Albumin/Mitotane Interaction Affects Drug Activity in Adrenocortical Carcinoma Cells: Smoke and Mirrors on Mitotane Effect with Possible Implications for Patients’ Management
Abstract
1. Introduction
2. Results
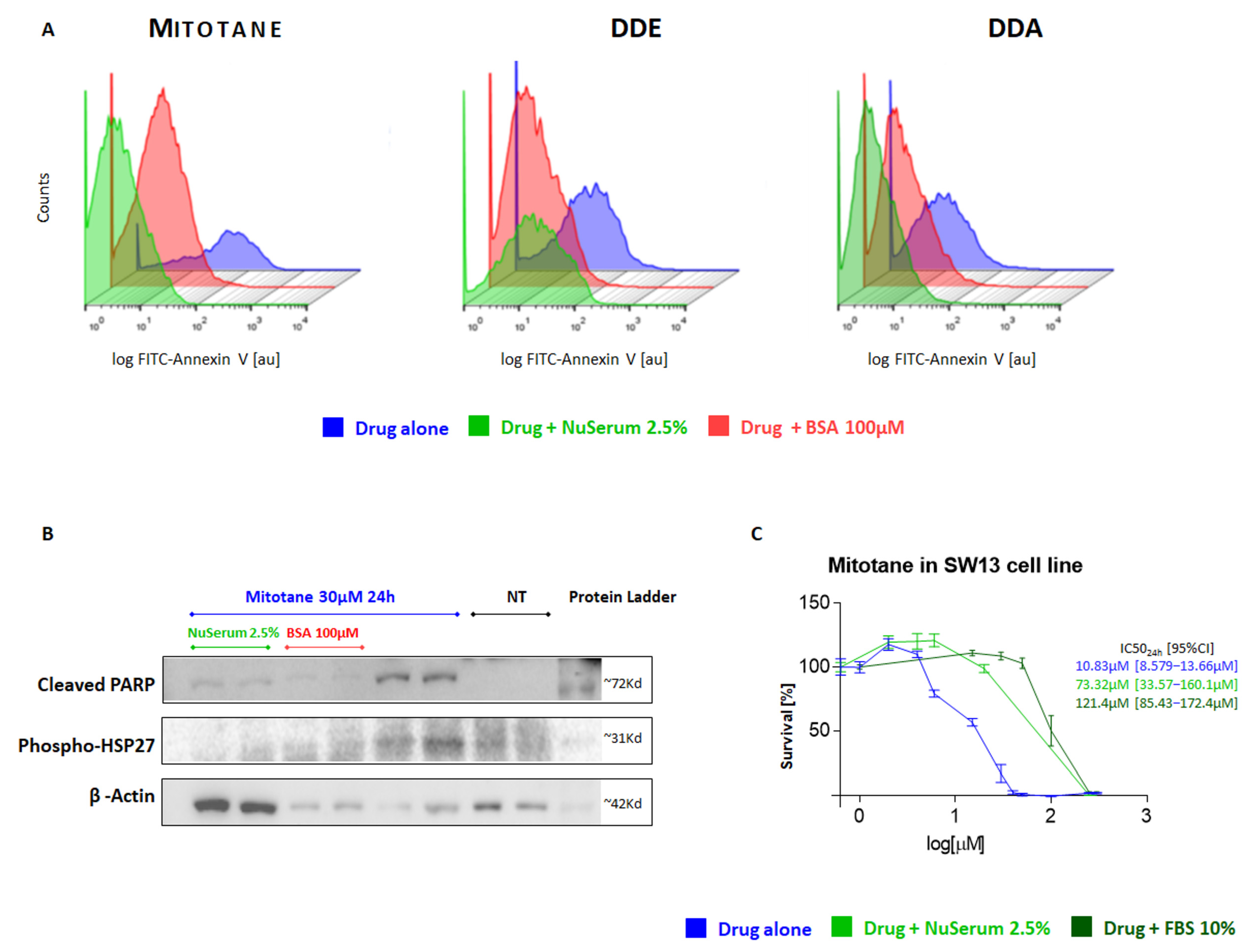
2.1. Study of the Influence of BSA and Serum on the Pharmacological Effect of Mitotane and Its Metabolites
2.2. Study of the Mitotane/BSA Interaction
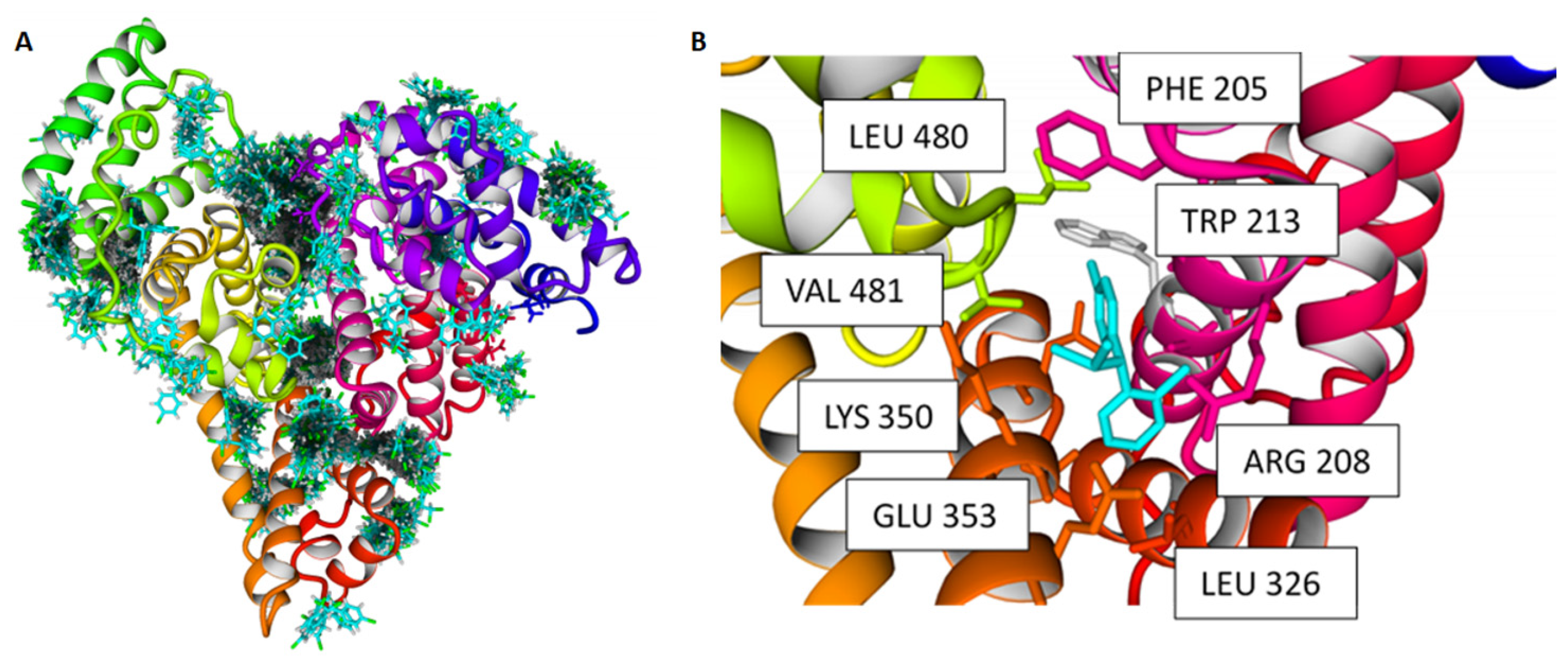
2.3. Docking Experiments
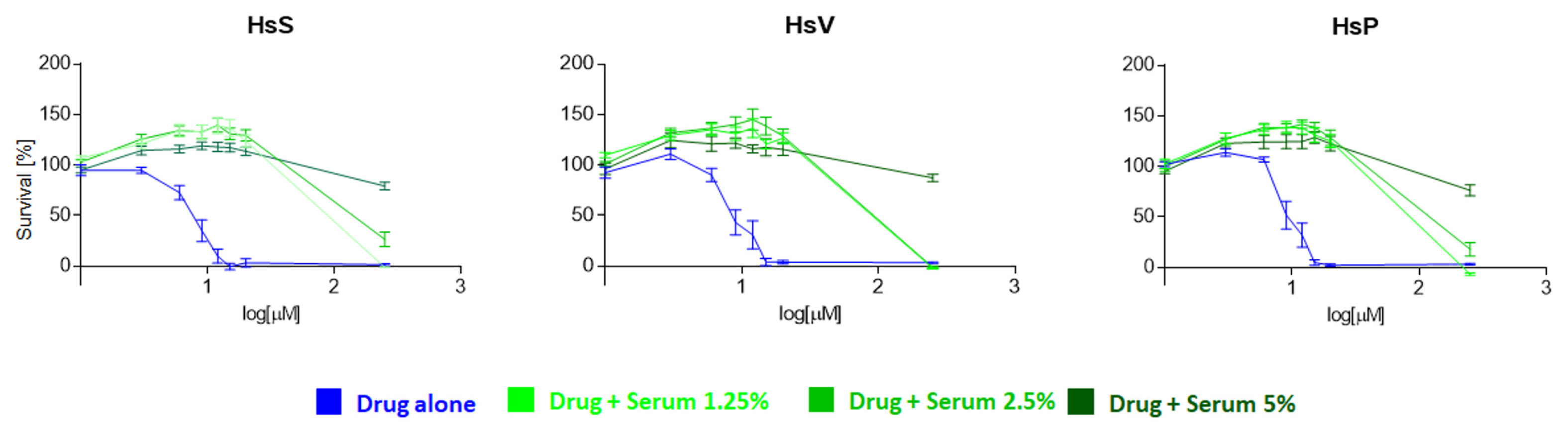
2.4. Study of the Influence of Human Serum on the Pharmacological Effect of Mitotane
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergenstal, D.M.; Lipsett, M.B.; Moy, R.H.; Hertz, R. Regression of Adrenal Cancer and Suppression of Adrenal Function in Man by o,p′-DDD1; Proceedings of a Conference Sponsored by the Cancer Chemotherapy National Service Center, National Cancer Institute, National Institutes of Health, U.S. Department of Health, Education and Welfare; Academic Press: Cambridge, MA, USA, 1960; pp. 463–475. [Google Scholar]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef]
- Terzolo, M.; Fassnacht, M. Endocrine Tumours: Our experience with the management of patients with non-metastatic adrenocortical carcinoma. Eur. J. Endocrinol. 2022, 187, R27–R40. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Zou, Z.; Liang, J.; Lu, Y.; Zhu, Y. Benefits of Adjuvant Mitotane after Resection of Adrenocortical Carcinoma: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9362108. [Google Scholar] [CrossRef] [PubMed]
- Schteingart, D.E. Adjuvant mitotane therapy of adrenal cancer-use and controversy. N. Engl. J. Med. 2007, 356, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Schteingart, D.E.; Motazedi, A.; Noonan, R.A.; Thompson, N.W. Treatment of adrenal carcinomas. Arch. Surg. 1982, 117, 1142–1146. [Google Scholar] [CrossRef]
- Young, R.B.; Bryson, M.J.; Sweat, M.L.; Street, J.C. Complexing of DDT and o,p′DDD with adrenal cytochrome P-450 hydroxylating systems. J. Steroid Biochem. 1973, 4, 585–591. [Google Scholar] [CrossRef]
- Nelson, A.A.; Woodard, G. Severe adrenal cortical atrophy (cytotoxic) and hepatic damage produced in dogs by feeding 2,2-bis(parachlorophenyl)-1,1-dichloroethane (DDD or TDE). Arch. Pathol. 1949, 48, 387–394. [Google Scholar]
- Corso, C.R.; Acco, A.; Bach, C.; Bonatto, S.J.R.; de Figueiredo, B.C.; de Souza, L.M. Pharmacological profile and effects of mitotane in adrenocortical carcinoma. Brit. J. Clin. Pharmacol. 2021, 87, 2698–2710. [Google Scholar] [CrossRef]
- Bach, C.; Corso, C.R.; Veiga, A.A.; Paraizo, M.M.; de Souza, L.M. Effects of o,p′-DDE, a Mitotane Metabolite, in an Adrenocortical Carcinoma Cell Line. Pharmaceuticals 2022, 15, 1486. [Google Scholar] [CrossRef]
- Hescot, S.; Paci, A.; Seck, A.; Slama, A.; Viengchareun, S.; Trabado, S.; Brailly-Tabard, S.; Al Ghuzlan, A.; Young, J.; Baudin, E.; et al. The lack of antitumor effects of o,p′DDA excludes its role as an active metabolite of mitotane for adrenocortical carcinoma treatment. Horm. Cancer 2014, 5, 312–323. [Google Scholar] [CrossRef]
- Sbiera, S.; Leich, E.; Liebisch, G.; Sbiera, I.; Schirbel, A.; Wiemer, L.; Matysik, S.; Eckhardt, C.; Gardill, F.; Gehl, A.; et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid-Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology 2015, 156, 3895–3908. [Google Scholar] [CrossRef]
- Lo Iacono, M.; Puglisi, S.; Perotti, P.; Saba, L.; Petiti, J.; Giachino, C.; Reimondo, G.; Terzolo, M. Molecular Mechanisms of Mitotane Action in Adrenocortical Cancer Based on In Vitro Studies. Cancers 2021, 13, 5255. [Google Scholar] [CrossRef]
- Hescot, S.; Seck, A.; Guerin, M.; Cockenpot, F.; Huby, T.; Broutin, S.; Young, J.; Paci, A.; Baudin, E.; Lombes, M. Lipoprotein-Free Mitotane Exerts High Cytotoxic Activity in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2015, 100, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Boulate, G.; Amazit, L.; Naman, A.; Seck, A.; Paci, A.; Lombes, A.; Pussard, E.; Baudin, E.; Lombes, M.; Hescot, S. Potentiation of mitotane action by rosuvastatin: New insights for adrenocortical carcinoma management. Int. J. Oncol. 2019, 54, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Kurlbaum, M.; Sbiera, S.; Kendl, S.; Martin Fassnacht, M.; Kroiss, M. Steroidogenesis in the NCI-H295 Cell Line Model is Strongly Affected By Culture Conditions and Substrain. Exp. Clin. Endocrinol. Diabetes 2020, 128, 672–680. [Google Scholar] [PubMed]
- Seidel, E.; Walenda, G.; Messerschmidt, C.; Obermayer, B.; Peitzsch, M.; Wallace, P.; Bahethi, R.; Yoo, T.; Choi, M.; Schrade, P.; et al. Generation and characterization of a mitotane-resistant adrenocortical cell line. Endocr. Connect. 2020, 9, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Weigand, I.; Schreiner, J.; Rohrig, F.; Sun, N.; Landwehr, L.S.; Urlaub, H.; Kendl, S.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Angeli, J.P.F.; et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Warde, K.M.; Schoenmakers, E.; Ribes Martinez, E.; Lim, Y.J.; Leonard, M.; Lawless, S.J.; O’Shea, P.; Chatterjee, K.V.; Gurnell, M.; Hantel, C.; et al. Liver X receptor inhibition potentiates mitotane-induced adrenotoxicity in ACC. Endocr. Relat. Cancer 2020, 27, 361–373. [Google Scholar] [CrossRef]
- Germano, A.; Rapa, I.; Volante, M.; Lo Buono, N.; Carturan, S.; Berruti, A.; Terzolo, M.; Papotti, M. Cytotoxic activity of gemcitabine, alone or in combination with mitotane, in adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2014, 382, 1–7. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yano, H.; Aoki, K. Differential Scanning Calorimetric Studies on Bovine Serum-Albumin: I. Effects of Ph and Ionic-Strength. Int. J. Biol. Macromol. 1990, 12, 263–268. [Google Scholar] [CrossRef]
- Stirpe, A.; Pantusa, M.; Rizzuti, B.; De Santo, M.P.; Sportelli, L.; Bartucci, R.; Guzzi, R. Resveratrol induces thermal stabilization of human serum albumin and modulates the early aggregation stage. Int. J. Biol. Macromol. 2016, 92, 1049–1056. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, N.; Kale, A.P.; Mondal, R.; Anand, U.; Ghosh, S.; Tiwari, V.K.; Kapur, M.; Mukherjee, S. Temperature Induced Morphological Transitions from Native to Unfolded Aggregated States of Human Serum Albumin. J. Phys. Chem. B 2014, 118, 7267–7276. [Google Scholar] [CrossRef]
- Zaidi, N.; Ajmal, M.R.; Rabbani, G.; Ahmad, E.; Khan, R.H. A Comprehensive Insight into Binding of Hippuric Acid to Human Serum Albumin: A Study to Uncover Its Impaired Elimination through Hemodialysis. PLoS ONE 2013, 8, e71422. [Google Scholar] [CrossRef]
- Yu, S.F.; Wang, S.Y.; Lu, M.; Zuo, L. Review of MEMS differential scanning calorimetry for biomolecular study. Front. Mech. Eng. 2017, 12, 526–538. [Google Scholar] [CrossRef]
- Durowoju, I.B.; Bhandal, K.S.; Hu, J.; Carpick, B.; Kirkitadze, M. Differential Scanning Calorimetry—A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J. Vis. Exp. 2017, 121, 55262. [Google Scholar]
- Kuwajima, K. The Molten Globule, and Two-State vs. Non-Two-State Folding of Globular Proteins. Biomolecules 2020, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Tsytlonok, M.; Itzhaki, L.S. The how’s and why’s of protein folding intermediates. Arch. Biochem. Biophys. 2013, 531, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Barrick, D. What have we learned from the studies of two-state folders, and what are the unanswered questions about two-state protein folding? Phys. Biol. 2009, 6, 015001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Figueroa, S.M.; Araos, P.; Reyes, J.; Gravez, B.; Barrera-Chimal, J.; Amador, C.A. Oxidized Albumin as a Mediator of Kidney Disease. Antioxidants 2021, 10, 404. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Gaggero, N. Albumin as a promiscuous biocatalyst in organic synthesis. RSC Adv. 2015, 5, 10588–10598. [Google Scholar] [CrossRef]
- Weaving, G.; Batstone, G.F.; Jones, R.G. Age and sex variation in serum albumin concentration: An observational study. Ann. Clin. Biochem. 2016, 53, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Catucci, G.; Ciaramella, A.; Di Nardo, G.; Zhang, C.; Castrignano, S.; Gilardi, G. Molecular Lego of Human Cytochrome P450: The Key Role of Heme Domain Flexibility for the Activity of the Chimeric Proteins. Int. J. Mol. Sci. 2022, 23, 3618. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Cimicata, G.; Baravalle, R.; Dell’Angelo, V.; Ciaramella, A.; Catucci, G.; Ugliengo, P.; Gilardi, G. Working at the membrane interface: Ligand-induced changes in dynamic conformation and oligomeric structure in human aromatase. Biotechnol. Appl. Biochem. 2018, 65, 46–53. [Google Scholar] [CrossRef]
- Catucci, G.; Aramini, D.; Sadeghi, S.J.; Gilardi, G. Ligand stabilization and effect on unfolding by polymorphism in human flavin-containing monooxygenase 3. Int. J. Biol. Macromol. 2020, 162, 1484–1493. [Google Scholar] [CrossRef]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and immunologic characterization of bovine, horse, and rabbit serum albumins. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—A self-parameterizing force field. Proteins 2002, 47, 393–402. [Google Scholar] [CrossRef]
- Gao, C.; Catucci, G.; Di Nardo, G.; Gilardi, G.; Sadeghi, S.J. Human flavin-containing monooxygenase 3: Structural mapping of gene polymorphisms and insights into molecular basis of drug binding. Gene 2016, 593, 91–99. [Google Scholar] [CrossRef][Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Haider, M.S.; Ahmad, T.; Groll, J.; Scherf-Clavel, O.; Kroiss, M.; Luxenhofer, R. The Challenging Pharmacokinetics of Mitotane: An Old Drug in Need of New Packaging. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 575–593. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavon, A.; Saba, L.; Catucci, G.; Petiti, J.; Puglisi, S.; Borin, C.; Reimondo, G.; Gilardi, G.; Giachino, C.; Terzolo, M.; et al. Albumin/Mitotane Interaction Affects Drug Activity in Adrenocortical Carcinoma Cells: Smoke and Mirrors on Mitotane Effect with Possible Implications for Patients’ Management. Int. J. Mol. Sci. 2023, 24, 16701. https://doi.org/10.3390/ijms242316701
Schiavon A, Saba L, Catucci G, Petiti J, Puglisi S, Borin C, Reimondo G, Gilardi G, Giachino C, Terzolo M, et al. Albumin/Mitotane Interaction Affects Drug Activity in Adrenocortical Carcinoma Cells: Smoke and Mirrors on Mitotane Effect with Possible Implications for Patients’ Management. International Journal of Molecular Sciences. 2023; 24(23):16701. https://doi.org/10.3390/ijms242316701
Chicago/Turabian StyleSchiavon, Aurora, Laura Saba, Gianluca Catucci, Jessica Petiti, Soraya Puglisi, Chiara Borin, Giuseppe Reimondo, Gianfranco Gilardi, Claudia Giachino, Massimo Terzolo, and et al. 2023. "Albumin/Mitotane Interaction Affects Drug Activity in Adrenocortical Carcinoma Cells: Smoke and Mirrors on Mitotane Effect with Possible Implications for Patients’ Management" International Journal of Molecular Sciences 24, no. 23: 16701. https://doi.org/10.3390/ijms242316701
APA StyleSchiavon, A., Saba, L., Catucci, G., Petiti, J., Puglisi, S., Borin, C., Reimondo, G., Gilardi, G., Giachino, C., Terzolo, M., & Lo Iacono, M. (2023). Albumin/Mitotane Interaction Affects Drug Activity in Adrenocortical Carcinoma Cells: Smoke and Mirrors on Mitotane Effect with Possible Implications for Patients’ Management. International Journal of Molecular Sciences, 24(23), 16701. https://doi.org/10.3390/ijms242316701










