In Vivo Serotonin 5-HT2A Receptor Availability and Its Relationship with Aggression Traits in Healthy Individuals: A Positron Emission Tomography Study with C-11 MDL100907
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Clinical Assessment
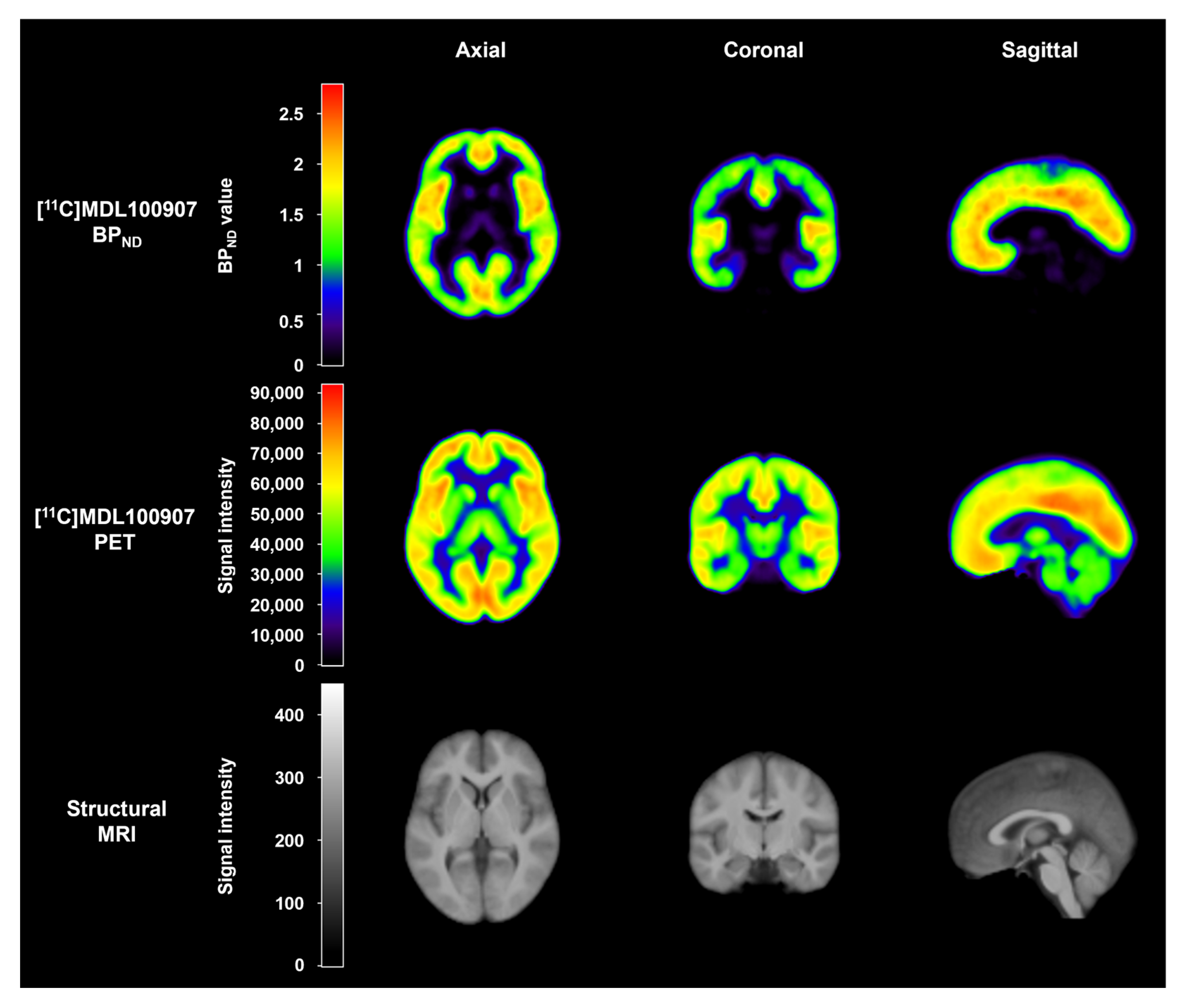
4.3. Image Acquisition
4.4. Image analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manhães de Castro, R.; Barreto Medeiros, J.M.; Mendes da Silva, C.; Ferreira, L.M.; Guedes, R.C.; Cabral Filho, J.E.; Costa, J.A. Reduction of Intraspecific Aggression in Adult Rats by Neonatal Treatment with a Selective Serotonin Reuptake Inhibitor. Braz. J. Med. Biol. Res. 2001, 34, 121–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giammanco, M.; Tabacchi, G.; Giammanco, S.; Di Majo, D.; La Guardia, M. Testosterone and Aggressiveness. Med. Sci. Monit. 2005, 11, RA136–RA145. [Google Scholar]
- da Cunha-Bang, S.; Knudsen, G.M. The Modulatory Role of Serotonin on Human Impulsive Aggression. Biol. Psychiatry 2021, 90, 447–457. [Google Scholar] [CrossRef]
- Bortolato, M.; Pivac, N.; Seler, D.M.; Perkovic, M.N.; Pessia, M.; Di Giovanni, G. The Role of Serotonergic System at the Interface of Aggression and Suicide. Neuroscience 2013, 236, 160–185. [Google Scholar] [CrossRef]
- Rylands, A.J.; Hinz, R.; Jones, M.; Holmes, S.E.; Feldmann, M.; Brown, G.; McMahon, A.W.; Talbot, P.S. Pre- and Postsynaptic Serotonergic Differences in Males with Extreme Levels of Impulsive Aggression without Callous Unemotional Traits: A Positron Emission Tomography Study Using 11C-DASB and 11C-MDL100907. Biol. Psychiatry 2012, 72, 1004–1011. [Google Scholar] [CrossRef]
- Rosell, D.R.; Thompson, J.L.; Slifstein, M.; Xu, X.; Frankle, W.G.; New, A.S.; Goodman, M.; Weinstein, S.R.; Laruelle, M.; Abi-Dargham, A.; et al. Increased Serotonin 2A Receptor Availability in the Orbitofrontal Cortex of Physically Aggressive Personality Disordered Patients. Biol. Psychiatry 2010, 67, 1154–1162. [Google Scholar] [CrossRef]
- Meyer, J.H.; Wilson, A.A.; Rusjan, P.; Clark, M.; Houle, S.; Woodside, S.; Arrowood, J.; Martin, K.; Colleton, M. Serotonin2A Receptor Binding Potential in People with Aggressive and Violent Behaviour. J. Psychiatry Neurosci. 2008, 33, 499–508. [Google Scholar]
- da Cunha-Bang, S.; Hjordt, L.V.; Perfalk, E.; Beliveau, V.; Bock, C.; Lehel, S.; Thomsen, C.; Sestoft, D.; Svarer, C.; Knudsen, G.M. Serotonin 1B Receptor Binding Is Associated with Trait Anger and Level of Psychopathy in Violent Offenders. Biol. Psychiatry 2017, 82, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Frankle, W.G.; Lombardo, I.; New, A.S.; Goodman, M.; Talbot, P.S.; Huang, Y.; Hwang, D.-R.; Slifstein, M.; Curry, S.; Abi-Dargham, A.; et al. Brain Serotonin Transporter Distribution in Subjects with Impulsive Aggressivity: A Positron Emission Study with [11C]McN 5652. Am. J. Psychiatry 2005, 162, 915–923. [Google Scholar] [CrossRef] [PubMed]
- van de Giessen, E.; Rosell, D.R.; Thompson, J.L.; Xu, X.; Girgis, R.R.; Ehrlich, Y.; Slifstein, M.; Abi-Dargham, A.; Siever, L.J. Serotonin Transporter Availability in Impulsive Aggressive Personality Disordered Patients: A PET Study with [11C]DASB. J. Psychiatr. Res. 2014, 58, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; George, D.T.; Fujita, M.; Liow, J.-S.; Ichise, M.; Hibbeln, J.; Ghose, S.; Sangare, J.; Hommer, D.; Innis, R.B. PET [11C]DASB Imaging of Serotonin Transporters in Patients with Alcoholism. Alcohol. Clin. Exp. Res. 2007, 31, 28–32. [Google Scholar] [CrossRef]
- Braccagni, G.; Scheggi, S.; Bortolato, M. Elevated Levels of Serotonin 5-HT2A Receptors in the Orbitofrontal Cortex of Antisocial Individuals. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Greitemeyer, T.; Sagioglou, C. Increasing Wealth Inequality May Increase Interpersonal Hostility: The Relationship between Personal Relative Deprivation and Aggression. J. Soc. Psychol. 2017, 157, 766–776. [Google Scholar] [CrossRef]
- Bernards, S.; Graham, K. The Cross-Cultural Association Between Marital Status and Physical Aggression between Intimate Partners. J. Fam. Violence 2013, 28, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Bushman, B.J.; Huesmann, L.R. Short-Term and Long-Term Effects of Violent Media on Aggression in Children and Adults. Arch. Pediatr. Adolesc. Med. 2006, 160, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Bushman, B.J. Media Violence and the General Aggression Model. J. Soc. Issues 2018, 74, 386–413. [Google Scholar] [CrossRef]
- Khurana, A.; Bleakley, A.; Ellithorpe, M.E.; Hennessy, M.; Jamieson, P.E.; Weitz, I. Media Violence Exposure and Aggression in Adolescents: A Risk and Resilience Perspective. Aggress. Behav. 2019, 45, 70–81. [Google Scholar] [CrossRef]
- Korfine, L.; Hooley, J.M. Detecting Individuals with Borderline Personality Disorder in the Community: An Ascertainment Strategy and Comparison with a Hospital Sample. J. Pers. Disord. 2009, 23, 62–75. [Google Scholar] [CrossRef][Green Version]
- Fonseca-Pedrero, E.; Paino, M.; Lemos-Giráldez, S.; Sierra-Baigrie, S.; González, M.P.G.-P.; Bobes, J.; Muňiz, J. Borderline Personality Traits in Nonclinical Young Adults. J. Pers. Disord. 2011, 25, 542–556. [Google Scholar] [CrossRef]
- Lim, Y.-O.; Suh, K.-H. Development and Validation of a Measure of Passive Aggression Traits: The Passive Aggression Scale (PAS). Behav. Sci. 2022, 12, 273. [Google Scholar] [CrossRef]
- Parsey, R.V.; Oquendo, M.A.; Simpson, N.R.; Ogden, R.T.; Van Heertum, R.; Arango, V.; Mann, J.J. Effects of Sex, Age, and Aggressive Traits in Man on Brain Serotonin 5-HT1A Receptor Binding Potential Measured by PET Using [C-11]WAY-100635. Brain Res. 2002, 954, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Flöel, A.; Stein, P.; Savli, M.; Mien, L.-K.; Wadsak, W.; Spindelegger, C.; Moser, U.; Fink, M.; Hahn, A.; et al. Aggression Is Related to Frontal Serotonin-1A Receptor Distribution as Revealed by PET in Healthy Subjects. Hum. Brain Mapp. 2009, 30, 2558–2570. [Google Scholar] [CrossRef] [PubMed]
- da Cunha-Bang, S.; Stenbæk, D.S.; Holst, K.; Licht, C.L.; Jensen, P.S.; Frokjaer, V.G.; Mortensen, E.L.; Knudsen, G.M. Trait Aggression and Trait Impulsivity Are Not Related to Frontal Cortex 5-HT2A Receptor Binding in Healthy Individuals. Psychiatry Res. 2013, 212, 125–131. [Google Scholar] [CrossRef] [PubMed]
- da Cunha-Bang, S.; Mc Mahon, B.; MacDonald Fisher, P.; Jensen, P.S.; Svarer, C.; Moos Knudsen, G. High Trait Aggression in Men Is Associated with Low 5-HT Levels, as Indexed by 5-HT4 Receptor Binding. Soc. Cogn. Affect. Neurosci. 2016, 11, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Giegling, I.; Hartmann, A.M.; Möller, H.-J.; Rujescu, D. Anger- and Aggression-Related Traits Are Associated with Polymorphisms in the 5-HT-2A Gene. J. Affect. Disord. 2006, 96, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Mikolajewski, A.J. Preliminary Evidence That Specific Candidate Genes Are Associated with Adolescent-Onset Antisocial Behavior. Aggress. Behav. 2008, 34, 437–445. [Google Scholar] [CrossRef]
- Buss, A.H.; Perry, M. The Aggression Questionnaire. J. Personal. Social. Psychol. 1992, 63, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Frau, R.; Pardu, A.; Godar, S.; Bini, V.; Bortolato, M. Combined Antagonism of 5-HT2 and NMDA Receptors Reduces the Aggression of Monoamine Oxidase a Knockout Mice. Pharmaceuticals 2022, 15, 213. [Google Scholar] [CrossRef]
- Godar, S.C.; Mosher, L.J.; Scheggi, S.; Devoto, P.; Moench, K.M.; Strathman, H.J.; Jones, C.M.; Frau, R.; Melis, M.; Gambarana, C.; et al. Gene-Environment Interactions in Antisocial Behavior Are Mediated by Early-Life 5-HT2A Receptor Activation. Neuropharmacology 2019, 159, 107513. [Google Scholar] [CrossRef]
- Sakaue, M.; Ago, Y.; Sowa, C.; Sakamoto, Y.; Nishihara, B.; Koyama, Y.; Baba, A.; Matsuda, T. Modulation by 5-HT2A Receptors of Aggressive Behavior in Isolated Mice. Jpn. J. Pharmacol. 2002, 89, 89–92. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Berggård, C.; Damberg, M.; Longato-Stadler, E.; Hallman, J.; Oreland, L.; Garpenstrand, H. The Serotonin 2A-1438 G/A Receptor Polymorphism in a Group of Swedish Male Criminals. Neurosci. Lett. 2003, 347, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Nomura, Y. Psychological, Neuroimaging, and Biochemical Studies on Functional Association between Impulsive Behavior and the 5-HT2A Receptor Gene Polymorphism in Humans. Ann. N. Y. Acad. Sci. 2006, 1086, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Sequeira, A.; Gingras, Y.; Séguin, M.; Lesage, A.; Tousignant, M.; Chawky, N.; Vanier, C.; Lipp, O.; Benkelfat, C.; et al. Suicide and Serotonin: Study of Variation at Seven Serotonin Receptor Genes in Suicide Completers. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003, 118B, 36–40. [Google Scholar] [CrossRef]
- Khait, V.D.; Huang, Y.; Zalsman, G.; Oquendo, M.A.; Brent, D.A.; Harkavy-Friedman, J.M.; Mann, J.J. Association of Serotonin 5-HT2A Receptor Binding and the T102C Polymorphism in Depressed and Healthy Caucasian Subjects. Neuropsychopharmacology 2005, 30, 166–172. [Google Scholar] [CrossRef]
- Cupaioli, F.A.; Zucca, F.A.; Caporale, C.; Lesch, K.-P.; Passamonti, L.; Zecca, L. The Neurobiology of Human Aggressive Behavior: Neuroimaging, Genetic, and Neurochemical Aspects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110059. [Google Scholar] [CrossRef]
- Gregg, T.R.; Siegel, A. Brain Structures and Neurotransmitters Regulating Aggression in Cats: Implications for Human Aggression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 91–140. [Google Scholar] [CrossRef]
- Raine, A.; Ishikawa, S.S.; Arce, E.; Lencz, T.; Knuth, K.H.; Bihrle, S.; LaCasse, L.; Colletti, P. Hippocampal Structural Asymmetry in Unsuccessful Psychopaths. Biol. Psychiatry 2004, 55, 185–191. [Google Scholar] [CrossRef]
- Critchley, H.D.; Simmons, A.; Daly, E.M.; Russell, A.; van Amelsvoort, T.; Robertson, D.M.; Glover, A.; Murphy, D.G. Prefrontal and Medial Temporal Correlates of Repetitive Violence to Self and Others. Biol. Psychiatry 2000, 47, 928–934. [Google Scholar] [CrossRef]
- Soloff, P.H.; Price, J.C.; Meltzer, C.C.; Fabio, A.; Frank, G.K.; Kaye, W.H. 5HT2A Receptor Binding Is Increased in Borderline Personality Disorder. Biol. Psychiatry 2007, 62, 580–587. [Google Scholar] [CrossRef]
- Soloff, P.H.; Chiappetta, L.; Mason, N.S.; Becker, C.; Price, J.C. Effects of Serotonin-2A Receptor Binding and Gender on Personality Traits and Suicidal Behavior in Borderline Personality Disorder. Psychiatry Res. Neuroimaging 2014, 222, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Seidenwurm, D.; Pounds, T.R.; Globus, A.; Valk, P.E. Abnormal Temporal Lobe Metabolism in Violent Subjects: Correlation of Imaging and Neuropsychiatric Findings. AJNR Am. J. Neuroradiol. 1997, 18, 625–631. [Google Scholar] [PubMed]
- Bufkin, J.L.; Luttrell, V.R. Neuroimaging Studies of Aggressive and Violent Behavior: Current Findings and Implications for Criminology and Criminal Justice. Trauma. Violence Abus. 2005, 6, 176–191. [Google Scholar] [CrossRef] [PubMed]
- Siever, L.J. Neurobiology of Aggression and Violence. Am. J. Psychiatry 2008, 165, 429–442. [Google Scholar] [CrossRef]
- Tonkonogy, J.M.; Geller, J.L. Hypothalamic Lesions and Intermittent Explosive Disorder. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 45–50. [Google Scholar] [CrossRef]
- Ito, M.; Okazaki, M.; Takahashi, S.; Muramatsu, R.; Kato, M.; Onuma, T. Subacute Postictal Aggression in Patients with Epilepsy. Epilepsy Behav. 2007, 10, 611–614. [Google Scholar] [CrossRef]
- Ueltzhöffer, K.; Herpertz, S.C.; Krauch, M.; Schmahl, C.; Bertsch, K. Whole-Brain Functional Connectivity during Script-Driven Aggression in Borderline Personality Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 46–54. [Google Scholar] [CrossRef]
- Bjork, J.M.; Moeller, F.G.; Dougherty, D.M.; Swann, A.C.; Machado, M.A.; Hanis, C.L. Serotonin 2a Receptor T102C Polymorphism and Impaired Impulse Control. Am. J. Med. Genet. 2002, 114, 336–339. [Google Scholar] [CrossRef]
- Sumner, B.E.H.; D’Eath, R.B.; Farnworth, M.J.; Robson, S.; Russell, J.A.; Lawrence, A.B.; Jarvis, S. Early Weaning Results in Less Active Behaviour, Accompanied by Lower 5-HT1A and Higher 5-HT2A Receptor mRNA Expression in Specific Brain Regions of Female Pigs. Psychoneuroendocrinology 2008, 33, 1077–1092. [Google Scholar] [CrossRef]
- Rios, M.; Lambe, E.K.; Liu, R.; Teillon, S.; Liu, J.; Akbarian, S.; Roffler-Tarlov, S.; Jaenisch, R.; Aghajanian, G.K. Severe Deficits in 5-HT2A -Mediated Neurotransmission in BDNF Conditional Mutant Mice. J. Neurobiol. 2006, 66, 408–420. [Google Scholar] [CrossRef]
- Talbot, P.S.; Slifstein, M.; Hwang, D.-R.; Huang, Y.; Scher, E.; Abi-Dargham, A.; Laruelle, M. Extended Characterisation of the Serotonin 2A (5-HT2A) Receptor-Selective PET Radiotracer 11C-MDL100907 in Humans: Quantitative Analysis, Test-Retest Reproducibility, and Vulnerability to Endogenous 5-HT Tone. Neuroimage 2012, 59, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Paterson, L.M.; Tyacke, R.J.; Nutt, D.J.; Knudsen, G.M. Measuring Endogenous 5-HT Release by Emission Tomography: Promises and Pitfalls. J. Cereb. Blood Flow. Metab. 2010, 30, 1682–1706. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine2A Serotonin Receptors in the Primate Cerebral Cortex: Possible Site of Action of Hallucinogenic and Antipsychotic Drugs in Pyramidal Cell Apical Dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Goldman-Rakic, P.S. Segregation of Serotonin 5-HT2A and 5-HT3 Receptors in Inhibitory Circuits of the Primate Cerebral Cortex. J. Comp. Neurol. 2000, 417, 337–348. [Google Scholar] [CrossRef]
- Farde, L.; Plavén-Sigray, P.; Borg, J.; Cervenka, S. Brain Neuroreceptor Density and Personality Traits: Towards Dimensional Biomarkers for Psychiatric Disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170156. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.T.; Bhagwagar, Z.; Cowen, P.J.; Cunningham, V.J.; Grasby, P.M.; Hinz, R. Simplified Quantification of 5-HT2A Receptors in the Human Brain with [11C]MDL 100,907 PET and Non-Invasive Kinetic Analyses. Neuroimage 2010, 50, 984–993. [Google Scholar] [CrossRef]
- Slifstein, M.; Laruelle, M. Models and Methods for Derivation of In Vivo Neuroreceptor Parameters with PET and SPECT Reversible Radiotracers. Nucl. Med. Biol. 2001, 28, 595–608. [Google Scholar] [CrossRef]
- Hinz, R.; Bhagwagar, Z.; Cowen, P.J.; Cunningham, V.J.; Grasby, P.M. Validation of a Tracer Kinetic Model for the Quantification of 5-HT(2A) Receptors in Human Brain with [(11)C]MDL 100,907. J. Cereb. Blood Flow. Metab. 2007, 27, 161–172. [Google Scholar] [CrossRef]
- Barrett, F.S.; Zhou, Y.; Carbonaro, T.M.; Roberts, J.M.; Smith, G.S.; Griffiths, R.R.; Wong, D.F. Human Cortical Serotonin 2A Receptor Occupancy by Psilocybin Measured Using [11C]MDL 100,907 Dynamic PET and a Resting-State FMRI-Based Brain Parcellation. Front. Neuroergon. 2022, 2, 45. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33; quiz 34–57. [Google Scholar]
- Yoo, S.-W.; Kim, Y.-S.; Noh, J.-S.; Oh, K.-S.; Kim, C.-H.; NamKoong, K.; Chae, J.-H.; Lee, G.-C.; Jeon, S.-I.; Min, K.-J. Validity of Korean Version of the Mini-International Neuropsychiatric Interview. Anxiety Mood 2006, 2, 50–55. [Google Scholar]
- Seo, S.-G.; Kwon, S. Validation study of the Korean version of the Aggression Questionnaire. Korean J. Clin. Psychol. 2002, 21, 487–501. [Google Scholar] [CrossRef]
- Lammertsma, A.A.; Hume, S.P. Simplified Reference Tissue Model for PET Receptor Studies. NeuroImage 1996, 4, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.N.; Lammertsma, A.A.; Hume, S.P.; Cunningham, V.J. Parametric Imaging of Ligand-Receptor Binding in PET Using a Simplified Reference Region Model. NeuroImage 1997, 6, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Archer, J. Sex Differences in Aggression in Real-World Settings: A Meta-Analytic Review. Rev. General. Psychol. 2004, 8, 291–322. [Google Scholar] [CrossRef]
- Leonard, R.L. Aggression: Relationships with Sex, Gender Role Identity, and Gender Role Stress. Doctoral Dissertation, East Tennessee State University, Johnson City, TN, USA, 2005. [Google Scholar]
- Archer, J. Does Sexual Selection Explain Human Sex Differences in Aggression? Behav. Brain Sci. 2009, 32, 249–266. [Google Scholar] [CrossRef]
- Soloff, P.H.; Price, J.C.; Mason, N.S.; Becker, C.; Meltzer, C.C. Gender, Personality, and Serotonin-2A Receptor Binding in Healthy Subjects. Psychiatry Res. 2010, 181, 77–84. [Google Scholar] [CrossRef]
- Moses-Kolko, E.L.; Price, J.C.; Shah, N.; Berga, S.; Sereika, S.M.; Fisher, P.M.; Coleman, R.; Becker, C.; Mason, N.S.; Loucks, T.; et al. Age, Sex, and Reproductive Hormone Effects on Brain Serotonin-1A and Serotonin-2A Receptor Binding in a Healthy Population. Neuropsychopharmacology 2011, 36, 2729–2740. [Google Scholar] [CrossRef]
- Horga, G.; Parellada, E.; Lomeña, F.; Fernández-Egea, E.; Mané, A.; Font, M.; Falcón, C.; Konova, A.B.; Pavia, J.; Ros, D.; et al. Differential Brain Glucose Metabolic Patterns in Antipsychotic-Naïve First-Episode Schizophrenia with and without Auditory Verbal Hallucinations. J. Psychiatry Neurosci. 2011, 36, 312–321. [Google Scholar] [CrossRef]
- Chiotis, K.; Saint-Aubert, L.; Savitcheva, I.; Jelic, V.; Andersen, P.; Jonasson, M.; Eriksson, J.; Lubberink, M.; Almkvist, O.; Wall, A.; et al. Imaging In-Vivo Tau Pathology in Alzheimer’s Disease with THK5317 PET in a Multimodal Paradigm. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1686–1699. [Google Scholar] [CrossRef]
- Magis, D.; D’Ostilio, K.; Thibaut, A.; De Pasqua, V.; Gerard, P.; Hustinx, R.; Laureys, S.; Schoenen, J. Cerebral Metabolism before and after External Trigeminal Nerve Stimulation in Episodic Migraine. Cephalalgia 2017, 37, 881–891. [Google Scholar] [CrossRef]
| Variable | Mean (SD)/Number (%) |
|---|---|
| Demographic characteristics | |
| Age (years) | 30.9 (8.3) |
| Male | 33.3 (8.6) |
| Female | 29.8 (8.2) |
| Sex | |
| Male | 10 (30.3%) |
| Female | 23 (69.7%) |
| Education (years) | 15.6 (0.8) |
| Clinical characteristics | |
| BPAQ | |
| Physical aggression | 13.3 (2.5) |
| Verbal aggression | 9.0 (2.8) |
| Anger | 9.6 (2.3) |
| Hostility | 10.8 (2.2) |
| Total | 42.6 (6.5) |
| [11C]MDL100907 PET scan information | |
| Injected dose (MBq) | 688.0 (52.5) |
| Specific activity (GBq/μmol) | 65.0 (32.9) |
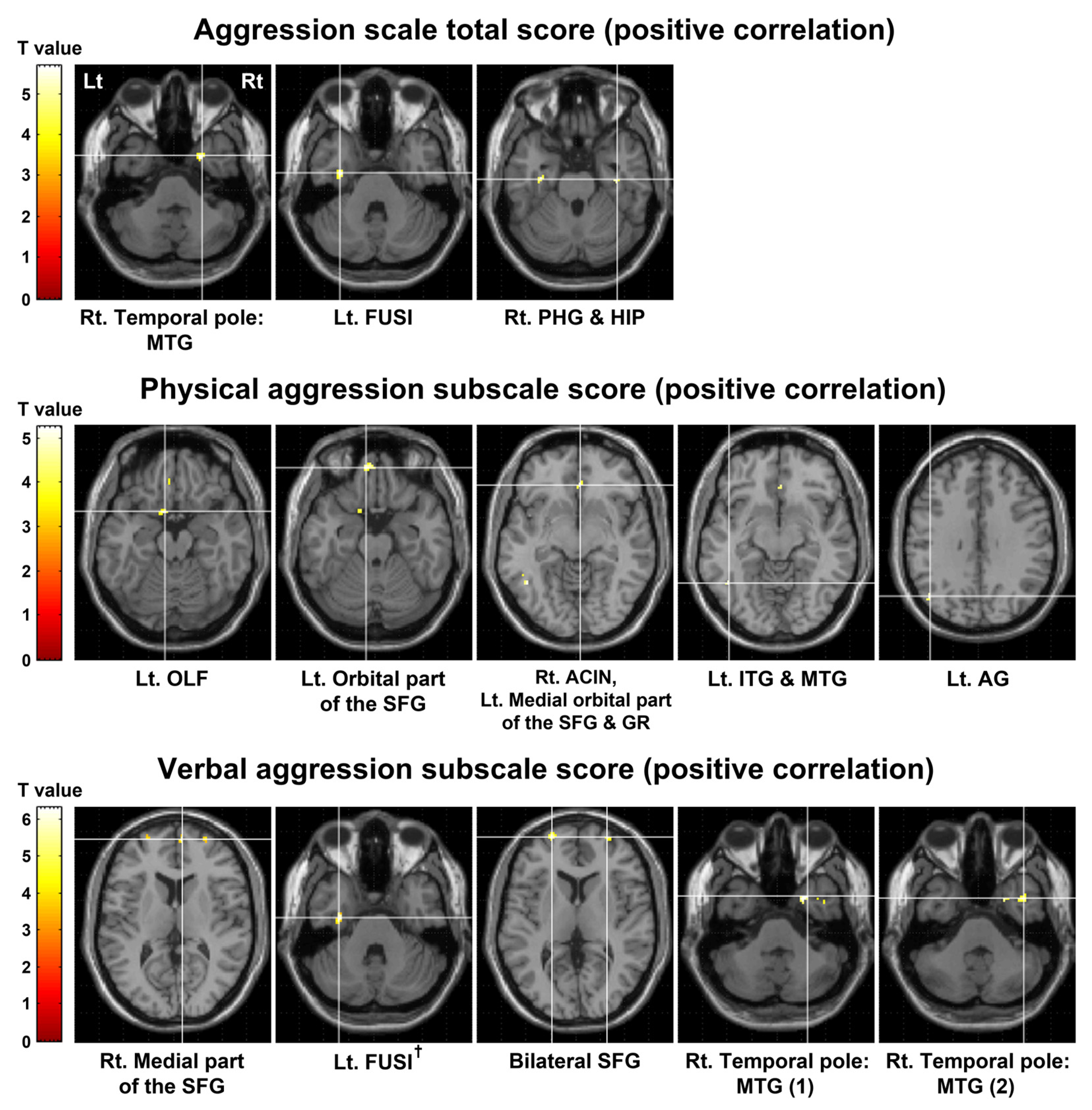
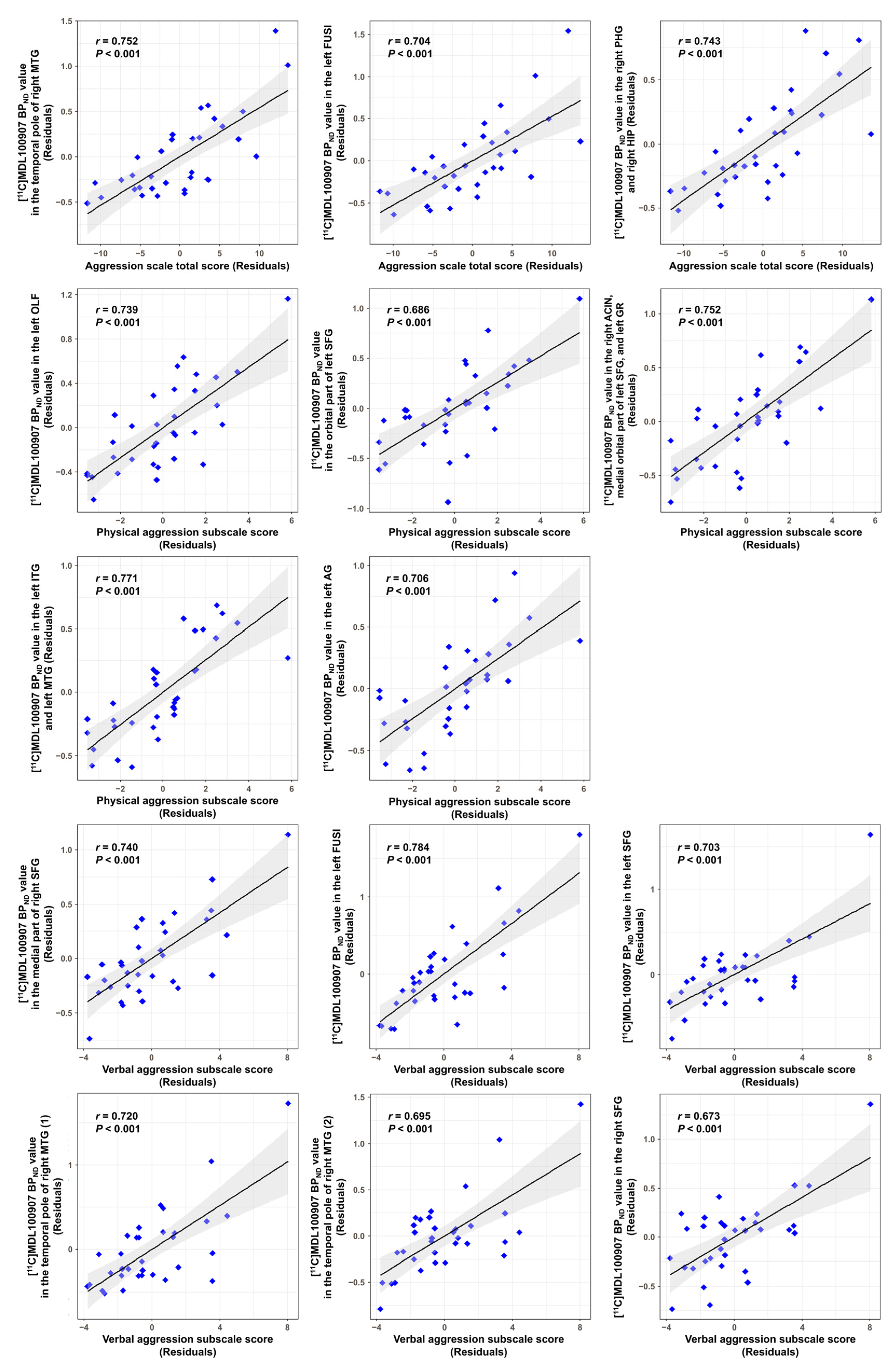
| Clinical Variable | MNI Coordinate (x, y, z) | Brain Region | Cluster Size (Voxels) | Peak-Level | |
|---|---|---|---|---|---|
| T Value | p Value | ||||
| Aggression scale total score | |||||
| Positive correlation | 26, 6, −40 | Rt. Temporal pole: MTG | 25 | 5.67 | <0.0001 |
| −32, −10, −32 | Lt. FUSI | 51 | 4.89 | ||
| 38, −16, −26 | Rt. PHG | 23 | 4.45 | 0.0001 | |
| 34, −20, −16 | Rt. HIP | 4.14 | |||
| Physical aggression subscale score | |||||
| Positive correlation | −8, 10, −18 | Lt. OIF | 30 | 5.25 | <0.0001 |
| −8, 50, −22 | Lt. Orbital part of the SFG | 26 | 5.13 | ||
| 4, 34, −8 | Rt. ACIN | 34 | 4.66 | ||
| −2, 30, −12 | Lt. Medial orbital part of the SFG | 4.2 | 0.0001 | ||
| −4, 38, −18 | Lt. GR | 3.88 | 0.0003 | ||
| −44, −56, −10 | Lt. ITG | 27 | 4.64 | <0.0001 | |
| −48, −48, −6 | Lt. MTG | 4.63 | |||
| −44, −68, 32 | Lt. AG | 20 | 4.54 | 0.0001 | |
| Verbal aggression subscale score | |||||
| Positive correlation | 8, 60, 10 | Rt. Medial part of the SFG | 23 | 6.3 | <0.0001 |
| −32, −12, −32 | Lt. FUSI † | 51 | 6.17 | ||
| −22, 62, 6 | Lt. SFG | 71 | 5.46 | ||
| 28, 8, −40 | Rt. Temporal pole: MTG (1) | 21 | 4.92 | ||
| 42, 6, −38 | Rt. Temporal pole: MTG (2) | 23 | 4.6 | ||
| 30, 62, 8 | Rt. SFG | 30 | 4.52 | 0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Kim, H.-K.; Son, Y.-D.; Kim, J.-H. In Vivo Serotonin 5-HT2A Receptor Availability and Its Relationship with Aggression Traits in Healthy Individuals: A Positron Emission Tomography Study with C-11 MDL100907. Int. J. Mol. Sci. 2023, 24, 15697. https://doi.org/10.3390/ijms242115697
Kim J-H, Kim H-K, Son Y-D, Kim J-H. In Vivo Serotonin 5-HT2A Receptor Availability and Its Relationship with Aggression Traits in Healthy Individuals: A Positron Emission Tomography Study with C-11 MDL100907. International Journal of Molecular Sciences. 2023; 24(21):15697. https://doi.org/10.3390/ijms242115697
Chicago/Turabian StyleKim, Jeong-Hee, Hang-Keun Kim, Young-Don Son, and Jong-Hoon Kim. 2023. "In Vivo Serotonin 5-HT2A Receptor Availability and Its Relationship with Aggression Traits in Healthy Individuals: A Positron Emission Tomography Study with C-11 MDL100907" International Journal of Molecular Sciences 24, no. 21: 15697. https://doi.org/10.3390/ijms242115697
APA StyleKim, J.-H., Kim, H.-K., Son, Y.-D., & Kim, J.-H. (2023). In Vivo Serotonin 5-HT2A Receptor Availability and Its Relationship with Aggression Traits in Healthy Individuals: A Positron Emission Tomography Study with C-11 MDL100907. International Journal of Molecular Sciences, 24(21), 15697. https://doi.org/10.3390/ijms242115697





