Molecular Profiling of Lymphatic Endothelial Cell Activation In Vitro
Abstract
:1. Introduction
2. Results
2.1. In Vitro Activation of HDLECs and Preparation of RNAseq Samples
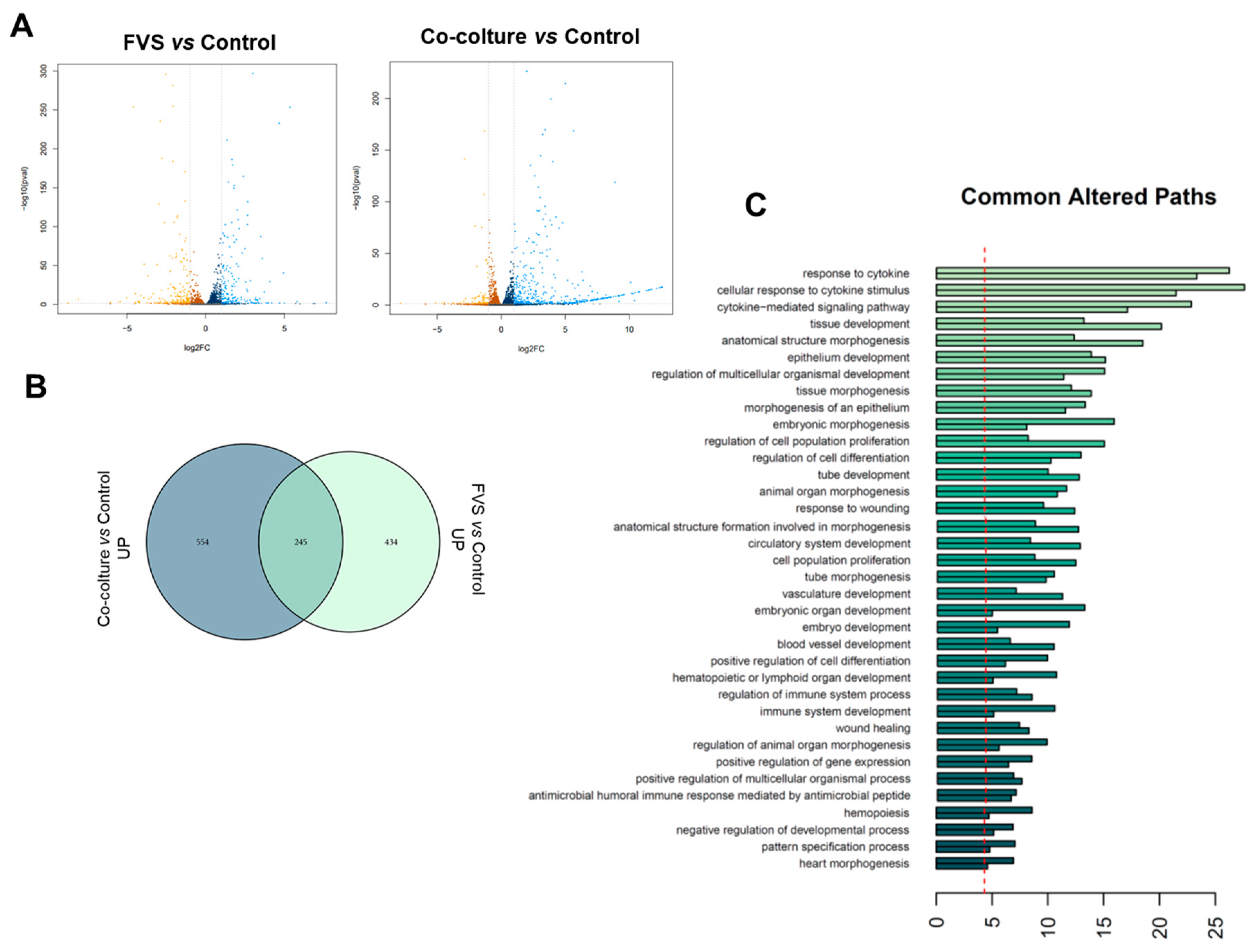
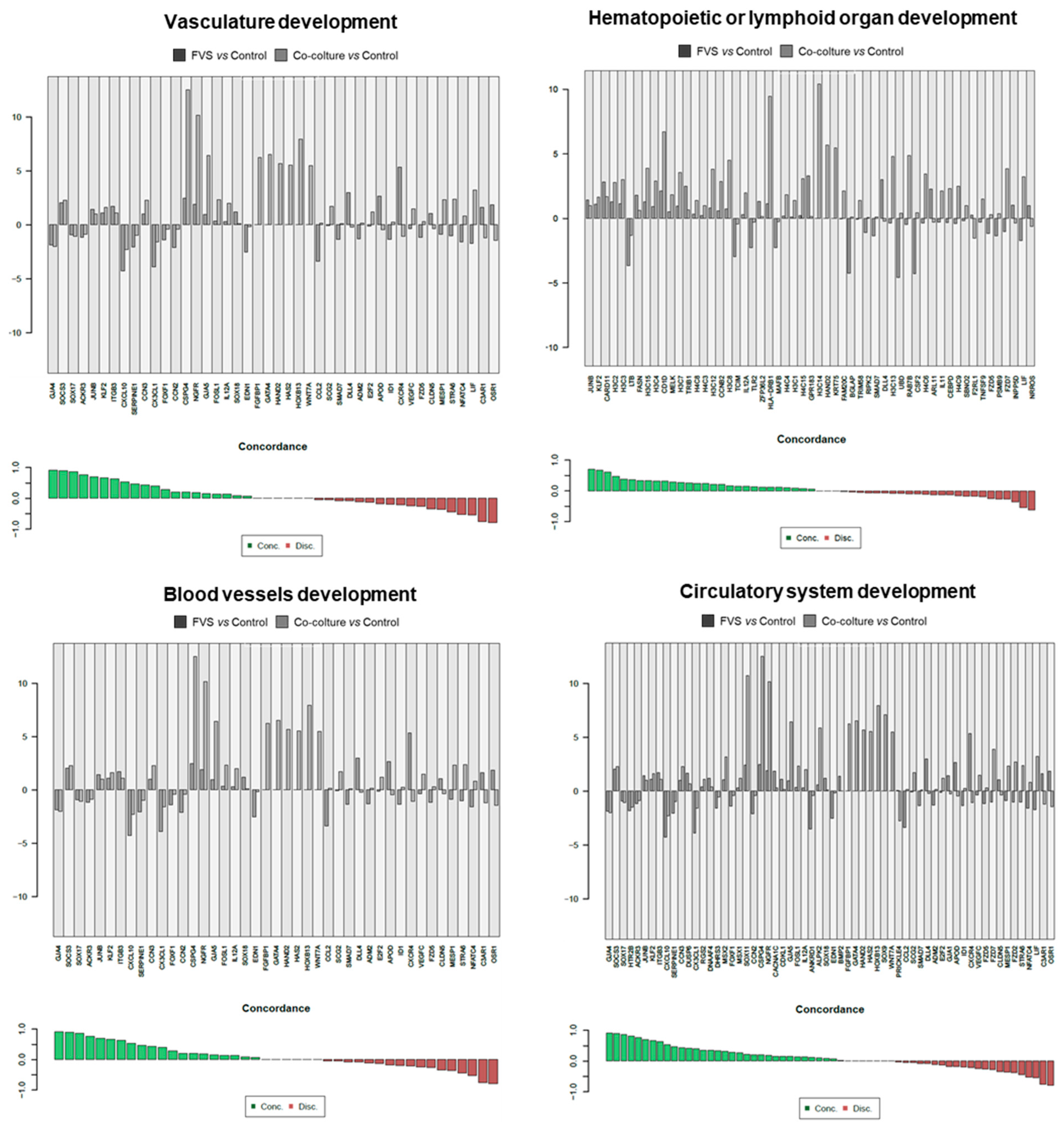
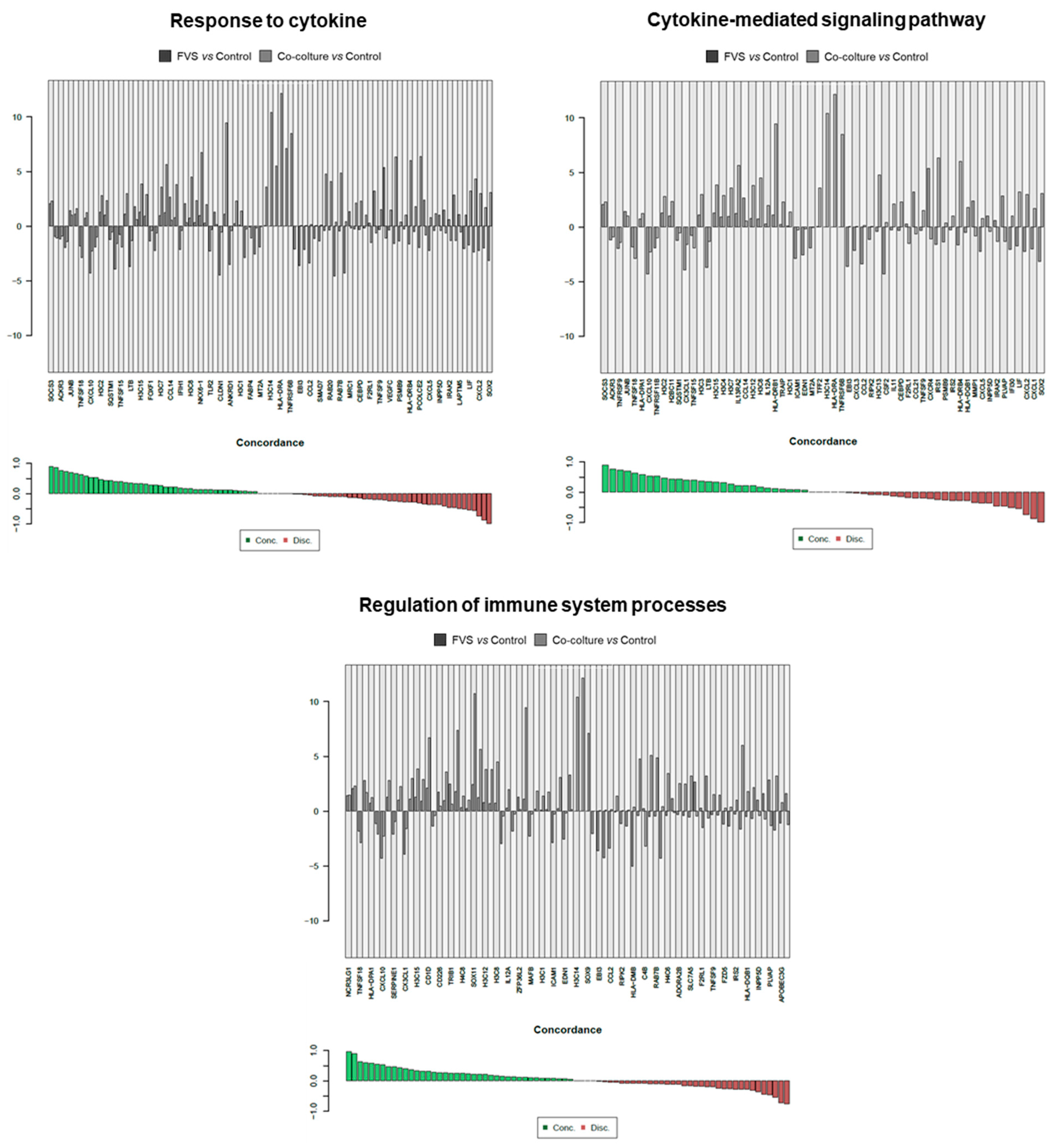
2.2. Transcriptional Comparison of HDLEC Activation by VFS and Human Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Cell Proliferation Assay
4.3. Spheroid Sprouting Assay
4.4. In Vitro HDLEC Stimulation and RNA Extraction
4.5. RNA Sequencing
4.6. In Silico Analysis
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swartz, M.A. The physiology of the lymphatic system. Adv. Drug Deliv. Rev. 2001, 50, 3–20. [Google Scholar] [CrossRef]
- Rezzola, S.; Sigmund, E.C.; Halin, C.; Ronca, R. The lymphatic vasculature: An active and dynamic player in cancer progression. Med. Res. Rev. 2022, 42, 576–614. [Google Scholar] [CrossRef]
- Cote, B.; Rao, D.; Alany, R.G.; Kwon, G.S.; Alani, A.W.G. Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors. Adv. Drug Deliv. Rev. 2019, 144, 16–34. [Google Scholar] [CrossRef]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef]
- Commerford, C.D.; Dieterich, L.C.; He, Y.; Hell, T.; Montoya-Zegarra, J.A.; Noerrelykke, S.F.; Russo, E.; Röcken, M.; Detmar, M. Mechanisms of Tumor-Induced Lymphovascular Niche Formation in Draining Lymph Nodes. Cell Rep. 2018, 25, 3554–3563.e4. [Google Scholar] [CrossRef]
- Swartz, M.A.; Skobe, M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc. Res. Tech. 2001, 55, 92–99. [Google Scholar] [CrossRef]
- Tacconi, C.; Correale, C.; Gandelli, A.; Spinelli, A.; Dejana, E.; D’alessio, S.; Danese, S. Vascular Endothelial Growth Factor C Disrupts the Endothelial Lymphatic Barrier to Promote Colorectal Cancer Invasion. Gastroenterology 2015, 148, 1438–1451.e8. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Bago-Horvath, Z.; Rudas, M.; Sexl, V.; Schneckenleithner, C.; Wolbank, S.; Bartel, G.; Krieger, S.; Kalt, R.; Hantusch, B.; et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Investig. 2011, 121, 2000–2012. [Google Scholar] [CrossRef]
- Das, S.; Skobe, M. Lymphatic Vessel Activation in Cancer. Ann. N. Y. Acad. Sci. 2008, 1131, 235–241. [Google Scholar] [CrossRef]
- Alitalo, A.; Detmar, M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene 2012, 31, 4499–4508. [Google Scholar] [CrossRef]
- Lund, A.W.; Duraes, F.V.; Hirosue, S.; Raghavan, V.R.; Nembrini, C.; Thomas, S.N.; Issa, A.; Hugues, S.; Swartz, M.A. VEGF-C Promotes Immune Tolerance in B16 Melanomas and Cross-Presentation of Tumor Antigen by Lymph Node Lymphatics. Cell Rep. 2012, 1, 191–199. [Google Scholar] [CrossRef]
- Rinderknecht, M.; Detmar, M. Tumor lymphangiogenesis and melanoma metastasis. J. Cell. Physiol. 2008, 216, 347–354. [Google Scholar] [CrossRef]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef]
- Christiansen, A.; Detmar, M. Lymphangiogenesis and Cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef]
- Raica, M.; Jitariu, A.-A.; Cimpean, A.M. Lymphangiogenesis and Anti-lymphangiogenesis in Cutaneous Melanoma. Anticancer. Res. 2016, 36, 4427–4436. [Google Scholar] [CrossRef]
- Turati, M.; Giacomini, A.; Rezzola, S.; Maccarinelli, F.; Gazzaroli, G.; Valentino, S.; Bottazzi, B.; Presta, M.; Ronca, R. The natural FGF-trap long pentraxin 3 inhibits lymphangiogenesis and lymphatic dissemination. Exp. Hematol. Oncol. 2022, 11, 84. [Google Scholar] [CrossRef]
- Schulz, M.M.P.; Reisen, F.; Zgraggen, S.; Fischer, S.; Yuen, D.; Kang, G.J.; Chen, L.; Schneider, G.; Detmar, M. Phenotype-based high-content chemical library screening identifies statins as inhibitors of in vivo lymphangiogenesis. Proc. Natl. Acad. Sci. 2012, 109, E2665–E2674. [Google Scholar] [CrossRef]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Bosisio, D.; Ronca, R.; Salvi, V.; Presta, M.; Sozzani, S. Dendritic cells in inflammatory angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018, 53, 180–186. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Issa, A.; Le, T.X.; Shoushtari, A.N.; Shields, J.D.; Swartz, M.A. Vascular Endothelial Growth Factor-C and C-C Chemokine Receptor 7 in Tumor Cell–Lymphatic Cross-talk Promote Invasive Phenotype. Cancer Res 2009, 69, 349–357. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turati, M.; Mattei, G.; Boaretto, A.; Magi, A.; Calvani, M.; Ronca, R. Molecular Profiling of Lymphatic Endothelial Cell Activation In Vitro. Int. J. Mol. Sci. 2023, 24, 16587. https://doi.org/10.3390/ijms242316587
Turati M, Mattei G, Boaretto A, Magi A, Calvani M, Ronca R. Molecular Profiling of Lymphatic Endothelial Cell Activation In Vitro. International Journal of Molecular Sciences. 2023; 24(23):16587. https://doi.org/10.3390/ijms242316587
Chicago/Turabian StyleTurati, Marta, Gianluca Mattei, Alessia Boaretto, Alberto Magi, Maura Calvani, and Roberto Ronca. 2023. "Molecular Profiling of Lymphatic Endothelial Cell Activation In Vitro" International Journal of Molecular Sciences 24, no. 23: 16587. https://doi.org/10.3390/ijms242316587
APA StyleTurati, M., Mattei, G., Boaretto, A., Magi, A., Calvani, M., & Ronca, R. (2023). Molecular Profiling of Lymphatic Endothelial Cell Activation In Vitro. International Journal of Molecular Sciences, 24(23), 16587. https://doi.org/10.3390/ijms242316587






