Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study
Abstract
:1. Introduction
2. Results
3. Discussion
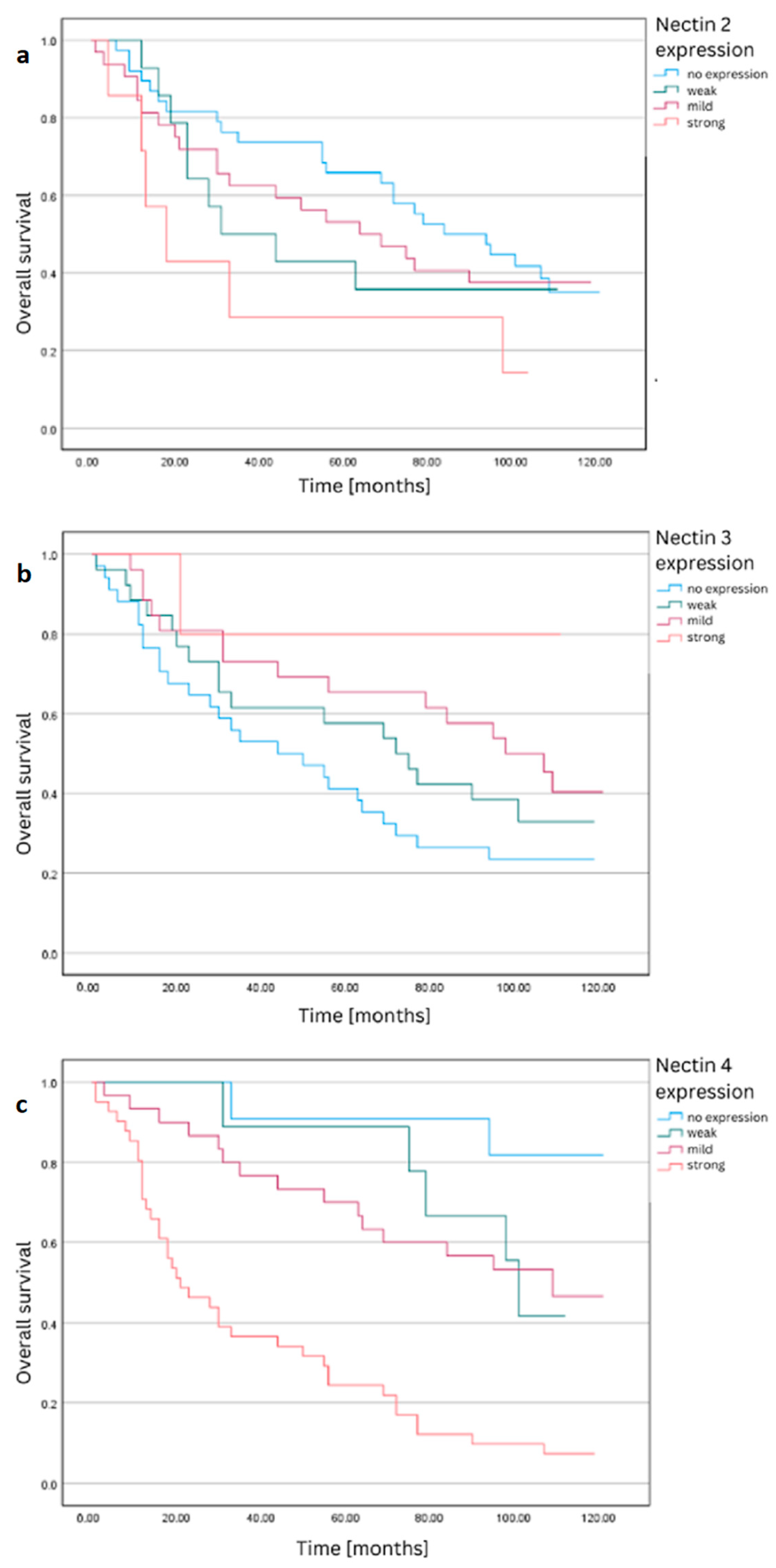
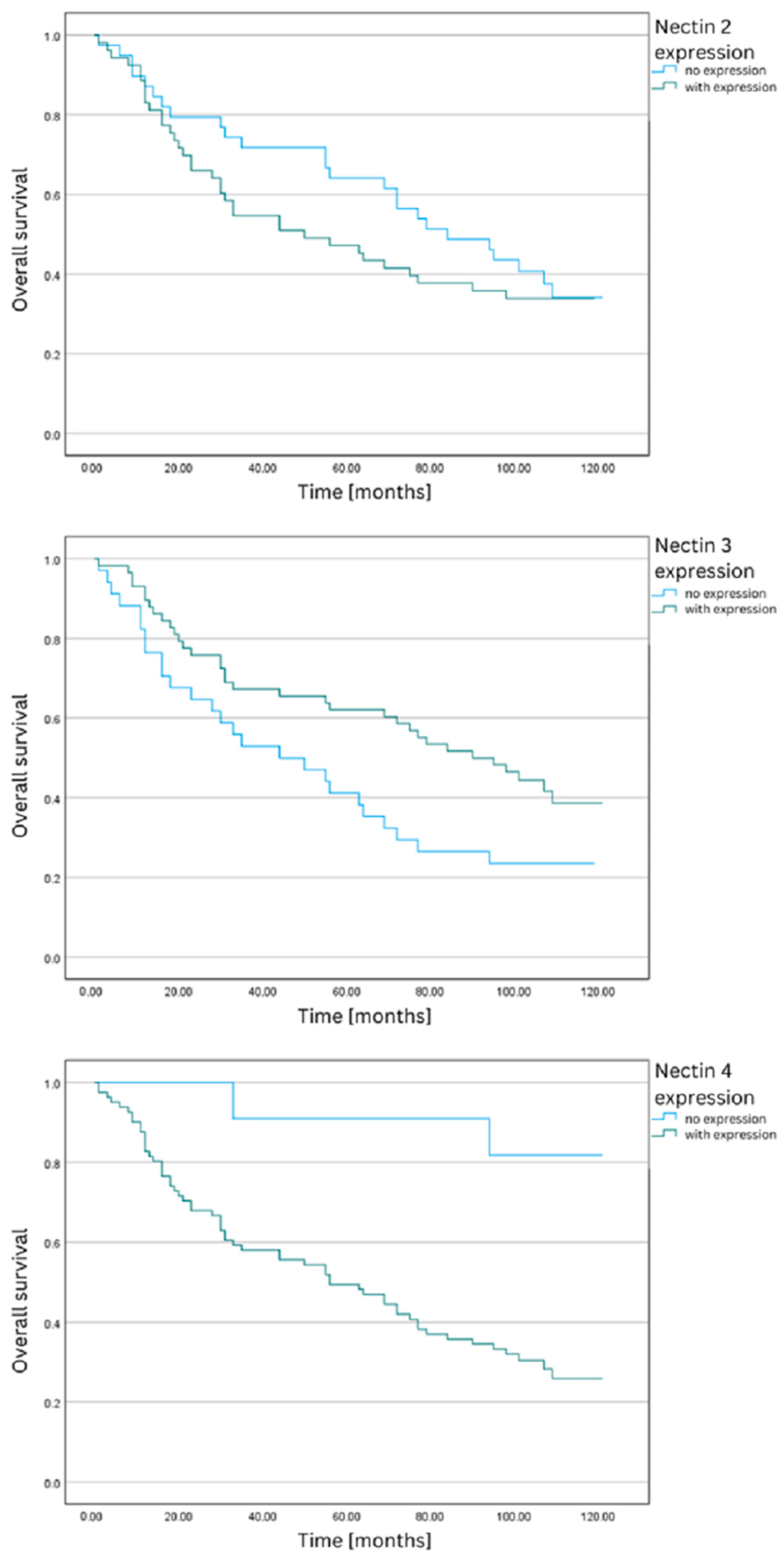
3.1. Nectin-2
3.2. Nectin-3
3.3. Nectin-4
4. Materials and Methods
4.1. Study Design
4.2. Patient Selection and Data Collection
4.3. IHC Analysis
4.4. Statistical Analysis
5. Conclusions
- A heterogeneous expression of nectins was observed in the tumor specimens, with nectin-4 showing the highest expression.
- A higher expression of nectin-4 was associated with poorer 5-year and overall survival rates, suggesting its potential as a negative prognostic marker.
- Conversely, the loss of nectin-3 expression was linked to a worse prognosis, highlighting its importance in CRC progression.
- Nectin-4’s expression showed a positive correlation with Carcinoembryonic Antigen (CEA) levels and advanced disease stages, while nectin-3’s expression was inversely related to these factors.
- The potential of nectins, especially nectin-4, as targets for therapeutic interventions is promising, given their significant expression in most CRC cells.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef]
- Sifaki-Pistolla, D.; Poimenaki, V.; Fotopoulou, I.; Saloustros, E.; Mavroudis, D.; Vamvakas, L.; Lionis, C. Significant Rise of Colorectal Cancer Incidence in Younger Adults and Strong Determinants: 30 Years Longitudinal Differences between under and over 50s. Cancers 2022, 14, 4799. [Google Scholar] [CrossRef]
- Del Vecchio Blanco, G.; Calabrese, E.; Biancone, L.; Monteleone, G.; Paoluzi, O.A. The Impact of COVID-19 Pandemic in the Colorectal Cancer Prevention. Int. J. Color. Dis. 2020, 35, 1951–1954. [Google Scholar] [CrossRef]
- Dulskas, A.; Gaizauskas, V.; Kildusiene, I.; Samalavicius, N.E.; Smailyte, G. Improvement of Survival over Time for Colorectal Cancer Patients: A Population-Based Study. J. Clin. Med. 2020, 9, 4038. [Google Scholar] [CrossRef]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, Micrornas and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef]
- Sideris, M.; Papagrigoriadis, S. Molecular Biomarkers and Classification Models in the Evaluation of the Prognosis of Colorectal Cancer. Anticancer Res. 2014, 34, 2061–2068. [Google Scholar]
- Seo, K.J.; Kim, M.; Kim, J. Prognostic Implications of Adhesion Molecule Expression in Colorectal Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 4148–4157. [Google Scholar]
- Dymicka-Piekarska, V.; Kemona, H. Does Colorectal Cancer Clinical Advancement Affect Adhesion Molecules (SP- Selectin, SE- Selectin and ICAM-1) Concentration? Thromb. Res. 2009, 124, 80–83. [Google Scholar] [CrossRef]
- Takai, Y.; Miyoshi, J.; Ikeda, W.; Ogita, H. Nectins and Nectin-like Molecules: Roles in Contact Inhibition of Cell Movement and Proliferation. Nat. Rev. Mol. Cell Biol. 2008, 9, 603–615. [Google Scholar] [CrossRef]
- Sakisaka, T.; Takai, Y. Biology and Pathology of Nectins and Nectin-like Molecules. Curr. Opin. Cell Biol. 2004, 16, 513–521. [Google Scholar] [CrossRef]
- Duraivelan, K.; Dash, S.; Samanta, D. An Evolutionarily Conserved Charged Residue Dictates the Specificity of Heterophilic Interactions among Nectins. Biochem. Biophys. Res. Commun. 2021, 534, 504–510. [Google Scholar] [CrossRef]
- Klekowski, J.; Zielińska, D.; Hofman, A.; Zajdel, N.; Gajdzis, P.; Chabowski, M. Clinical Significance of Nectins in HCC and Other Solid Malignant Tumors: Implications for Prognosis and New Treatment Opportunities—A Systematic Review. Cancers 2023, 15, 3983. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Fong, C.F.; Corman, M.L. History of Right Colectomy for Cancer. Ann. Laparosc. Endosc. Surg. 2019, 4, 49. [Google Scholar] [CrossRef]
- Malik, A. A History of Colorectal Surgery. EMJ 2020, 12–13. [Google Scholar]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. Consensus Molecular Subtypes Classification of Colorectal Cancer as a Predictive Factor for Chemotherapeutic Efficacy against Metastatic Colorectal Cancer. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef]
- Hoorn, S.T.; De Back, T.R.; Sommeijer, D.W.; Vermeulen, L. Clinical Value of Consensus Molecular Subtypes in Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2022, 114, 503–516. [Google Scholar] [CrossRef]
- Oshima, T.; Sato, S.; Kato, J.; Ito, Y.; Watanabe, T.; Tsuji, I.; Hori, A.; Kurokawa, T.; Kokubo, T. Nectin-2 Is a Potential Target for Antibody Therapy of Breast and Ovarian Cancers. Mol. Cancer 2013, 12, 60. [Google Scholar] [CrossRef]
- Liang, S.; Yang, Z.; Li, D.; Miao, X.; Yang, L.; Zou, Q.; Yuan, Y. The Clinical and Pathological Significance of Nectin-2 and DDX3 Expression in Pancreatic Ductal Adenocarcinomas. Dis. Markers 2015, 2015, 379568. [Google Scholar] [CrossRef]
- Bekes, I.; Löb, S.; Holzheu, I.; Janni, W.; Baumann, L.; Wöckel, A.; Wulff, C. Nectin-2 in Ovarian Cancer: How Is It Expressed and What Might Be Its Functional Role? Cancer Sci. 2019, 110, 1872–1882. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The Human Protein Atlas: A Spatial Map of the Human Proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Mandai, K.; Rikitake, Y.; Mori, M.; Takai, Y. Nectins and Nectin-Like Molecules in Development and Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 112. [Google Scholar]
- Miyake, M.; Miyamoto, T.; Shimizu, T.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Oda, Y.; Morizawa, Y.; Hori, S.; Gotoh, D.; et al. Tumor Expression of Nectin-1–4 and Its Clinical Implication in Muscle Invasive Bladder Cancer: An Intra-Patient Variability of Nectin-4 Expression. Pathol. Res. Pract. 2022, 237, 154072. [Google Scholar] [CrossRef]
- Miyoshi, J.; Takai, Y. Nectin and Nectin-like Molecules: Biology and Pathology. Am. J. Nephrol. 2007, 27, 590–604. [Google Scholar] [CrossRef]
- Martin, T.A.; Lane, J.; Harrison, G.M.; Jiang, W.G. The Expression of the Nectin Complex in Human Breast Cancer and the Role of Nectin-3 in the Control of Tight Junctions during Metastasis. PLoS ONE 2013, 8, e82696. [Google Scholar] [CrossRef]
- Duraivelan, K.; Samanta, D. Emerging Roles of the Nectin Family of Cell Adhesion Molecules in Tumour-Associated Pathways. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188589. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, T.; He, L.; Wang, H.; Ji, G.; Liu, X.; Zhang, Y.; Dong, G. Identification and Functional Analysis of Ligands for Natural Killer Cell Activating Receptors in Colon Carcinoma. Tohoku J. Exp. Med. 2012, 226, 59–68. [Google Scholar] [CrossRef]
- Karabulut, M.; Gunaldi, M.; Alis, H.; Afsar, C.U.; Karabulut, S.; Serilmez, M.; Akarsu, C.; Seyit, H.; Aykan, N.F. Serum Nectin-2 Levels Are Diagnostic and Prognostic in Patients with Colorectal Carcinoma. Clin. Transl. Oncol. 2016, 18, 160–171. [Google Scholar] [CrossRef]
- Erturk, K.; Karaman, S.; Dagoglu, N.; Serilmez, M.; Duranyildiz, D.; Tas, F. Serum Nectin-2 and Nectin-4 Are Diagnostic in Lung Cancer: Which Is Superior? Wien. Klin. Wochenschr. 2019, 131, 419–426. [Google Scholar] [CrossRef]
- Koomägi, R.; Volm, M. Expression of Fas (CD95/APO-1) and Fas Ligand in Lung Cancer, Its Prognostic and Predictive Relevance. Int. J. Cancer 1999, 84, 239–243. [Google Scholar] [CrossRef]
- Lim, D.R.; Kuk, J.K.; Kim, T.; Shin, E.J. Comparison of Oncological Outcomes of Right-Sided Colon Cancer versus Left-Sided Colon Cancer after Curative Resection. Medicine 2017, 96, e8241. [Google Scholar] [CrossRef]
- Yin, J.; Cohen, R.; Jin, Z.; Liu, H.; Pederson, L.; Adams, R.; Grothey, A.; Maughan, T.S.; Venook, A.; Van Cutsem, E.; et al. Prognostic and Predictive Impact of Primary Tumor Sidedness for Previously Untreated Advanced Colorectal Cancer. JNCI: J. Natl. Cancer Inst. 2021, 113, 1705–1713. [Google Scholar] [CrossRef]
- Mangone, L.; Pinto, C.; Mancuso, P.; Ottone, M.; Bisceglia, I.; Chiaranda, G.; Michiara, M.; Vicentini, M.; Carrozzi, G.; Ferretti, S.; et al. Colon Cancer Survival Differs from Right Side to Left Side and Lymph Node Harvest Number Matter. BMC Public. Health 2021, 21, 906. [Google Scholar] [CrossRef]
- Puccini, A.; Marshall, J.L.; Salem, M.E. Molecular Variances Between Right- and Left-Sided Colon Cancers. Curr. Color. Cancer Rep. 2018, 14, 152–158. [Google Scholar] [CrossRef]
- Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Nectins and Nectin-like Molecules in Colorectal Cancer: Role in Diagnostics, Prognostic Values, and Emerging Treatment Options: A Literature Review. Diagnostics 2022, 12, 3076. [Google Scholar] [CrossRef]
- Lee, J.; Warnken, U.; Schnölzer, M.; Gebert, J.; Kopitz, J. A New Method for Detection of Tumor Driver-Dependent Changes of Protein Sialylation in a Colon Cancer Cell Line Reveals Nectin-3 as TGFBR2 Target. Protein Sci. 2015, 24, 1686–1694. [Google Scholar] [CrossRef]
- LaFrance, M.E.; Farrow, M.A.; Chandrasekaran, R.; Sheng, J.; Rubin, D.H.; Lacy, D.B. Identification of an Epithelial Cell Receptor Responsible for Clostridium Difficile TcdB-Induced Cytotoxicity. Proc. Natl. Acad. Sci. USA 2015, 112, 7073–7078. [Google Scholar] [CrossRef]
- Maniwa, Y.; Nishio, W.; Okita, Y.; Yoshimura, M. Expression of Nectin-3: Novel Prognostic Marker of Lung Adenocarcinoma. Thorac. Cancer 2012, 3, 175–181. [Google Scholar] [CrossRef]
- Hirabayashi, K.; Tajiri, T.; Bosch, D.E.; Morimachi, M.; Miyaoka, M.; Inomoto, C.; Nakamura, N.; Yeh, M.M. Loss of Nectin-3 Expression as a Marker of Tumor Aggressiveness in Pancreatic Neuroendocrine Tumor. Pathol. Int. 2020, 70, 84–91. [Google Scholar] [CrossRef]
- Izumi, H.; Hirabayashi, K.; Nakamura, N.; Nakagohri, T. Nectin Expression in Pancreatic Adenocarcinoma: Nectin-3 Is Associated with a Poor Prognosis. Surg. Today 2015, 45, 487–494. [Google Scholar] [CrossRef]
- Tsai, P.L.; Su, W.J.; Leung, W.H.; Lai, C.T.; Liu, C.K. Neutrophil-Lymphocyte Ratio and CEA Level as Prognostic and Predictive Factors in Colorectal Cancer: A Systematic Review and Meta-Analysis. J. Cancer Res. Ther. 2016, 12, 582–589. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Alese, O.B.; Zhou, W.; Jiang, R.; Zakka, K.; Huang, Z.; Okoli, C.; Shaib, W.L.; Akce, M.; Diab, M.; Wu, C.; et al. Predictive and Prognostic Effects of Primary Tumor Size on Colorectal Cancer Survival. Front. Oncol. 2021, 11, 728076. [Google Scholar] [CrossRef]
- Dai, W.; Mo, S.; Xiang, W.; Han, L.; Li, Q.; Wang, R.; Xu, Y.; Cai, G. The Critical Role of Tumor Size in Predicting Prognosis for T1 Colon Cancer. Oncologist 2020, 25, 244–251. [Google Scholar] [CrossRef]
- Dai, W.; Li, Y.; Meng, X.; Cai, S.; Li, Q.; Cai, G. Does Tumor Size Have Its Prognostic Role in Colorectal Cancer? Re-Evaluating Its Value in Colorectal Adenocarcinoma with Different Macroscopic Growth Pattern. Int. J. Surg. 2017, 45, 105–112. [Google Scholar] [CrossRef]
- Li, J.; Guo, B.C.; Sun, L.R.; Wang, J.W.; Fu, X.H.; Zhang, S.Z.; Poston, G.; Ding, K.F. TNM Staging of Colorectal Cancer Should Be Reconsidered by T Stage Weighting. World J. Gastroenterol. 2014, 20, 5104–5112. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Heath, E.I.; Rosenberg, J.E. The Biology and Rationale of Targeting Nectin-4 in Urothelial Carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef]
- Sethy, C.; Goutam, K.; Nayak, D.; Pradhan, R.; Molla, S.; Chatterjee, S.; Rout, N.; Wyatt, M.D.; Narayan, S.; Kundu, C.N. Clinical Significance of a Pvrl 4 Encoded Gene Nectin-4 in Metastasis and Angiogenesis for Tumor Relapse. J. Cancer Res. Clin. Oncol. 2020, 146, 245–259. [Google Scholar] [CrossRef]
- Moretto, R.; Germani, M.M.; Giordano, M.; Conca, V.; Proietti, A.; Niccoli, C.; Pietrantonio, F.; Lonardi, S.; Tamburini, E.; Zaniboni, A.; et al. Trop-2 and Nectin-4 Immunohistochemical Expression in Metastatic Colorectal Cancer: Searching for the Right Population for Drugs’ Development. Br. J. Cancer 2023, 128, 1391–1399. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, K.; Wang, K.; Zhang, L.; Ma, W.; Qiu, Z.; Wang, W. Prognostic Value of Nectin-4 in Human Cancers: A Meta-Analysis. Front. Oncol. 2023, 13, 1081655. [Google Scholar] [CrossRef]
- Tarantino, P.; Carmagnani Pestana, R.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.; Curigliano, G. Antibody–Drug Conjugates: Smart Chemotherapy Delivery across Tumor Histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef]
- Hofmann, M.; Stoss, O.; Shi, D.; Büttner, R.; Van De Vijver, M.; Kim, W.; Ochiai, A.; Rüschoff, J.; Henkel, T. Assessment of a HER2 Scoring System for Gastric Cancer: Results from a Validation Study. Histopathology 2008, 52, 797–805. [Google Scholar] [CrossRef]
- Specht, E.; Kaemmerer, D.; Sänger, J.; Wirtz, R.M.; Schulz, S.; Lupp, A. Comparison of Immunoreactive Score, HER2/Neu Score and H Score for the Immunohistochemical Evaluation of Somatostatin Receptors in Bronchopulmonary Neuroendocrine Neoplasms. Histopathology 2015, 67, 368–377. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different Approaches for Interpretation and Reporting of Immunohistochemistry Analysis Results in the Bone Tissue—A Review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef]
| Nectin and Expression Method | Covariate(B) | HR (95% CI) | Wald Test | p-Value |
|---|---|---|---|---|
| Nectin-2 Expression According to IRS Classification. | 0.17 | 1.2 (0.9–1.6) | 1.5 | 0.22 |
| Nectin-3 expression according to alternative IRS Classification (0–1) vs. (2–12). | −0.61 | 0.54 (0.31–0.96) | 4.4 | 0.036 |
| Nectin-3 Expression According to IRS Classification. | −0.16 | 0.29 (0.3–0.76) | 2.1 | 0.28 |
| Nectin-4 Expression According to IRS Classification. | 1.4 | 4 (2.4–6.8) | 27 | <0.001 |
| 95% CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nectin | Expression | No. of Patients | No. of Deaths | 5-Year Survival | M | SE | LLCI | ULCI | χ2 |
| No expression | 39 | 14 | 64.10% | 47.44 | 3.25 | 41.07 | 53.80 | 5.98 | |
| Weak expression | 14 | 8 | 42.86% | 39.29 | 5.07 | 29.35 | 49.22 | ||
| 2 | Mild expression | 32 | 15 | 53.13% | 42.69 | 3.82 | 35.21 | 50.17 | |
| Strong expression | 7 | 5 | 28.57% | 28.71 | 8.06 | 12.93 | 44.50 | ||
| No expression | 34 | 20 | 41.18% | 38.35 | 3.82 | 30.86 | 45.85 | 5.17 | |
| Weak expression | 26 | 11 | 57.69% | 43.96 | 4.15 | 35.82 | 52.10 | ||
| 3 | Mild expression | 26 | 9 | 65.38% | 47.73 | 3.73 | 40.42 | 55.04 | |
| Strong expression | 5 | 1 | 80.00% | 52.20 | 6.98 | 38.53 | 65.87 | ||
| No expression | 11 | 1 | 90.91% | 57.55 | 2.34 | 52.96 | 62.13 | 31.90 *** | |
| Weak expression | 9 | 1 | 88.89% | 56.78 | 3.04 | 50.82 | 62.73 | ||
| 4 | Mild expression | 30 | 9 | 70.00% | 50.17 | 3.16 | 43.98 | 56.35 | |
| Strong expression | 41 | 31 | 24.39% | 30.68 | 3.37 | 24.08 | 37.29 | ||
| 95% CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nectin | Expression | No. of Patients | No. of Deaths | 5-Year Survival | M | SE | LLCI | ULCI | χ2 |
| 2 | No expression | 39 | 14 | 64.10% | 47.44 | 3.25 | 41.07 | 53.80 | 2.56 |
| With expression | 53 | 28 | 47.17% | 39.94 | 2.94 | 34.18 | 45.70 | ||
| 3 | No expression | 34 | 20 | 41.18% | 38.35 | 3.82 | 30.86 | 45.85 | 3.98 * |
| With expression | 58 | 22 | 62.07% | 45.91 | 2.64 | 40.74 | 51.09 | ||
| 4 | No expression | 11 | 1 | 90.91% | 57.55 | 2.34 | 52.96 | 62.13 | 5.56 * |
| With expression | 81 | 41 | 49.38% | 41.16 | 2.42 | 36.43 | 45.90 | ||
| Nectin-2 | Nectin-3 | Nectin-4 | ||
|---|---|---|---|---|
| CEA | ρ-value | 0.13 | −0.33 | 0.45 |
| p-value | 0.233 | 0.003 | <0.001 | |
| Tumor size | ρ-value | −0.08 | −0.30 | 0.09 |
| p-value | 0.478 | 0.003 | 0.386 | |
| Stage | ρ-value | 0.07 | −0.28 | 0.43 |
| p-value | 0.485 | 0.007 | <0.001 | |
| T | ρ-value | −0.02 | −0.09 | 0.20 |
| p-value | 0.867 | 0.382 | 0.053 |
| Nectin-2 Expression | χ2 | ||||
|---|---|---|---|---|---|
| No Expression | With Expression | ||||
| Tumor location | Left side | n | 32 | 29 | 7.51 ** |
| % | 34.8% | 31.5% | |||
| (2.7) | (−2.7) | ||||
| Right side | n | 7 | 24 | ||
| % | 7.6% | 26.1% | |||
| (−2.7) | (2.7) | ||||
| M | SD | Me | |
|---|---|---|---|
| Age | 67.93 | 10.21 | 66.00 |
| BMI | 25.48 | 4.45 | 24.59 |
| n | % | ||
| Gender | Female | 37 | 40.22 |
| Male | 55 | 59.78 | |
| CRC location | Left side | 61 | 66.30 |
| Right side | 31 | 33.70 | |
| Smoking | No | 56 | 60.87 |
| Yes | 36 | 39.13 | |
| Alcohol abuse | No | 86 | 93.48 |
| Yes | 6 | 6.52 | |
| Type of surgery | - Abdominoperineal resection of the rectum | 2 | 2.17 |
| - Anterior resection of the rectum | 43 | 46.74 | |
| - Left hemicolectomy | 11 | 11.96 | |
| - Right hemicolectomy | 29 | 31.52 | |
| - Sigmoidectomy | 4 | 4.35 | |
| - Transverse colectomy | 3 | 3.26 | |
| Laparoscopic procedure | No | 59 | 64.13 |
| Yes | 33 | 35.87 | |
| Stage according to AJCC 8th edition | 1 | 27 | 29.35 |
| 2 | 32 | 34.78 | |
| 3 | 22 | 23.91 | |
| 4 | 11 | 11.96 | |
| Positive family history of colorectal cancer | No | 86 | 93.48 |
| Yes | 6 | 6.52 |
| Percentage of Positive Cells | X | Intensity of Staining |
| 0—No visible cells with expression 1—<10% 2—10–50% 3—51–80% 4—>80% | 0—No visible reaction 1—Weak staining 2—Mild staining 3—Intense staining | |
| IRS Classification | ||
| 0–1 pts.—no expression 2–3 pts.—weak expression 4–8 pts.—mild expression 9–12 pts.—strong expression | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobecki, J.; Gajdzis, P.; Mazur, G.; Chabowski, M. Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study. Int. J. Mol. Sci. 2023, 24, 15900. https://doi.org/10.3390/ijms242115900
Kobecki J, Gajdzis P, Mazur G, Chabowski M. Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study. International Journal of Molecular Sciences. 2023; 24(21):15900. https://doi.org/10.3390/ijms242115900
Chicago/Turabian StyleKobecki, Jakub, Paweł Gajdzis, Grzegorz Mazur, and Mariusz Chabowski. 2023. "Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study" International Journal of Molecular Sciences 24, no. 21: 15900. https://doi.org/10.3390/ijms242115900
APA StyleKobecki, J., Gajdzis, P., Mazur, G., & Chabowski, M. (2023). Prognostic Potential of Nectin Expressions in Colorectal Cancer: An Exploratory Study. International Journal of Molecular Sciences, 24(21), 15900. https://doi.org/10.3390/ijms242115900








