li, Y.; Liu, J.; Yue, Z.; Yuan, B.; Sun, J.; Xie, C.; Li, L.; et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J. 2020, 34, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- Viatchenko-Karpinski, V.; Kong, L.; Weng, H.R. Activation of microglial GPR109A alleviates thermal hyperalgesia in female lupus mice by suppressing IL-18 and glutamatergic synaptic activity. Glia 2022, 70, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, W.; Lu, M.; Ni, B.; Yang, J. D-β-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br. J. Pharmacol. 2017, 174, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Safari, A.; Badeli-Sarkala, H.; Namavar, M.R.; Kargar-Abarghouei, E.; Anssari, N.; Izadi, S.; Borhani-Haghighi, A. Neuroprotective effect of dimethyl fumarate in stroke: The role of nuclear factor erythroid 2-related factor 2. Iran. J. Neurol. 2019, 18, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Shehadah, A.; Chen, J.; Zacharek, A.; Cui, Y.; Ion, M.; Roberts, C.; Kapke, A.; Chopp, M. Niaspan treatment induces neuroprotection after stroke. Neurobiol. Dis. 2010, 40, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yuan, B.; Wu, Y.; Gong, Y.; Guo, W.; Fu, S.; Luan, Y.; Wang, W. Sodium Butyrate Protects N2a Cells against A. Mediators Inflamm. 2020, 2020, 7605160. [Google Scholar] [CrossRef]
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Gong, Y.; Yang, R.; Chen, Z.; Hu, W.; Wu, Y.; Gao, M.; Xu, X.; Qin, Y.; et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 2018, 111, 12–25. [Google Scholar] [CrossRef]
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004, 141, 874–880. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr. Res. 2015, 61, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Faller, C.J.; Ceretta, R.A.; Petronilho, F.; Lopes-Borges, J.; Valvassori, S.S.; Quevedo, J. Sodium Butyrate Prevents Memory Impairment by Re-establishing BDNF and GDNF Expression in Experimental Pneumococcal Meningitis. Mol. Neurobiol. 2015, 52, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Mosienko, V.; Teschemacher, A.G.; Kasparov, S. Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow. Metab. 2015, 35, 1069–1075. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhou, C.J.; Zheng, P.; Cheng, K.; Wang, H.Y.; Li, J.; Zeng, L.; Xie, P. Differential urinary metabolites related with the severity of major depressive disorder. Behav. Brain Res. 2017, 332, 280–287. [Google Scholar] [CrossRef]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Cassé, F.; Carron, C.; Burlet-Godinot, S.; Toni, N.; Magistretti, P.J.; Martin, J.L. Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol. Psychiatry 2021, 26, 6723–6735. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233. [Google Scholar] [CrossRef] [PubMed]
- Margineanu, M.B.; Mahmood, H.; Fiumelli, H.; Magistretti, P.J. L-Lactate Regulates the Expression of Synaptic Plasticity and Neuroprotection Genes in Cortical Neurons: A Transcriptome Analysis. Front. Mol. Neurosci. 2018, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Zheng, T.; Dai, G.; Wang, M.; Zhou, L.; Zheng, Y.; Chen, G. Associations between the kynurenine pathway and the brain in patients with major depressive disorder-A systematic review of neuroimaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110675. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smith, D.G.; Smythe, G.A.; Armati, P.J.; Brew, B.J. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003, 527, 105–112. [Google Scholar]
- Orhan, F.; Bhat, M.; Sandberg, K.; Ståhl, S.; Piehl, F.; Svensson, C.; Erhardt, S.; Schwieler, L.; Schizophrenia Project (KaSP) Consortium. Tryptophan Metabolism Along the Kynurenine Pathway Downstream of Toll-like Receptor Stimulation in Peripheral Monocytes. Scand. J. Immunol. 2016, 84, 262–271. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112 Pt B, 399–412. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Béguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; Zhao, L.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization—Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Verhoeven, J.E.; Han, L.K.M.; Lever-van Milligen, B.A.; Hu, M.X.; Révész, D.; Hoogendoorn, A.W.; Batelaan, N.M.; van Schaik, D.J.F.; van Balkom, A.J.L.M.; van Oppen, P.; et al. Antidepressants or running therapy: Comparing effects on mental and physical health in patients with depression and anxiety disorders. J. Affect. Disord. 2023, 329, 19–29. [Google Scholar] [CrossRef]
- Hu, M.X.; Turner, D.; Generaal, E.; Bos, D.; Ikram, M.K.; Ikram, M.A.; Cuijpers, P.; Penninx, B.W.J.H. Exercise interventions for the prevention of depression: A systematic review of meta-analyses. BMC Public Health 2020, 20, 1255. [Google Scholar] [CrossRef]
- Cangin, C.; Harris, R.; Binkley, P.; Schwartzbaum, J.; Focht, B. Anaerobic muscle strengthening physical activity and depression severity among USA adults. Prev. Med. Rep. 2018, 10, 299–303. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef]
- Zhao, J.L.; Jiang, W.T.; Wang, X.; Cai, Z.D.; Liu, Z.H.; Liu, G.R. Exercise, brain plasticity, and depression. CNS Neurosci. Ther. 2020, 26, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Miskowiak, K.W.; Köhler, C.A.; Maes, M.; Fernandes, B.S.; Berk, M.; Carvalho, A.F. Cognitive remission: A novel objective for the treatment of major depression? BMC Med. 2016, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Kim, M. Effects of exercise therapy on global cognitive function and, depression in older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 106, 104855. [Google Scholar] [CrossRef]
- Ren, F.F.; Alderman, B.L.; Wang, W.G.; Chen, F.T.; Zhou, W.S.; Zong, W.J.; Liang, W.M.; Chang, Y.K. Effects of Exercise Training on Executive Functioning in Adults with Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2023, 53, 1765–1788. [Google Scholar] [CrossRef] [PubMed]
- Salehi, I.; Hosseini, S.M.; Haghighi, M.; Jahangard, L.; Bajoghli, H.; Gerber, M.; Pühse, U.; Holsboer-Trachsler, E.; Brand, S. Electroconvulsive therapy (ECT) and aerobic exercise training (AET) increased plasma BDNF and ameliorated depressive symptoms in patients suffering from major depressive disorder. J. Psychiatr. Res. 2016, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moraes, H.S.; Silveira, H.S.; Oliveira, N.A.; Matta Mello Portugal, E.; Araújo, N.B.; Vasques, P.E.; Bergland, A.; Santos, T.M.; Engedal, K.; Coutinho, E.S.; et al. Is Strength Training as Effective as Aerobic Training for Depression in Older Adults? A Randomized Controlled Trial. Neuropsychobiology 2020, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gujral, S.; Aizenstein, H.; Reynolds, C.F.; Butters, M.A.; Grove, G.; Karp, J.F.; Erickson, K.I. Exercise for Depression: A Feasibility Trial Exploring Neural Mechanisms. Am. J. Geriatr. Psychiatry 2019, 27, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Vints, W.A.J.; Levin, O.; Fujiyama, H.; Verbunt, J.; Masiulis, N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocrinol. 2022, 66, 100993. [Google Scholar] [CrossRef]
- Gubert, C.; Hannan, A.J. Exercise mimetics: Harnessing the therapeutic effects of physical activity. Nat. Rev. Drug Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S. A new perspective of the hippocampus in the origin of exercise-brain interactions. Brain Struct. Funct. 2018, 223, 2527–2545. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bilek, F.; Cetisli-Korkmaz, N.; Ercan, Z.; Deniz, G.; Demir, C.F. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord 2022, 61, 103742. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, L. An exercise-induced messenger boosts memory in Alzheimer’s disease. Nat. Med. 2019, 25, 20–21. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell’Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605. [Google Scholar] [CrossRef]
- Siteneski, A.; Olescowicz, G.; Pazini, F.L.; Camargo, A.; Fraga, D.B.; Brocardo, P.S.; Gil-Mohapel, J.; Cunha, M.P.; Rodrigues, A.L.S. Antidepressant-like and pro-neurogenic effects of physical exercise: The putative role of FNDC5/irisin pathway. J. Neural. Transm. 2020, 127, 355–370. [Google Scholar] [CrossRef]
- Wrann, C.D. FNDC5/irisin—Their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015, 1, 55–61. [Google Scholar] [CrossRef]
- Flores, A.D.; Yu, W.S.; Fung, M.L.; Lim, L.W. Neuromodulation and hippocampal neurogenesis in depression: A scoping review. Brain Res. Bull. 2022, 188, 92–107. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Siteneski, A.; Cunha, M.P.; Lieberknecht, V.; Pazini, F.L.; Gruhn, K.; Brocardo, P.S.; Rodrigues, A.L.S. Central irisin administration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84 Pt A, 294–303. [Google Scholar] [CrossRef]
- Mohammadi, S.; Oryan, S.; Komaki, A.; Eidi, A.; Zarei, M. Effects of intra-dentate gyrus microinjection of myokine irisin on long-term potentiation in male rats. Arq. Neuropsiquiatr. 2019, 77, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Gökçe, E.; Gün, N. The Relationship Between Exercise, Cathepsin B, and Cognitive Functions: Systematic Review. Percept. Mot. Skills 2023, 130, 1366–1385. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gaitán, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; van Praag, H. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front Endocrinol 2021, 12, 660181. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Kortas, J.A.; Gmiat, A.; Jaworska, J.; Kozlowska, M.; Lysak-Radomska, A.; Rodziewicz-Flis, E.; Zychowska, M.; Ziemann, E. Habitually inactive physically—A proposed procedure of counteracting cognitive decline in women with diminished insulin sensitivity through a high-intensity circuit training program. Physiol. Behav. 2021, 229, 113235. [Google Scholar] [CrossRef]
- Gökçe, E.; Güneş, E.; Arı, F.; Hayme, S.; Nalçacı, E. Comparison of the effects of open- and closed-skill exercise on cognition and peripheral proteins: A cross-sectional study. PLoS ONE 2021, 16, e0251907. [Google Scholar] [CrossRef]
- de Sousa Fernandes, M.S.; Ordônio, T.F.; Santos, G.C.J.; Santos, L.E.R.; Calazans, C.T.; Gomes, D.A.; Santos, T.M. Effects of Physical Exercise on Neuroplasticity and Brain Function: A Systematic Review in Human and Animal Studies. Neural Plast. 2020, 2020, 8856621. [Google Scholar] [CrossRef]
- Liang, J.; Wang, H.; Zeng, Y.; Qu, Y.; Liu, Q.; Zhao, F.; Duan, J.; Jiang, Y.; Li, S.; Ying, J.; et al. Physical exercise promotes brain remodeling by regulating epigenetics, neuroplasticity and neurotrophins. Rev. Neurosci. 2021, 32, 615–629. [Google Scholar] [CrossRef]
- Reddy, I.; Yadav, Y.; Dey, C.S. Cellular and Molecular Regulation of Exercise-A Neuronal Perspective. Cell Mol. Neurobiol. 2023, 43, 1551–1571. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth factors as clinical biomarkers of prognosis and diagnosis in psychiatric disorders. Cytokine Growth Factor. Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef]
- McPhee, G.M.; Downey, L.A.; Stough, C. Neurotrophins as a reliable biomarker for brain function, structure and cognition: A systematic review and meta-analysis. Neurobiol. Learn. Mem. 2020, 175, 107298. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chang, Y.; Gao, X.L.; Li, H.; Zhao, P. Dynamic Expression and the Role of BDNF in Exercise-induced Skeletal Muscle Regeneration. Int. J. Sports Med. 2017, 38, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Pinho, R.A.; Aguiar, A.S.; Radák, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef]
- Carro, E.; Nuñez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, Y.; Wang, D. Endurance and resistance training mitigate the negative consequences of depression on synaptic plasticity through different molecular mechanisms. Int. J. Neurosci. 2020, 130, 541–550. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Q.; Wu, H.; Dong, Y.; Peng, Z.; Guo, X.; Jiang, N. The role of IGF-1 in exercise to improve obesity-related cognitive dysfunction. Front. Neurosci. 2023, 17, 1229165. [Google Scholar] [CrossRef]
- Berg, U.; Bang, P. Exercise and circulating insulin-like growth factor I. Horm. Res. 2004, 62 (Suppl. S1), 50–58. [Google Scholar] [CrossRef]
- Cetinkaya, C.; Sisman, A.R.; Kiray, M.; Camsari, U.M.; Gencoglu, C.; Baykara, B.; Aksu, I.; Uysal, N. Positive effects of aerobic exercise on learning and memory functioning, which correlate with hippocampal IGF-1 increase in adolescent rats. Neurosci. Lett. 2013, 549, 177–181. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors 2007, 25, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Annenkov, A. The insulin-like growth factor (IGF) receptor type 1 (IGF1R) as an essential component of the signalling network regulating neurogenesis. Mol. Neurobiol. 2009, 40, 195–215. [Google Scholar] [CrossRef]
- Zheng, W.H.; Quirion, R. Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J. Neurochem. 2004, 89, 844–852. [Google Scholar] [CrossRef]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef]
- Kuang, W.H.; Dong, Z.Q.; Tian, L.T.; Li, J. IGF-1 defends against chronic-stress induced depression in rat models of chronic unpredictable mild stress through the PI3K/Akt/FoxO3a pathway. Kaohsiung J. Med. Sci. 2018, 34, 370–376. [Google Scholar] [CrossRef]
- Al-Jarrah, M.; Jamous, M.; Al Zailaey, K.; Bweir, S.O. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson’s Disease. NeuroRehabilitation 2010, 26, 369–373. [Google Scholar] [CrossRef]
- Tang, K.; Xia, F.C.; Wagner, P.D.; Breen, E.C. Exercise-induced VEGF transcriptional activation in brain, lung and skeletal muscle. Respir. Physiol. Neurobiol. 2010, 170, 16–22. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage 2016, 131, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Cheng, J.; Pan, G.; Wu, S.; Hu, Q.; Jiang, H.; Wang, Y.; Xiong, J.; Pang, Q.; Chen, X. Treadmill exercise ameliorates focal cerebral ischemia/reperfusion-induced neurological deficit by promoting dendritic modification and synaptic plasticity via upregulating caveolin-1/VEGF signaling pathways. Exp. Neurol. 2019, 313, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Algaidi, S.A.; Eldomiaty, M.A.; Elbastwisy, Y.M.; Almasry, S.M.; Desouky, M.K.; Elnaggar, A.M. Effect of voluntary running on expression of myokines in brains of rats with depression. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419833533. [Google Scholar] [CrossRef]
- Kiuchi, T.; Lee, H.; Mikami, T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience 2012, 207, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Andersson, K.A.; Haugen, Ø.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, F.; Zhan, L.; Lin, W.; Liang, C.; Pang, Y.; Zhang, J.; Mu, Z. Exercise Regulates the Lactate Receptor HCAR1 and ERK1/2-PI3K/Akt Pathways to Promote Cerebral Angiogenesis. Iran. J. Public Health 2022, 51, 2298–2307. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Liang, L.; Liu, P.; Deng, Y.; Li, J.; Zhao, S. L- lactate inhibits lipopolysaccharide-induced inflammation of microglia in the hippocampus. Int. J. Neurosci. 2022, 26, 1–8. [Google Scholar] [CrossRef]
- Kouba, B.R.; Gil-Mohapel, J.; Rodrigues, A.L.S. NLRP3 Inflammasome: From Pathophysiology to Therapeutic Target in Major Depressive Disorder. Int. J. Mol. Sci. 2022, 24, 133. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Inman, D.M. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflamm. 2018, 15, 313. [Google Scholar] [CrossRef]
- Shi, T.; Shi, Y.; Gao, H.; Ma, Y.; Wang, Q.; Shen, S.; Shao, X.; Gong, W.; Chen, X.; Qin, J.; et al. Exercised accelerated the production of muscle-derived kynurenic acid in skeletal muscle and alleviated the postmenopausal osteoporosis through the Gpr35/NFκB p65 pathway. J. Orthop. Translat 2022, 35, 1–12. [Google Scholar] [CrossRef]
- Dai, Y.; Pang, J.; Gong, H.; Fan, W.; Zhang, T.M. Roles and tissue source of adiponectin involved in lifestyle modifications. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 117–128. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.R.; Lee, T.M.; Xu, A.; So, K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Verma, S.; Göhring, I.; Bobbert, T.; Seifert, J.; Sindler, A.L.; Pfeiffer, A.; Hileman, S.M.; Tschöp, M.; Banks, W.A. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 2006, 55, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Tu, H.; Kastin, A.J. Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin, and adiponectin. Peptides 2006, 27, 911–916. [Google Scholar] [CrossRef]
- Wang, P.; Liang, Y.; Chen, K.; Yau, S.Y.; Sun, X.; Cheng, K.K.; Xu, A.; So, K.F.; Li, A. Potential Involvement of Adiponectin Signaling in Regulating Physical Exercise-Elicited Hippocampal Neurogenesis and Dendritic Morphology in Stressed Mice. Front. Cell Neurosci. 2020, 14, 189. [Google Scholar] [CrossRef]
- You, J.; Sun, L.; Wang, J.; Sun, F.; Wang, W.; Wang, D.; Fan, X.; Liu, D.; Xu, Z.; Qiu, C.; et al. Role of Adiponectin-Notch pathway in cognitive dysfunction associated with depression and in the therapeutic effect of physical exercise. Aging Cell 2021, 20, e13387. [Google Scholar] [CrossRef]
- Pousti, F.; Ahmadi, R.; Mirahmadi, F.; Hosseinmardi, N.; Rohampour, K. Adiponectin modulates synaptic plasticity in hippocampal dentate gyrus. Neurosci. Lett. 2018, 662, 227–232. [Google Scholar] [CrossRef]
- Wang, M.; Jo, J.; Song, J. Adiponectin improves long-term potentiation in the 5XFAD mouse brain. Sci. Rep. 2019, 9, 8918. [Google Scholar] [CrossRef]
- Xu, Z.P.; Gan, G.S.; Liu, Y.M.; Xiao, J.S.; Liu, H.X.; Mei, B.; Zhang, J.J. Adiponectin Attenuates Streptozotocin-Induced Tau Hyperphosphorylation and Cognitive Deficits by Rescuing PI3K/Akt/GSK-3β Pathway. Neurochem. Res. 2018, 43, 316–323. [Google Scholar] [CrossRef]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M.N.D.N. The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Front. Neurosci. 2018, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Waki, H.; Iwabu, M.; Okada-Iwabu, M.; Nakamura, M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef]
- Ferraro, E.; Giammarioli, A.M.; Chiandotto, S.; Spoletini, I.; Rosano, G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: Redox signaling and role of autophagy. Antioxid. Redox Signal 2014, 21, 154–176. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef]
- Sharma, V.; Kaur, A.; Singh, T.G. Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer’s disease. Biomed. Pharmacother. 2020, 129, 110373. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Ignácio, Z.M.; da Silva, R.S.; Plissari, M.E.; Quevedo, J.; Réus, G.Z. Physical Exercise and Neuroinflammation in Major Depressive Disorder. Mol. Neurobiol. 2019, 56, 8323–8335. [Google Scholar] [CrossRef]
- Qu, H.; Liu, R.; Chen, J.; Zheng, L.; Chen, R. Aerobic Exercise Inhibits CUMS-Depressed Mice Hippocampal Inflammatory Response via Activating Hippocampal miR-223/TLR4/MyD88-NF-κB Pathway. Int. J. Environ. Res. Public Health 2020, 17, 2676. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Sheng, H.; Ni, X.; Lu, J. Exercise amelioration of depression-like behavior in OVX mice is associated with suppression of NLRP3 inflammasome activation in hippocampus. Behav. Brain Res. 2016, 307, 18–24. [Google Scholar] [CrossRef]
- Sadeghi, M.; Peeri, M.; Hosseini, M.J. Adolescent voluntary exercise attenuated hippocampal innate immunity responses and depressive-like behaviors following maternal separation stress in male rats. Physiol. Behav. 2016, 163, 177–183. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.; Pérez, S. PGC-1. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [PubMed]
- Eldomiaty, M.A.; Almasry, S.M.; Desouky, M.K.; Algaidi, S.A. Voluntary running improves depressive behaviours and the structure of the hippocampus in rats: A possible impact of myokines. Brain Res. 2017, 1657, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Kim, S.H.; Yang, Y.R.; Song, P.; Yu, H.S.; Park, H.G.; Hwang, O.; Lee-Kwon, W.; Seo, J.K.; Hwang, D.; et al. Macrophage migration inhibitory factor mediates the antidepressant actions of voluntary exercise. Proc. Natl. Acad. Sci. USA 2012, 109, 13094–13099. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.A.; Gallagher, D.; Fatt, M.P.; Simonetta, J.V.; Kaplan, D.R.; Miller, F.D. Interleukin-6 Regulates Adult Neural Stem Cell Numbers during Normal and Abnormal Post-natal Development. Stem Cell Reports 2018, 10, 1464–1480. [Google Scholar] [CrossRef]
- Bowen, K.K.; Dempsey, R.J.; Vemuganti, R. Adult interleukin-6 knockout mice show compromised neurogenesis. Neuroreport 2011, 22, 126–130. [Google Scholar] [CrossRef]
- Schlittler, M.; Goiny, M.; Agudelo, L.Z.; Venckunas, T.; Brazaitis, M.; Skurvydas, A.; Kamandulis, S.; Ruas, J.L.; Erhardt, S.; Westerblad, H.; et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. Cell Physiol. 2016, 310, C836–C840. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; Wiatr, M.; Ciałowicz, M.; Gomes de Assis, G.; Borowicz, W.; Rocha-Rodrigues, S.; Paprocka-Borowicz, M.; Marques, A. BDNF Impact on Biological Markers of Depression-Role of Physical Exercise and Training. Int. J. Environ. Res. Public Health 2021, 18, 7553. [Google Scholar] [CrossRef]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.J.; Lee, T.T.; Gil-Mohapel, J.; Gorzalka, B.B.; Hillard, C.J.; Christie, B.R. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can Physical Activity Support the Endocannabinoid System in the Preventive and Therapeutic Approach to Neurological Disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef]
- Siebers, M.; Biedermann, S.V.; Fuss, J. Do Endocannabinoids Cause the Runner’s High? Evidence and Open Questions. Neuroscientist 2023, 29, 352–369. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport. Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2021, 43, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Tarracchini, C.; Fontana, F.; Lugli, G.A.; Mancabelli, L.; Alessandri, G.; Turroni, F.; Ventura, M.; Milani, C. Investigation of the Ecological Link between Recurrent Microbial Human Gut Communities and Physical Activity. Microbiol. Spectr. 2022, 10, e0042022. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Morck, D.W.; Ghosh, S.; Quin, C.; Pither, J.; Barnett, J.A.; Gill, S.K.; Gibson, D.L. Physical Activity Shapes the Intestinal Microbiome and Immunity of Healthy Mice but Has No Protective Effects against Colitis in MUC2. mSystems 2020, 5, e00515-20. [Google Scholar] [CrossRef]
- Li, R.; Liu, R.; Chen, L.; Wang, G.; Qin, L.; Yu, Z.; Wan, Z. Microbiota from Exercise Mice Counteracts High-Fat High-Cholesterol Diet-Induced Cognitive Impairment in C57BL/6 Mice. Oxid. Med. Cell Longev. 2023, 2023, 2766250. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Mohamed Elfadil, O.; Mundi, M.S.; Abdelmagid, M.G.; Patel, A.; Patel, N.; Martindale, R. Butyrate: More Than a Short Chain Fatty Acid. Curr. Nutr. Rep. 2023, 12, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Allahverdi, A.; Yang, R.; Korolev, N.; Fan, Y.; Davey, C.A.; Liu, C.F.; Nordenskiöld, L. The effects of histone H4 tail acetylations on cation-induced chromatin folding and self-association. Nucleic Acids Res. 2011, 39, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Li, T.; Li, M.; Huang, S.; Qiu, Y.; Feng, R.; Zhang, S.; Chen, M.; Xiong, L.; Zeng, Z. Systematic Review and Meta-analysis: Short-Chain Fatty Acid Characterization in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Pindus, D.M.; Khan, N.A.; Burd, N.A.; Holscher, H.D. Associations between Accelerometer-Measured Physical Activity and Fecal Microbiota in Adults with Overweight and Obesity. Med. Sci. Sports Exerc. 2023, 55, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Rosa, J.C.; Pimentel, G.D.; Souza, H.A.; Caperuto, E.C.; Carnevali, L.C.; Seelaender, M.; Damaso, A.R.; Oyama, L.M.; de Mello, M.T.; et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 2010, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, C.; Zhang, C.; Ren, Y.; Guo, L.; Wang, T.; Chen, F.; Li, Y.; Zhang, X.; Wang, H.; et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed. Pharmacother. 2023, 160, 114308. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P.; et al. Butyrate ameliorates quinolinic acid-induced cognitive decline in obesity models. J. Clin. Investig. 2023, 133, e154612. [Google Scholar] [CrossRef]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut-Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef]
- Kang, S.S.; Jeraldo, P.R.; Kurti, A.; Miller, M.E.; Cook, M.D.; Whitlock, K.; Goldenfeld, N.; Woods, J.A.; White, B.A.; Chia, N.; et al. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Wisniewski, P.J.; Noji, M.; McGuinness, L.R.; Häggblom, M.M.; Lightfoot, S.A.; Joseph, L.B.; Kerkhof, L.J. The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 2016, 11, e0150502. [Google Scholar] [CrossRef] [PubMed]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhu, H.; Sun, Y.; Hang, T.; Zhang, M. The modified outer membrane protein Amuc_1100 of Akkermansia muciniphila improves chronic stress-induced anxiety and depression-like behavior in mice. Food Funct. 2022, 13, 10748–10758. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Song, J.; Wang, H.; Shi, F.; Zhou, N.; Jiang, J.; Xu, Y.; Zhang, L.; Yang, L.; Zhou, M. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. 2019, 225, 88–97. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M.G.; Maté-Muñoz, J.L.; Domínguez, R.; Moreno, D.; Larrosa, M. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 2017, 12, e0171352. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbó, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietilä, S.; Hollmén, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Martínez-Camblor, P.; Villar, C.J.; Tomás-Zapico, C.; Fernández-García, B.; Lombó, F. Resistance and Endurance Exercise Training Induce Differential Changes in Gut Microbiota Composition in Murine Models. Front. Physiol. 2021, 12, 748854. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, L.; Jing, X.; Wang, Y.; An, C. Features of gut microbiota and short-chain fatty acids in patients with first-episode depression and their relationship with the clinical symptoms. Front. Psychol. 2023, 14, 1088268. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lai, J.; Zhang, P.; Ding, J.; Jiang, J.; Liu, C.; Huang, H.; Zhen, H.; Xi, C.; Sun, Y.; et al. Multi-omics analyses of serum metabolome, gut microbiome and brain function reveal dysregulated microbiota-gut-brain axis in bipolar depression. Mol. Psychiatry 2022, 27, 4123–4135. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Hoshino, Y.; Hosokawa, M.; Takeyama, H.; Higuchi, M. Effects of short-term endurance exercise on gut microbiota in elderly men. Physiol. Rep. 2018, 6, e13935. [Google Scholar] [CrossRef] [PubMed]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Siddharth, J.; Chakrabarti, A.; Pannérec, A.; Karaz, S.; Morin-Rivron, D.; Masoodi, M.; Feige, J.N.; Parkinson, S.J. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging 2017, 9, 1698–1720. [Google Scholar] [CrossRef]
- Maruta, H.; Yamashita, H. Acetic acid stimulates G-protein-coupled receptor GPR43 and induces intracellular calcium influx in L6 myotube cells. PLoS ONE 2020, 15, e0239428. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guo, Q.; Zhou, X.; Duan, Y.; Yang, Y.; Gong, S.; Han, M.; Liu, Y.; Yang, Z.; Chen, Q.; et al. Role of brain-gut-muscle axis in human health and energy homeostasis. Front. Nutr. 2022, 9, 947033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, T.; Randall, W.R.; Schneider, M.F. Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J. Muscle Res. Cell Motil. 2005, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Nunomiya, A.; Kitajima, Y.; Dan, T.; Miyata, T.; Nagatomi, R. Prolyl hydroxylase domain 2 deficiency promotes skeletal muscle fiber-type transition via a calcineurin/NFATc1-dependent pathway. Skelet. Muscle 2016, 6, 5. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Verheggen, R.J.H.M.; Konstanti, P.; Smidt, H.; Hermus, A.R.M.M.; Thijssen, D.H.J.; Hopman, M.T.E. Eight-week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity 2021, 29, 1615–1624. [Google Scholar] [CrossRef]
- Domingo, M.C.; Huletsky, A.; Boissinot, M.; Bernard, K.A.; Picard, F.J.; Bergeron, M.G. Ruminococcus gauvreauii sp. nov., a glycopeptide-resistant species isolated from a human faecal specimen. Int. J. Syst. Evol. Microbiol. 2008, 58 Pt 6, 1393–1397. [Google Scholar] [CrossRef]
- Schwiertz, A.; Hold, G.L.; Duncan, S.H.; Gruhl, B.; Collins, M.D.; Lawson, P.A.; Flint, H.J.; Blaut, M. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate-utilising, butyrate-producing bacterium from human faeces. Syst. Appl. Microbiol. 2002, 25, 46–51. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of Gut Microbiota to Metabolite Changes Induced by Endurance Exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport. Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, T.Y.; Kim, Y.; Lee, S.H.; Kim, S.; Kang, S.W.; Yang, J.Y.; Baek, I.J.; Sung, Y.H.; Park, Y.Y.; et al. Microbiota-Derived Lactate Accelerates Intestinal Stem-Cell-Mediated Epithelial Development. Cell Host Microbe 2018, 24, 833–846.e836. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Shanmugam, A.; Swafford, D.; Suryawanshi, A.; Bhattacharjee, P.; Hussein, M.S.; Koni, P.A.; Prasad, P.D.; Kurago, Z.B.; Thangaraju, M.; et al. GPR81, a Cell-Surface Receptor for Lactate, Regulates Intestinal Homeostasis and Protects Mice from Experimental Colitis. J. Immunol. 2018, 200, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.X.; Cai, X.T.; Yang, J.N.; Wang, H.C.; Li, Y.X.; Dai, Z.F.; Yang, R.L.; Lin, X.H. Irisin attenuates inflammation in a mouse model of ulcerative colitis by altering the intestinal microbiota. Exp. Ther. Med. 2021, 22, 1433. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Y.; Li, G.; Guo, T.; Jin, M.; Xi, D.; Wang, S.; Liu, X.; Guo, S.; Liu, H.; et al. Irisin ameliorates myocardial ischemia-reperfusion injury by modulating gut microbiota and intestinal permeability in rats. PLoS ONE 2023, 18, e0291022. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, B.; Lee, C.; Joung, H.; Kim, B.K.; Choi, I.S.; Hyun, C.K. Comprehensive amelioration of high-fat diet-induced metabolic dysfunctions through activation of the PGC-1α pathway by probiotics treatment in mice. PLoS ONE 2020, 15, e0228932. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Xu, T.; Yuan, Q.; Hu, N.; Sun, W.; Bai, Y.; Liu, L.; Feng, J.; Yi, Q. Fndc5/irisin deficiency leads to dysbiosis of gut microbiota contributing to the depressive-like behaviors in mice. Brain Res. 2023, 1819, 148537. [Google Scholar] [CrossRef]
- Tabone, M.; Bressa, C.; García-Merino, J.A.; Moreno-Pérez, D.; Van, E.C.; Castelli, F.A.; Fenaille, F.; Larrosa, M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci. Rep. 2021, 11, 3558. [Google Scholar] [CrossRef]
- Merino, E.; Jensen, R.A.; Yanofsky, C. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 2008, 11, 78–86. [Google Scholar] [CrossRef]
- Manaf, F.A.; Lawler, N.G.; Peiffer, J.J.; Maker, G.L.; Boyce, M.C.; Fairchild, T.J.; Broadhurst, D. Characterizing the plasma metabolome during and following a maximal exercise cycling test. J. Appl. Physiol. (1985) 2018, 125, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Kummerhoff, F.; Koliamitra, C.; Schenk, A.; Walzik, D.; Hardt, L.; Knoop, A.; Thevis, M.; Kiesl, D.; Metcalfe, A.J.; et al. Exercise and the Kynurenine pathway: Current state of knowledge and results from a randomized cross-over study comparing acute effects of endurance and resistance training. Exerc. Immunol. Rev. 2020, 26, 24–42. [Google Scholar] [PubMed]
- Glavin, G.B.; Bose, R.; Pinsky, C. Kynurenic acid protects against gastroduodenal ulceration in mice injected with extracts from poisonous Atlantic shellfish. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Tiszlavicz, Z.; Németh, B.; Fülöp, F.; Vécsei, L.; Tápai, K.; Ocsovszky, I.; Mándi, Y. Different inhibitory effects of kynurenic acid and a novel kynurenic acid analogue on tumour necrosis factor-α (TNF-α) production by mononuclear cells, HMGB1 production by monocytes and HNP1-3 secretion by neutrophils. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 447–455. [Google Scholar] [CrossRef]
- Martin, K.S.; Azzolini, M.; Lira Ruas, J. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. Cell Physiol. 2020, 318, C818–C830. [Google Scholar] [CrossRef] [PubMed]
- Divorty, N.; Mackenzie, A.E.; Nicklin, S.A.; Milligan, G. G protein-coupled receptor 35: An emerging target in inflammatory and cardiovascular disease. Front. Pharmacol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef]
- Kang, C.S.; Ban, M.; Choi, E.J.; Moon, H.G.; Jeon, J.S.; Kim, D.K.; Park, S.K.; Jeon, S.G.; Roh, T.Y.; Myung, S.J.; et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Siqueira, I.R.; Palazzo, R.P.; Cechinel, L.R. Circulating extracellular vesicles delivering beneficial cargo as key players in exercise effects. Free Radic. Biol. Med. 2021, 172, 273–285. [Google Scholar] [CrossRef]
- Dos Santos, J.A.C.; Veras, A.S.C.; Batista, V.R.G.; Tavares, M.E.A.; Correia, R.R.; Suggett, C.B.; Teixeira, G.R. Physical exercise and the functions of microRNAs. Life Sci. 2022, 304, 120723. [Google Scholar] [CrossRef] [PubMed]
- Mei, T.; Liu, Y.; Wang, J.; Zhang, Y. miR-340-5p: A potential direct regulator of Nrf2 expression in the post-exercise skeletal muscle of mice. Mol. Med. Rep. 2019, 19, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Packer, N.; Hoffman-Goetz, L. Apoptotic and inflammatory cytokine protein expression in intestinal lymphocytes after acute treadmill exercise in young and old mice. J. Sports Med. Phys. Fitness 2012, 52, 202–211. [Google Scholar]
- Hoffman-Goetz, L.; Pervaiz, N.; Packer, N.; Guan, J. Freewheel training decreases pro- and increases anti-inflammatory cytokine expression in mouse intestinal lymphocytes. Brain Behav. Immun. 2010, 24, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Quiroga, R.; Nistal, E.; Estébanez, B.; Porras, D.; Juárez-Fernández, M.; Martínez-Flórez, S.; García-Mediavilla, M.V.; de Paz, J.A.; González-Gallego, J.; Sánchez-Campos, S.; et al. Exercise training modulates the gut microbiota profile and impairs inflammatory signaling pathways in obese children. Exp. Mol. Med. 2020, 52, 1048–1061. [Google Scholar] [CrossRef]
- Chevalier, G.; Siopi, E.; Guenin-Macé, L.; Pascal, M.; Laval, T.; Rifflet, A.; Boneca, I.G.; Demangel, C.; Colsch, B.; Pruvost, A.; et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 2020, 11, 6363. [Google Scholar] [CrossRef]
- Dohnalová, L.; Lundgren, P.; Carty, J.R.E.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.P.; Litichevskiy, L.; Descamps, H.C.; et al. A microbiome-dependent gut-brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Boisseau, N.; Barnich, N.; Koechlin-Ramonatxo, C. The Nutrition-Microbiota-Physical Activity Triad: An Inspiring New Concept for Health and Sports Performance. Nutrients 2022, 14, 924. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, M.; Chavanelle, V.; Chassaing, B.; Perriere, F.; Etienne, M.; Plissonneau, C.; Boscaro, A.; Barnich, N.; Pialoux, V.; Maugard, T.; et al. The TOTUM-63 Supplement and High-Intensity Interval Training Combination Limits Weight Gain, Improves Glycemic Control, and Influences the Composition of Gut Mucosa-Associated Bacteria in Rats on a High Fat Diet. Nutrients 2021, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Plissonneau, C.; Capel, F.; Chassaing, B.; Dupuit, M.; Maillard, F.; Wawrzyniak, I.; Combaret, L.; Dutheil, F.; Etienne, M.; Mairesse, G.; et al. High-Intensity Interval Training and α-Linolenic Acid Supplementation Improve DHA Conversion and Increase the Abundance of Gut Mucosa-Associated. Nutrients 2021, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wang, I.L.; Zhu, X.Y.; Chiu, W.C.; Chiu, Y.S. Red Clover Isoflavones Influence Estradiol Concentration, Exercise Performance, and Gut Microbiota in Female Mice. Front. Nutr. 2021, 8, 623698. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Palma, J.; Bytowska, Z.K.; Kujach, S.; Kaczor, J.J. Combined probiotics with vitamin D. Front. Nutr. 2023, 10, 1256226. [Google Scholar] [CrossRef]
- Bonomini-Gnutzmann, R.; Plaza-Díaz, J.; Jorquera-Aguilera, C.; Rodríguez-Rodríguez, A.; Rodríguez-Rodríguez, F. Effect of Intensity and Duration of Exercise on Gut Microbiota in Humans: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9518. [Google Scholar] [CrossRef]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.A.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, P.B.d.; de Araujo Borba, L.; Castro de Jesus, L.; Valverde, A.P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise. Int. J. Mol. Sci. 2023, 24, 16870. https://doi.org/10.3390/ijms242316870
Souza PBd, de Araujo Borba L, Castro de Jesus L, Valverde AP, Gil-Mohapel J, Rodrigues ALS. Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise. International Journal of Molecular Sciences. 2023; 24(23):16870. https://doi.org/10.3390/ijms242316870
Chicago/Turabian StyleSouza, Pedro Borges de, Laura de Araujo Borba, Louise Castro de Jesus, Ana Paula Valverde, Joana Gil-Mohapel, and Ana Lúcia S. Rodrigues. 2023. "Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise" International Journal of Molecular Sciences 24, no. 23: 16870. https://doi.org/10.3390/ijms242316870
APA StyleSouza, P. B. d., de Araujo Borba, L., Castro de Jesus, L., Valverde, A. P., Gil-Mohapel, J., & Rodrigues, A. L. S. (2023). Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise. International Journal of Molecular Sciences, 24(23), 16870. https://doi.org/10.3390/ijms242316870






