Phylogenetics and Population Genetics of the Petrolisthes lamarckii–P. haswelli Complex in China: Old Lineage and New Species
Abstract
1. Introduction
2. Results
2.1. Genetic Diversity
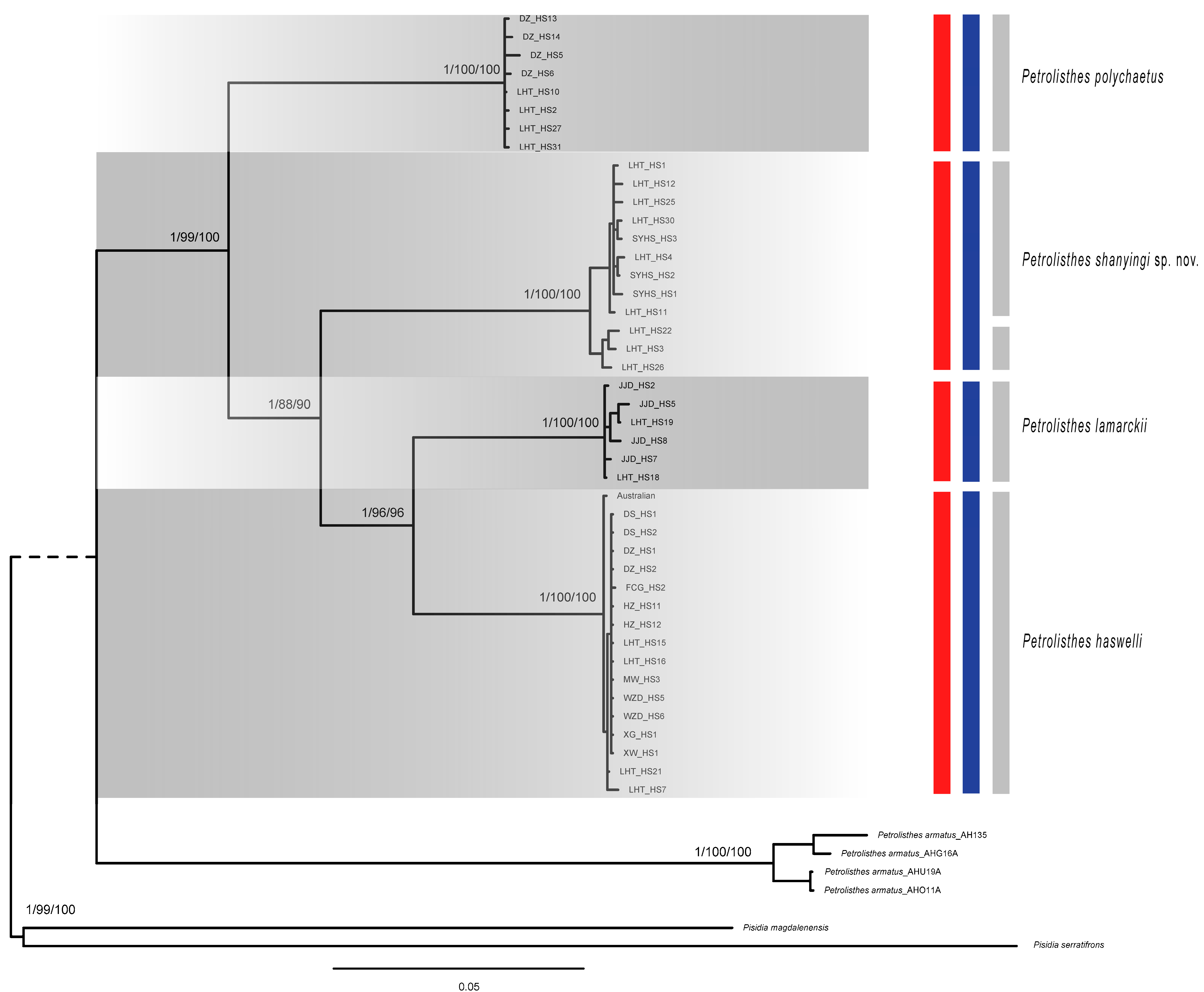
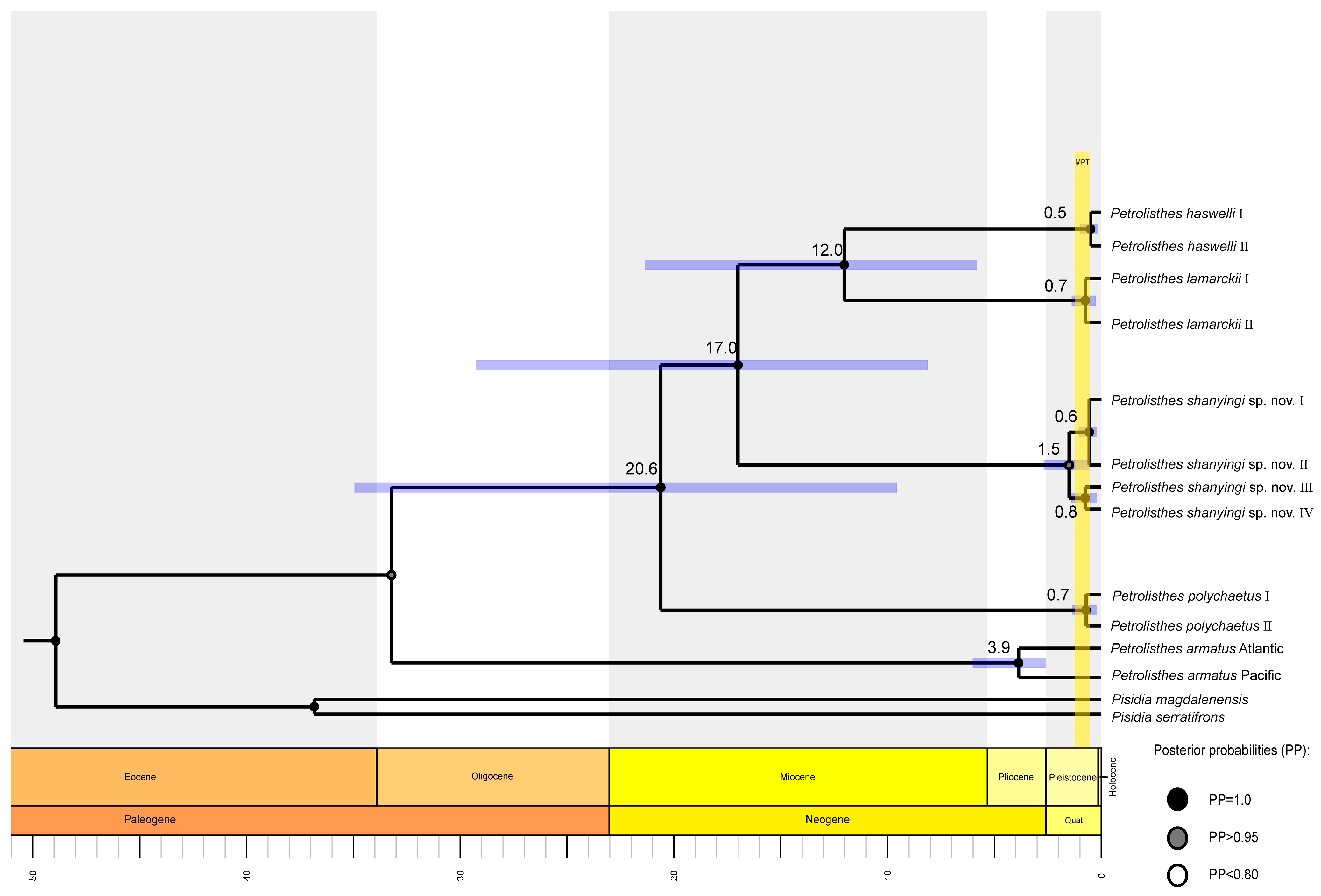
2.2. Phylogeny, Species Delimitation and Divergence Time Estimation
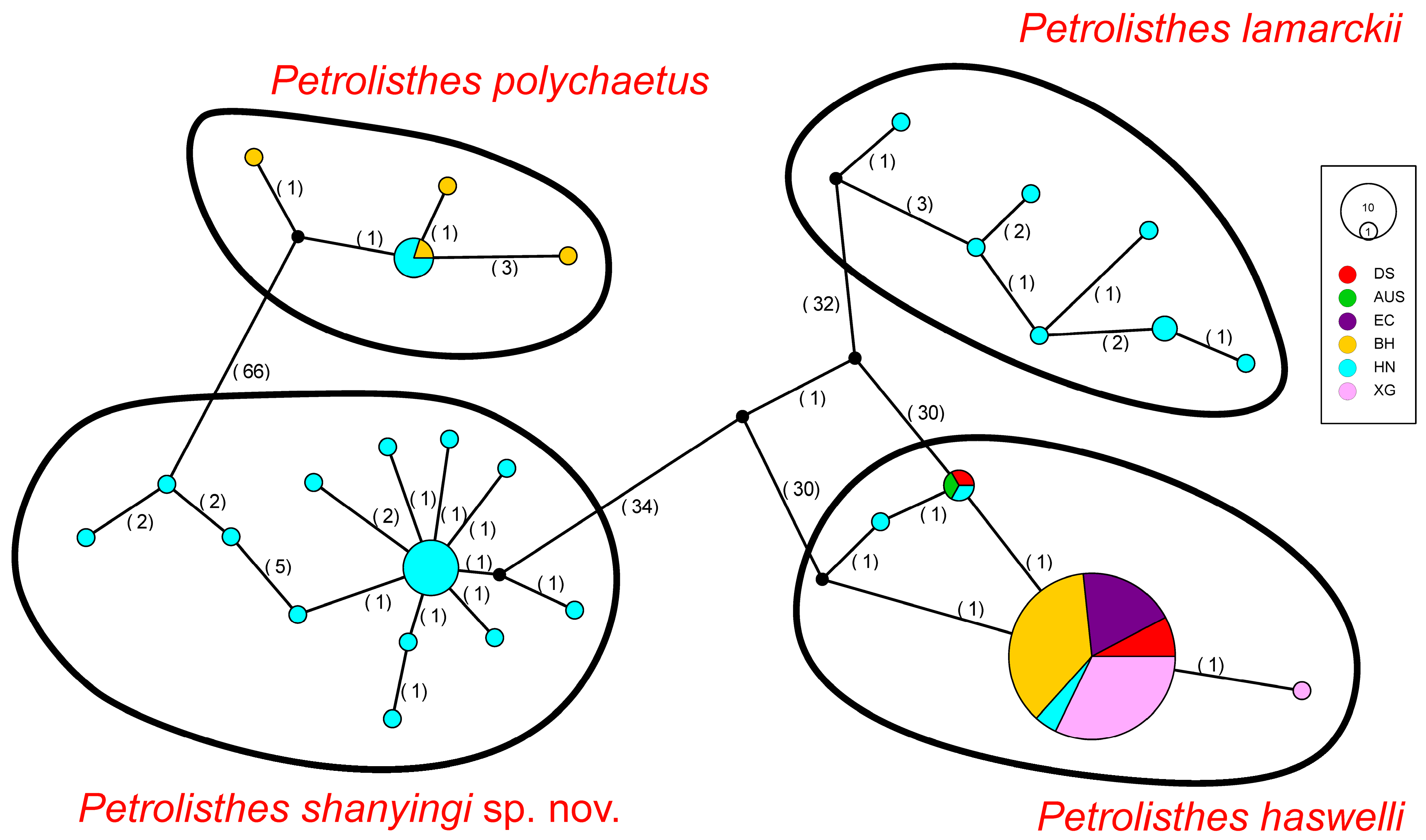
2.3. Geographical Distribution of Genetic Biodiversity
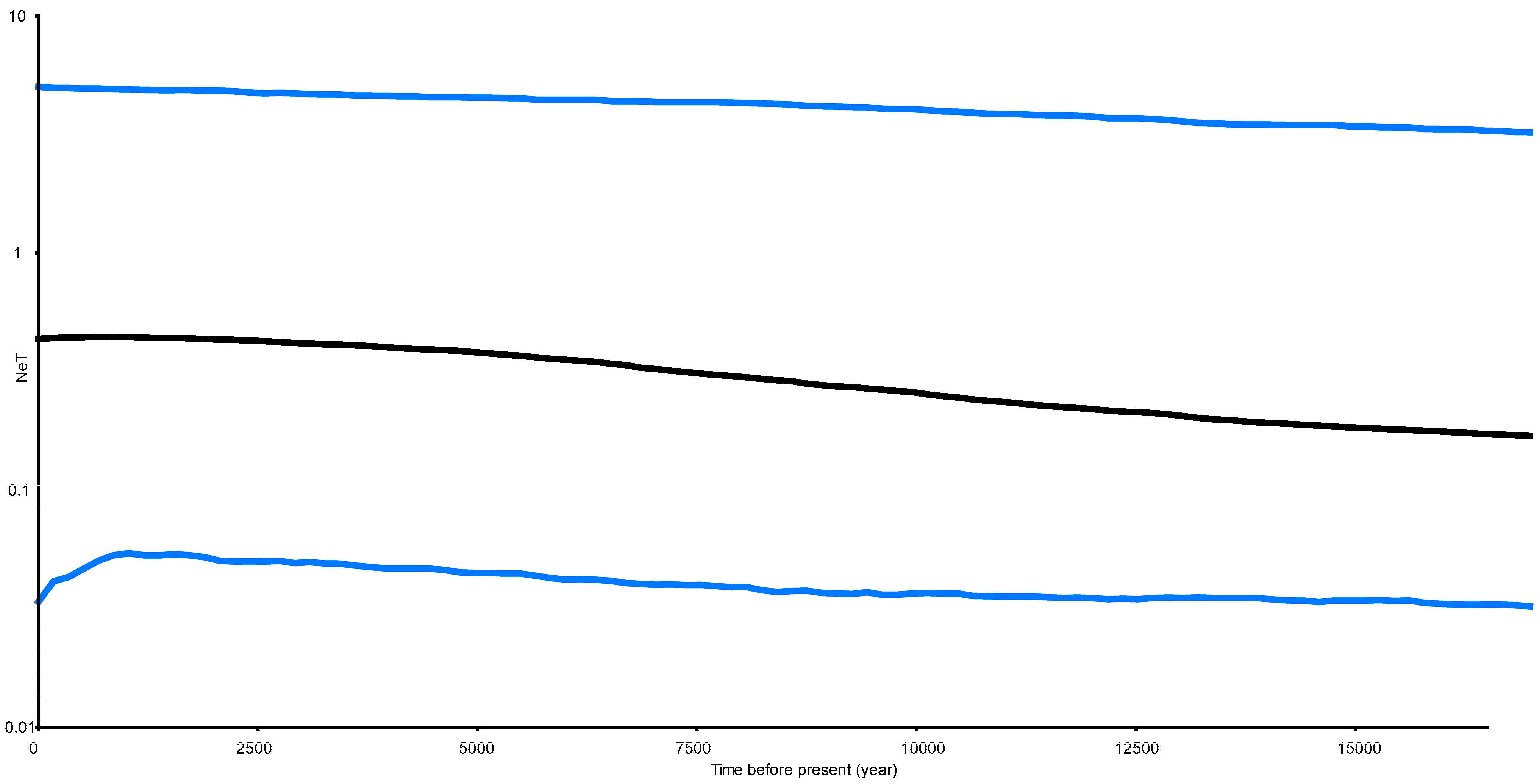
2.4. Demographic History and Gene Flow in P. haswelli
3. Systematics
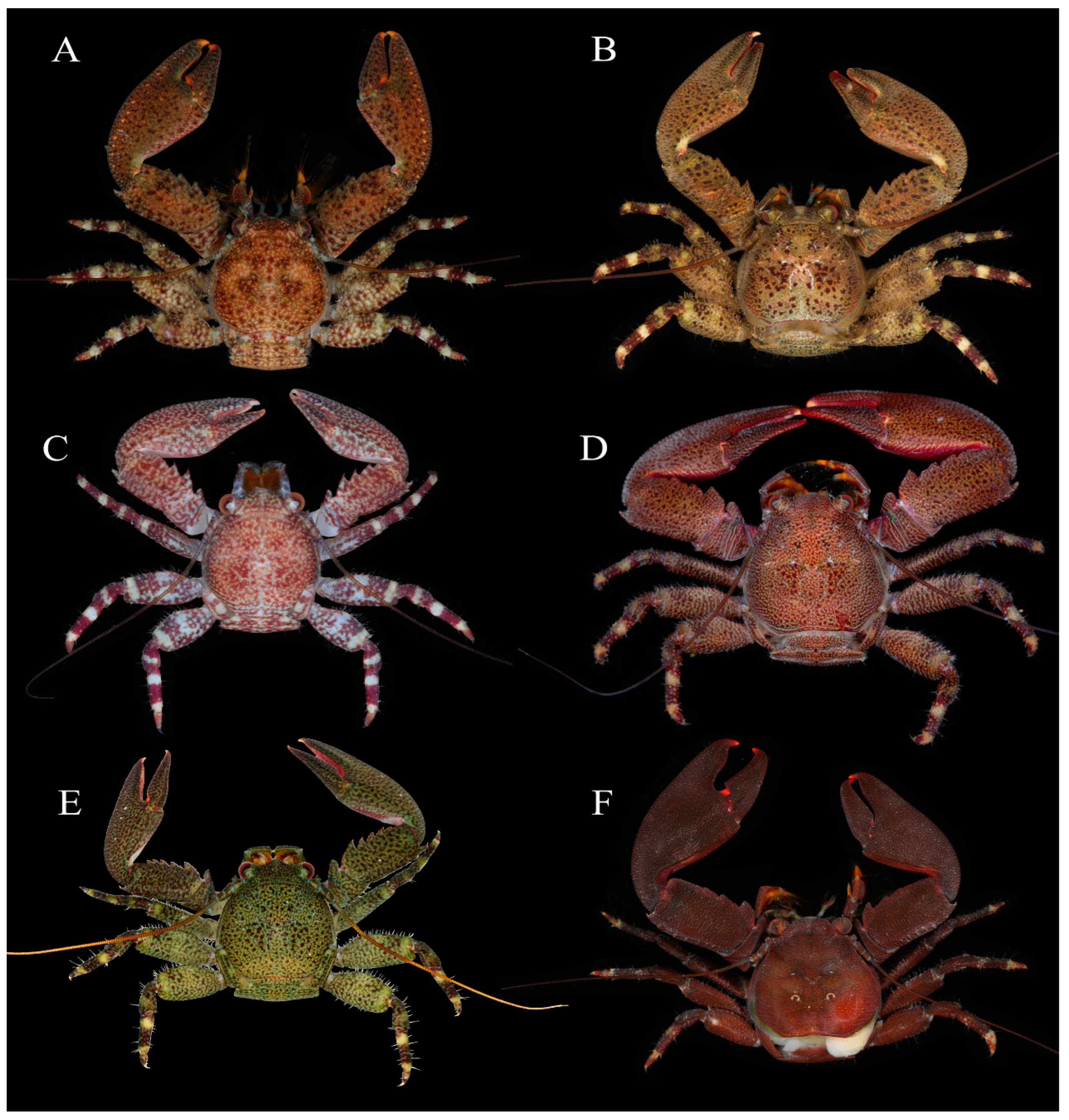
3.1. Petrolisthes lamarckii (Leach, 1821)
3.1.1. Synonymy
3.1.2. Material Examined
3.1.3. Diagnose
3.1.4. Distribution
3.1.5. Remarks
3.2. Petrolisthes shanyingi sp. nov.
3.2.1. Synonymy
3.2.2. Material Examined
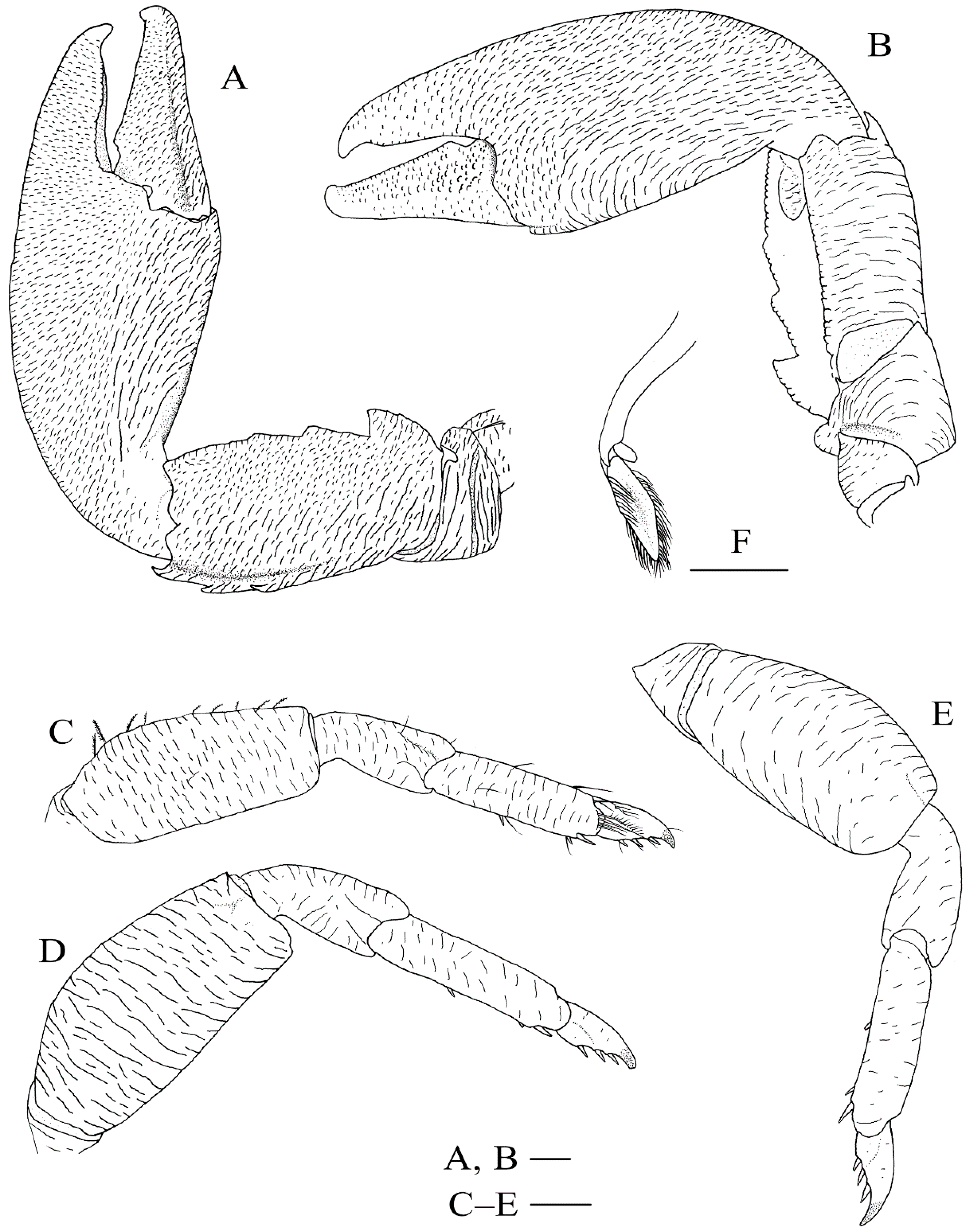
3.2.3. Description
3.2.4. Distribution
3.2.5. Etymology
3.2.6. Remarks
4. Discussion
4.1. Genetic Diversity and Population Structure
4.2. Phylogeny and Divergence Time Estimation
4.3. Demographic History and Gene Flow
5. Materials and Methods
5.1. Collections and Morphological Examination
5.2. DNA Extraction, Sequencing and Molecular Data
5.3. Population Genetics
5.4. Phylogenetic Analyses and Species Delimitation
5.5. Divergence Time Estimation
5.6. Demographic History and Gene Flow of Petrolisthes haswelli
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamaki, K.; Honza, E. Global tectonics and formation of marginal basins: Role of the western pacific. Episodes 1991, 14, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.F.; Li, Q. Genetic evidence for the existence of cryptic species in an endangered clam coelomactra antiquata. Mar. Biol. 2009, 156, 1507–1515. [Google Scholar] [CrossRef]
- Perez-Ponce De Leon, G.; Poulin, R. Taxonomic distribution of cryptic diversity among metazoans: Not so homogeneous after all. Biol. Lett. 2016, 12, 20160371. [Google Scholar] [CrossRef]
- Santamaria, C.; Koch, M. Cryptic genetic diversity in the coastal isopod alloniscus oahuensis from the pacific ocean. Zool. Stud. 2023, 62, e14. [Google Scholar]
- Schön, I.; Pinto, R.; Halse, S.; Smith, A.; Martens, K.; Birky, C. Cryptic species in putative ancient asexual darwinulids (crustacea, ostracoda). PLoS ONE 2012, 7, e39844. [Google Scholar] [CrossRef]
- Hickerson, M.J.; Carstens, B.C.; Cavender-Bares, J.; Crandall, K.A.; Graham, C.H.; Johnson, J.B.; Rissler, L.; Victoriano, P.F.; Yoder, A.D. Phylogeography’s past, present, and future: 10 years after avise, 2000. Mol. Phylogenetics Evol. 2010, 54, 291–301. [Google Scholar] [CrossRef]
- Mráz, P.; Ronikier, M. Biogeography of the carpathians: Evolutionary and spatial facets of biodiversity. Biol. J. Linn. Soc. 2016, 119, 528–559. [Google Scholar] [CrossRef]
- Hiller, A.; Lessios, H.A. Marine species formation along the rise of central america: The anomuran crab megalobrachium. Mol. Ecol. 2020, 29, 413–428. [Google Scholar] [CrossRef]
- He, L.J.; Zhang, A.B.; Zhu, C.D.; Liu, J.Y. Genetic imprints of paleo-oceanographic conditions in the chinese seas: Population bottlenecks of scylla paramamosain and periophthalmus modestus inferred from mitochondrial genes. J. Earth Sci. 2010, 21, 237–240. [Google Scholar] [CrossRef]
- Liu, J.-X.; Gao, T.; Yokogawa, K.; Zhang, Y.-P. Differential population structuring and demographic history of two closely related fish species, japanese sea bass (Lateolabrax japonicus) and spotted sea bass (Lateolabrax maculatus) in northwestern pacific. Mol. Phylogenetics Evol. 2006, 39, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, B.; Zheng, J.; Cheng, W.; Heqian, Z.; Wang, J.; Su, Y.; Xu, P.; Mao, Y. Fine-scale population genetic structure and parapatric cryptic species of kuruma shrimp (Marsupenaeus japonicus), along the northwestern pacific coast of China. Front. Genet. 2020, 11, 118. [Google Scholar] [CrossRef]
- Arranz Martinez, V.; Thakur, V.; Lavery, S. Demographic history, not larval dispersal potential, explains differences in population structure of two new zealand intertidal species. Mar. Biol. 2021, 168, 105. [Google Scholar] [CrossRef]
- Hiller, A.; Lessios, H.A. Phylogeography of petrolisthes armatus, an invasive species with low dispersal ability. Sci. Rep. 2017, 7, 3359. [Google Scholar] [CrossRef]
- Dawson, M.N.; Hamner, W.M. A biophysical perspective on dispersal and the geography of evolution in marine and terrestrial systems. J. R. Soc. Interface 2008, 5, 135–150. [Google Scholar] [CrossRef]
- Bowen, B.W.; Rocha, L.A.; Toonen, R.J.; Karl, S.A.; ToBo, L. The origins of tropical marine biodiversity. Trends Ecol. Evol. 2013, 28, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kong, L.; Yu, H. Cryptic diversity of marine gastropod monodonta labio (trochidae): Did the early pleistocene glacial isolation and sea surface temperature gradient jointly drive diversification of sister species and/or subspecies in the northwestern pacific? Mar. Ecol. 2017, 38, e12443. [Google Scholar] [CrossRef]
- Brown, C.J.; Harborne, A.R.; Paris, C.B.; Mumby, P.J. Uniting paradigms of connectivity in marine ecology. Ecology 2016, 97, 2447–2457. [Google Scholar] [CrossRef]
- Ni, G.; Kong, L.; Yu, H. Comparative phylogeography in marginal seas of the northwestern pacific. Mol. Ecol. 2014, 23, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Response of western pacific marginal seas to glacial cycles: Paleoceanographic and sedimentological features. Mar. Geol. 1999, 156, 5–39. [Google Scholar] [CrossRef]
- Dasen, C.; Bo, C.; Jinhui, Y.A.N.; Huifen, X.U. The seasonal variation characteristics of residual currents in the qiongzhou strait. Trans. Oceanol. Limnol. 2006, 2, 12–17. [Google Scholar]
- Hu, J.; Kawamura, H.; Li, C.; Hong, H.; Jiang, Y. Review on current and seawater volume transport through the taiwan strait. J. Oceanogr. 2010, 66, 591–610. [Google Scholar] [CrossRef]
- Hiller, A.; Werding, B. A new species of petrolisthes (crustacea, anomura, porcellanidae) inhabiting vermetid formations mollusca, gastropoda, vermetidae) in the southern caribbean sea. Zookeys 2019, 876, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M. Porcellanidae (crustacea: Decapoda: Anomura) from new caledonia and the loyalty islands. Zootaxa 2007, 1548, 1. [Google Scholar] [CrossRef]
- Werding, B.; Hiller, A. The porcellanidae (crustacea: Decapoda: Anomura) of the red sea with description of a new species of petrolisthes. Zootaxa 2007, 1460, 1–24. [Google Scholar] [CrossRef]
- Osawa, M.; Chan, T.Y. Porcellanidae (Porcelain crabs). In Crustacean Fauna of Taiwan: Crab-like Anomurans (Hippoidea, Lithodea, Porcellanidae); Chan, T.-Y., Ed.; National Taiwan Ocean University: Keelung, Taiwan, 2010; pp. 67–181. [Google Scholar]
- Borradaile, L.A. On some crustaceans from the South Pacific.-Part II. Macrura Anomala. In Proceedings of the General Meetings for Scientific Business of the Zoological Society of London; Blackwell Publishing Ltd.: Oxford, UK, 1898; Volume 31, pp. 457–468. [Google Scholar]
- Kropp, R.K. Three new species of Porcellanidae (Crustacea: Anomura) from the Mariana Islands and a discussion of Borradaile’s Petrolisthes lamarckii complex. Micronesica 1984, 19, 91–106. [Google Scholar]
- Haig, J. Hong Kong’s porcellanid crabs. In Proceedings of the Fourth International Marine Biological Workshop; Morton, B., Ed.; The Marine Flora and Fauna of Hong Kong and Southern China III; Hong Kong University Press: Hong Kong, China, 1992; pp. 303–327. [Google Scholar]
- Petrick, B.; Martínez-García, A.; Auer, G.; Reuning, L.; Auderset, A.; Deik, H.; Takayanagi, H.; De Vleeschouwer, D.; Iryu, Y.; Haug, G. Glacial indonesian throughflow weakening across the mid-pleistocene climatic transition. Sci. Rep. 2019, 9, 16995. [Google Scholar] [CrossRef]
- Haworth, A.H. A new binary arrangement of the brachyurous crustacea. Philos. Mag. 1825, 65, 105–106. [Google Scholar] [CrossRef]
- Stimpson, W. Prodromus descriptionis animalium evertebratorum, quae in expeditione ad oceanum pacificum septentrionalem, a republica federata missa, Cadwaladaro Ringgold et Johanne Rodgers ducibus, observavit et descripsit. Pars VII. Crustac. Anomura. Proc. Acad. Nat. Sci. Phila. 1858, 10, 225–252. [Google Scholar]
- Leach, W.E. Galatéadées. Dictionnaire des Sciences Naturelles; Levrault, F.G., Ed.; Strasbourg and Le Normant: Paris, France, 1821; Volume 18, pp. 49–56. [Google Scholar]
- Milne, E.H. Histoire Naturelle des Crustacés, Comprenant L’anatomie, la Physiologie et la Classification de ces Animaux; Librairie Encylopédique de Roret: Paris, France, 1837; Volume 2, 532p. [Google Scholar]
- White, A. List of the specimens of crustacea in the collection of the british museum. Br. Mus. Lond. 1847, i–viii, 1–141. [Google Scholar]
- Dana, J.D. Crustacea, Part I. In United States Exploring Expedition. During the Years 1838, 1839, 1840, 1841, 1842. under the Command of Charles Wilkes; U.S.N.C. Sherman: Phildelphia, PA, USA, 1852; Volume 13, pp. i–viii, +1–685. [Google Scholar]
- Dana, J.D. Crustacea, Atlas. In United States Exploring Expedition during the Years 1838, 1839, 1840, 1841, 1842 under the Command of Charles Wilkes; U.S.N.C. Sherman: Phildelphia, PA, USA, 1855; Volume 13, pp. 1–96. [Google Scholar]
- Heller, C. Reise der österreichischen fregatte novara um die erde in den jahren 1857, 58unt, 59 er den befehlen des commodors b. Von wüllerstorf-urbair. Zool. Theil Zveiter Band Dritte Abtheilung Crustac. Viena 1865, 4, 1–280. [Google Scholar]
- Miyake, S. Studies on the decapod crustaceans of Micronesia. III. Porcellanidae. Palau Trop. Biol. Stn. Stud. 1942, 2, 329–379. [Google Scholar]
- Miyake, S. Studies on the crab-shaped Anomura of Nippon and adjacent waters. J. Dep. Agric. Kyushu Imp. Univ. 1943, 7, 49–158. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, B.F.; Chan, T.Y.; Yu, H.P. On the porcellanid crabs (Crustacea: Decapoda: Porcellanidae) of Taiwan. Ann. Taiwan Mus. 1997, 40, 275–359. [Google Scholar]
- Beleem, I.; Poriya, P.; Gohil, B. Porcelain crabs (crustacea: Decapoda: Anomura) of western coast of india. Mar. Biodivers. Rec. 2016, 9, 1–7. [Google Scholar] [CrossRef][Green Version]
- Yang, S. Preliminary report on the Porcellanidae (Crustacea, Anomura) of Xisha Islands; Memoirs of Beijing Natural History Museum: Guangdong, China, 1983; Volume 24, pp. 1–9. [Google Scholar]
- Yang, S.; Sun, X. The Porcellanidae (Crustacea: Anomura) of Hainan Island, China with description of a new species. Nat. Sci. Mus. 2005, 1, 1–30. [Google Scholar]
- Prakash, S.; Ajith-Kumar, T.; Gopi, M.; Balasubramanian, T. First records of four species of Petrolisthes (Decapoda: Anomura: Porcellanidae) in Lakshadweep, India. Mar. Biodivers. Rec. 2013, 6, E47. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Coykendall, D.K.; Nizinski, M.S.; Morrison, C.L. A phylogenetic perspective on diversity of galatheoidea (munida, munidopsis) from cold-water coral and cold seep communities in the western north atlantic ocean. Deep Sea Res. Part Ⅱ Top. Stud. Oceanogr. 2017, 137, 258–272. [Google Scholar] [CrossRef]
- Hague, M.; Routman, E. Does population size affect genetic diversity? A test with sympatric lizard species. Heredity 2015, 116, 92–98. [Google Scholar] [CrossRef]
- Bertacini Moraes, J.C.; Terossi, M.; Buranelli, R.C.; Tavares, M.; Mantelatto, F.L.; De Siqueira Bueno, S.L. Morphological and molecular data reveal the cryptic diversity among populations of Aegla paulensis (Decapoda, Anomura, Aeglidae), with descriptions of four new species and comments on dispersal routes and conservation status. Zootaxa 2016, 4193, 1–48. [Google Scholar]
- Crivellaro, M.; Zimmermann, B.; Bartholomei-Santos, M.; Crandall, K.; Pérez-Losada, M.; Bond-Buckup, G.; Santos, S. Looks can be deceiving: Species delimitation reveals hidden diversity in the freshwater crab aegla longirostri (decapoda: Anomura). Zool. J. Linn. Soc. 2018, 182, 24–37. [Google Scholar] [CrossRef]
- Gore, R. Petrolisthes armatus: A redescription of larval development under laboratory conditions (decapoda, porcellanidae). Crustaceana 1970, 18, 75–89. [Google Scholar] [CrossRef]
- Rodríguez Flores, P.; Machordom, A.; Abello, P.; Cuesta, J.; Macpherson, E. Species delimitation and multi-locus species tree solve an old taxonomic problem for european squat lobsters of the genus munida leach, 1820. Mar. Biodivers. 2019, 49, 1751–1773. [Google Scholar] [CrossRef]
- Rodríguez Flores, P.; Seid, C.; Rouse, G.; Giribet, G. Cosmopolitan abyssal lineages? A systematic study of east pacific deep-sea squat lobsters (decapoda: Galatheoidea: Munidopsidae). Invertebr. Syst. 2023, 37, 14–60. [Google Scholar] [CrossRef]
- Meyer, C. Molecular systematics of cowries (gastropoda: Cypraeidae) and diversification patterns in the tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef]
- Renema, W.; Bellwood, D.; Braga, J.; Bromfield, K.; Hall, R.; Johnson, K.; Lunt, P.; Meyer, C.; McMonagle, L.; Morley, R.; et al. Hopping hotspots: Global shifts in marine biodiversity. Science 2008, 321, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.C. Coincident biogeographic patterns: Indo-west pacific ocean. Evolution 1999, 53, 326–335. [Google Scholar] [CrossRef]
- Rodríguez Flores, P.; Buckley, D.; Macpherson, E.; Corbari, L.; Machordom, A. Deep-sea squat lobster biogeography (munidopsidae: Leiogalathea) unveils tethyan vicariance and evolutionary patterns shared by shallow-water relatives. Zool. Scr. 2020, 49, 340–356. [Google Scholar] [CrossRef]
- Bermingham, E.; Martin, A.P. Comparative mtdna phylogeography of neotropical freshwater fishes: Testing shared history to infer the evolutionary landscape of lower central america. Mol. Ecol. 1998, 7, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Hays, J.D.; Imbrie, J.; Shackleton, N.J. Variations in the Earth’s Orbit: Pacemaker of the Ice Ages. Science 1976, 194, 1121–1132. [Google Scholar] [CrossRef]
- Cabezas, P.; Sanmartin, I.; Paulay, G.; Macpherson, E.; Machordom, A. Deep under the sea: Unraveling the evolutionary history of the deep-sea squat lobster paramunida (decapoda, munididae). Evol. Int. J. Org. Evol. 2012, 66, 1878–1896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Kong, X.Y.; Yu, Z.N.; Kong, J.; Ma, S.; Chen, L.M. Genetic diversity and historical demography of Chinese shrimp Feneropenaeus chinensis in Yellow Sea and Bohai Sea based on mitochondrial DNA analysis. Afr. J. Biotechnol. 2009, 8, 1193–1202. [Google Scholar]
- Ma, P.; Liu, P.; Li, J.; Li, J.; Chen, P. The genetic diversity and phylogenetic analysis of coi gene in mitochondrial DNA of three populations of exopalaemon carinicauda. Prog. Fish. Sci. 2011, 32, 50–56. [Google Scholar]
- Hewitt, G.M. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol. J. Linn. Soc. 1996, 58, 247–276. [Google Scholar] [CrossRef]
- Hewitt, G.M. The genetic legacy of the quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Millar, C.I.; Libby, W.J. Strategies for conserving clinal, ecotypic, and disjunct population diversity in widespread species. In Conference on the Genetics and Conservation of Rare Plants; Oxford University Press: Oxford, UK, 1989; pp. 149–170. [Google Scholar]
- Whitlock, M.; McCauley, D. Indirect measures of gene flow and migration: Fst≠1/(4 nm + 1). Heredity 1999, 82, 117–125. [Google Scholar] [CrossRef]
- Ichikawa, H.; Beardsley, R.C. The current system in the yellow and east china seas. J. Oceanogr. 2002, 58, 77–92. [Google Scholar] [CrossRef]
- Guan, B.X.; Fang, G.H. Winter counter-wind currents off the southeastern china coast: A review. J. Oceanogr. 2006, 62, 1–24. [Google Scholar] [CrossRef]
- Hu, J.; Kawamura, H.; Hong, H.; Qi, Y. A review on the currents in the south china sea: Seasonal circulation, south china sea warm current and kuroshio intrusion. J. Oceanogr. 2000, 56, 607–624. [Google Scholar] [CrossRef]
- Tsang, L.M.; Chan, B.; Ma, K.; Chu, K.H. Genetic differentiation, hybridization and adaptive divergence in two subspecies of the acorn barnacle, tetraclita japonica, in nw pacific. Mol. Ecol. 2008, 17, 4151–4163. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Crenshaw, H. Plankton community dynamics of the central great barrier reef lagoon: Analysis of data from ikeda et al. Mar. Biol. 1984, 82, 167–180. [Google Scholar] [CrossRef]
- Wu, F.-X.; Gu, Y.-G.; Liu, Q.-X.; Zhang, S.-F.; Rao, Y.-Y.; Liu, H.-X.; Dai, M.; Wang, Y.-G.; Huang, H.-H. Research on the seasonal variation of zooplankton community in daya bay, south china sea. Front. Mar. Sci. 2023, 10, 1110160. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, C.; Chen, Z.; Ye, L.; Chen, W.; Wang, W.; Sun, X. Structural characteristics of zooplankton populations and their annual changes in zhanjiang bay. Mar. Sci. 2015, 39, 46–55. [Google Scholar]
- Zhou, F.L.; Jiang, S.G.; Jiang, Y.J.; Huang, H.; Ma, Z.M. Population genetic structure of the tiger prawn (penaeus monodon) in the coastal waters of south china, based on mitochondrial DNA control region sequences. J. Appl. Ichthyol. 2009, 25, 411–416. [Google Scholar] [CrossRef]
- Xia, H.Y.; Li, S.H.; Shi, M.C. Three-D numerical simulation of wind-driven current and density current in the Beibu Gulf. Acta Oceanol. Sin 2001, 20, 455–472. [Google Scholar]
- Zhang, Q.; Allen, S.; Reece, K. Genetic variation in wild and hatchery stocks of suminoe oyster (Crassostrea ariakensis) assessed by pcr-rflp and microsatellite markers. Mar. Biotechnol. 2005, 7, 588–599. [Google Scholar] [CrossRef]
- Dong, D.; Li, X.Z.; Osawa, M. Petrolisthes polychaetus n. Sp., a new species of porcellanidae (decapoda, anomura) from hainan island, china. Crustaceana 2010, 83, 1507–1517. [Google Scholar] [CrossRef]
- Palumbi, S.R. Nucleic acids II: The polymerase chain reaction. In Molecular Systematics, 2nd ed.; Hillis, D.M., Moritz, C., Mable, B.K., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 1996; pp. 205–247. [Google Scholar]
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit i from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Merritt, T.; Shi, L.; Chase, M.; Rex, M.; Etter, R.; Quattro, J. Universal cytochrome b primers facilitate intraspecific studies in molluscan taxa. Mol. Mar. Biol. Biotechnol. 1998, 7, 7–11. [Google Scholar]
- Whiting, M.; Carpenter, J.; Wheeler, Q.; Wheeler, W.C. The stresiptera problem: Phylogeny of the holometabolous insect orders inferred from 18s and 28s ribosomal DNA sequences and morphology. Syst. Biol. 1997, 46, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Colgan, D.J.; McLauchlan, A.; Wilson, G.D.F.; Livingston, S.P.; Edgecombe, G.D.; Macaranas, J.; Cassis, G.; Gray, M.R. Histone h3 and u2 snrna DNA sequences and arthropod molecular evolution. Aust. J. Zool. 1998, 46, 419–437. [Google Scholar] [CrossRef]
- Burland, T. Dnastar’s lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [PubMed]
- Tan, M.H.; Gan, H.M.; Lee, Y.P.; Austin, C. The complete mitogenome of the porcelain crab petrolisthes haswelli miers, 1884 (crustacea: Decapoda: Anomura). Mitochondrial DNA 2016, 27, 1–2. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Thompson, J.; Higgins, D.; Gibson, T. W: Clustal. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. Mafft multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.; Wang, G. Phylosuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.; Sánchez-Gracia, A. Dnasp 6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New tools for the analysis of genome-wide snp data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 16 December 2022).
- Leigh, J.; Bryant, D. Popart: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Tajima, F.V. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Minin, V.; Bloomquist, E.; Suchard, M. Minin vn, bloomquist ew, suchard ma. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol. Biol. Evol. 2008, 25, 1459–1471. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kuhnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. Beast 2: A software platform for bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. Modelfinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. Partitionfinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.; von Haeseler, A.; Minh, B. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Mark, P.; Ayres, D.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Glassell, S.A. New porcellanids and pinnotherids from tropical North American waters. Trans. San Diego Soc. Nat. Hist. 1936, 8, 277–304. [Google Scholar]
- Rambaut, A. Figtree Version 1.4.2. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 4 June 2023).
- Letunic, I.; Bork, P. Interactive tree of life (itol) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Barraclough, T.; Gómez-Zurita, J.; Cardoso, A.; Duran, D.; Hazell, S.; Kamoun, S.; Sumlin, W.; Vogler, A. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Gibbes, L.R. On the carcinological collections of the united states, and an enumeration of species contained in them, with notes on the most remarkable, and descriptions of new species. Proc. Am. Assoc. Adv. Sci. 1850, 3, 165–201. [Google Scholar]
- Hiller, A.; Kraus, H.; Almon, M.; Werding, B. The petrolisthes galathinus complex: Species boundaries based on color pattern, morphology and molecules, and evolutionary interrelationships between this complex and other porcellanidae (crustacea: Decapoda: Anomura). Mol. Phylogenetics Evol. 2006, 40, 547–569. [Google Scholar] [CrossRef]
- Coates, A.G. The geologic evolution of the Central American Isthmus. In Evolution and Environment in Tropical America; Jackson, J.B.C., Coates, A.G., Budd, A., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; pp. 21–56. [Google Scholar]
- O’Dea, A.; Lessios, H.; Coates, A.; Eytan, R.; Restrepo-Moreno, S.; Cione, A.; Collins, L.; Queiroz, A.; Farris, D.; Norris, R.; et al. Formation of the isthmus of panama. Sci. Adv. 2016, 2, e1600883. [Google Scholar] [CrossRef]
- Montes, C.; Cardona, A.; Jaramillo, C.; Pardo-Trujillo, A.; Silva Tamayo, J.-C.; Valencia, V.; Ayala, C.; Pérez-Angel, L.; Rodriguez-Parra, L.; Ramirez, V.; et al. Middle miocene closure of the central american seaway. Science 2015, 348, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.; Xie, D.; Baele, G.; Suchard, M. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Bell, M.A.; Lloyd, G.T. Strap: An r package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 2015, 58, 379–389. [Google Scholar] [CrossRef]
- Revell, L. Phytools: An r package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Samarasin, P.; Shuter, B.; Rodd, F. The problem of estimating recent genetic connectivity in a changing world. Conserv. Biol. 2017, 31, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Beerli, P. Comparison of bayesian and maximum-likelihood inference of population genetic parameters. Bioinformatics 2006, 22, 341–345. [Google Scholar] [CrossRef]
- Beerli, P. Migrate Documentation 2012. Available online: http://popgen.sc.fsu.edu/migratedoc.pdf (accessed on 20 July 2023).
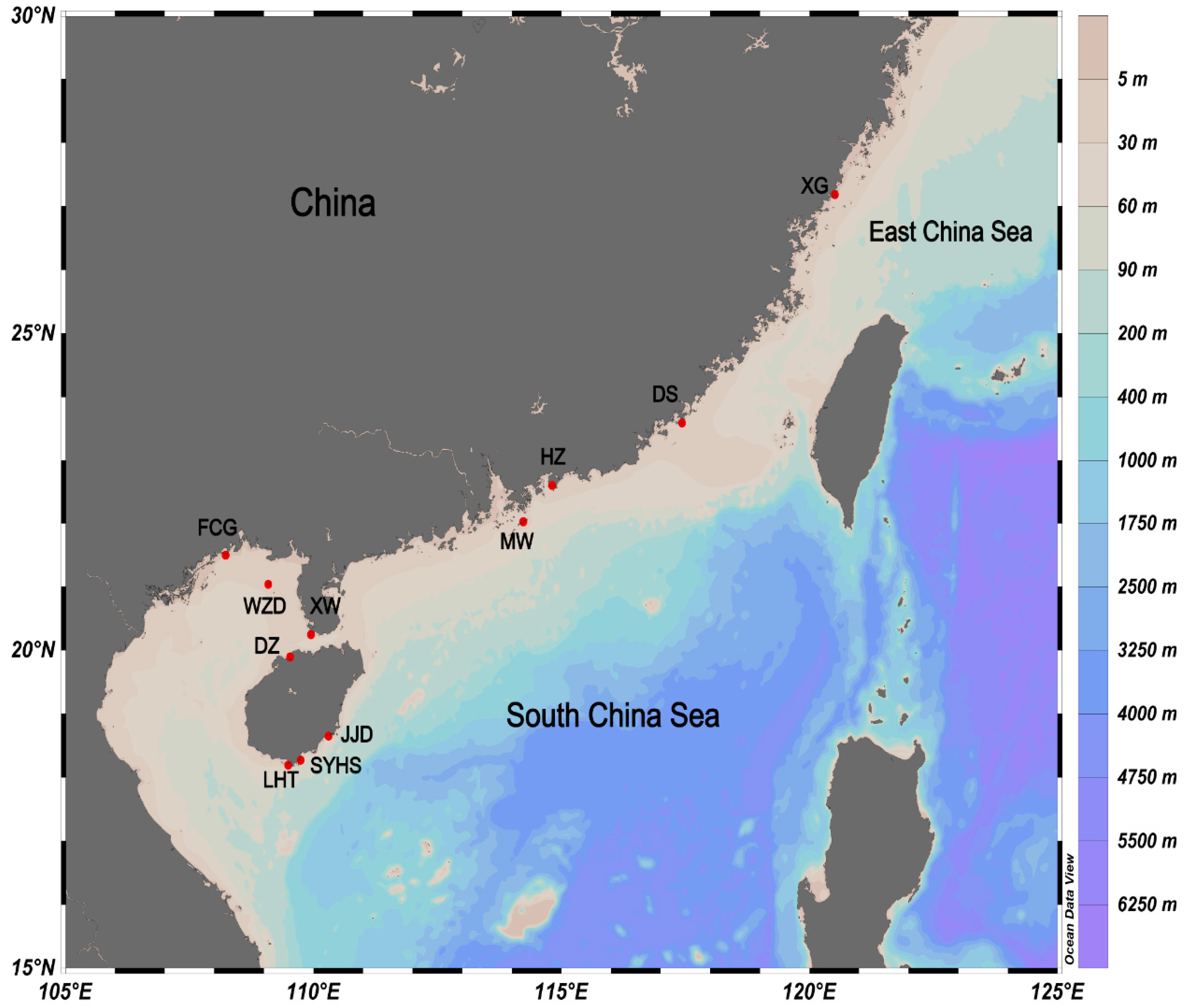


| Locality | S | Latitude, Longitude | Taxon | COI | 16S | CYTB | 18S | H3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | H1 | Hd1 | π1 | N2 | H2 | Hd2 | π2 | N3 | H3 | Hd3 | π3 | N4 | H4 | Hd4 | π4 | N5 | H5 | Hd5 | π5 | ||||
| Xiaguan (XG) | 33 | 27.19° N 120.51° E | P. haswelli | 30 | 2 | 0.067 | 0.00012 | 22 | 1 | 0 | 0 | 33 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 21 | 1 | 0 | 0 |
| Dongshan (DS) | 8 | 23.59° N 117.43° E | P. haswelli | 8 | 2 | 0.250 | 0.00046 | 8 | 2 | 0.250 | 0.00057 | 8 | 1 | 0 | 0 | 7 | 1 | 0 | 0 | 8 | 1 | 0 | 0 |
| Huizhou (HZ) | 11 | 22.60° N 114.81° E | P. haswelli | 11 | 1 | 0 | 0 | 11 | 1 | 0 | 0 | 8 | 2 | 0.250 | 0.00067 | 2 | 1 | 0 | 0 | 5 | 1 | 0 | 0 |
| Miaowan (MW) | 6 | 22.03° N 114.23° E | P. haswelli | 6 | 1 | 0 | 0 | 6 | 1 | 0 | 0 | 6 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Fangcheng (FCG) | 4 | 21.50° N 108.23° E | P. haswelli | 4 | 1 | 0 | 0 | 4 | 1 | 0 | 0 | 2 | 2 | 1.000 | 0.00265 | 2 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Weizhou (WZD) | 12 | 21.04° N 109.09° E | P. haswelli | 12 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 4 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Xuwen (XW) | 7 | 20.25° N 109.95° E | P. haswelli | 7 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 |
| Danzhou (DZ) | 14 | 19.90° N 109.53° E | P. haswelli | 10 | 1 | 0 | 0 | 6 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 5 | 1 | 0 | 0 |
| P. polychaetus | 4 | 4 | 1.000 | 0.00548 | 4 | 2 | 0.500 | 0.00113 | 4 | 4 | 1.000 | 0.00663 | 4 | 1 | 0 | 0 | 4 | 1 | 0 | 0 | |||
| Jiajingdao (JJD) | 6 | 18.65° N 110.30° E | P. haswelli | 1 | 1 | 0 | 0 | ||||||||||||||||
| P. lamarckii | 5 | 5 | 1.000 | 0.00841 | 5 | 5 | 1.000 | 0.00363 | 4 | 3 | 0.833 | 0.00531 | 5 | 1 | 0 | 0 | 5 | 1 | 0 | 0 | |||
| Houhai (SYHS) | 3 | 18.27° N 109.74° E | P. shanyingi sp. nov. | 3 | 3 | 1.000 | 0.00366 | 3 | 1 | 0 | 0 | 3 | 3 | 1.000 | 0.00884 | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Luhuitou (LHT) | 31 | 18.19° N 109.49° E | P. haswelli | 5 | 3 | 0.700 | 0.00184 | 5 | 3 | 0.700 | 0.00272 | 5 | 2 | 0.400 | 0.00106 | 4 | 1 | 0 | 0 | 5 | 1 | 0 | 0 |
| P. lamarckii | 3 | 3 | 1.000 | 0.00366 | 3 | 1 | 0 | 0 | 2 | 2 | 1.000 | 0.00531 | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | |||
| P. polychaetus | 4 | 1 | 0 | 0 | 4 | 1 | 0 | 0 | 4 | 4 | 1.000 | 0.00398 | 4 | 1 | 0 | 0 | 4 | 1 | 0 | 0 | |||
| P. shanyingi sp. nov. | 19 | 11 | 0.789 | 0.00552 | 19 | 8 | 0.673 | 0.00223 | 13 | 11 | 0.962 | 0.01051 | 12 | 1 | 0 | 0 | 19 | 1 | 0 | 0 | |||
| Total | 135 | P. haswelli | 94 | 4 | 0.084 | 0.00019 | 67 | 3 | 0.088 | 0.00027 | 68 | 4 | 0.087 | 0.00023 | 26 | 1 | 0 | 0 | 53 | 1 | 0 | 0 | |
| P. lamarckii | 8 | 7 | 0.964 | 0.00620 | 8 | 5 | 0.786 | 0.00227 | 6 | 4 | 0.800 | 0.00460 | 8 | 1 | 0 | 0 | 8 | 1 | 0 | 0 | |||
| P. polychaetus | 8 | 4 | 0.643 | 0.00274 | 8 | 2 | 0.250 | 0.00057 | 8 | 7 | 0.964 | 0.00531 | 8 | 1 | 0 | 0 | 8 | 1 | 0 | 0 | |||
| P. shanyingi sp. nov. | 22 | 13 | 0.805 | 0.00530 | 22 | 8 | 0.602 | 0.00194 | 16 | 14 | 0.975 | 0.01017 | 15 | 1 | 0 | 0 | 22 | 1 | 0 | 0 | |||
| XG | DS | EC | BH | HN | |
|---|---|---|---|---|---|
| XG | 0.000 | 0.000 | 0.000 | 0.001 | |
| DS | 0.042 | 0.000 | 0.000 | 0.001 | |
| EC | −0.021 | 0.102 | 0.000 | 0.001 | |
| BH | 0.003 | 0.213 | 0.000 | 0.001 | |
| HN | 0.288 | −0.057 | 0.320 | 0.482 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Dong, D.; Yang, M.; Li, X. Phylogenetics and Population Genetics of the Petrolisthes lamarckii–P. haswelli Complex in China: Old Lineage and New Species. Int. J. Mol. Sci. 2023, 24, 15843. https://doi.org/10.3390/ijms242115843
Fang X, Dong D, Yang M, Li X. Phylogenetics and Population Genetics of the Petrolisthes lamarckii–P. haswelli Complex in China: Old Lineage and New Species. International Journal of Molecular Sciences. 2023; 24(21):15843. https://doi.org/10.3390/ijms242115843
Chicago/Turabian StyleFang, Xuefeng, Dong Dong, Mei Yang, and Xinzheng Li. 2023. "Phylogenetics and Population Genetics of the Petrolisthes lamarckii–P. haswelli Complex in China: Old Lineage and New Species" International Journal of Molecular Sciences 24, no. 21: 15843. https://doi.org/10.3390/ijms242115843
APA StyleFang, X., Dong, D., Yang, M., & Li, X. (2023). Phylogenetics and Population Genetics of the Petrolisthes lamarckii–P. haswelli Complex in China: Old Lineage and New Species. International Journal of Molecular Sciences, 24(21), 15843. https://doi.org/10.3390/ijms242115843





