Changes in the Level of DNA Methylation in Candida albicans under the Influence of Physical and Chemical Factors
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Testing of the Influence of Radiation on the Degree of Cytosine Methylation in DNA
4.1.1. Electromagnetic Radiation (EMR)
4.1.2. Microwave Radiation (MR)
4.1.3. Infrared Radiation (IR)
4.1.4. Light Amplification by Stimulated Emission of Radiation (LASER)
4.1.5. UVC Radiation
4.1.6. X-ray Radiation
4.2. Testing of the Influence of Temperature on the Degree of Cytosine Methylation in DNA
4.3. Testing of the Influence of Controlled Atmosphere on the Degree of Cytosine Methylation in DNA
4.4. Isolation of DNA from C. albicans ATCC 10231 Cells and DNA Methylation Determination
- A450S—average absorbance of the sample;
- A450B—average absorbance of the blank;
- A450MC—average absorbance of the methylated control.
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crisp, P.A.; Ganguly, D.; Eichten, S.R.; Borevitz, J.O.; Pogson, B.J. Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics. Sci. Adv. 2016, 2, e1501340. [Google Scholar] [CrossRef]
- Verhoeven, K.J.F.; vonHoldt, B.M.; Sork, V.L. Epigenetics in ecology and evolution: What we know and what we need to know. Mol. Ecol. 2016, 25, 1631–1638. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Epigenetics and transgenerational transfer: A physiological perspective. J. Exp. Biol. 2010, 213, 3–16. [Google Scholar] [CrossRef]
- Moen, E.L.; Mariani, C.J.; Zullow, H.; Jeff-Eke, M.; Litwin, E.; Nikitas, J.N.; Godley, L.A. New themes in the biological functions of 5-methylcytosine and 5-hydroxymethylcytosine. Immunol. Rev. 2015, 263, 36–49. [Google Scholar] [CrossRef]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. Biochim. Biophys. Acta 2007, 1775, 138–162. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- Hattman, S.; Kenny, C.; Berger, L.; Pratt, K. Comparative study of DNA methylation in three unicellular eucaryotes. J. Bacteriol. 1978, 135, 1156–1157. [Google Scholar] [CrossRef]
- Gryzinska, M.; Andraszek, K.; Jezewska-Witkowska, G. Estimation of Global Content of 5-methylcytosine in DNA during Allantoic and Pulmonary Respiration in the Chicken Embryo. Folia Biol. 2014, 62, 97–101. [Google Scholar] [CrossRef]
- Gryzinska, M.; Błaszczak, E.; Strachecka, A.; Jeżewska-Witkowska, G. Analysis of Age-Related Global DNA Methylation in Chicken. Biochem. Genet. 2013, 51, 554–563. [Google Scholar] [CrossRef]
- Gryzinska, M.; Jakubczak, A.; Listos, P.; Dudko, P.; Abramowicz, K.; Jeżewska-Witkowska, G. Association between body weight and age of dogs and global DNA methylation. Med. Weter 2016, 72, 64–67. [Google Scholar]
- Andraszek, K.; Gryzinska, M.; Wójcik, E.; Knaga, S.; Smalec, E. Age-dependent change in the morphology of nucleoli and methylation of genes of the nucleolar organizer region in the Japanese quail model Coturnix japonica (Temminck and Schlegel, 1849) (Galliformes: Aves). Folia Biol. 2014, 62, 293–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lan, H.; Sun, R.; Fan, K.; Yang, K.; Zhang, F.; Nie, X.Y.; Wang, X.; Zhuang, Z.; Wang, S. The Aspergillus flavus Histone Acetyltransferase Afl GcnE Regulates Morphogenesis, Aflatoxin Biosynthesis, and Pathogenicity. Front. Microbiol. 2016, 7, 1324. [Google Scholar] [CrossRef] [PubMed]
- Martienssen, R.A.; Colot, V. DNA methylation and epigenetic inheritance in plants and filamentous fungi. Science 2001, 293, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Weil, C.; Martienssen, R. Epigenetic interactions between transposons and genes: Lessons from plants. Curr. Opin. Genet. Dev. 2008, 18, 188–192. [Google Scholar] [CrossRef]
- Aparicio, J.G.; Viggiani, C.J.; Gibson, D.G.; Aparicio, O.M. The Rpd3-Sin3 histone deacetylase regulates replication timing and enables intra-S origin control in Saccharomyces cerevisiae. Mol. Cell. Biol. 2004, 24, 4769–4780. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Volpe, T.A. Regulation of Heterochromatic Silencing and Histone H3 Lysine-9 Methylation by RNAi. Science 2002, 297, 1833–1837. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Czechowicz, P.; Nowicka, J.; Gościniak, G. Virulence Factors of Candida spp. and Host Immune Response Important in the Pathogenesis of Vulvovaginal Candidiasis. Int. J. Mol. Sci. 2022, 23, 5895. [Google Scholar] [CrossRef]
- Huang, G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Hirakawa, M.P.; Martinez, D.A.; Sakthikumar, S.; Anderson, M.; Berlin, S.; Gujja, S.; Zeng, Q.; Zisson, E.; Wang, J.M.; Greenberg, J.M.; et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015, 3, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, X.D.; Wang, Y.; Yuan, B.F.; Feng, Y.Q. Widespread Existence of Cytosine Methylation in Yeast DNA Measured by Gas Chromatography/Mass Spectrometry. Anal. Chem. 2012, 84, 7249–7255. [Google Scholar] [CrossRef]
- Turchetti, B.; Marconi, G.; Sannino, C.; Buzzini, P.; Albertini, E. DNA Methylation Changes Induced by Cold in Psychrophilic and Psychrotolerant Naganishia Yeast Species. Microorganisms 2020, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.P.; Pandey, G.; Sharma, N.; Puranik, S.; Muthamilarasan, M.; Prasad, M. Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep. 2013, 32, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Colasanti, J.; Rothstein, S.J. The role of epigenetic processes in controlling flowering time in plants exposed to stress. J. Exp. Bot. 2011, 62, 3727–3735. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.L.; Alonso, C.; Becker, C.; Bossdorf, O.; Bucher, E.; Colomé-Tatché, M.; Durka, W.; Engelhardt, J.; Gaspar, B.; Gogol-Doring, A.; et al. Ecological plant epigenetics: Evidence from model and non-model species, and the way forward. Ecol. Lett. 2017, 20, 1576–1590. [Google Scholar] [CrossRef]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure Associated DNA Methylation in Human Populations. Annu. Rev. Public Health 2018, 39, 309–333. [Google Scholar] [CrossRef]
- Gryzińska, M.; Wlazło, Ł.; Nowakowicz-Dębek, B.; Jeżewska-Witkowska, G.; Jakubczak, A. DNA methylation in yeast-like fungi of the species Candida albicans induced by different lengths of exposure to ozone. Rus. J. Genet. 2019, 55, 396–398. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Irvine, R.F.; Schell, M.J. Back in the water: The return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2001, 2, 327–338. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Danielsson, A.; Barreau, K.; Kling, T.; Tisell, M.; Carén, H. Accumulation of DNA methylation alterations in paediatric glioma stem cells following fractionated dose irradiation. Clin. Epigenet. 2020, 12, 26. [Google Scholar] [CrossRef]
- Dabin, J.; Fortuny, A.; Polo, S.E. Epigenome maintenance in response to DNA damage. Mol. Cell. 2016, 62, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Tan, L.; Fu, X. The pivotal role of DNA methylation in the radio-sensitivity of tumor radiotherapy. Cancer Med. 2018, 7, 3812–3819. [Google Scholar] [CrossRef]
- Antwih, D.A.; Gabbara, K.M.; Lancaster, W.D.; Ruden, D.M.; Zielske, S.P. Radiation-induced epigenetic DNA methylation modification of radiation-response pathways. Epigenetics 2013, 8, 839–848. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Chie, E.K.; Young, P.D.; Kim, I.A.; Kim, I.H. DNMT (DNA methyltransferase) inhibitors radiosensitize human cancer cells by suppressing DNA repair activity. Radiat. Oncol. 2012, 7, 39. [Google Scholar] [CrossRef]
- Zamani, A.R.N.; Saberianpour, S.; Geranmayeh, M.H.; Bani, F.; Haghighi, L.; Rahbarghazi, R. Modulatory effect of photobiomodulation on stem cell epigenetic memory: A highlight on differentiation capacity. Lasers Med. Sci. 2020, 35, 299–306. [Google Scholar] [CrossRef]
- Gomes, M.V.d.M.; Manfredo, M.H.; Toffoli, L.V.; Castro-Alves, D.C.; Lucas Magnoni do Nascimento, L.; da Silva, W.R.; Kashimoto, R.K.; Rodrigues, G.M., Jr.; Estrada, V.B.; Andraus, R.A.; et al. Effects of the led therapy on the global DNA methylation and the expression of Dnmt1 and Dnmt3a genes in a rat model of skin wound healing. Lasers Med. Sci. 2016, 31, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Costello, J. DNA methylation: An epigenetic mark of cellular memory. Exp. Mol. Med. 2017, 49, e322. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.Y.; Murray, V. The influence of DNA methylation on the sequence specificity of UVB- and UVC-induced DNA damage. J. Photochem. Photobiol. B 2021, 221, 112225. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.F.P.; de Souza, B.F.; de Castro Coêlho, M. UV Radiation and Its Relation to DNA Methylation in Epidermal Cells: A Review. Epigenomes 2020, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, E.; Schinkel, C.C.F.; Klatt, S.; Hörandl, E. Effects of Temperature Treatments on Cytosine-Methylation Profiles of Diploid and Autotetraploid Plants of the Alpine Species Ranunculus kuepferi (Ranunculaceae). Front. Plant Sci. 2020, 11, 435. [Google Scholar] [CrossRef]
- Naydenov, M.; Baev, V.; Apostolova, E.; Gospodinova, N.; Sablok, G.; Gozmanova, M.; Yahubyan, G. High-temperature effect on genes engaged in DNA methylation and affected by DNA methylation in Arabidopsis. Plant Physiol. Biochem. 2015, 87, 102–108. [Google Scholar] [CrossRef]
- Lallias, D.; Bernard, M.; Ciobotaru, C.; Dechamp, N.; Labbé, L.; Goardon, L.; Le Calvez, J.M.; Bideau, M.; Fricot, A.; Prézelin, A.; et al. Sources of variation of DNA methylation in rainbow trout: Combined effects of temperature and genetic background. Epigenetics 2021, 16, 1031–1052. [Google Scholar] [CrossRef]
- Metzger, D.C.H.; Schulte, P.M. Persistent and plastic effects of temperature on DNA methylation across the genome of threespine stickleback (Gasterosteus aculeatus). Proc. Biol. Sci. 2017, 284, 20171667. [Google Scholar] [CrossRef]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell type switches and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef]
- Williams, S.; Cleary, I.; Thomas, D. Anaerobic conditions are a major influence on Candida albicans chlamydospore formation. Folia Microbiol. 2023, 68, 321–324. [Google Scholar] [CrossRef]
- Bartelli, T.F.; Bruno, D.C.F.; Briones, M.R.S. Evidence for Mitochondrial Genome Methylation in the Yeast Candida albicans: A Potential Novel Epigenetic Mechanism Affecting Adaptation and Pathogenicity? Front. Genet. 2018, 9, 166. [Google Scholar] [CrossRef] [PubMed]
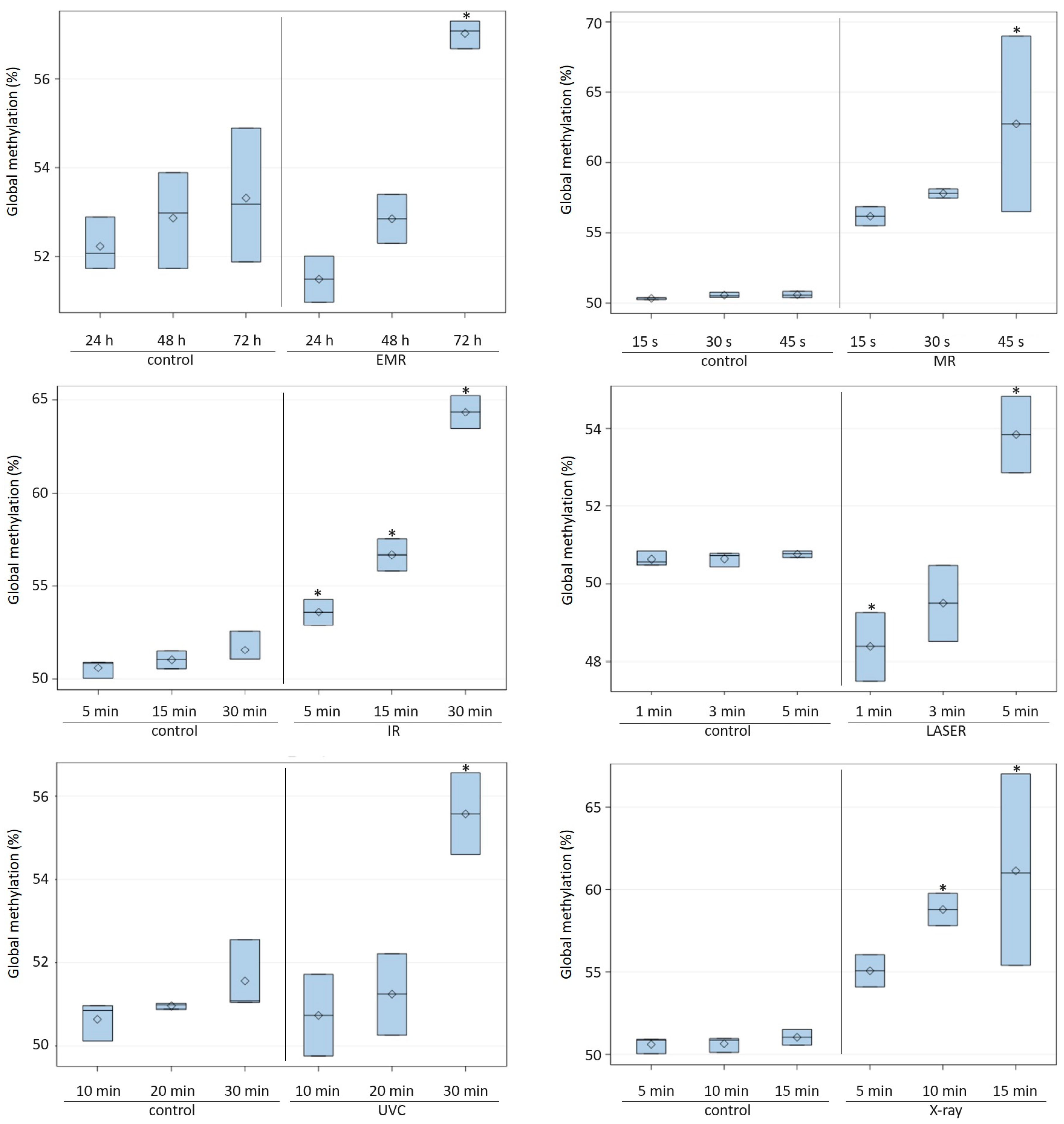

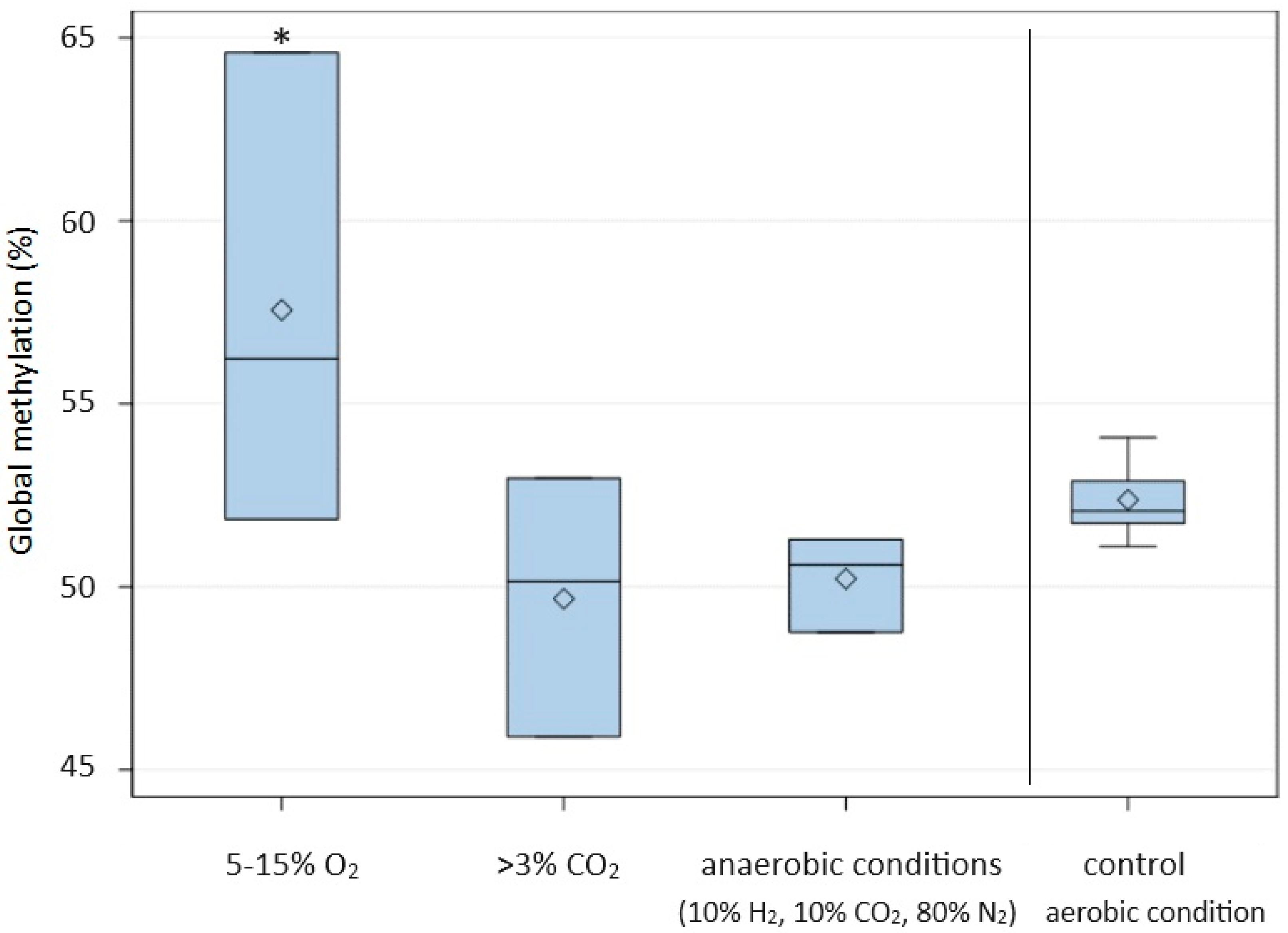
| Factor Type | Factor 1 | Exposure Time | Global DNA Methylation (%) | |
|---|---|---|---|---|
| Control Samples | Experimental Samples | |||
| Radiation | EMR | 24 h | 52.23 ± 0.60 | 51.49 b ± 0.52 |
| 48 h | 52.87 ± 1.08 | 52.85 b ± 0.55 | ||
| 72 h | 53.32 ± 1.51 | 57.08 a,* ± 0.31 | ||
| MR | 15 s | 50.34 ± 0.08 | 56.18 ± 0.68 | |
| 30 s | 50.57 ± 0.19 | 57.80 ± 0.33 | ||
| 45 s | 50.60 ± 0.23 | 62.75 * ± 6.24 | ||
| IR | 5 min | 50.60 ± 0.49 | 53.59 c,* ± 0.70 | |
| 15 min | 51.04 ± 0.48 | 56.68 b,* ± 0.88 | ||
| 30 min | 51.56 ± 0.87 | 64.36 a,* ± 0.88 | ||
| LASER | 1 min | 50.63 ± 0.18 | 48.39 b,* ± 0.88 | |
| 3 min | 50.65 ± 0.18 | 49.50 b ± 0.98 | ||
| 5 min | 50.76 ± 0.09 | 53.84 a,* ± 0.98 | ||
| UVC | 10 min | 50.65 ± 0.46 | 50.74 b ± 0.98 | |
| 20 min | 50.96 ± 0.07 | 51.24 b ± 0.98 | ||
| 30 min | 51.56 ± 0.87 | 55.57 a,* ± 0.98 | ||
| X-ray | 5 min | 50.60 ± 0.49 | 55.07 ± 0.98 | |
| 10 min | 50.65 ± 0.46 | 58.79 * ± 0.98 | ||
| 15 min | 51.04 ± 0.48 | 61.14 * ± 5.81 | ||
| Temperature | 37 °C | 90 min | 51.07 ± 0.46 | 54.95 b,* ± 0.98 |
| 180 min | 51.36 ± 0.64 | 56.68 b,* ± 0.98 | ||
| 360 min | 51.42 ± 1.24 | 61.51 a,* ± 1.53 | ||
| 42 °C | 90 min | 51.07 ± 0.46 | 57.67 c,* ± 0.98 | |
| 180 min | 51.36 ± 0.64 | 63.00 b,* ± 1.53 | ||
| 360 min | 51.42 ± 1.24 | 69.55 a,* ± 1.53 | ||
| Controlled atmosphere | 5–15% O2, 30 °C | 24 h | 52.23 ± 0.60 | 57.56 a,* ± 6.48 |
| >3% CO2, 30 °C | 24 h | 52.23 ± 0.60 | 49.68 b ± 3.55 | |
| (10% H2, 10% CO2, 80% N2) **, 30 °C | 24 h | 52.23 ± 0.60 | 50.23 b ± 1.31 | |
| Factor Type | Factor * | Exposure Time |
|---|---|---|
| Radiation | EMR | 24, 48, 72 h |
| MR | 15, 30, 45 s | |
| IR | 5, 15, 30 min | |
| LASER | 1, 3, 5 min | |
| UVC | 10, 20, 30 min | |
| X-ray | 5, 10, 15 min | |
| Temperature | 37 °C | 90, 180, 360 min |
| 42 °C | 90, 180, 360 min | |
| Controlled atmosphere | 5–15% O2, 30 °C | 24 h |
| >3% CO2, 30 °C | 24 h | |
| (10% H2, 10% CO2 and 80% N2) **, 30 °C | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryzinska, M.; Kot, B.; Dudzinska, E.; Biernasiuk, A.; Jakubczak, A.; Malm, A.; Andraszek, K. Changes in the Level of DNA Methylation in Candida albicans under the Influence of Physical and Chemical Factors. Int. J. Mol. Sci. 2023, 24, 15873. https://doi.org/10.3390/ijms242115873
Gryzinska M, Kot B, Dudzinska E, Biernasiuk A, Jakubczak A, Malm A, Andraszek K. Changes in the Level of DNA Methylation in Candida albicans under the Influence of Physical and Chemical Factors. International Journal of Molecular Sciences. 2023; 24(21):15873. https://doi.org/10.3390/ijms242115873
Chicago/Turabian StyleGryzinska, Magdalena, Barbara Kot, Ewa Dudzinska, Anna Biernasiuk, Andrzej Jakubczak, Anna Malm, and Katarzyna Andraszek. 2023. "Changes in the Level of DNA Methylation in Candida albicans under the Influence of Physical and Chemical Factors" International Journal of Molecular Sciences 24, no. 21: 15873. https://doi.org/10.3390/ijms242115873
APA StyleGryzinska, M., Kot, B., Dudzinska, E., Biernasiuk, A., Jakubczak, A., Malm, A., & Andraszek, K. (2023). Changes in the Level of DNA Methylation in Candida albicans under the Influence of Physical and Chemical Factors. International Journal of Molecular Sciences, 24(21), 15873. https://doi.org/10.3390/ijms242115873






