Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies
Abstract
:1. Introduction
2. Results
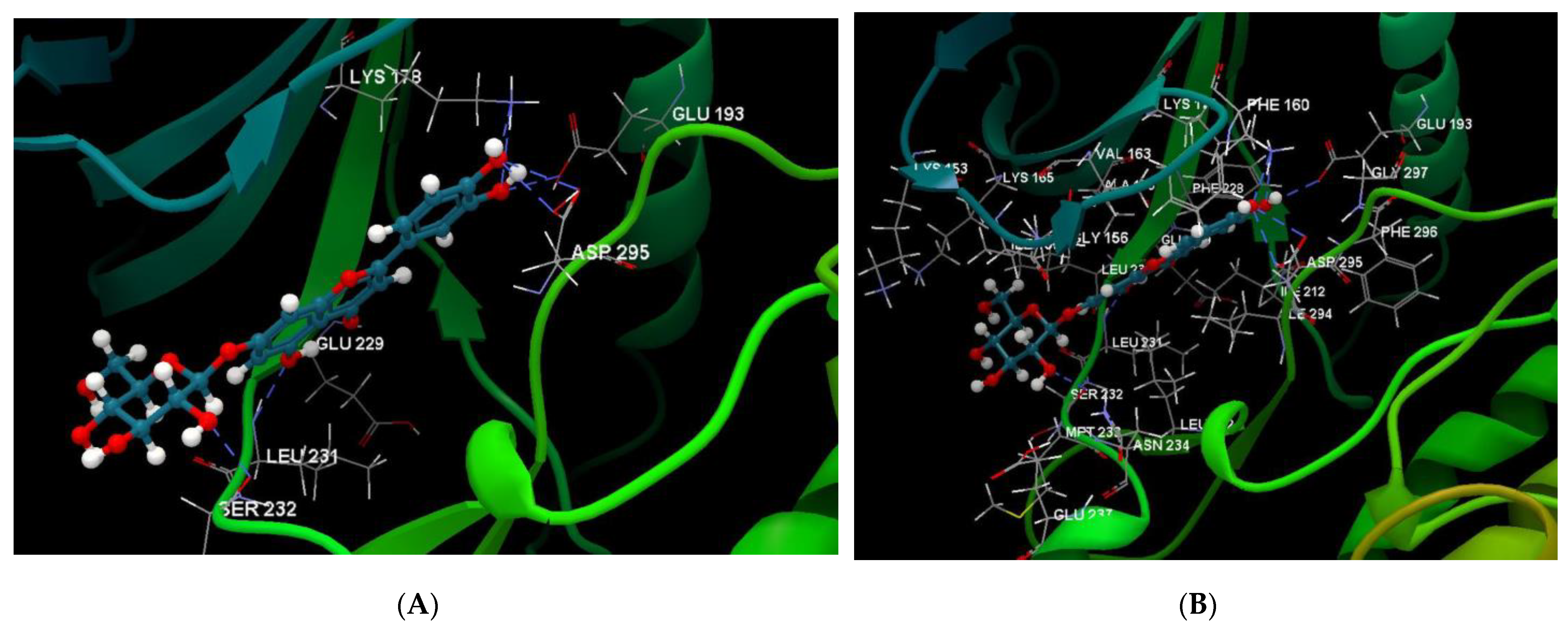
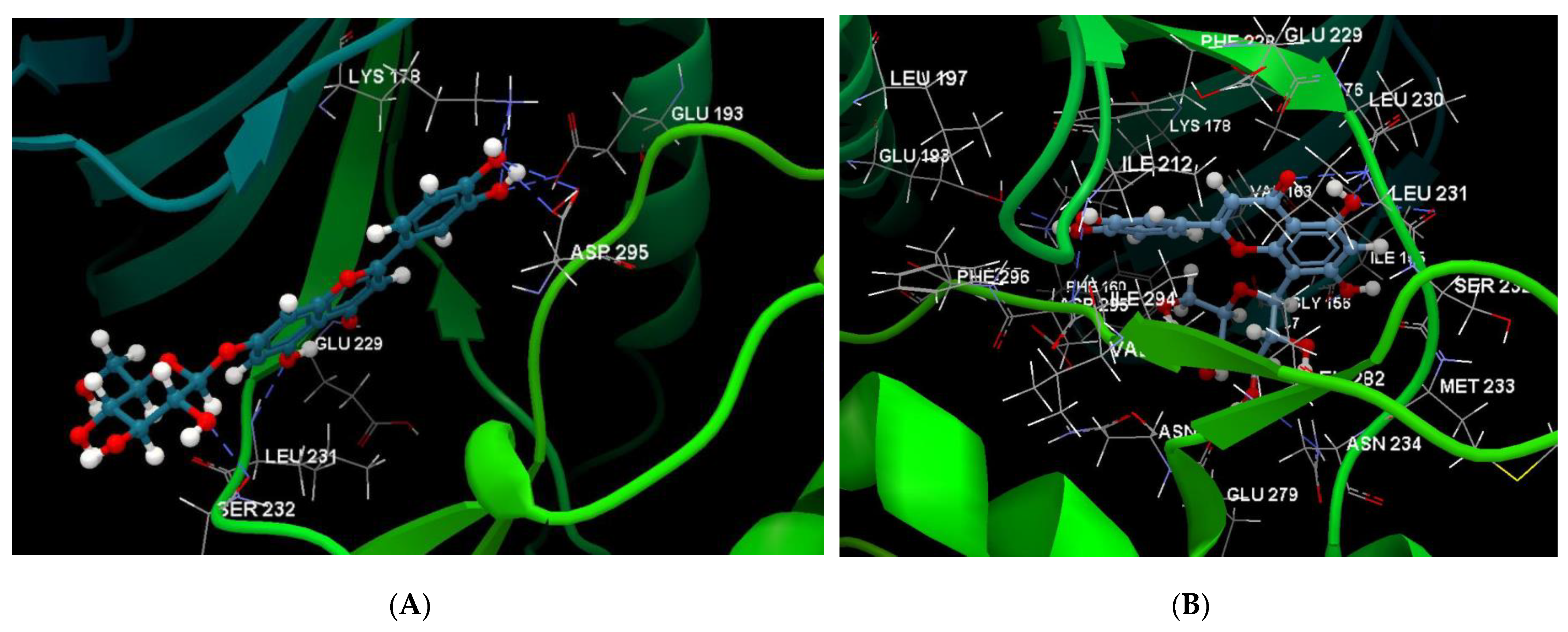
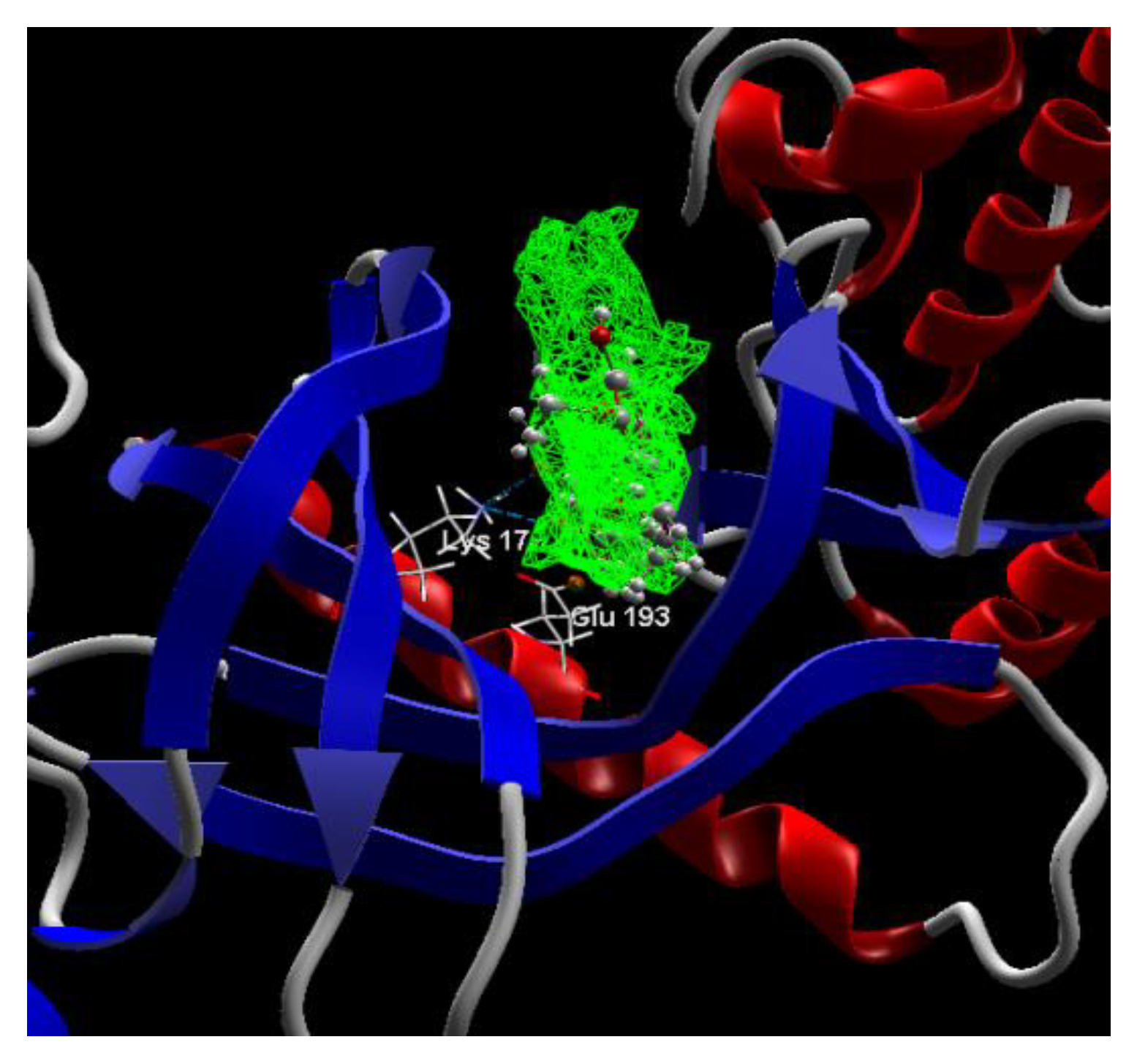
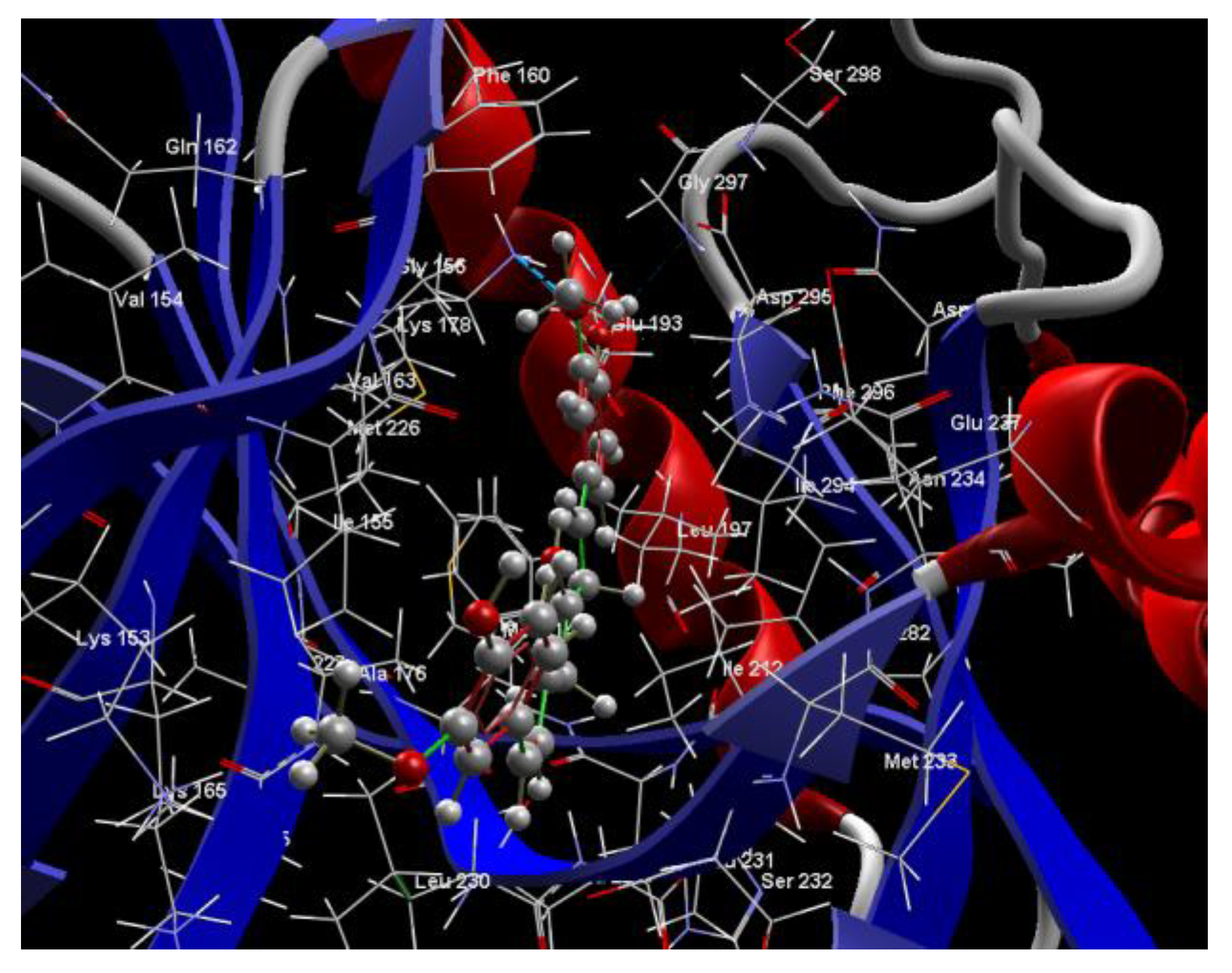
2.1. In Silico Studies
| Ligand Name/Chemical Structure * | Score/ RMSD | Amino Acids Group Interaction | (HB) | HB Length (Å) |
|---|---|---|---|---|
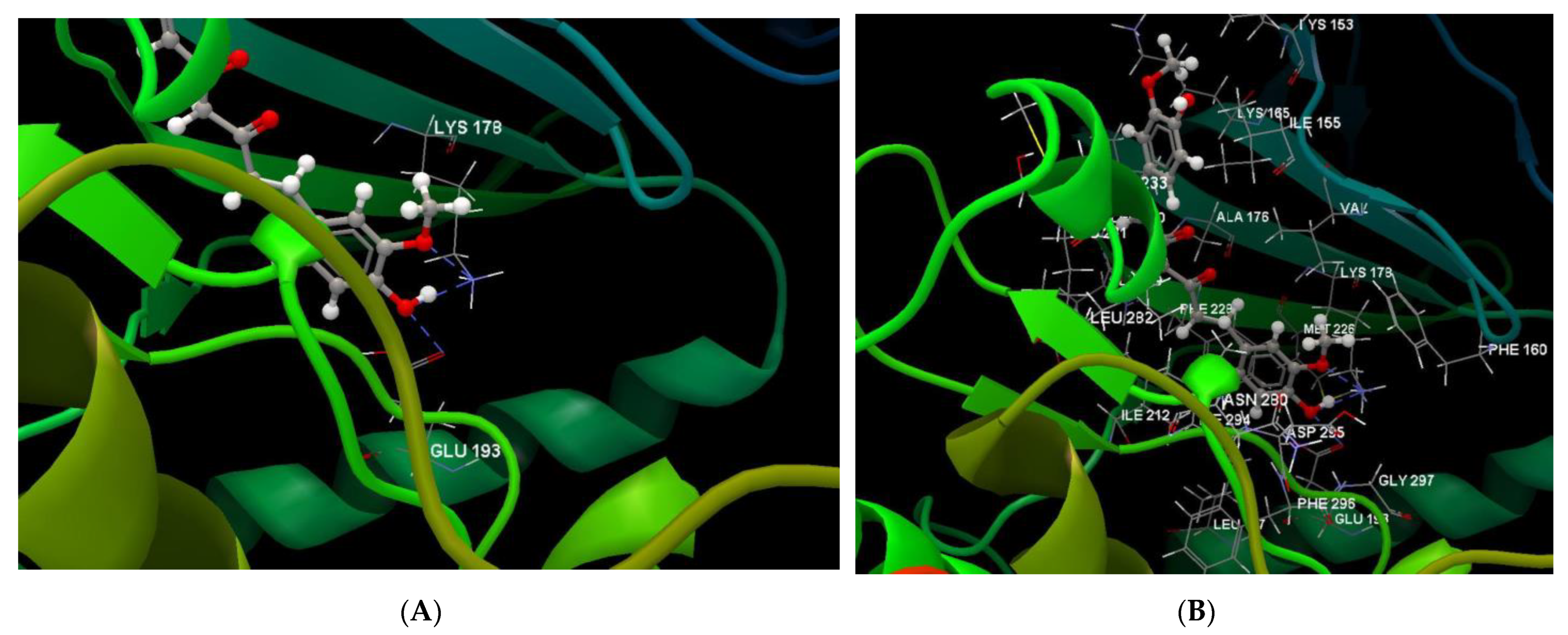
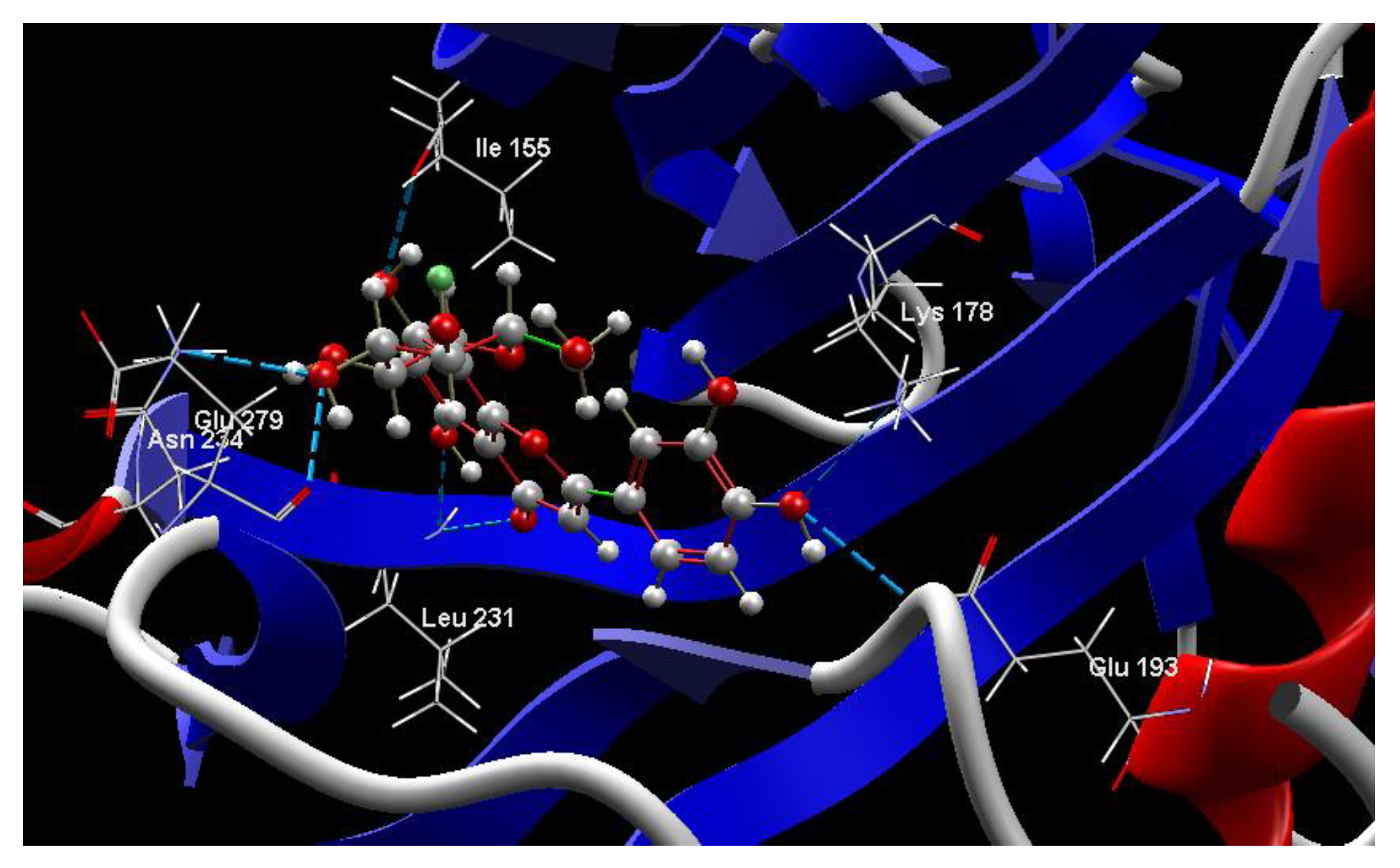
Co-crystallized CUR A501, CURCUMIN | −3.92/1.99 | PHE 160, GLY 197, GLU 193, PHE 296, LEU 197, ASP 295, MET 226, LYS 178, VAL 163, ILE 155, ASN 280, ILE 294, ILE 212, LEU 282, PHE 228, ALA 176, ILE 155, LYS 153, LYS 165, SER 232, LEU 231, LEU 230, GLU 229, ILE 212, LEU 282, MET 233. | O sp3 (O1)–N sp3 LYS 178 O sp3 (O3)–N sp3 LYS 178 O sp3(O3)–O sp2 GLU 193 | 3.102 2.661 3.163 |
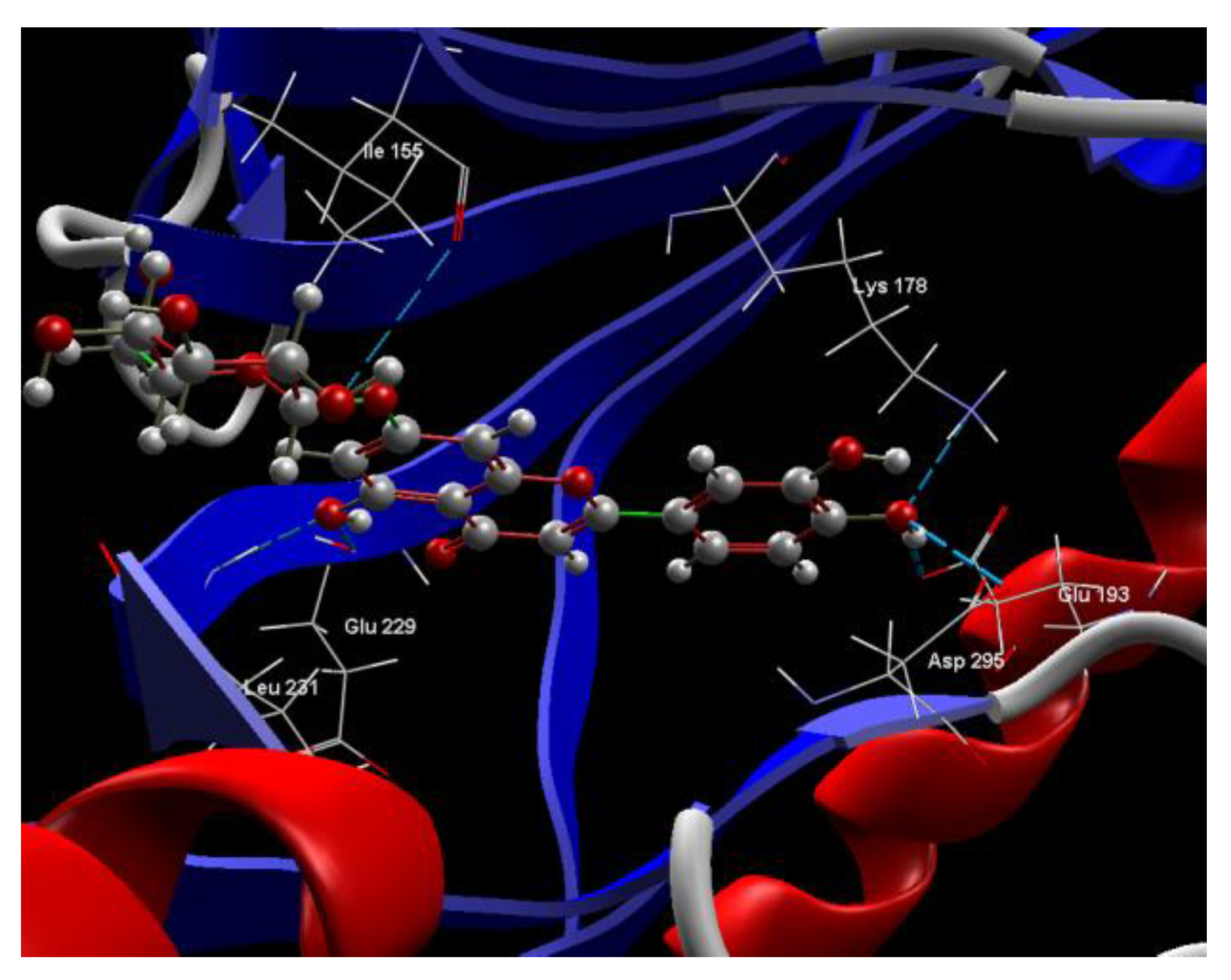
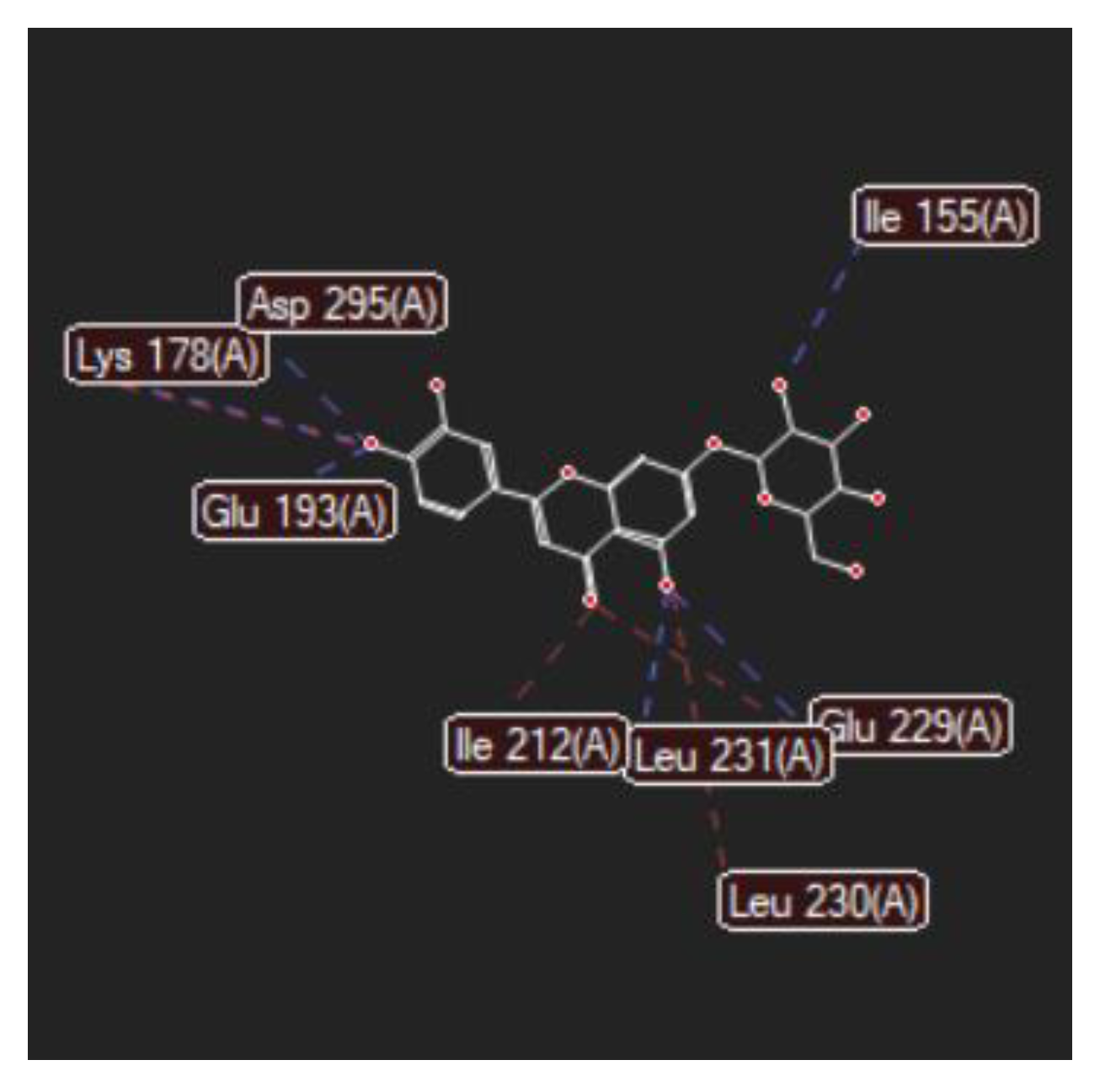
| Luteolin-7-O–glucoside (Cynaroside) (7-Lut)  | −81.35/0.28 | GLU 193, PFE 228, LYS 178, GLU 229, ALA 176, VAL 163, LEU 230, PHE 160, ILE 155, GLY 156, LYS 165, LYS 153, LEU 231, SER 232, MET 233, GLU 237, ASN 234, LEU 282, ILE 294, ASP 295, PHE 296, ILE 212, GLY 297 | O sp3 (O7)–O sp2 GLU 229 O sp3 (O7)–N sp2 LEU 231 O sp3 (O4)–O sp2 SER 232 O sp3 (O10)–O sp3 ASP 295 O sp3 (O10)–O sp2 ASP 295 O sp3 (O10)–N sp3 LYS 178 O sp3 (O9)–N sp3 LYS 178 O sp3 (O9)–O sp2 GLU 193 | 2.908 2.797 3.092 3.365 2.787 2.710 3.042 2.990 |
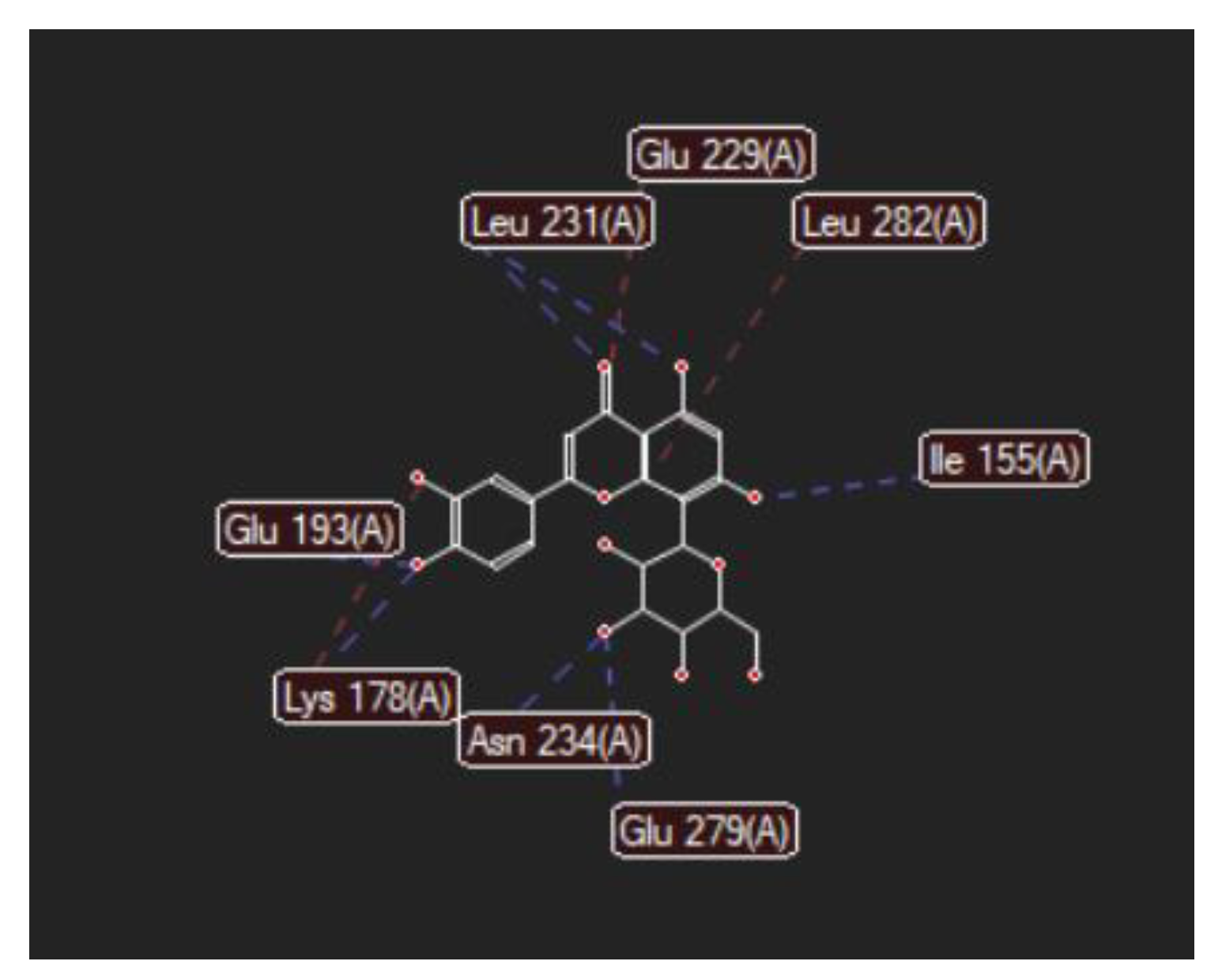
| Luteolin-8-C-glucoside (Orientin) (8-Lut)  | −86.61/0.06 | LEU 197, GLU 193, PHE 296, GLY 292, PHE 160, ASP 295, ILE 294, VAL 293, ASN 280, GLU 279, ASN 234, LEU 262, MET 233, SER 232, LEU 231, ILE 155, GLY 156, LYS 157, ILE 212, VAL 163, LEU 230, ALA 176, GLU 229, LYS 178, PHE 228. | O sp2 (O8)–N sp2 LEU 231 O sp3 (O7)–N sp2 LEU 231 O sp3 (O7)–O sp2 LEU 231 O sp3 (O6)–O sp2 ILE 155 O sp3 (O2)–O sp2 GLU 279 O sp3 (O2)–N sp2 ASN234 O sp3(O10)–N sp3 LYS 178 O sp3(O10)–O sp2 GLU 193 O sp3 (O9)–N sp2 ASP 295 | 3.039 2.623 3.314 2.642 2.557 3.130 3.218 3.064 2.936 |
| Ligand | Mol Dock Score | Molecule Contributions | HB | HB Length Å | Steric Interactions | Distance Å |
|---|---|---|---|---|---|---|
| CUR A501 Curcumin | −115.85 | ALA 176, ASP 295, GLU 193, GLU 229, GLY 297, ILE 155, ILE 212, ILE 294, LEU 197, LEU 230, LEU 231, LEU 282, LYS 153, LYS 165, LYS 178, MET 233, PHE 160, PHE 228, PPHE 296, SER 232, VAL 163 | O sp23(O1)–N sp3 LYS 178 O sp3 (O3)–N sp3 LYS 178 O sp3 (O3)–O sp3GLU 193 | 3.102 2.661 2.249 | C sp2 (C22)–Nsp2 GLU 193 C sp2 (C18)–Nsp2 GLU 193 O sp3 (O3)–C sp2 GLU 193 O sp2 (O3)–C sp3 LYS 178 C sp3 (C15)–O sp2 LEU 231 C sp3 (C23)–N sp2 LEU 231 | 3.12 3.05 3.03 3.13 2.80 2.74 |
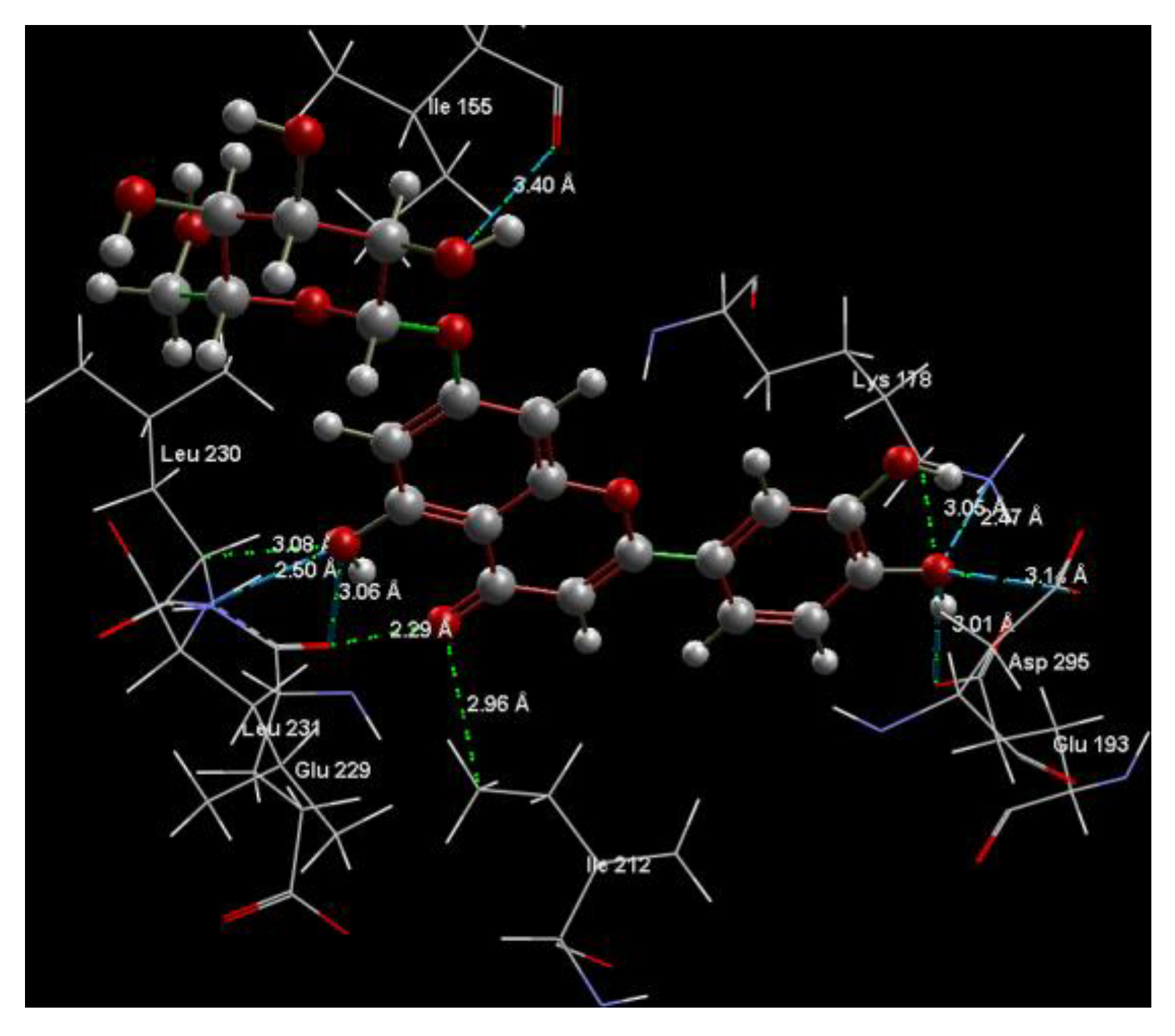
| 7-LUT | −128.51 | ALA 176, ASP 295, GLU 193, GLU 229, GLY 297, ILE 155, ILE 212, ILE 294, LEU 230, LEU 231, LEU 282, LYS 165, LYS 178, MET 233, PHE 160, PHE 228, PHE 296, SER 232, VAL 163 | O sp3 (O7)–O sp2 GLU 229 O sp3 (O7)–N sp2 LEU 231 O sp3 (O4)–O sp2 ILE 155 O sp3 (O10)–O sp3ASP 295 O sp3 (O10)–N sp3 LYS 178 O sp3(O10)–O sp3 GLU 193 | 3.056 2.500 3.339 3.137 2.470 3.014 | O sp2 (O8)–O sp2 GLU 229 O sp2 (O2)–C sp3 ILE 212 O sp3 (O7)–C sp3 LEU 230 O sp3 (O10)–C sp3 LYS 178 | 2.29 2.96 3.08 3.05 |
| 8-LUT | −126.66 | ALA 176, ASN 234, ASN 280, ASP 295, GLU 193, GLU 229, GLU 279, GLY 297, ILE 155, ILE 212, ILE 294, LEU 230, LEU 231, LEU 282, LYS 178, MET 233, PHE 160, PHE 228, PHE 296, SER 232, VAL 163 | O sp2 (O8)–N sp2 LEU 231 O sp3 (O7)–N sp2 LEU 231 O sp3 (O6)–O sp2 ILE 155 O sp3 (O2)–O sp2 GLU 279 O sp3 (O2)–N sp2 ASN 234 O sp3(O10)–N sp3 LYS 178 O sp3(O10)–Osp2 GLU 193 | 2.830 2.578 2.658 2.568 2.830 3.396 3.013 | O sp2 (O8)–Osp2 GLU 229 O sp3 (O9)–C sp3 LYS 178 C sp2 (C18)–C sp3 LEU 282 | 2.43 2.98 3.20 |
2.2. In Vitro Pharmacological Studies
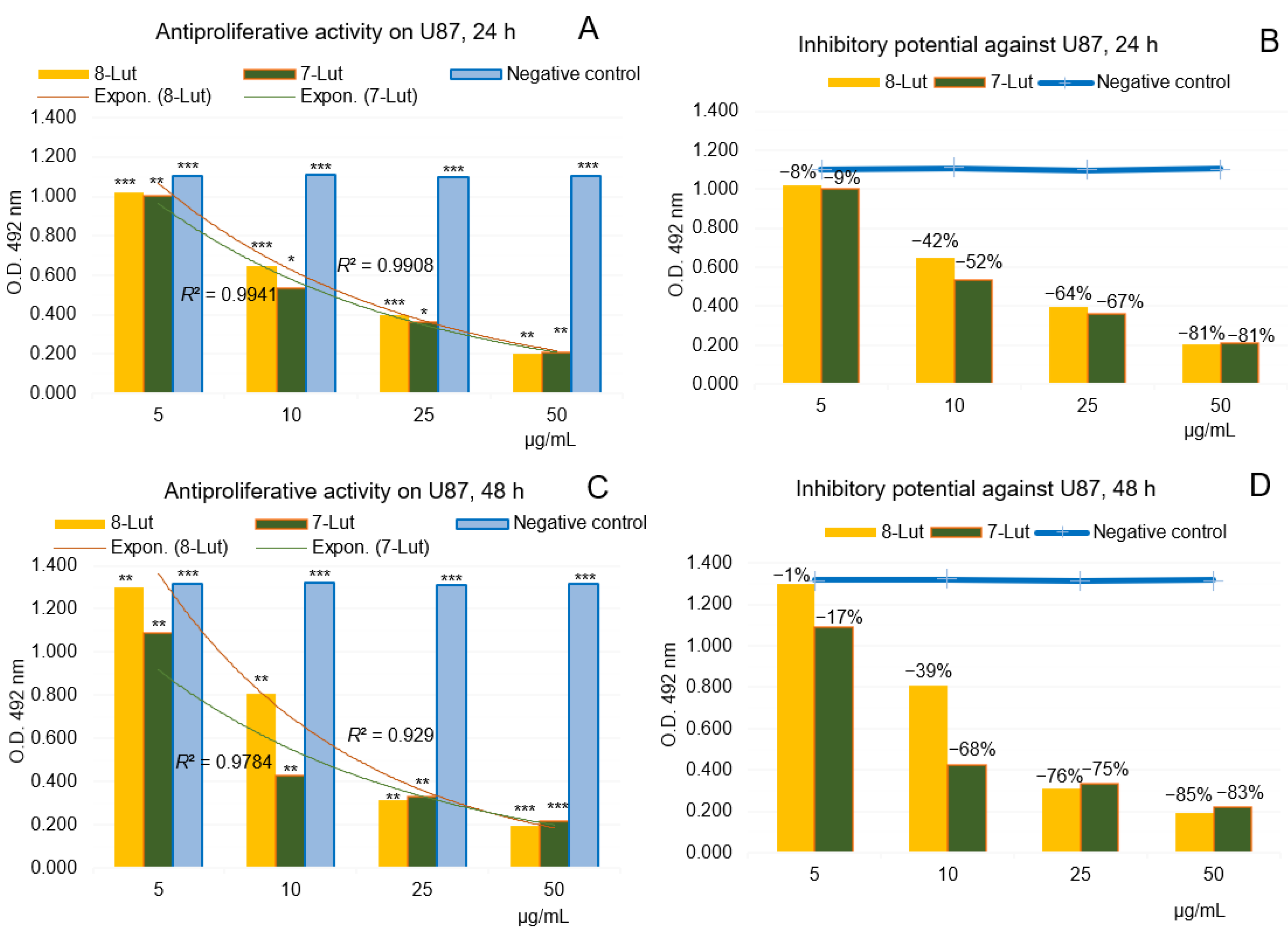
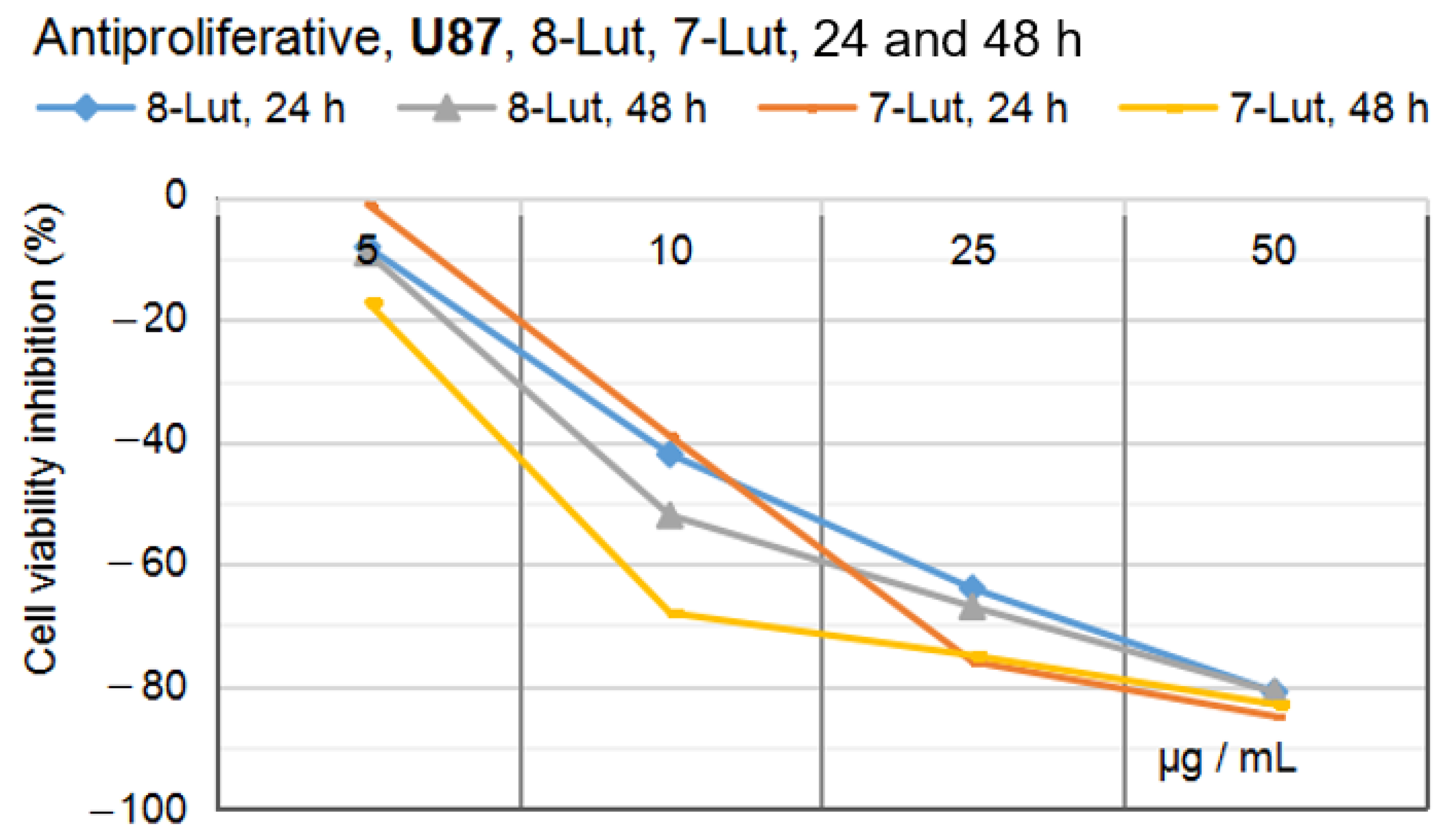
2.2.1. Anti-Proliferative Potential of 7-Lut and 8-Lut on U87 Human Glioblastoma Cells
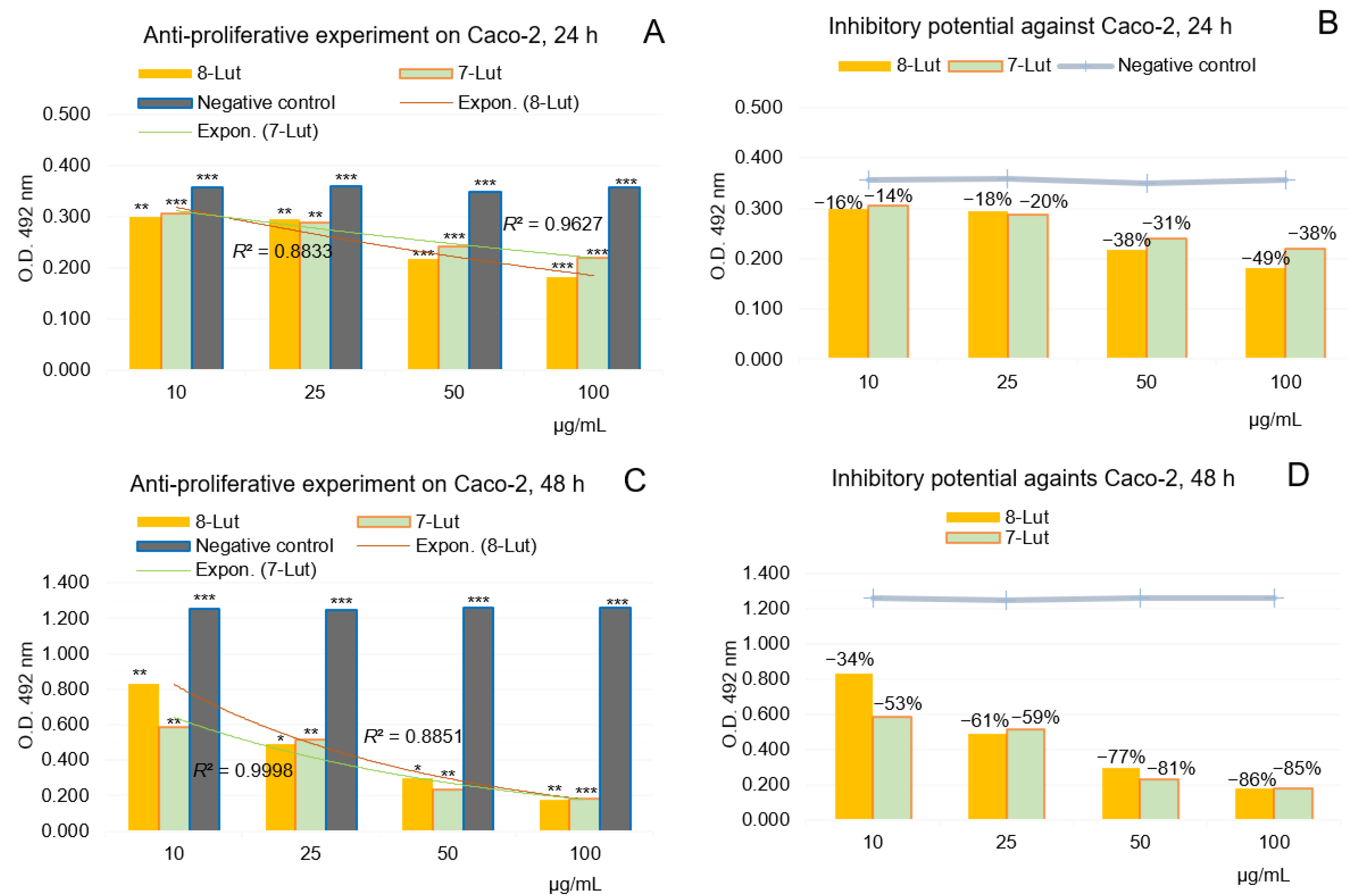
2.2.2. Anti-Proliferative Potential of 7-Lut and 8-Lut on Colon Carcinoma Cell Line Caco-2
3. Discussion
4. Materials and Methods
4.1. In Silico Assay
4.2. In Vitro Assay
4.3. Test Compounds Preparation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.K.; du Toit, L.C.; Choonara, Y.E. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoad-hesive tailored biopolymer-based nanoparticles: A review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Imeh, A.E.; Li, W. Polymeric nanoparticles—Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef] [PubMed]
- El-Hosari, D.G.; Hussein, W.M.; Elgendy, M.O.; Elgendy, S.O.; Ibrahim, A.R.N.; Fahmy, A.M.; Hassan, A.; Mokhtar, F.A.; Hussein, M.F.; Abdelrahim, M.E.A.; et al. Galangal–cinnamon spice mixture blocks the coronavirus infection pathway through inhibition of SARS-CoV-2 MPro, three HCoV-229E targets; quantum-chemical calculations support in vitro evaluation. Pharmaceuticals 2023, 16, 1378. [Google Scholar] [CrossRef] [PubMed]
- Stefaniu, A.; Pirvu, L. In silico study approach upon a series of 50 polyphenolic compounds in plants; a comparison on the bioavailability and bioactivity data. Molecules 2022, 27, 1413. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Lu, J.; Shang, X.; Zhong, W.; Xu, Y.; Shi, R.; Wang, X. New insights of CYP1A in endogenous metabolism: A focus on single nucleotide polymorphisms and diseases. Acta Pharm. Sin. B 2019, 10, 91–104. [Google Scholar] [CrossRef]
- Donadio, G.; Bellone, M.L.; Mensitieri, F.; Parisi, V.; Santoro, V.; Vitiello, M.; Piaz, F.D.; De Tommasi, N. Characterization of health beneficial components in discarded leaves of three escarole (Cichorium endivia L.) cultivar and study of their antioxidant and anti-inflammatory activities. Antioxidants 2023, 12, 1402. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D.D. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell. Biochem. 2004, 265, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Dijkgraaf, E.M.; Santegoets, S.J.A.M.; Reyners, A.K.L.; Goedemans, R.; Wouters, M.C.A.; Kenter, G.G.; van Erkel, A.R.; van Poelgeest, M.I.; Nijman, E.H.W.; van der Hoeven, J.J.M.; et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann. Oncol. 2015, 26, 2141–2149. [Google Scholar] [CrossRef]
- Kang, O.-H.; Choi, J.-G.; Lee, J.-H.; Kwon, D.-Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-κB and MAPKs activation pathways in HMC-1 cells. Molecules 2010, 15, 385–398. [Google Scholar] [CrossRef]
- Paganga, G.; A Rice-Evans, C. The identification of flavonoids as glycosides in human plasma. FEBS Lett. 1997, 401, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Braune, A.; Blaut, M. Intestinal bacterium Eubacterium cellulosolvens deglycosylates flavonoid C- and O-glucosides. Appl. Environ. Microbiol. 2012, 78, 8151–8153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Zheng, S.; Geng, D.; Liu, S.; Wang, Q.; Liu, S.; Wang, R. A newly isolated human intestinal bacterium strain capable of de-glycosylating flavone C-glycosides and its functional properties. Microb. Cell Factories 2019, 18, 94. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef]
- Limban, C.; Nuta, D.C.; Missir, A.V.; Roman, R.; Caproiu, M.T.; Dumitrascu, F.; Pintilie, L.; Stefaniu, A.; Chifiriuc, M.C.; Popa, M.; et al. Synthesis and Characterization of New Fluoro/Trifluoromethyl-Substituted Acylthiourea Derivatives with Promising Activity against Planktonic and Biofilm-Embedded Microbial Cells. Processes 2020, 8, 503. [Google Scholar] [CrossRef]
- Schroder, V.; Radu, N.; Cornea, P.C.; Coman, O.A.; Pirvu, L.C.; Mohammed, M.S.O.; Stefaniu, A.; Pintilie, L.; Bostan, M.; Caramihai, M.D.; et al. Studies Regarding the Antimicrobial Behavior of Clotrimazole and Limonene. Antibiotics 2022, 11, 1816. [Google Scholar] [CrossRef]
- Pirvu, L.C.; Neagu, G.; Albulescu, A.; Stefaniu, A.; Pintilie, L. Potential Benefits of Dietary Plant Compounds on Normal and Tumor Brain Cells in Humans: In Silico and In Vitro Approaches. Int. J. Mol. Sci. 2023, 24, 7404. [Google Scholar] [CrossRef]
- PubChem Open Chemistry Database at the National Institutes of Health (NIH), U.S. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 January 2021).
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J. A Guide to Molecular Mechanics and Quantum Chemical Calculations; Wavefunction, Inc.: Irvine, CA, USA, 2003; Available online: http://mms.dsfarm.unipd.it/files/Lezioni/PSF/PDF/AGuidetoMM&QM.pdf (accessed on 30 September 2023).
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Protocols & Applications Guide. Available online: www.promega.com (accessed on 8 August 2022).
- Zhou, P.; Li, L.-P.; Luo, S.-Q.; Jiang, H.-D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2007, 56, 296–300. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Yuan, Z.; Zhang, L.; Xu, L.; Cui, Y.; Duan, K. Pharmacokinetics and tissue distribution study of orientin in rat by liquid chromatography. J. Pharm. Biomed. Anal. 2008, 47, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hodek, P.; Trefil, P.; Stiborová, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
- Otake, Y.; Walle, T. Oxidation of the flavonoids galangin and kaempferide by human liver microsomes and CYP1A1, CYP1A2, and CYP2C9. Drug Metab. Dispos. 2002, 30, 103–105. [Google Scholar] [CrossRef]
- Lampe, J.W.; Chang, J.-L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Schär, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange juice–derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef]
- Felgines, C.; Fraisse, D.; Besson, C.; Vasson, M.-P.; Texier, O. Bioavailability of lemon verbena (Aloysia triphylla) polyphenols in rats: Impact of colonic inflammation. Br. J. Nutr. 2014, 111, 1773–1781. [Google Scholar] [CrossRef]
- Mukinda, J.T.; Syce, J.A.; Fisher, D.; Meyer, M. Effect of the plant matrix on the uptake of luteolin derivatives-containing Artemisia afra aqueous-extract in Caco-2 cells. J. Ethnopharmacol. 2010, 130, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and metabolism of luteolin in rats and humans in relation to in vitro anti-inflammatory effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef]
- Kure, A.; Nakagawa, K.; Kondo, M.; Kato, S.; Kimura, F.; Watanabe, A.; Shoji, N.; Hatanaka, S.; Tsushida, T.; Miyazawa, T. Metabolic fate of luteolin in rats: Its relationship to anti-inflammatory effect. J. Agric. Food Chem. 2016, 64, 4246–4254. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383. [Google Scholar] [CrossRef]
- Phenol-Explorer. Available online: https://phenol-explorer.eu/compounds/245 (accessed on 7 September 2023).
- Lugasi, A.; Hóvári, J. Flavonoid aglycones in foods of plant origin I. Vegetables. Acta Aliment. 2000, 29, 345–352. [Google Scholar] [CrossRef]
- Lugasi, A.; Takács, M. Flavonoid aglycons in foods of plant origin II. Fresh and dried fruits. Acta Aliment. 2002, 31, 63–71. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3.1. U.S. Department of Agriculture, Agricultural Re-search Service. Nutrient Data Laboratory. 2014. Available online: http://www.ars.usda.gov/nutrientdata/flav (accessed on 1 October 2023).
- Magalhães, S.C.; Taveira, M.; Cabrita, A.R.; Fonseca, A.J.; Valentão, P.; Andrade, P.B. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2016, 215, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Bramati, L.; Aquilano, F.; Pietta, P. Unfermented Rooibos Tea: Quantitative Characterization of Flavonoids by HPLC−UV and Determination of the Total Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 7472–7474. [Google Scholar] [CrossRef]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Ricoux, L.; Lamaison, J.L. The aromatic and polyphenolic composition of Roman chamomile tea. Fitoterapia 2004, 75, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Caristi, C.; Bellocco, E.; Gargiulli, C.; Toscano, G.; Leuzzi, U. Flavone-di-C-glycosides in citrus juices from Southern Italy. Food Chem. 2006, 95, 431–437. [Google Scholar] [CrossRef]
- Švehlíková, V.; Repčák, M. Apigenin chemotypes of Matricaria chamomilla L. Biochem. Syst. Ecol. 2006, 34, 654–657. [Google Scholar] [CrossRef]
- Gattuso, G.; Caristi, C.; Gargiulli, C.; Bellocco, E.; Toscano, G.; Leuzzi, U. Flavonoid glycosides in bergamot juice (Citrus bergamia Risso). J. Agric. Food Chem. 2006, 54, 3929–3935. [Google Scholar] [CrossRef]
- Lechtenberg, M.; Zumdick, S.; Gerhards, C.; Schmidt, T.J.; Hensel, A. Evaluation of analytical markers characterising different drying methods of parsley leaves (Petroselinum crispum L.). Pharmazie 2007, 62, 949–954. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. Comptes Rendus Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Dykes, L.; Peterson, G.C.; Rooney, W.L.; Rooney, L.W. Flavonoid composition of lemon-yellow sorghum genotypes. Food Chem. 2011, 128, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, G.Y.; Mares, D.J. Apigenin di-C-glycosides (ACG) content and composition in grains of bread wheat (Triticum aes-tivum) and related species. J. Cereal. Sci. 2012, 56, 260–267. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical profiles of black, red, brown, and white rice from the Ca-margue region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Singh, V.; Fang, Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 6, 21835. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Berrin, J.-G.; McLauchlan, W.R.; Needs, P.; Williamson, G.; Puigserver, A.; Kroon, P.A.; Juge, N. Functional expression of human liver cytosolic b-glucosidase in Pichia pastoris. Insights into its role in the metabolism of dietary glucosides. Eur. J. Biochem. 2002, 269, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Boersma, M.G.; van der Woude, H.; Bogaards, J.; Boeren, S.; Vervoort, J.; Cnubben, N.H.P.; van Iersel, M.L.P.S.; van Bladeren, P.J.; Rietjens, I.M.C.M. Regioselectivity of Phase II Metabolism of Luteolin and Quercetin by UDP-Glucuronosyl Transferases. Chem. Res. Toxicol. 2002, 15, 662–670. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Haller, D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-kB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 2006, 136, 664–671. [Google Scholar] [CrossRef]
- Kim, J.-A.; Kim, D.-K.; Kang, O.-H.; Choi, Y.-A.; Park, H.-J.; Choi, S.-C.; Kim, T.-H.; Yun, K.-J.; Nah, Y.-H.; Lee, Y.-M. Inhibitory effect of luteolin on TNF-α-induced IL-8 production in human colon epithelial cells. Int. Immunopharmacol. 2005, 5, 209–217. [Google Scholar] [CrossRef]
- Qiu, X.; Shen, C.; Zhao, W.; Zhang, X.; Zhao, D.; Wu, X.; Yang, L. A pan-cancer analysis of the oncogenic role of dual-specificity tyrosine (Y)-phosphorylation- regulated kinase 2 (DYRK2) in human tumors. Sci. Rep. 2022, 12, 15419. [Google Scholar] [CrossRef]
- Correa-Sáez, A.; Jiménez-Izquierdo, R.; Garrido-Rodríguez, M.; Morrugares, R.; Muñoz, E.; Calzado, M.A. Updating dual-specificity tyro-sine-phosphorylation-regulated kinase 2 (DYRK2): Molecular basis, functions and role in diseases. Cell. Mol. Life Sci. 2020, 77, 4747–4763. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.; de la Vega, L.; Banerjee, S. Emerging roles of DYRK2 in cancer. J. Biol. Chem. 2021, 296, 100233. [Google Scholar] [CrossRef] [PubMed]
- Boni, J.; Rubio-Perez, C.; López-Bigas, N.; Fillat, C.; De La Luna, S. The DYRK family of kinases in cancer: Molecular functions and therapeutic opportunities. Cancers 2020, 12, 2106. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, Y.; Li, M.; Ge, X. Dyrk2 mediated the release of proinflammatory cytokines in LPS-induced BV2 cells. Int. J. Biol. Macromol. 2018, 109, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]






| Quantum Parameter | 7-Lut, Cynaroside | Luteolin, Aglycone | 8-Lut, Orientin |
|---|---|---|---|
| EHOMO | −5.97 | −6.05 | −5.98 |
| ELUMO | −1.63 | −1.84 | −1.71 |
| ΔE | 4.34 | 4.21 | 4.27 |
| I | 5.97 | 6.05 | 5.98 |
| A | 1.63 | 1.84 | 1.71 |
| χ | 3.80 | 3.94 | 3.84 |
| η | 2.17 | 2.105 | 2135 |
| σ | 0.46 | 0.47 | 0.47 |
| μ | −3.80 | −3.94 | −3.84 |
| ω | 3.33 | 3.68 | 3.45 |
| Test Compounds | Atoms | Weight (Daltons) | Flexible Bonds | Lipinski Violations | Hydrogen Donors | Hydrogen Acceptors | logP |
|---|---|---|---|---|---|---|---|
| Curcumin | 49 | 370.40 | 9 | 0 | 2 | 6 | 3.00 |
| 7-Lut | 52 | 448.38 | 4 | 2 | 7 | 11 | 1.57 |
| 8-Lut | 52 | 448.38 | 3 | 2 | 8 | 11 | −0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, L.C.; Pintilie, L.; Albulescu, A.; Stefaniu, A.; Neagu, G. Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies. Int. J. Mol. Sci. 2023, 24, 16555. https://doi.org/10.3390/ijms242316555
Pirvu LC, Pintilie L, Albulescu A, Stefaniu A, Neagu G. Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies. International Journal of Molecular Sciences. 2023; 24(23):16555. https://doi.org/10.3390/ijms242316555
Chicago/Turabian StylePirvu, Lucia Camelia, Lucia Pintilie, Adrian Albulescu, Amalia Stefaniu, and Georgeta Neagu. 2023. "Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies" International Journal of Molecular Sciences 24, no. 23: 16555. https://doi.org/10.3390/ijms242316555
APA StylePirvu, L. C., Pintilie, L., Albulescu, A., Stefaniu, A., & Neagu, G. (2023). Anti-Proliferative Potential of Cynaroside and Orientin—In Silico (DYRK2) and In Vitro (U87 and Caco-2) Studies. International Journal of Molecular Sciences, 24(23), 16555. https://doi.org/10.3390/ijms242316555






