The Improvement of Fluorescence In Situ Hybridization Technique Based on Explorations of Symbionts in Cicadas
Abstract
:1. Introduction
2. Results
2.1. Similarity between rRNA-Targeted Oligonucleotide Probes and 16S rRNA/18S rRNA Gene Sequence of Targeted Microbes in Cicadas and Other Auchenorrhynchan Insects
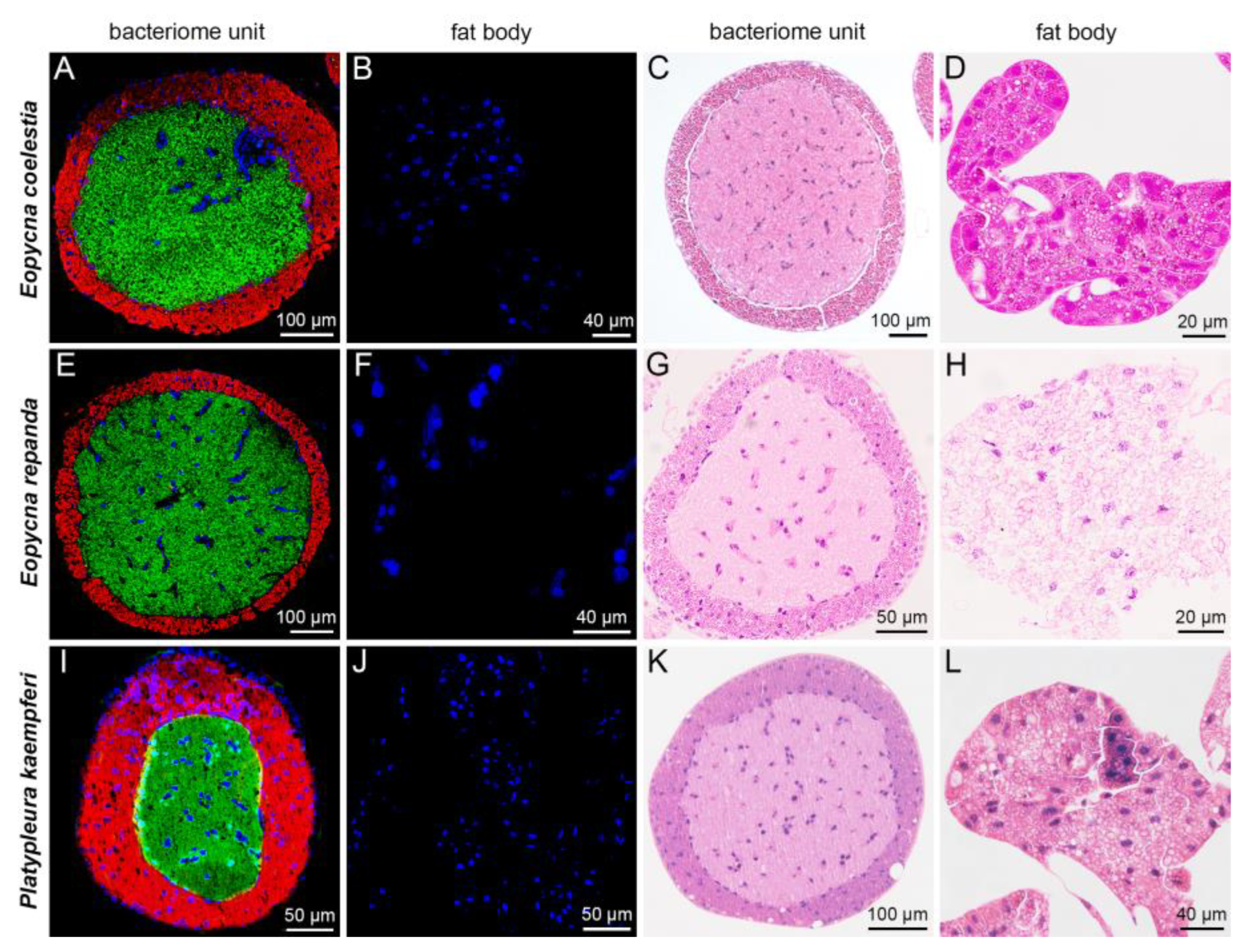
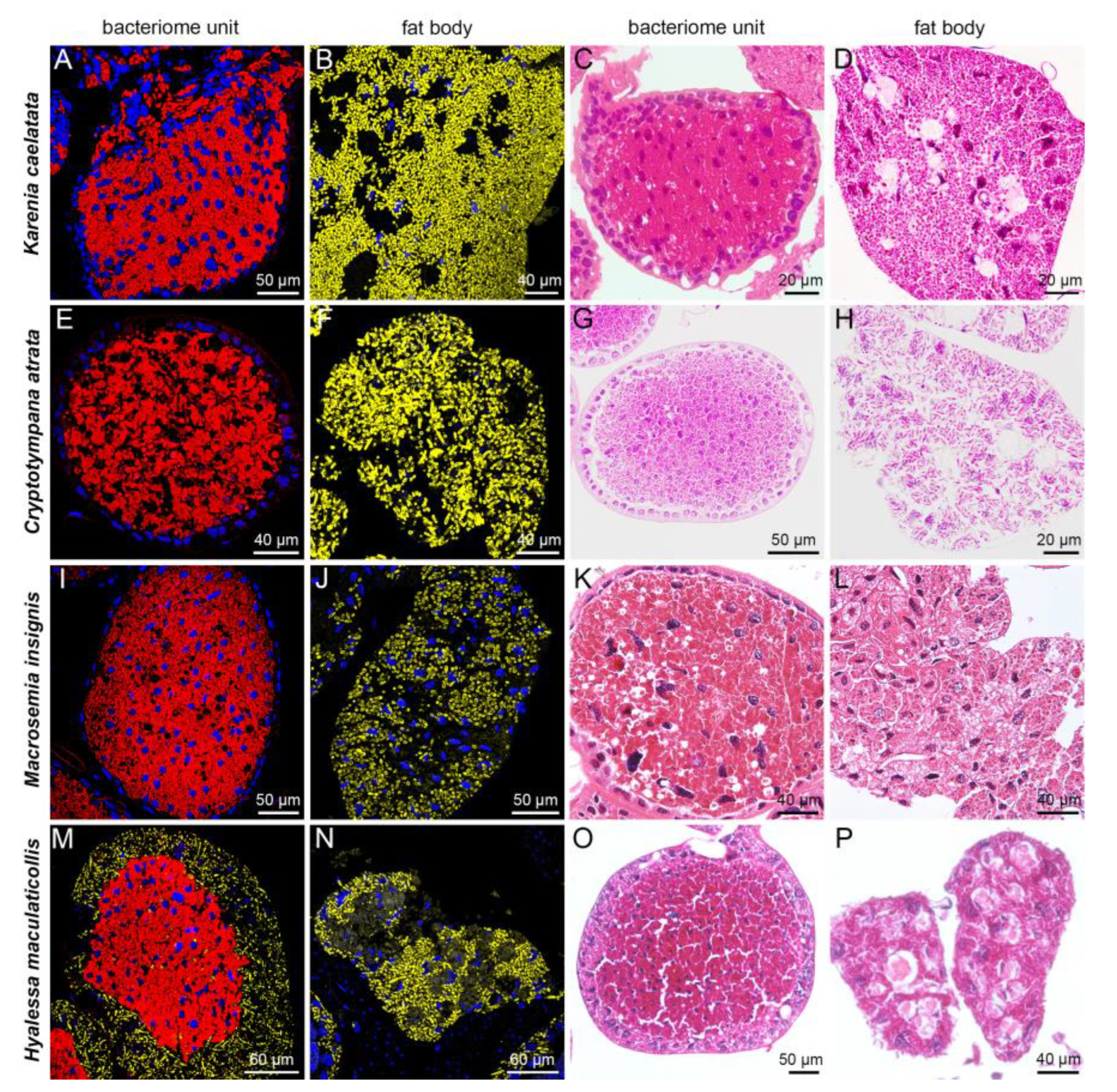
2.2. Distribution of Sulcia, Hodgkinia and YLS in Bacteriomes and Fat Bodies of Seven Representative Cicada Species
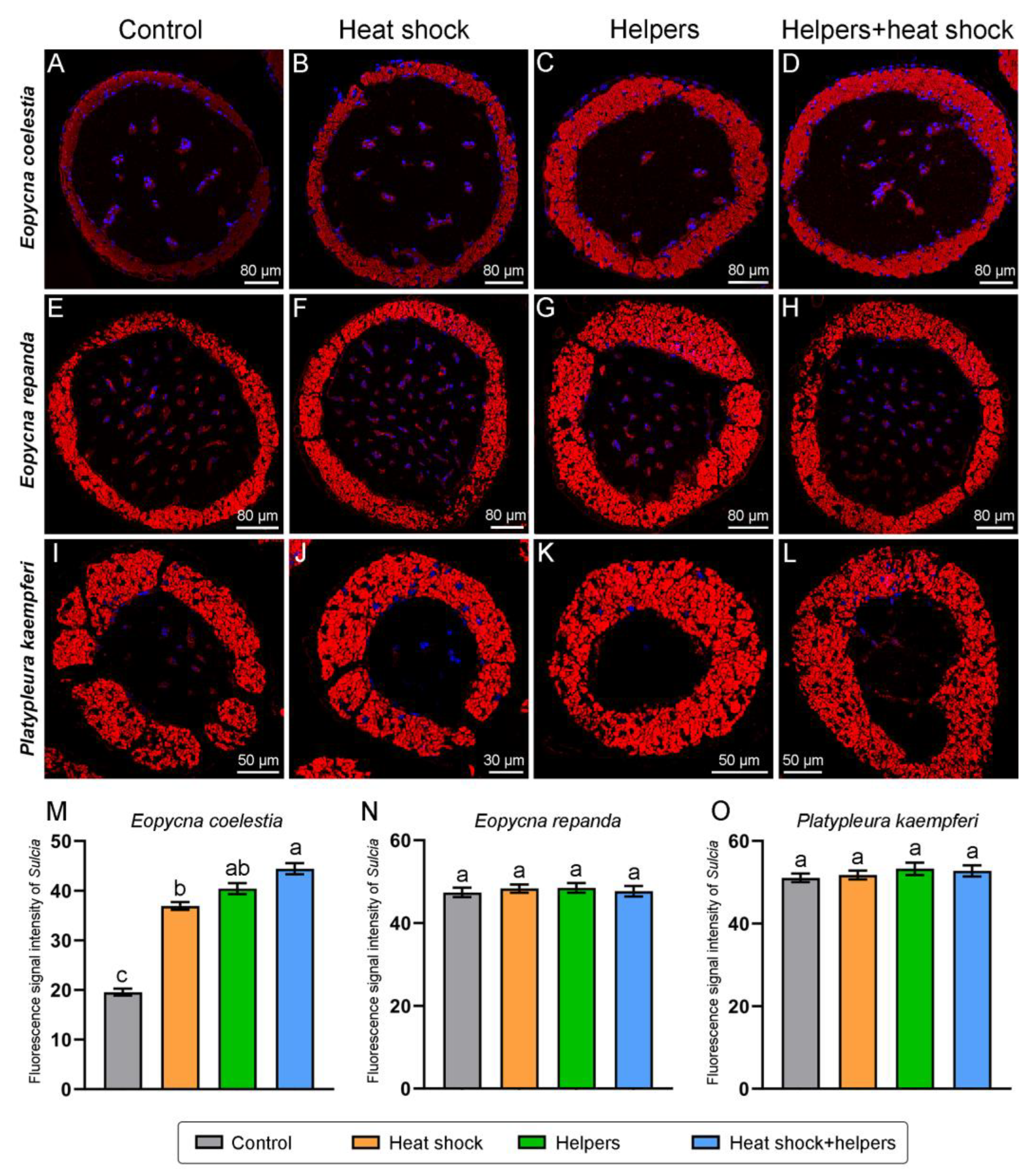
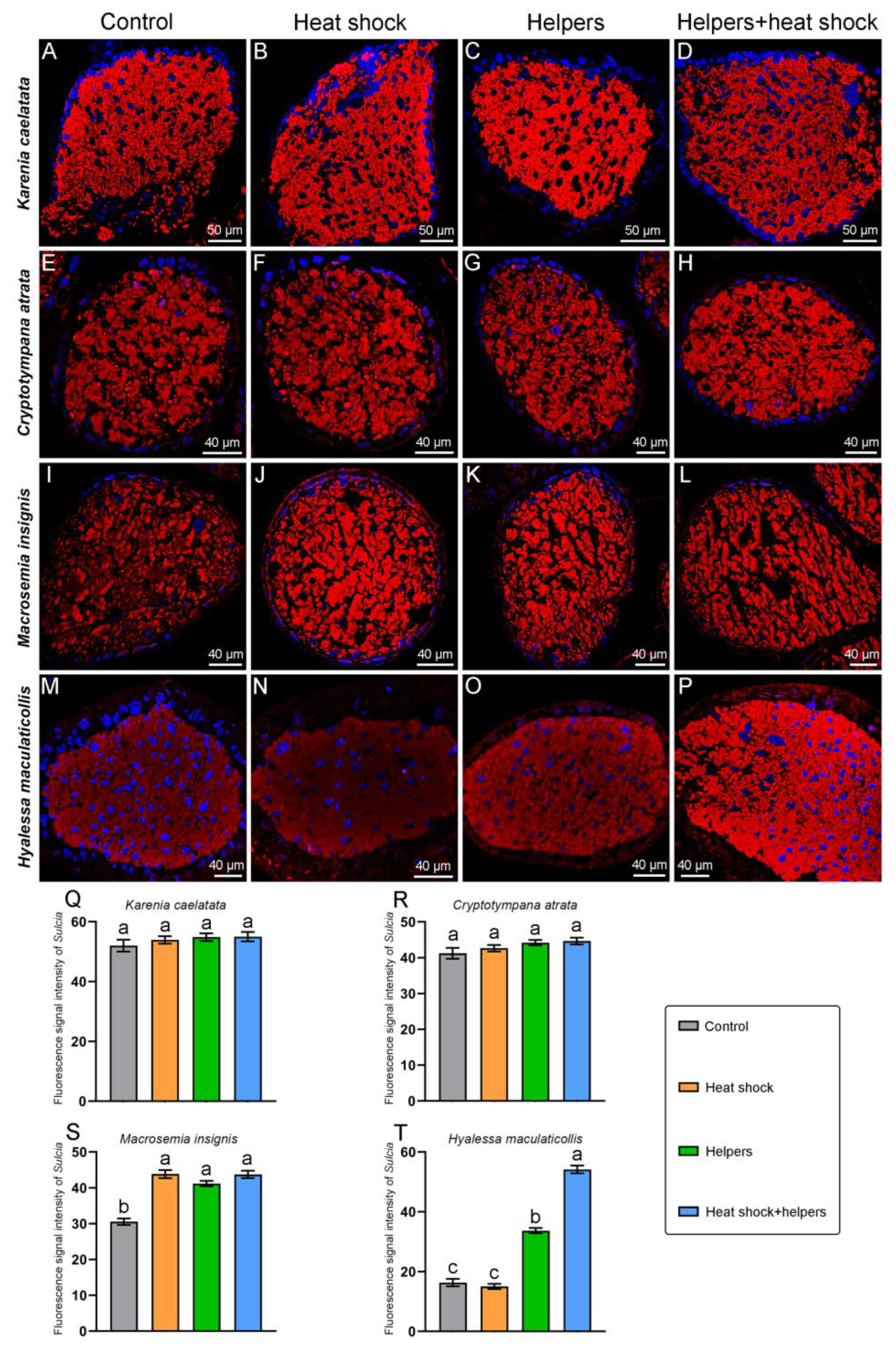
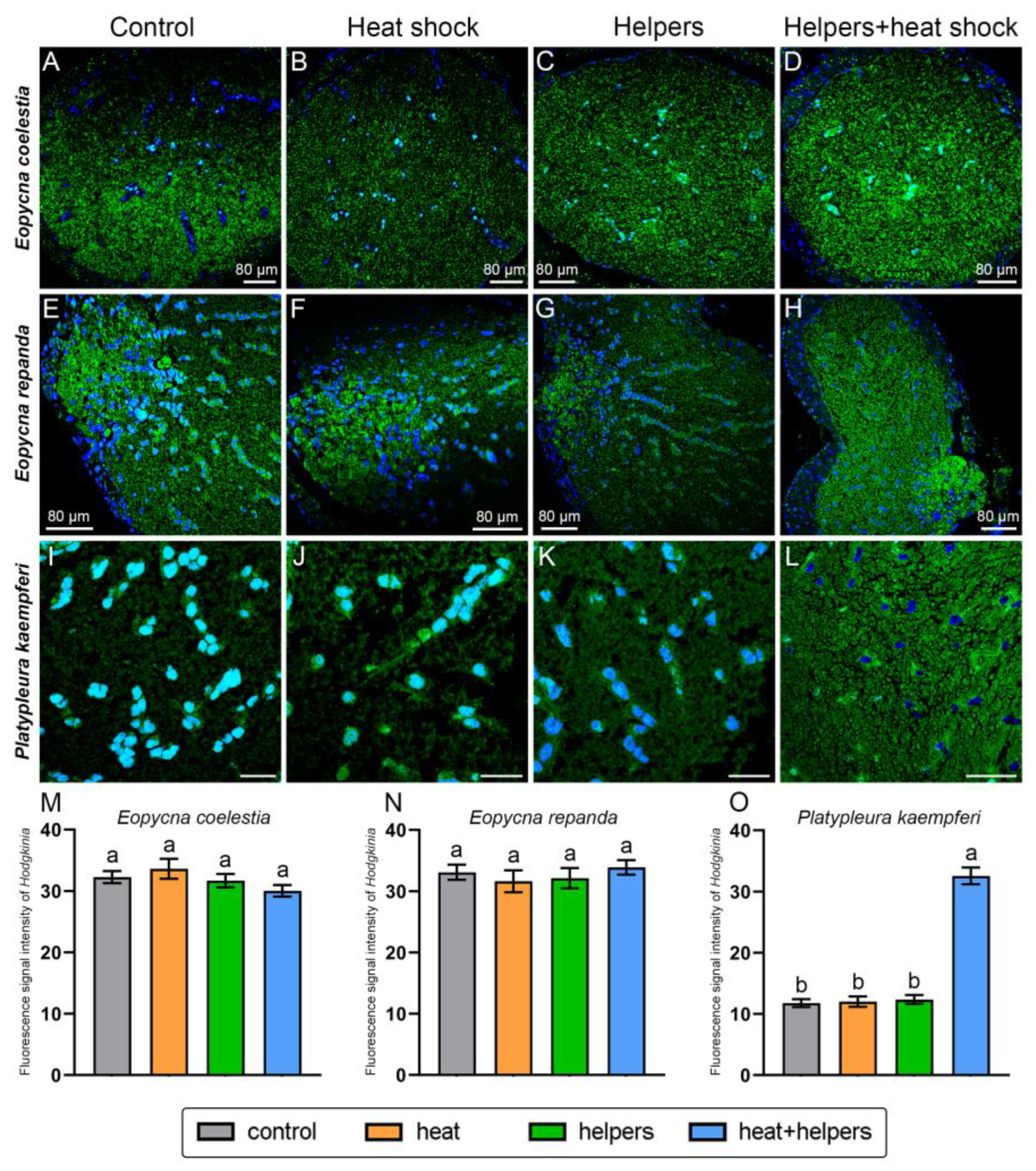
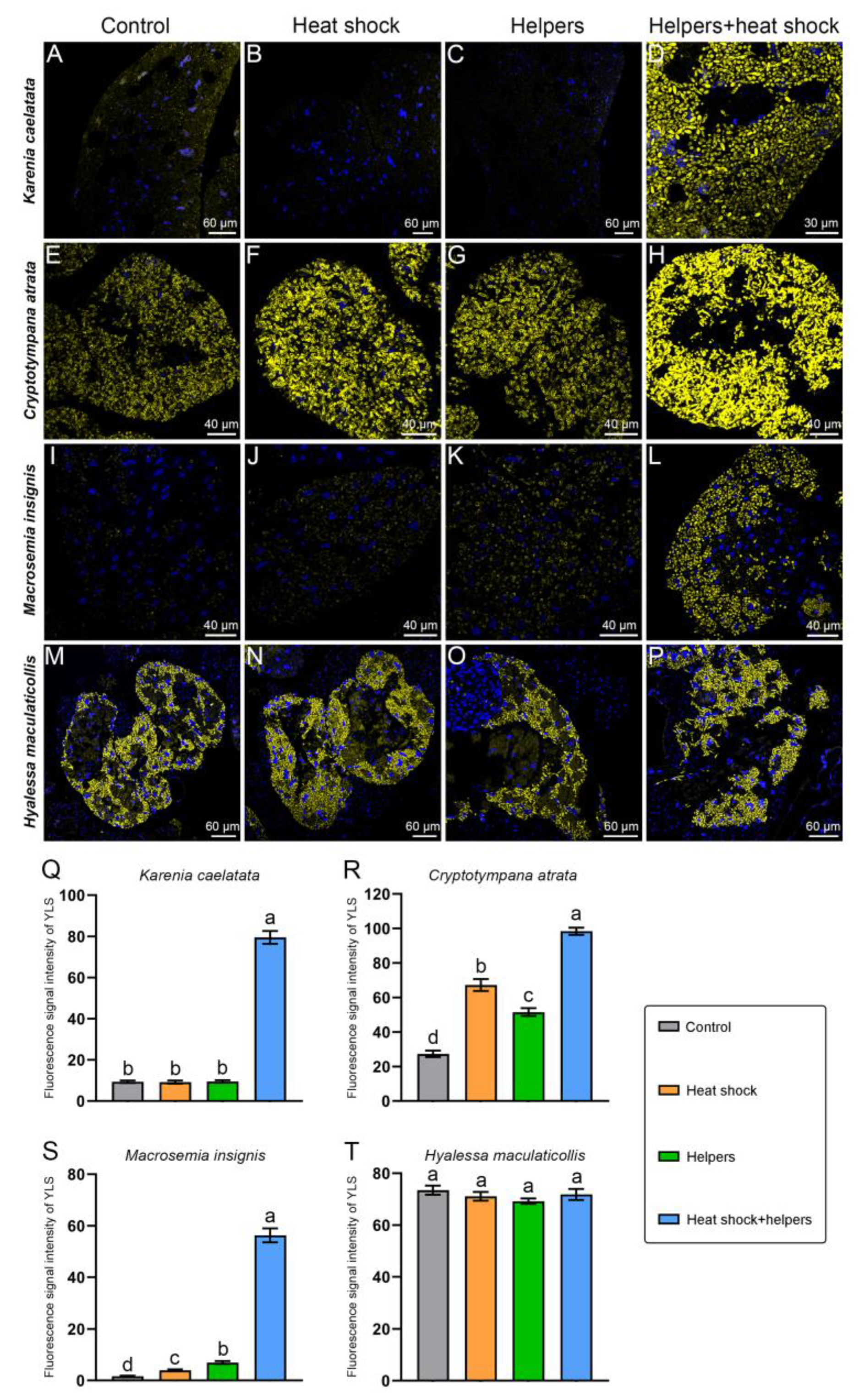
2.3. The Improvement of the FISH Signal Intensity of Symbionts by Addition of Unlabeled Helper Sequences and Heat Shock
2.4. The Predicted Secondary Structure of 16S rRNA/18S rRNA Gene Sequence of Symbionts in Seven Cicadas
3. Discussion
3.1. Sample Preservation and Paraffin Section
3.2. The Selection of rRNA-Targeted Probes for Hybridization Experiments
3.3. The Enhancement of Fluorescence Signal Intensity in Symbionts by Addition of Unlabeled Helper Sequences and Heat Shock
4. Materials and Methods
4.1. Sample Collection and Dissection
4.2. Compared the Similarity between rRNA-Targeted Oligonucleotide Probe and 16S rRNA/18S rRNA Gene Sequence of Targeted Microbes
4.3. Fluorescence In Situ Hybridization
4.4. The Prediction of Secondary Structure of 16S rRNA/18S rRNA Gene Sequence of Symbionts in Six Cicadas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, P.; James, R.R.; Koga, R.; Kwong, W.K.; McFrederick, Q.S.; Moran, N.A. Standard methods for research on Apis mellifera gut symbionts. J. Apic. Res. 2013, 52, 1–24. [Google Scholar] [CrossRef]
- Amann, R.; Fuchs, B.M. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 2008, 6, 339–348. [Google Scholar] [CrossRef]
- Moter, A.; Göbel, U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods 2000, 41, 85–112. [Google Scholar] [CrossRef]
- Masson, F.; Copete, S.C.; Schüpfer, F.; Garcia-Arraez, G.; Lemaitre, B. In vitro culture of the insect endosymbiont Spiroplasma poulsonii highlights bacterial genes involved in host-symbiont interaction. mBio 2018, 9, e00024-18. [Google Scholar] [CrossRef]
- Perreau, J.; Moran, N.A. Genetic innovations in animal-microbe symbioses. Nat. Rev. Genet. 2022, 23, 23–39. [Google Scholar] [CrossRef]
- Singh, B. Exploring microbial diversity for biotechnology: The way forward. Trends Biotechnol. 2010, 28, 111–116. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Brar, G.A.; Rouskin, S.; McGeachy, A.M.; Weissman, J.S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 2012, 7, 1534–1550. [Google Scholar] [CrossRef]
- Hollar, A.; Bursey, H.; Jabbari, H. Pseudoknots in RNA structure prediction. Curr. Protoc. 2023, 3, e661. [Google Scholar] [CrossRef]
- Greuter, D.; Loy, A.; Horn, M.; Rattei, T. ProbeBase—An online resource for rRNA-targeted oligonucleotide probes and primers: New features 2016. Nucleic Acids Res 2016, 44, D586–D589. [Google Scholar] [CrossRef]
- Loy, A.; Horn, M.; Wagner, M. ProbeBase: An online resource for rRNA-targeted oligonucleotide probes. Nucleic Acids Res. 2003, 31, 514–516. [Google Scholar] [CrossRef]
- Loy, A.; Arnold, R.; Tischler, P.; Rattei, T.; Wagner, M.; Horn, M. ProbeCheck: A central resource for evaluating oligonucleotide probe coverage and specificity. Environ. Microbiol. 2008, 10, 2894–2898. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, D.; Li, J.; Wei, C.; He, H. Transovarial transmission of bacteriome-associated symbionts in the cicada Pycna repanda (Hemiptera: Cicadidae). Appl. Environ. Microbiol. 2020, 86, e02957-19. [Google Scholar] [CrossRef]
- Luan, J.B.; Shan, H.W.; Isermann, P.; Huang, J.H.; Lammerding, J.; Liu, S.S.; Douglas, A.E. Cellular and molecular remodelling of a host cell for vertical transmission of bacterial symbionts. Proc. R. Soc. B 2016, 283, 20160580. [Google Scholar] [CrossRef]
- Michalik, A.; Franco, D.; Kobiałka, M.; Stroinski, A.; Łukasik, P. Alternative transmission patterns in independently acquired nutritional co-symbionts of Dictyopharidae planthoppers. mBio 2021, 12, e0122821. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Wiley Press: New York, NY, USA, 1965. [Google Scholar]
- Moran, N.A.; Tran, P.; Gerardo, N.M. Symbiosis and insect diversification: An ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 2005, 71, 8802–8810. [Google Scholar] [CrossRef]
- McCutcheon, J.P.; McDonald, B.R.; Moran, N.A. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc. Natl. Acad. Sci. USA 2009, 106, 15394–15399. [Google Scholar] [CrossRef]
- Łukasik, P.; Nazario, K.; Van Leuven, J.T.; Campbell, M.A.; Meyer, M.; Michalik, A.; Pessacq, P.; Simon, C.; Veloso, C.; McCutcheon, J.P. Multiple origins of interdependent endosymbiotic complexes in a genus of cicadas. Proc. Natl. Acad. Sci. USA 2018, 115, E226–E235. [Google Scholar] [CrossRef]
- Campbell, M.A.; Łukasik, P.; Meyer, M.M.; Buckner, M.; Simon, C.; Veloso, C.; Michalik, A.; McCutcheon, J.P. Changes in endosymbiont complexity drive host-level compensatory adaptations in cicadas. mBio 2018, 9, e02104-18. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Su, Y.; Wei, C. Bacterial communities in bacteriomes, ovaries and testes of three geographical populations of a sap-feeding insect, Platypleura kaempferi (Hemiptera: Cicadidae). Curr. Microbiol. 2021, 78, 1778–1791. [Google Scholar] [CrossRef]
- Matsuura, Y.; Moriyama, M.; Łukasik, P.; Vanderpool, D.; Tanahashi, M.; Meng, X.Y.; McCutcheon, J.P.; Fukatsu, T. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc. Natl. Acad. Sci. USA 2018, 115, E5970–E5979. [Google Scholar] [CrossRef]
- Wang, D.; Huang, Z.; Billen, J.; Zhang, G.; He, H.; Wei, C. Structural diversity of symbionts and related cellular mechanisms underlying vertical symbiont transmission in cicadas. Environ. Microbiol. 2021, 23, 6603–6621. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, J.; Zhang, Z.; He, H.; Wei, C. A study on symbiotic systems of cicadas provides new insights into distribution of microbial symbionts and improves fluorescence in situ hybridization technique. Int. J. Mol. Sci. 2023, 24, 2434. [Google Scholar] [CrossRef]
- Nishino, T.; Tanahashi, M.; Lin, C.P.; Koga, R.; Fukatsu, T. Fungal and bacterial endosymbionts of eared leafhoppers of the subfamily Ledrinae (Hemiptera: Cicadellidae). Appl. Entomol. Zool. 2016, 51, 465–477. [Google Scholar] [CrossRef]
- Van Leuven, J.T.; Meister, R.C.; Simon, C.; McCutcheon, J.P. Sympatric speciation in a bacterial endosymbiont results in two genomes with the functionality of one. Cell 2014, 158, 1270–1280. [Google Scholar] [CrossRef]
- Fuchs, B.M.; Wallner, G.; Beisker, W.; Schwippl, I.; Ludwig, W.; Amann, R. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 4973–4982. [Google Scholar] [CrossRef]
- Fuchs, B.M.; Glöckner, F.O.; Wulf, J.; Amann, R. Unlabeled helper oligonucleotides increase the in situ accessibility to 16S rRNA of fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 2000, 66, 3603–3607. [Google Scholar] [CrossRef]
- Timoshevskiy, V.A.; Sharma, A.; Sharakhov, I.V.; Sharakhova, M.V. Fluorescent in situ hybridization on mitotic chromosomes of mosquitoes. J. Vis. Exp. 2012, 67, e4215. [Google Scholar]
- Sharma, A.; Kinney, N.A.; Timoshevskiy, V.A.; Sharakhova, M.V.; Sharakhov, I.V. Structural variation of the X chromosome heterochromatin in the Anopheles gambiae complex. Genes 2020, 11, 327. [Google Scholar] [CrossRef]
- Procter, J.B.; Carstairs, G.M.; Soares, B.; Mourão, K.; Ofoegbu, T.C.; Barton, D.; Lui, L.; Menard, A.; Sherstnev, N.; Roldan-Martinez, D.; et al. Alignment of biological sequences with Jalview. Methods Mol. Biol. 2021, 2231, C1. [Google Scholar]
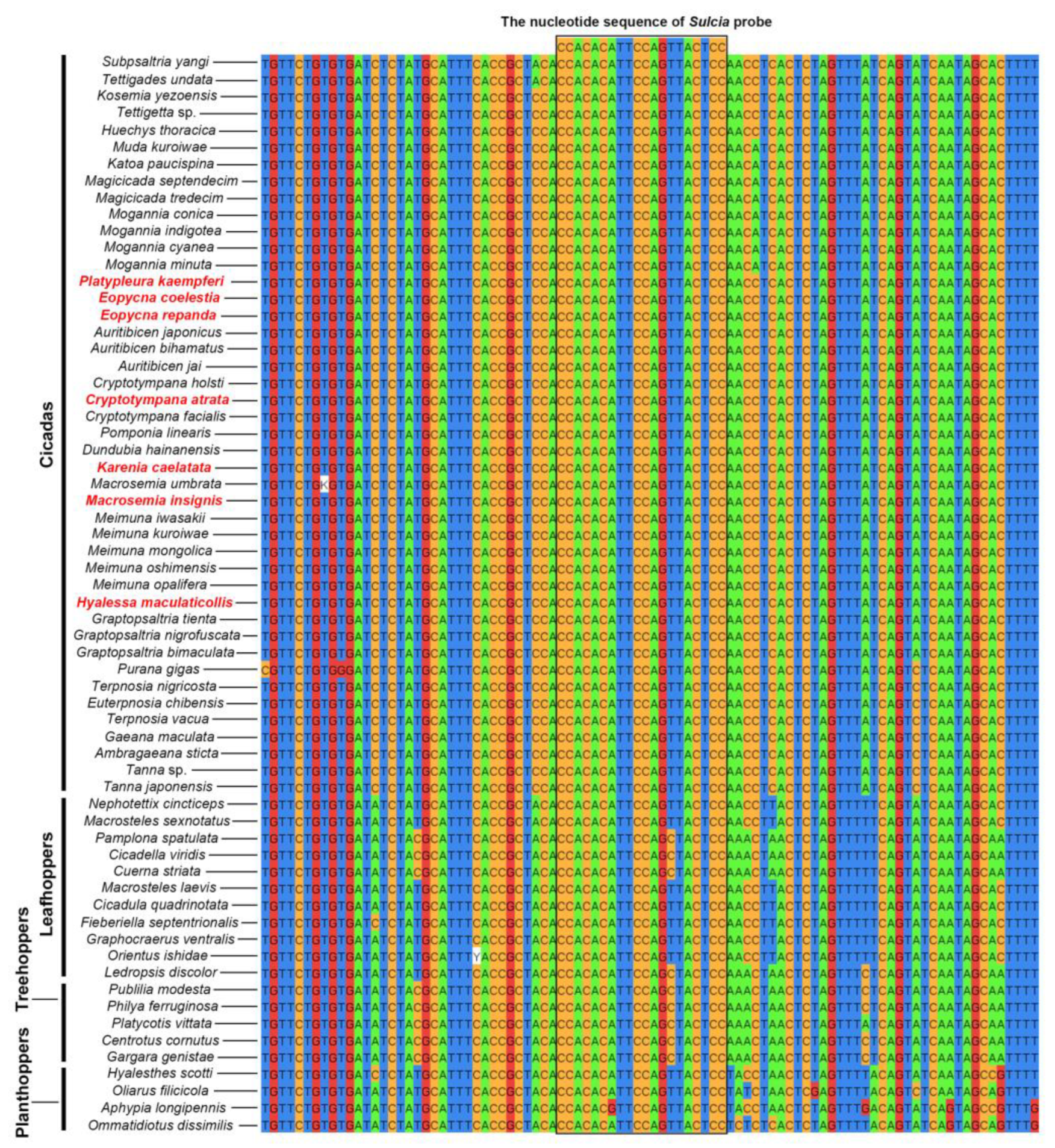
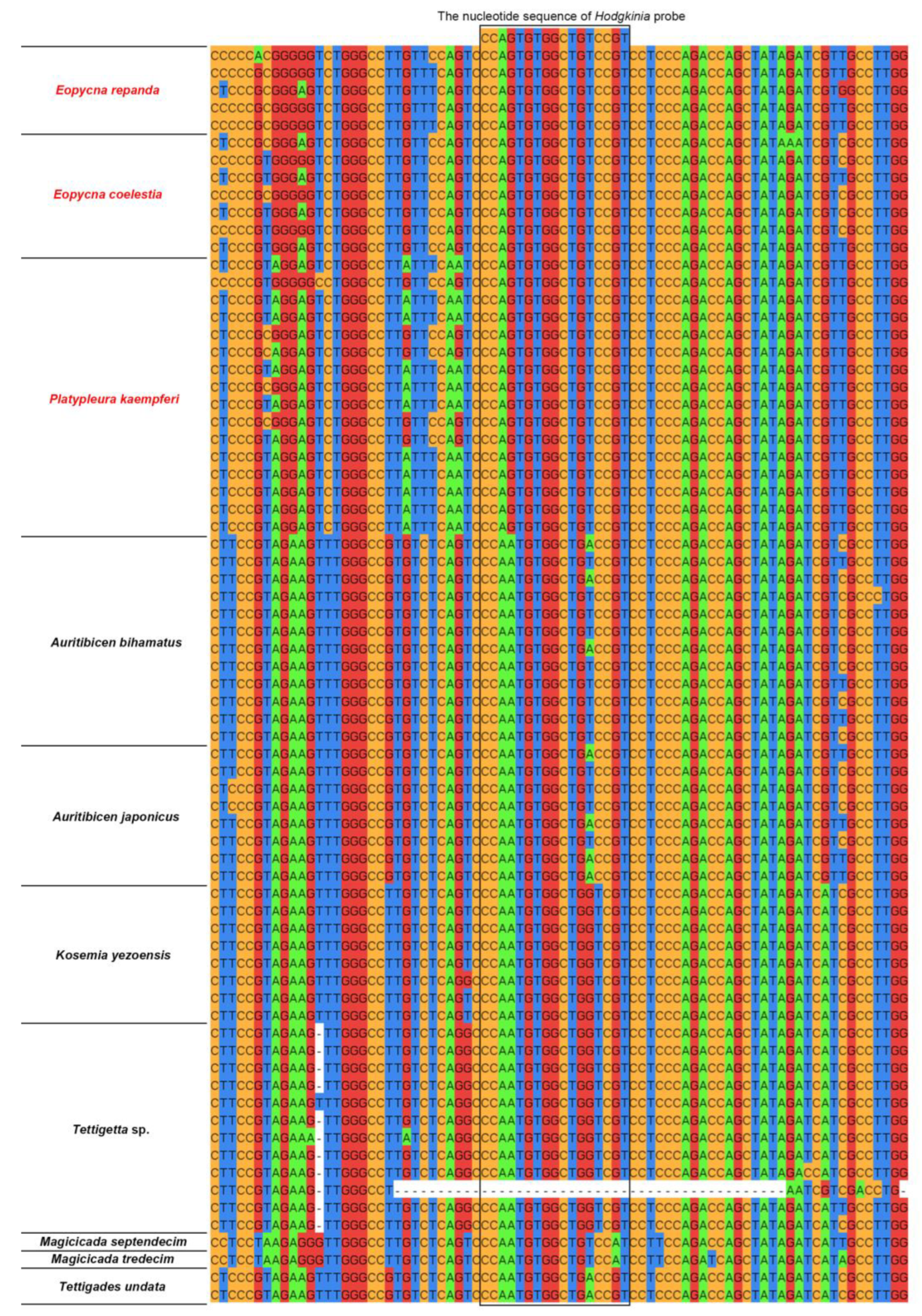
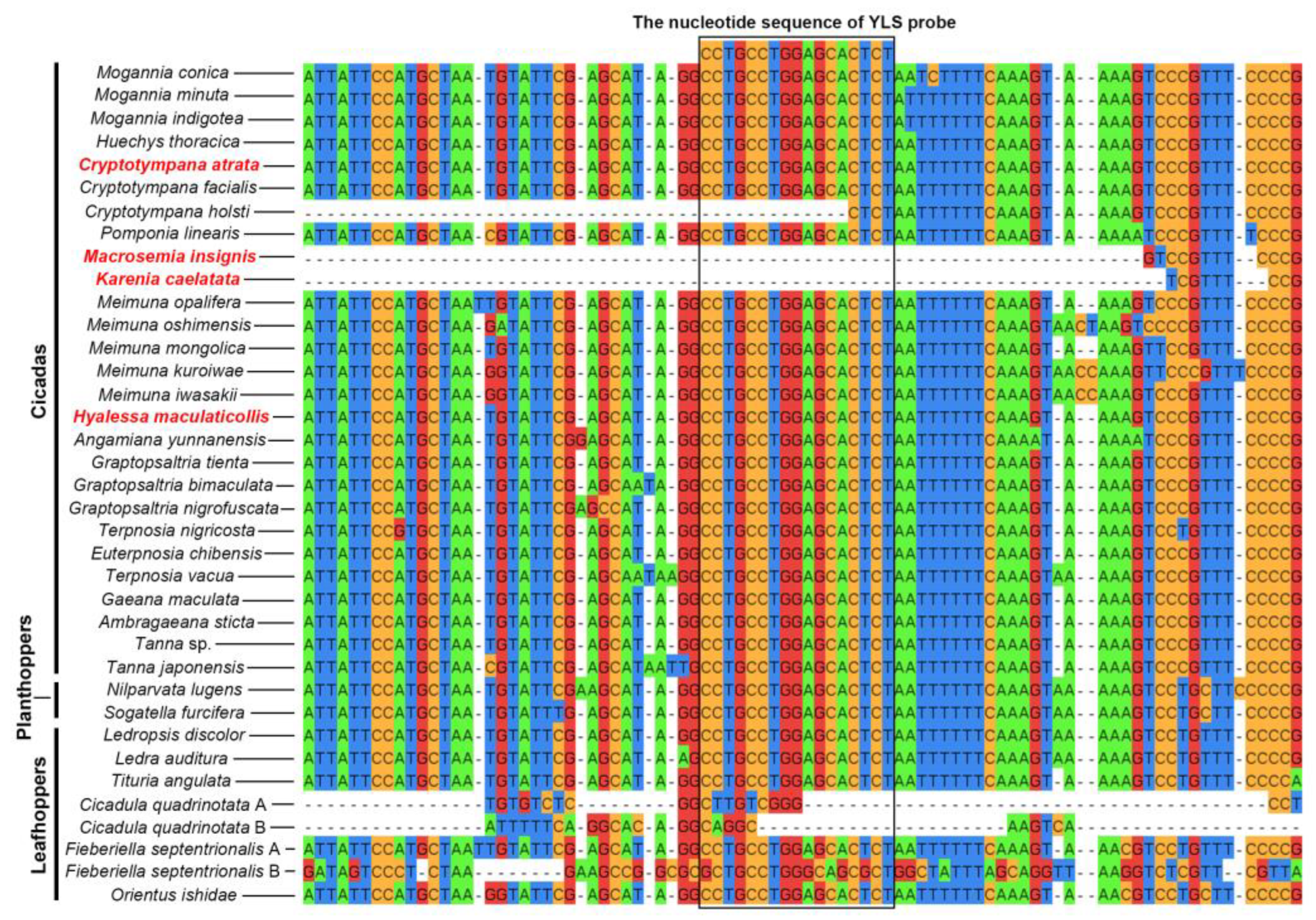
| Probe Name | Suitable for Host Cicadas | Primer Sequence (5′–3′) | References |
|---|---|---|---|
| Sulcia | All species | CCACACATTCCAGTTACTCC | [18] |
| YLS | All species | CCTGCCTGGAGCACTCT | [21] |
| Hodgkinia-Ple | Eopycna repanda, Eopycna coelestia, Platypleura kaempferi | CCAGT GTGGC TGTCC GT | This study |
| Hodgkinia-Tet | Tettigades undata, Diceroprocta semicincta | CCAAT GTGGC TGACC GT | [25] |
| Hodgkinia-Mag | Magicicada septendecim | CCAAT GTGGC TGTYC RT | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Wang, D.; Zhou, J.; He, H.; Wei, C. The Improvement of Fluorescence In Situ Hybridization Technique Based on Explorations of Symbionts in Cicadas. Int. J. Mol. Sci. 2023, 24, 15838. https://doi.org/10.3390/ijms242115838
Huang Z, Wang D, Zhou J, He H, Wei C. The Improvement of Fluorescence In Situ Hybridization Technique Based on Explorations of Symbionts in Cicadas. International Journal of Molecular Sciences. 2023; 24(21):15838. https://doi.org/10.3390/ijms242115838
Chicago/Turabian StyleHuang, Zhi, Dandan Wang, Jinrui Zhou, Hong He, and Cong Wei. 2023. "The Improvement of Fluorescence In Situ Hybridization Technique Based on Explorations of Symbionts in Cicadas" International Journal of Molecular Sciences 24, no. 21: 15838. https://doi.org/10.3390/ijms242115838
APA StyleHuang, Z., Wang, D., Zhou, J., He, H., & Wei, C. (2023). The Improvement of Fluorescence In Situ Hybridization Technique Based on Explorations of Symbionts in Cicadas. International Journal of Molecular Sciences, 24(21), 15838. https://doi.org/10.3390/ijms242115838







