lncRNA MSTRG4710 Promotes the Proliferation and Differentiation of Preadipocytes through miR-29b-3p/IGF1 Axis
Abstract
:1. Introduction
2. Results
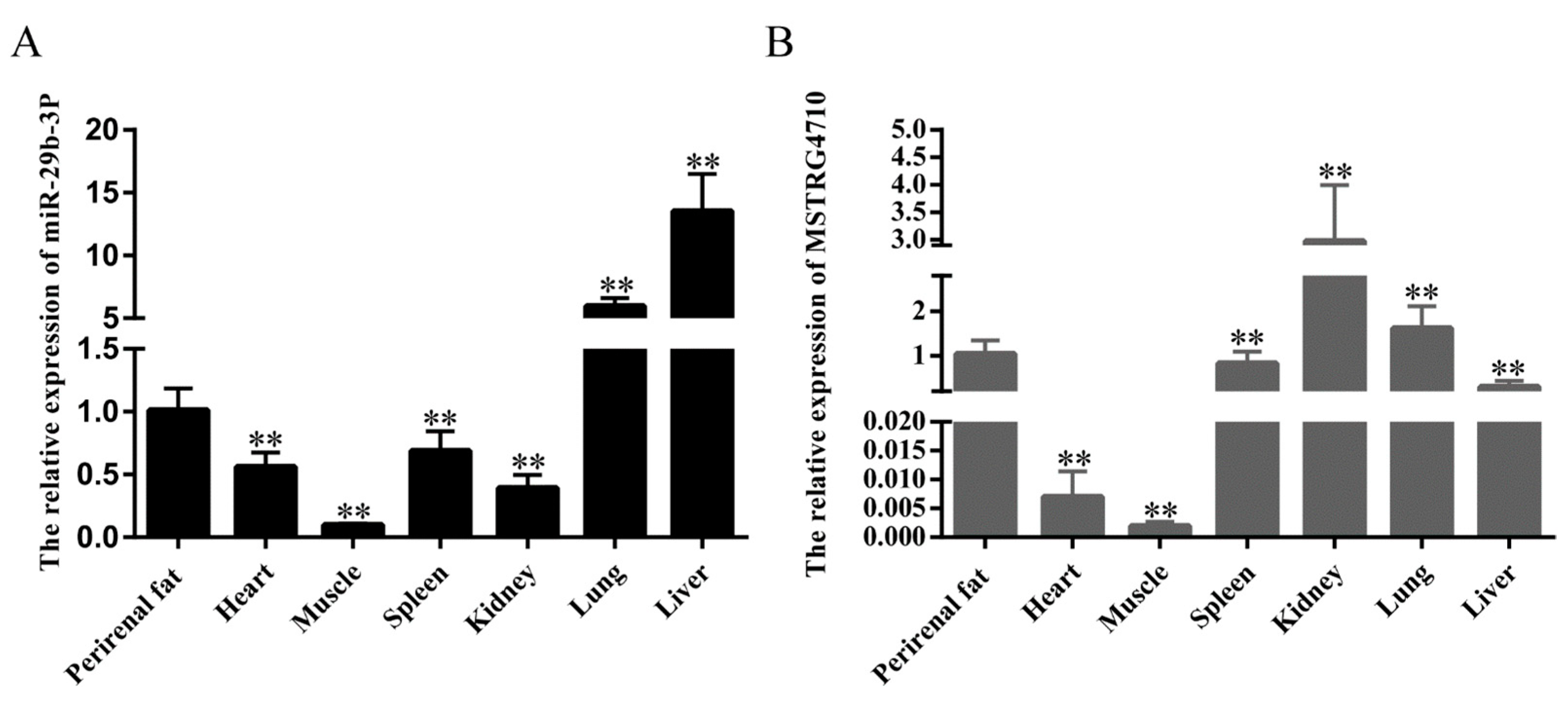
2.1. Tissue Expression Profiles of LncRNA-MSTRG4710 and miR-29b-3p in Rabbits
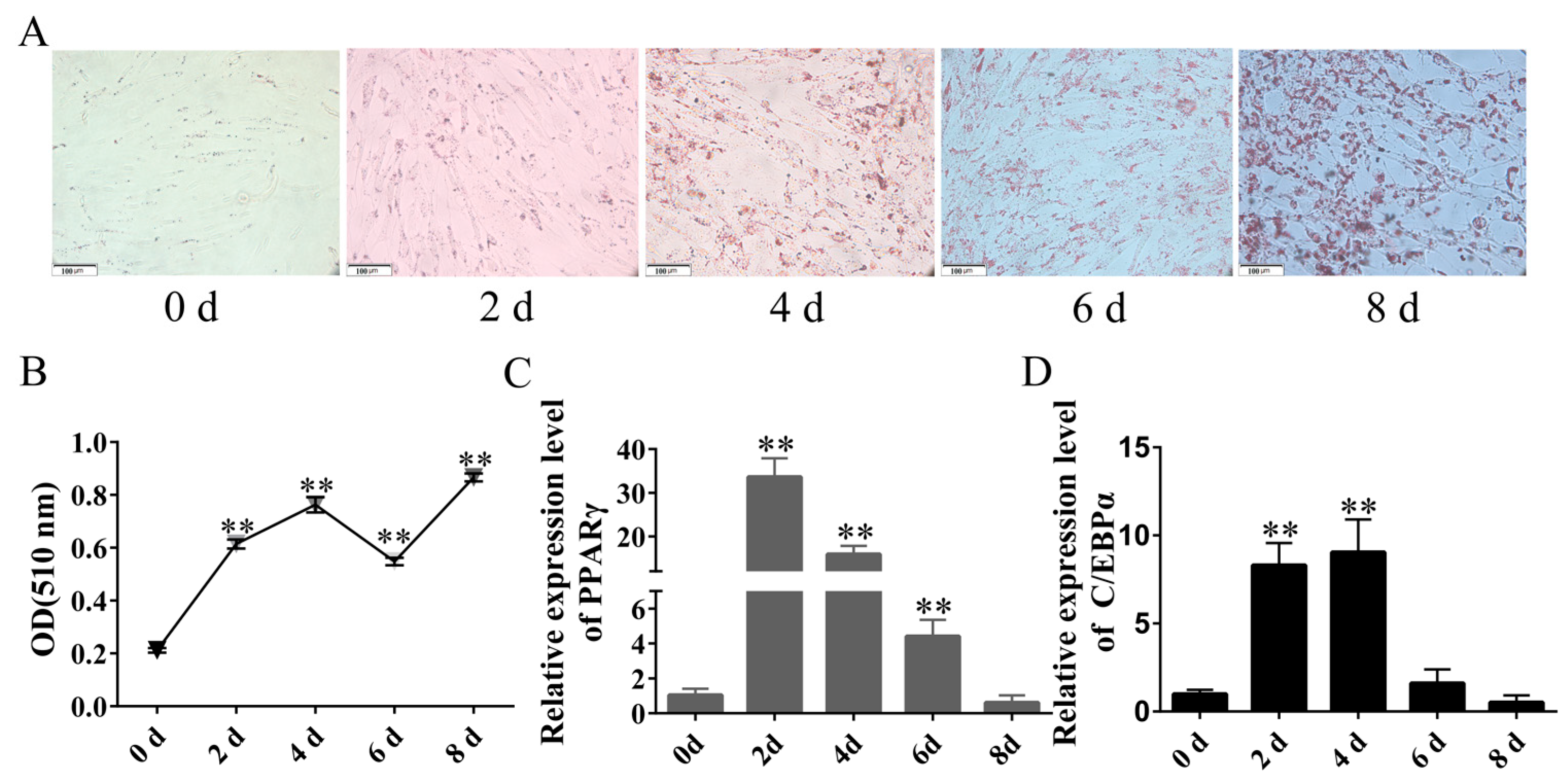
2.2. Isolation and Culture and Establishment of Rabbit Differentiation Model of Rabbit Preadipocytes
2.3. Effect of LncRNA-MSTRG4710 on Proliferation and Differentiation of Rabbit Preadipocytes
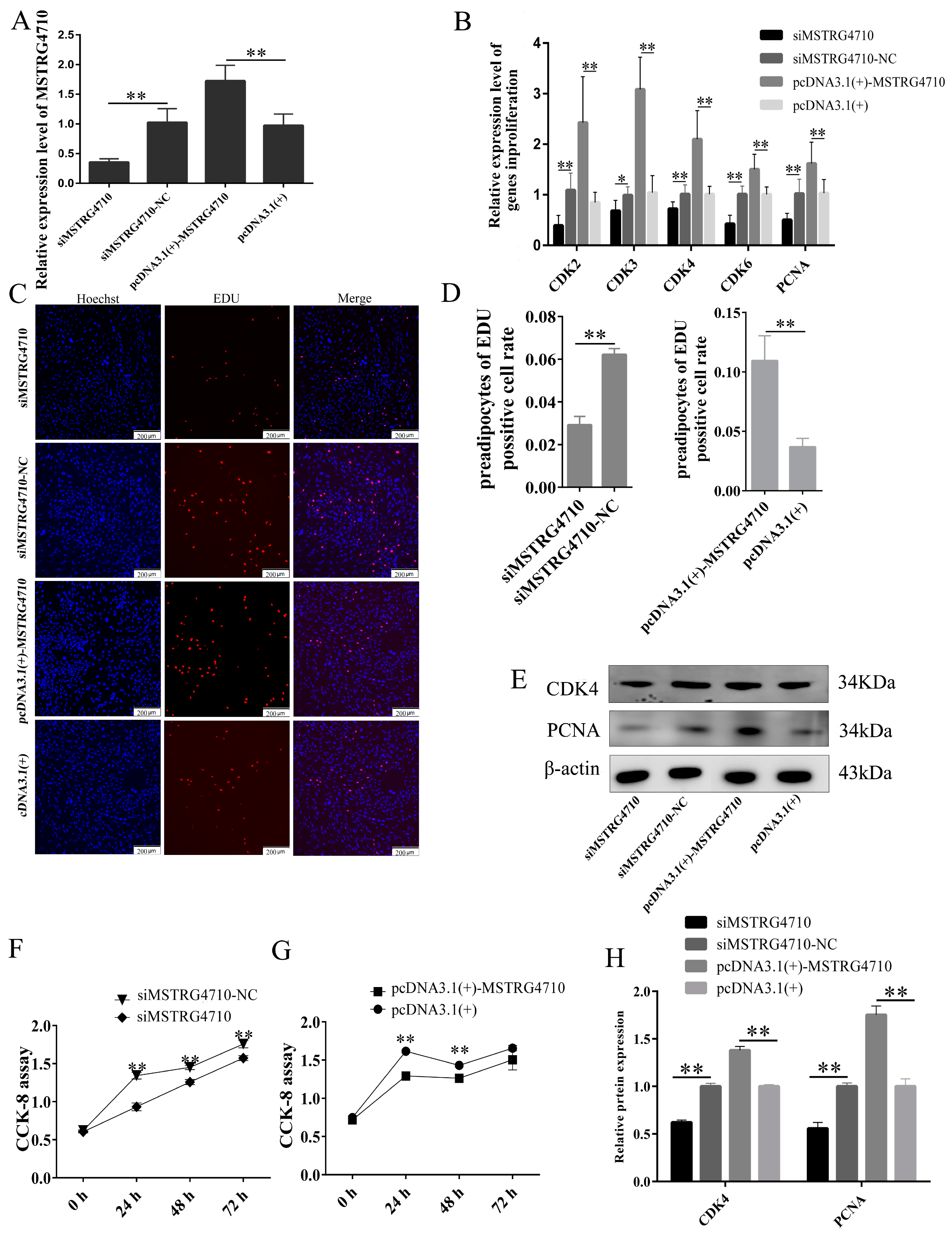
2.3.1. LncRNA-MSTRG4710 Promotes the Proliferation of Rabbit Preadipocytes
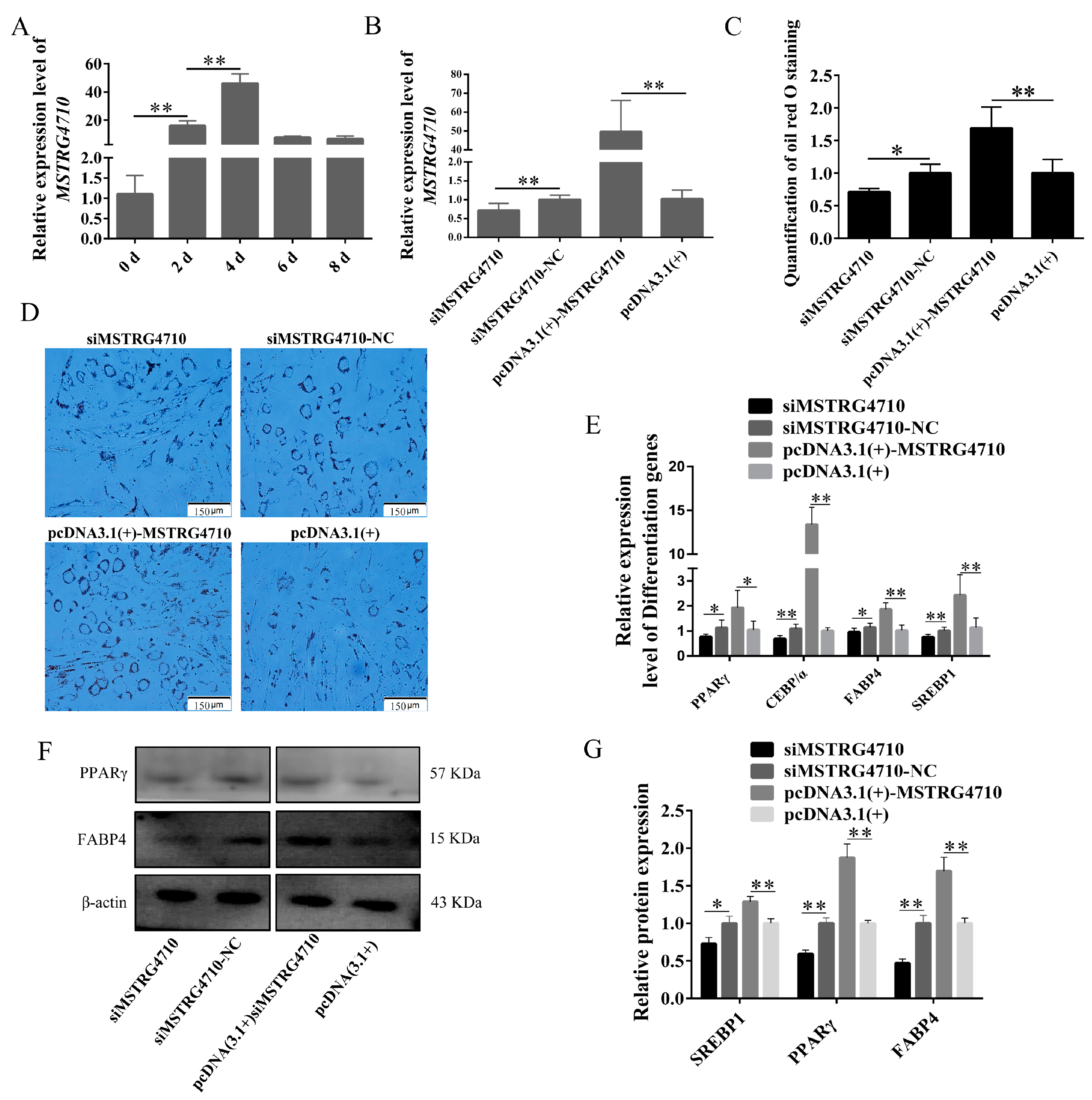
2.3.2. LncRNA-MSTRG4710 Promotes the Differentiation of Rabbit Preadipocytes
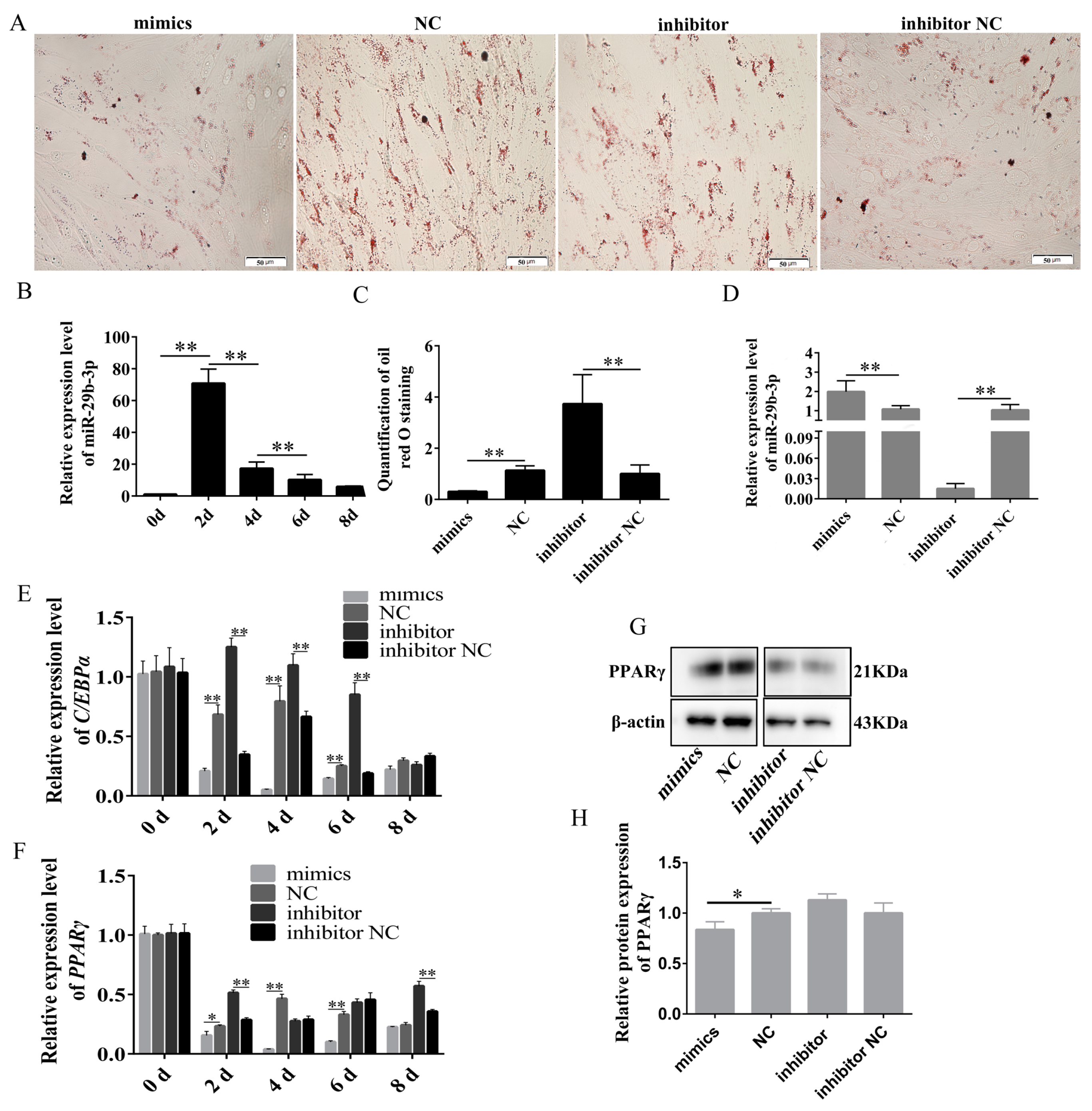
2.4. Effect of miR-29b-3p Proliferation and Differentiation of Rabbit Preadipocytes
2.4.1. miR-29b-3p Inhibited the Proliferation of Rabbit Preadipocytes
2.4.2. miR-29b-3p Inhibits the Differentiation of Rabbit Preadipocytes
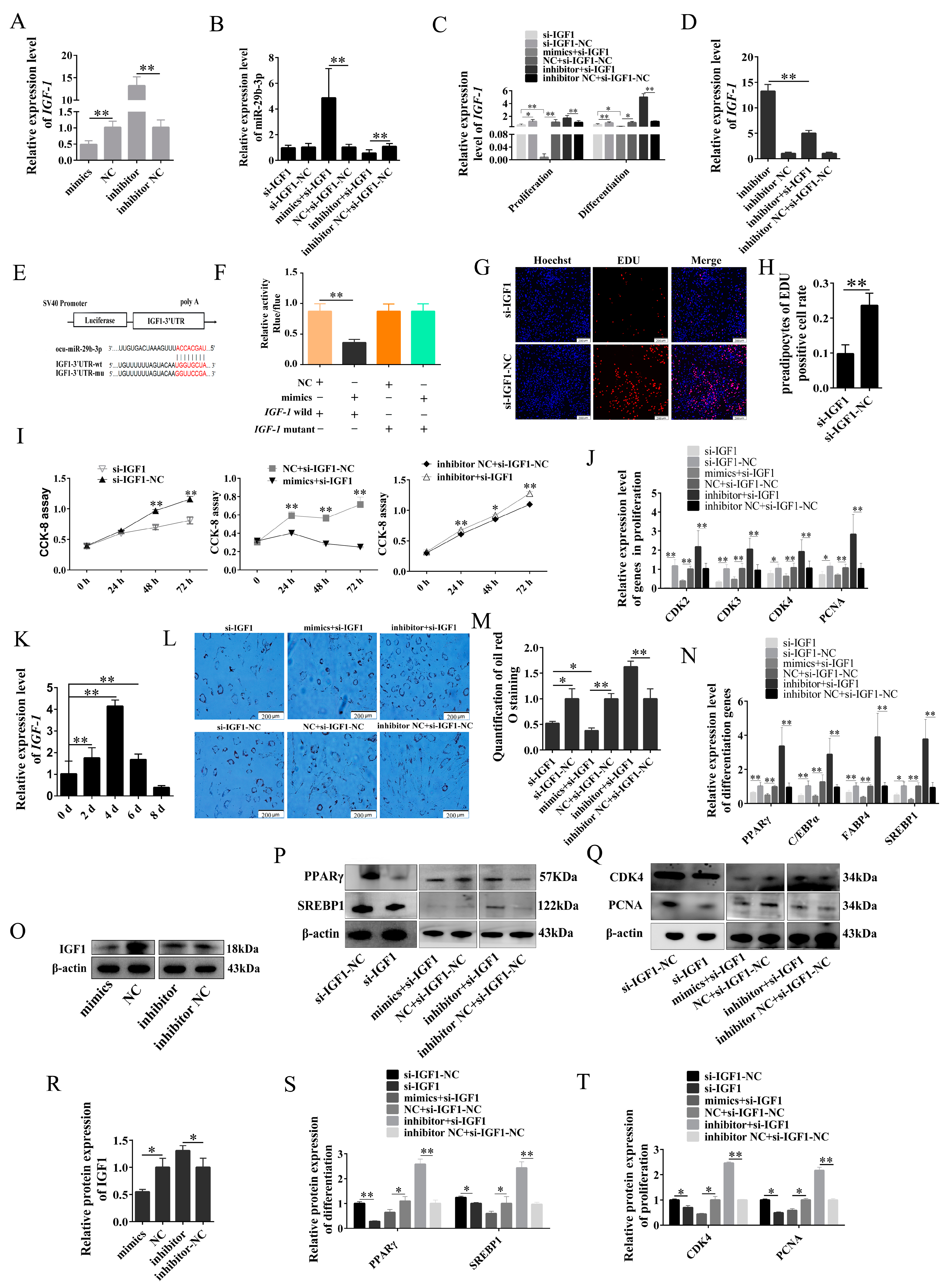
2.5. miR-29b-3p Inhibits the Proliferation and Differentiation of Rabbit Preadipocytes by Targeting IGF1
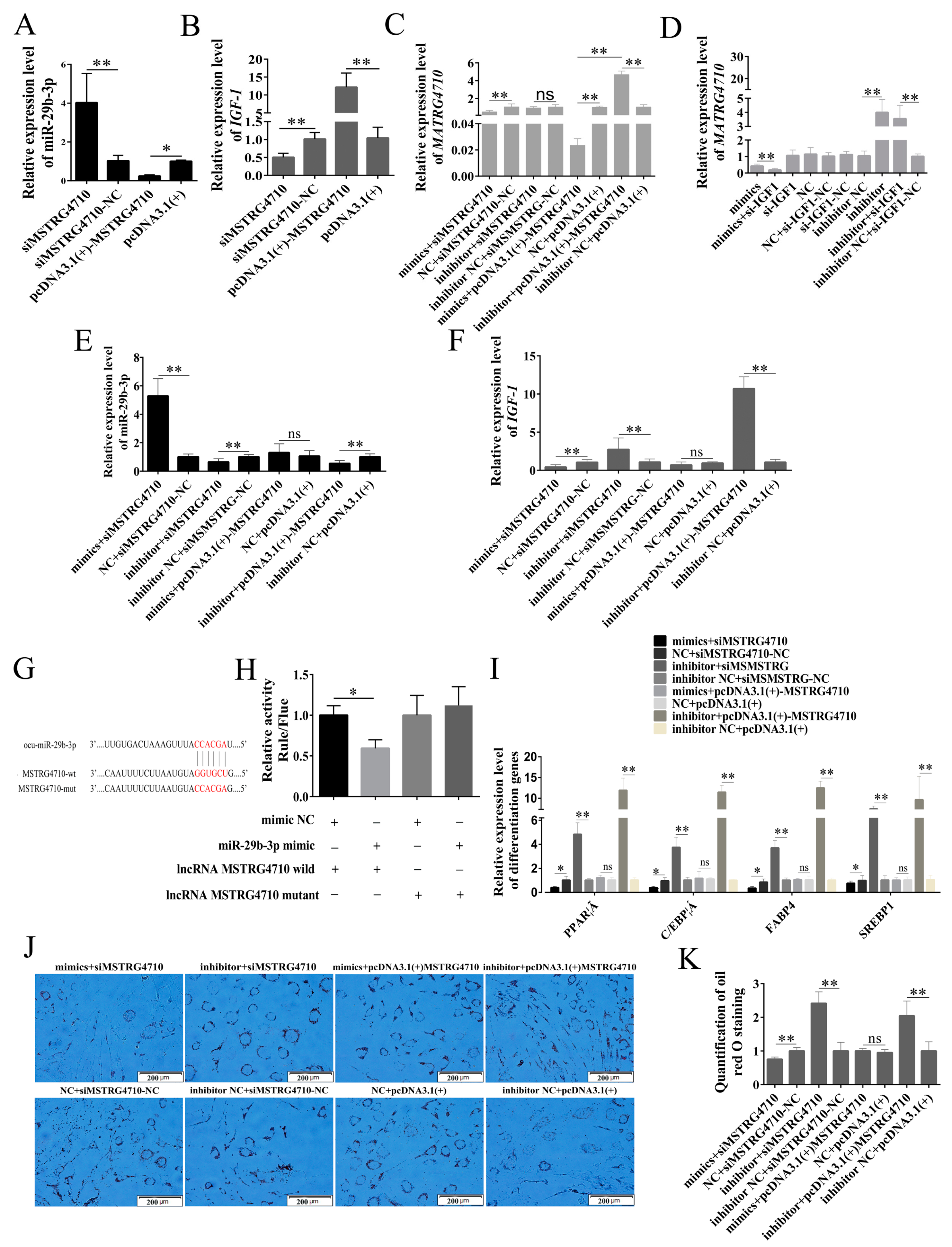
2.6. MSTRG4710 Promotes the Differentiation of Rabbit Preadipocytes via the miR-29b-3p/IGF1 Pathway
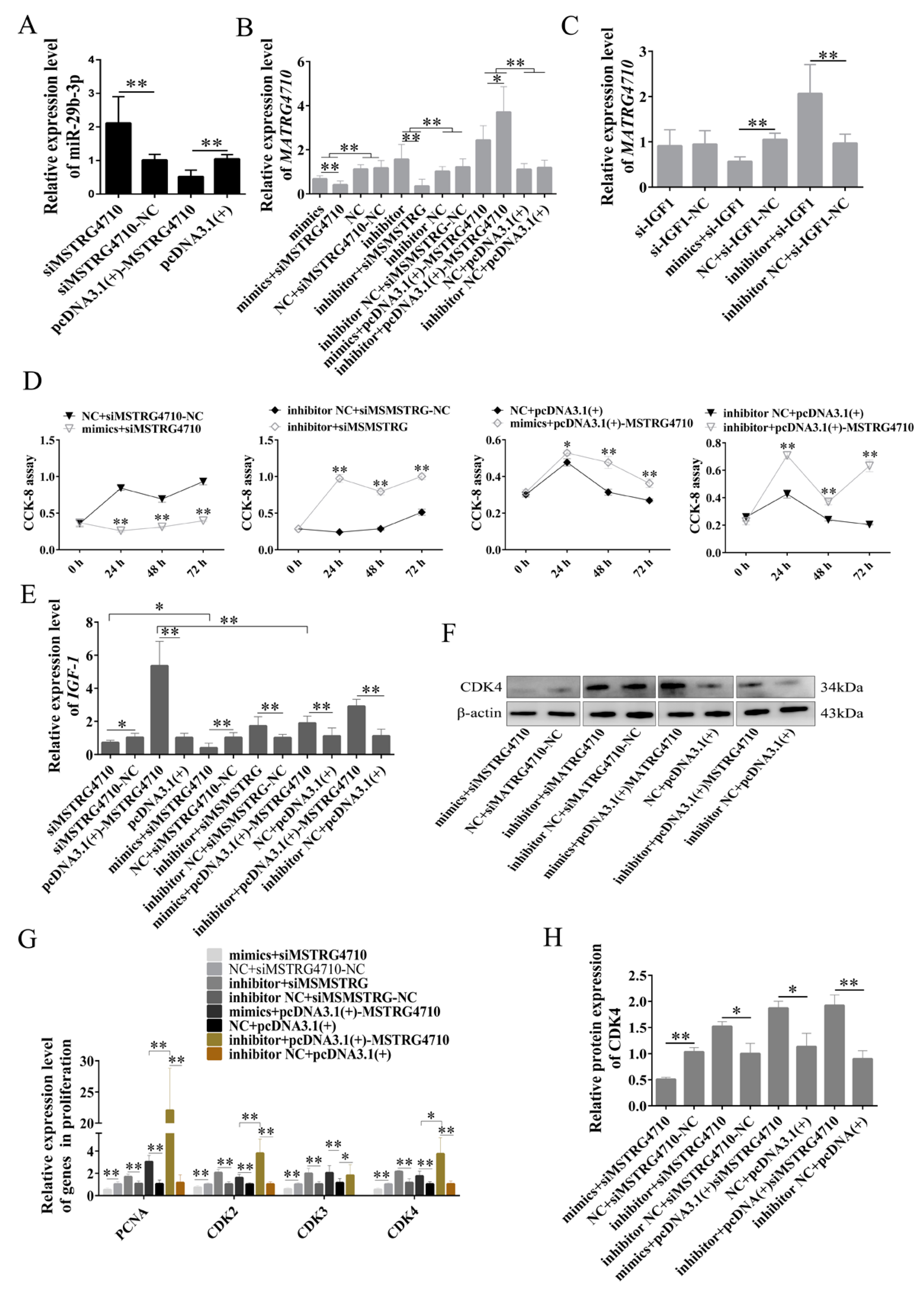
2.7. MSTRG4710 Promotes the Proliferation of Rabbit Preadipocytes via the miR-29b-3p/IGF1 Pathway
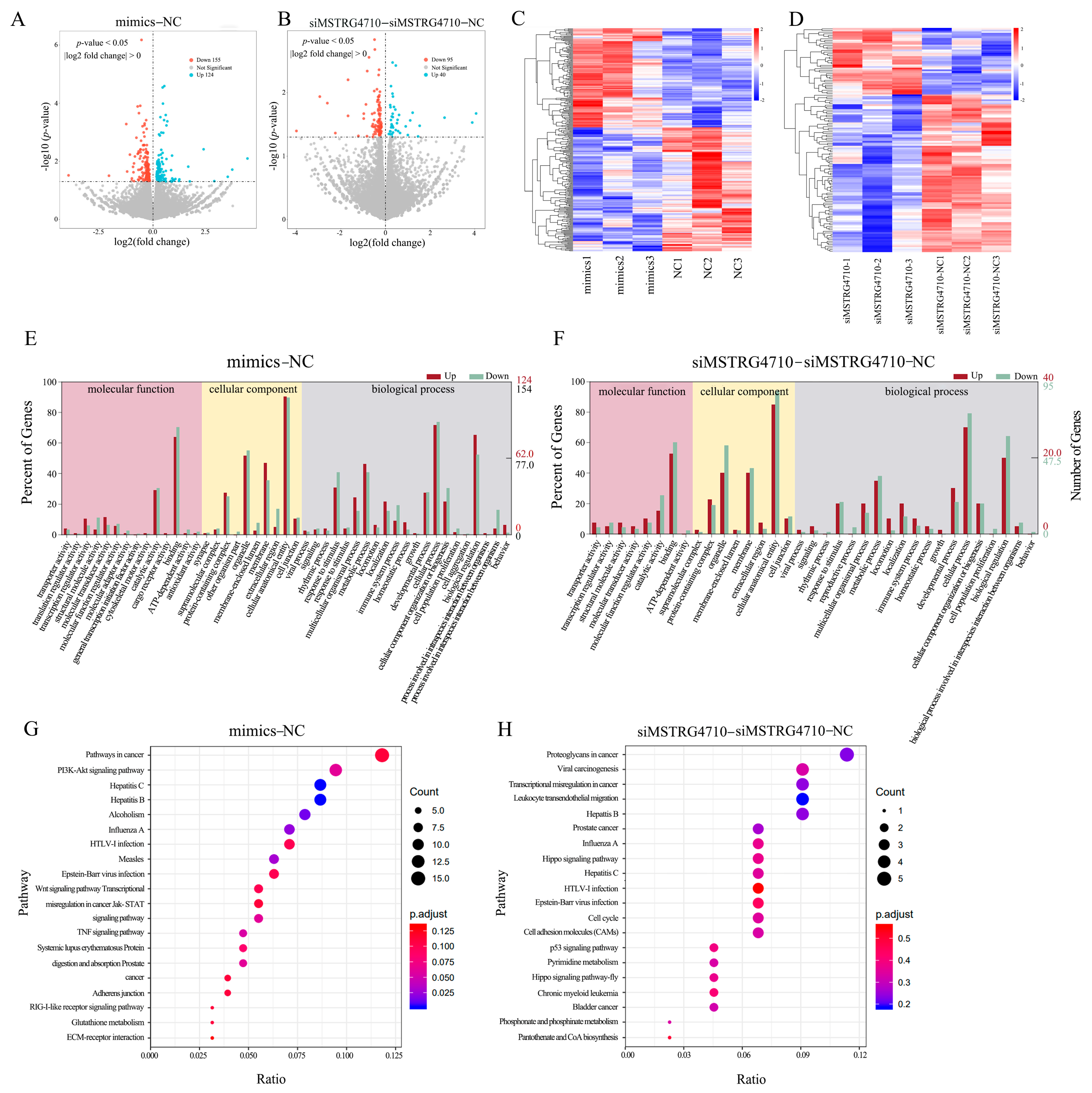
2.8. Analysis of Differentially Expressed Genes
3. Discussion
4. Materials and Methods
4.1. Animals and Sample Collection
4.2. Culture and Differentiation of Rabbit Preadipocytes
4.3. Plasmid Construction and Cell Transfection
4.4. Rapid Amplification of cDNA Ends Assay
4.5. Target Gene Prediction and Luciferase Reporter Assay
4.6. Oil Red O Staining and Lipid Quantification
4.7. Cell Proliferation Analysis CCK-8 and EDU Proliferation Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction Analysis
4.9. Western Blot Analysis
4.10. RNA-Seq
4.11. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public Health 2020, 53, 98–105. [Google Scholar] [CrossRef]
- Chu, D.-T.; Minh Nguyet, N.T.; Nga, V.T.; Thai Lien, N.V.; Vo, D.D.; Lien, N.; Nhu Ngoc, V.T.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 13, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, E.; Wing, A.; Holtrup, B.; Sebo, Z.; Kaplan, J.L.; Saavedra-Peña, R.; Church, C.D.; Colman, L.; Berry, R.; Rodeheffer, M.S. The Adipose Tissue Microenvironment Regulates Depot-Specific Adipogenesis in Obesity. Cell Metab. 2016, 24, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016, 26, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Jathar, S.; Kumar, V.; Srivastava, J.; Tripathi, V. Technological Developments in lncRNA Biology. Adv. Exp. Med. Biol. 2017, 1008, 283–323. [Google Scholar] [PubMed]
- Chen, X.; Sun, Y.; Cai, R.; Wang, G.; Shu, X.; Pang, W. Long noncoding RNA: Multiple players in gene expression. BMB Rep. 2018, 51, 280–289. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.-M.; Chang, Y.-N.; Hu, Z.-M.; Qi, H.-X.; Hong, W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene 2014, 547, 1–9. [Google Scholar] [CrossRef]
- Dey, B.K.; Mueller, A.C.; Dutta, A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription 2014, 5, e944014. [Google Scholar] [CrossRef]
- Li, P.; Ruan, X.; Yang, L.; Kiesewetter, K.; Zhao, Y.; Luo, H.; Chen, Y.; Gucek, M.; Zhu, J.; Cao, H. A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab. 2015, 21, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Chai, X.-R.; Yoon, M.J.; Kim, H.-J.; Lo, K.A.; Zhang, Z.-C.; Xu, D.; Siang, D.T.C.; Walet, A.C.E.; Xu, S.-H.; et al. Dynamic transcriptome changes during adipose tissue energy expenditure reveal critical roles for long noncoding RNA regulators. PLoS Biol. 2017, 15, e2002176. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Rajewsky, N. The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 2007, 8, 93–103. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Iqbal, A.; Ali, S.; Gao, Z.; Pan, Z.; Xia, L.; Yin, F.; Zhao, Z. MiR-485 targets the DTX4 gene to regulate milk fat synthesis in bovine mammary epithelial cells. Sci. Rep. 2021, 11, 7623. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Duan, Y.; Sang, Y.; Li, Y.; Zhang, H.; Liang, Y.; Liu, Y.; Zhang, N.; Yang, Q. LncRNA-CDC6 promotes breast cancer progression and function as ceRNA to target CDC6 by sponging microRNA-215. J. Cell Physiol. 2019, 234, 9105–9117. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Cai, H.; Sun, Y.; Plath, M.; Li, C.; Lan, X.; Lei, C.; Lin, F.; Bai, Y.; et al. Long non-coding RNA ADNCR suppresses adipogenic differentiation by targeting miR-204. Biochim. Biophys. Acta 2016, 1859, 871–882. [Google Scholar] [CrossRef]
- Wang, J.; Shao, J.; Li, Y.; Elzo, M.A.; Jia, X.; Lai, S. Genome-wide identification and characterization of perirenal adipose tissue microRNAs in rabbits fed a high-fat diet. Biosci. Rep. 2021, 41, BSR20204297. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Shao, J.; Liu, Z.; Fu, C.; Chen, G.; Zhao, K.; Li, H.; Sun, W.; Jia, X.; et al. Combined analysis of differentially expressed lncRNAs and miRNAs in liver tissues of high-fat fed rabbits by transcriptome sequencing. Front. Genet. 2022, 13, 1000574. [Google Scholar] [CrossRef]
- Wei, S.; Du, M.; Jiang, Z.; Hausman, G.J.; Zhang, L.; Dodson, M.V. Long noncoding RNAs in regulating adipogenesis: New RNAs shed lights on obesity. Cell. Mol. Life Sci. 2016, 73, 2079–2087. [Google Scholar] [CrossRef]
- Lea-Currie, Y.R.; Monroe, D.; Mcintosh, M.K. Dehydroepiandrosterone and related steroids alter 3T3-L1 preadipocyte proliferation and differentiation. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 123, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Zou, T.; Wang, B.; Yang, Q.; de Avila, J.M.; Zhu, M.-J.; You, J.; Chen, D.; Du, M. Raspberry promotes brown and beige adipocyte development in mice fed high-fat diet through activation of AMP-activated protein kinase (AMPK) α1. J. Nutr. Biochem. 2018, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.; Héon, E.; Zhen, M. Ciliary dysfunction and obesity. Clin. Genet. 2010, 77, 18–27. [Google Scholar] [CrossRef]
- Laudes, M. Role of WNT signaling in the determination of human mesemchymal stem cells into preadipocytes. J. Mol. Endocrinol. 2011, 46, 65–72. [Google Scholar]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; CDC National Center for Health Statistics: Hyattsville, MD, USA, 2020; pp. 1–8. [Google Scholar]
- He, H.; Cai, M.; Zhu, J.; Xiao, W.; Liu, B.; Shi, Y.; Yang, X.; Liang, X.; Zheng, T.; Hu, S.; et al. miR-148a-3p promotes rabbit preadipocyte differentiation by targeting PTEN. In Vitro Cell Dev. Biol. Anim. 2018, 54, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef]
- Anoushepour, A.; Mottaghian, P.; Sakha, M. The comparison of some biochemical parameters in hyperketonemic and normal ewes. iMedPub 2014, 4, 83–87. [Google Scholar]
- Rosen, E.D.; Spiegelman, B.M. PPARγ: A Nuclear Regulator of Metabolism, Differentiation, and Cell Growth. J. Biol. Chem. 2001, 276, 37731–37734. [Google Scholar] [CrossRef] [PubMed]
- Makowski, L.; Hotamisligil, G.S. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr. Opin. Infect. Dis. 2005, 16, 543–548. [Google Scholar] [CrossRef]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- Sun, L.; Goff, L.A.; Trapnell, C.; Alexander, R.; Lo, K.A.; Hacisuleyman, E.; Sauvageau, M.; Tazon-Vega, B.; Kelley, D.R.; Hendrickson, D.G.; et al. Long noncoding RNAs regulate adipogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 3387–3392. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, J.R.; Bai, Z.; Xu, D.; Yuan, B.; Lo, K.A.; Yoon, M.J.; Lim, Y.C.; Knoll, M.; Slavov, N.; Chen, S.; et al. De Novo reconstruction of adipose tissue transcriptomes reveals long non-coding RNA regulators of brown adipocyte development. Cell Metab. 2015, 21, 764–776. [Google Scholar] [CrossRef]
- Thunen, A.; La Placa, D.; Zhang, Z.; Shively, J.E. Role of lncRNA LIPE-AS1 in adipogenesis. Adipocyte 2022, 11, 11–27. [Google Scholar] [CrossRef]
- Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Calviello, L.; Hirsekorn, A.; de Pretis, S.; Pelizzola, M.; Ohler, U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat. Struct. Mol. Biol. 2017, 24, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Yao, B.; Li, Q.; Wang, L.; Wang, C.; Dou, C.; Xu, M.; Liu, Q.; Tu, K. Long non-coding RNA CASC2 suppresses epithelial-mesenchymal transition of hepatocellular carcinoma cells through CASC2/miR-367/FBXW7 axis. Mol. Cancer 2017, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Van Tienen, F.H.J.; Laeremans, H.; Van Der Kallen, C.J.H.; Smeets, H.J.M. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem. Biophys. Res. Commun. 2009, 387, 207–211. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Ji, M.; Rong, X.; Zhang, Y.; Yang, S.; Lu, C.; Cai, C.; Gao, P.; Guo, X.; et al. The long non-coding RNA lncMYOZ2 mediates an AHCY/MYOZ2 axis to promote adipogenic differentiation in porcine preadipocytes. BMC Genom. 2022, 23, 700. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Yang, Q.-Y.; Hu, Y.; Liu, X.-D.; de Avila, J.M.; Zhu, M.-J.; Nathanielsz, P.W.; Du, M. Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance. Nat. Commun. 2021, 12, 6845. [Google Scholar] [CrossRef]
- Xiao, T.; Liu, L.; Li, H.; Sun, Y.; Luo, H.; Li, T.; Wang, S.; Dalton, S.; Zhao, R.C.; Chen, R. Long noncoding RNA ADINR regulates adipogenesis by transcriptionally activating C/EBPα. Stem. Cell Rep. 2015, 5, 856–865. [Google Scholar] [CrossRef]
- Tsurimoto, T. PCNA binding proteins. Front. Biosci. 1999, 4, 849–858. [Google Scholar] [CrossRef]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, Y.M.; Su, Y.D.; Zuo, F.; Wu, B.; Nian, X. MiR-26a regulated adipogenic differentiation of ADSCs induced by insulin through CDK5/FOXC2 pathway. Mol. Cell Biochem. 2021, 476, 1705–1716. [Google Scholar] [CrossRef]
- Drummond, C.A.; Fan, X.; Haller, S.T.; Kennedy, D.J.; Liu, J.; Tian, J. Na/K-ATPase signaling mediates miR-29b-3p regulation and cardiac fibrosis formation in mice with chronic kidney disease. PLoS ONE 2018, 13, e0197688. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Lu, S. lncRNA H19 promotes viability and epithelial-mesenchymal transition of lung adenocarcinoma cells by targeting miR-29b-3p and modifying STAT3. Int. J. Oncol. 2019, 54, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Pan, X.; Zhang, M.; Huang, W.; Liu, Y.; Yang, H.; Cheng, Z.; Zhang, G.; Qie, M.; et al. LncRNA MIR99AHG enhances adipocyte differentiation by targeting miR-29b-3p to upregulate PPARγ. Mol. Cell Endocrinol. 2022, 550, 111648. [Google Scholar] [CrossRef]
- Szatkowski, C.; Vallet, J.; Dormishian, M.; Messaddeq, N.; Valet, P.; Boulberdaa, M.; Metzger, D.; Chambon, P.; Nebigil, C.G. Prokineticin receptor 1 as a novel suppressor of preadipocyte proliferation and differentiation to control obesity. PLoS ONE 2013, 8, e81175. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kamagate, A.; Qu, S.; Perdomo, G.; Su, D.; Kim, D.H.; Slusher, S.; Meseck, M.; Dong, H.H. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Investig. 2008, 118, 2347–2364. [Google Scholar] [CrossRef]
- Heald, A.H.; Kaushal, K.; Siddals, K.W.; Rudenski, A.S.; Anderson, S.G.; Gibson, J.M. Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp. Clin. Endocrinol. Diabetes 2006, 114, 371–376. [Google Scholar] [CrossRef]
- Holzenberger, M.; Hamard, G.; Zaoui, R.; Leneuve, P.; Ducos, B.; Beccavin, C.; Périn, L.; Le Bouc, Y. Experimental IGF-I receptor deficiency generates a sexually dimorphic pattern of organ-specific growth deficits in mice, affecting fat tissue in particular. Endocrinology 2001, 142, 4469–4478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T. Lessons from X-chromosome inactivation: Long ncRNA as guides and tethers to the epigenome. Genes Dev. 2009, 23, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wu, M.; Wang, C.; Liu, R.; Zhao, H.; Yang, L.; Liu, J.; Wang, Y.; Zhang, S.; Yuan, Z.; et al. Long Noncoding RNA Lnc-SEMT Modulates IGF2 Expression by Sponging miR-125b to Promote Sheep Muscle Development and Growth. Cell Physiol. Biochem. 2018, 49, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gerin, I.; Miao, H.; Vu-Phan, D.; Johnson, C.N.; Xu, R.; Chen, X.-W.; Cawthorn, W.P.; MacDougald, O.A.; Koenig, R.J. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS ONE 2010, 5, e14199. [Google Scholar] [CrossRef]
- Prestwich, T.C.; Macdougald, O.A. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Bree, A.J.; Yao, Y.; Du, B.; Hemati, N.; Martinez-Santibañez, G.; MacDougald, O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012, 50, 477–489. [Google Scholar] [CrossRef]
- Rawlings, J.S. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Leonard, W.J. Role of Jak kinases and STATs in cytokine signal transduction. Int. J. Hematol. 2001, 73, 271–277. [Google Scholar] [CrossRef]

| Sample | Clean Reads | Q30 (%) | GC (%) | Total Mapping (%) | Exon (%) | Intron (%) | Intergenic (%) |
|---|---|---|---|---|---|---|---|
| mimics 1 | 46,291,190 | 94.67 | 56.41 | 87.80 | 67.35 | 15.37 | 17.28 |
| mimics 2 | 67,471,608 | 95.06 | 56.73 | 87.88 | 68.92 | 14.23 | 16.85 |
| mimics 3 | 44,188,296 | 94.69 | 56.71 | 87.74 | 67.52 | 15.48 | 17.00 |
| NC1 | 57,343,036 | 94.83 | 56.15 | 88.39 | 67.38 | 15.72 | 16.89 |
| NC2 | 46,680,152 | 94.12 | 56.70 | 87.70 | 68.69 | 15.40 | 15.91 |
| NC3 | 58,249,758 | 94.89 | 56.64 | 88.07 | 67.69 | 15.08 | 17.22 |
| siMSTRG4710-1 | 75,961,000 | 95.25 | 56.64 | 88.43 | 65.86 | 16.63 | 17.51 |
| siMSTRG4710-2 | 75,068,706 | 95.47 | 57.32 | 87.75 | 66.61 | 16.80 | 16.59 |
| siMSTRG4710-3 | 46,202,572 | 94.50 | 56.96 | 87.20 | 67.67 | 14.71 | 17.62 |
| siMSTRG4710-NC1 | 46,667,670 | 94.33 | 56.50 | 87.85 | 66.54 | 16.60 | 16.86 |
| siMSTRG4710-NC2 | 44,768,540 | 94.78 | 56.78 | 87.89 | 66.95 | 16.78 | 16.27 |
| siMSTRG4710-NC3 | 74,122,238 | 95.43 | 56.85 | 88.02 | 67.69 | 15.45 | 16.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Jiang, G.; Shao, J.; Wang, M.; Zhang, X.; Xia, S.; Sun, W.; Jia, X.; Wang, J.; Lai, S. lncRNA MSTRG4710 Promotes the Proliferation and Differentiation of Preadipocytes through miR-29b-3p/IGF1 Axis. Int. J. Mol. Sci. 2023, 24, 15715. https://doi.org/10.3390/ijms242115715
Tang T, Jiang G, Shao J, Wang M, Zhang X, Xia S, Sun W, Jia X, Wang J, Lai S. lncRNA MSTRG4710 Promotes the Proliferation and Differentiation of Preadipocytes through miR-29b-3p/IGF1 Axis. International Journal of Molecular Sciences. 2023; 24(21):15715. https://doi.org/10.3390/ijms242115715
Chicago/Turabian StyleTang, Tao, Genglong Jiang, Jiahao Shao, Meigui Wang, Xiaoxiao Zhang, Siqi Xia, Wenqiang Sun, Xianbo Jia, Jie Wang, and Songjia Lai. 2023. "lncRNA MSTRG4710 Promotes the Proliferation and Differentiation of Preadipocytes through miR-29b-3p/IGF1 Axis" International Journal of Molecular Sciences 24, no. 21: 15715. https://doi.org/10.3390/ijms242115715
APA StyleTang, T., Jiang, G., Shao, J., Wang, M., Zhang, X., Xia, S., Sun, W., Jia, X., Wang, J., & Lai, S. (2023). lncRNA MSTRG4710 Promotes the Proliferation and Differentiation of Preadipocytes through miR-29b-3p/IGF1 Axis. International Journal of Molecular Sciences, 24(21), 15715. https://doi.org/10.3390/ijms242115715






