Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization
Abstract
:1. Introduction
2. Results
2.1. Porcine Teeth Are Similar to Human Teeth in Size and Shape
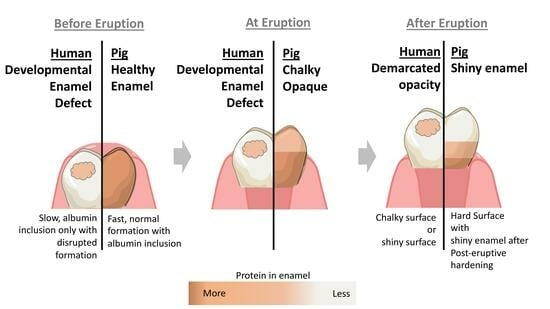
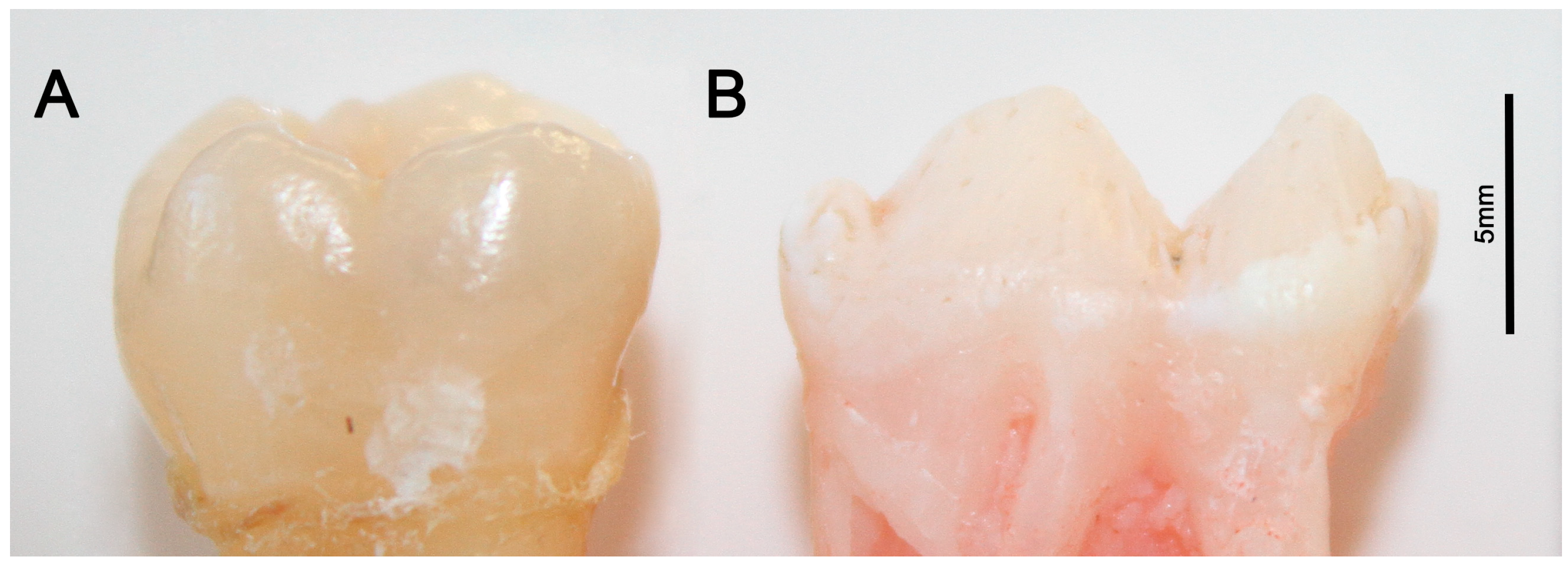
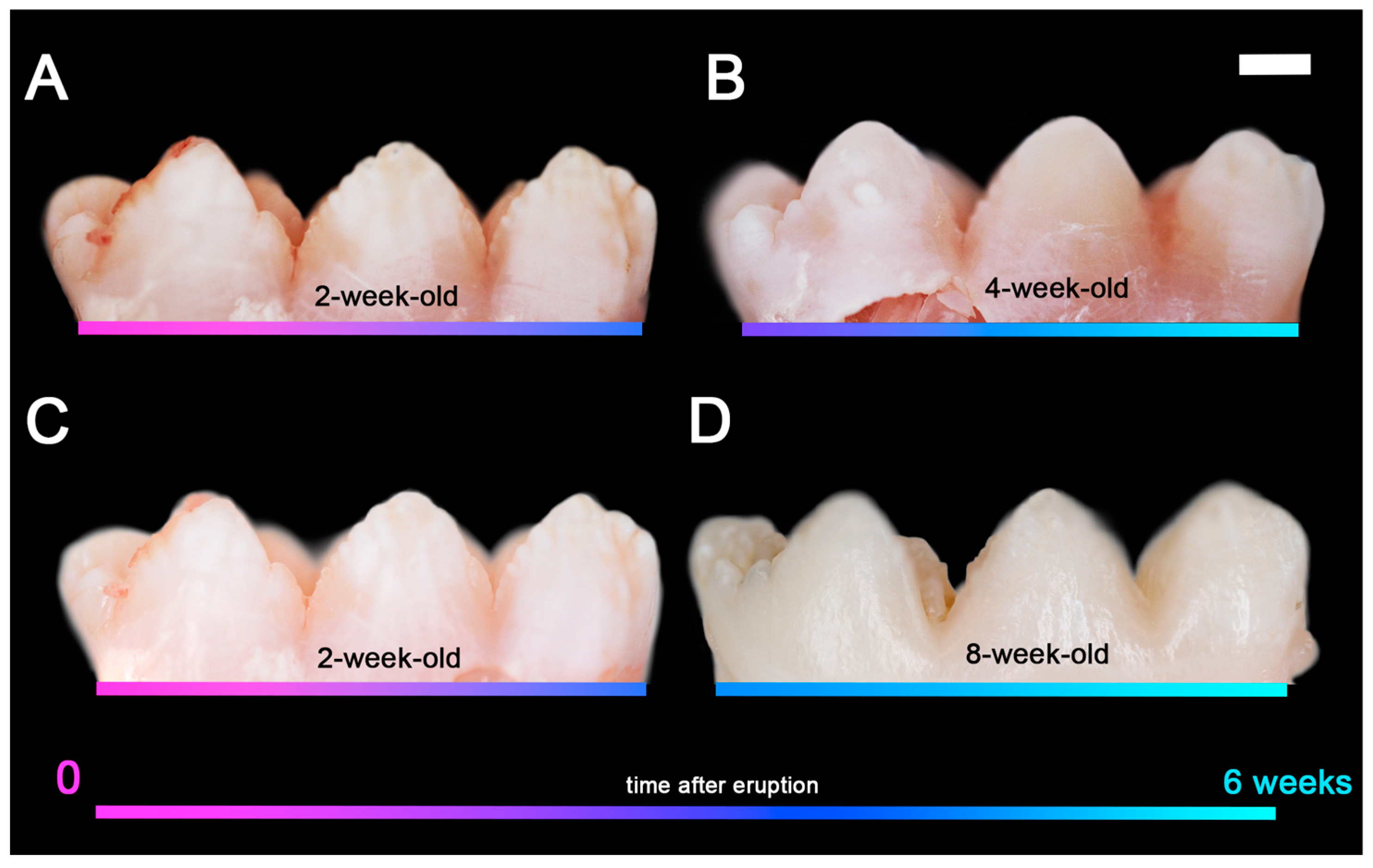
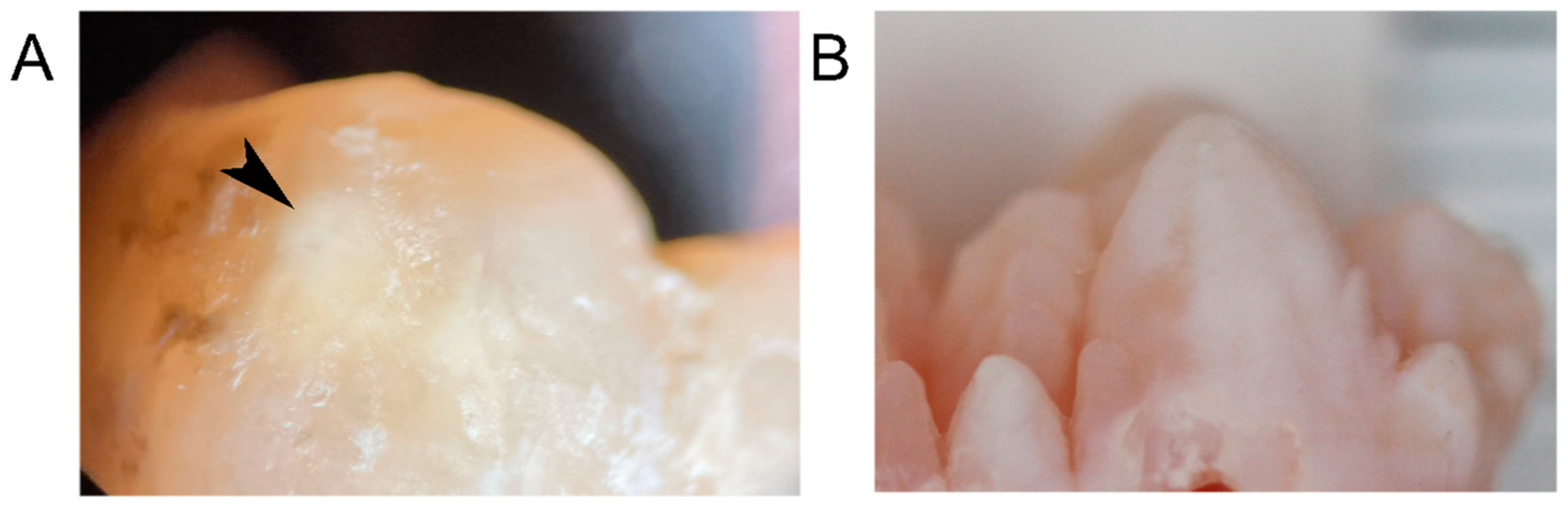
2.2. Porcine Teeth Show Opaque Enamel and Localized Opacities Similar to Demarcated Opacities in Human Teeth
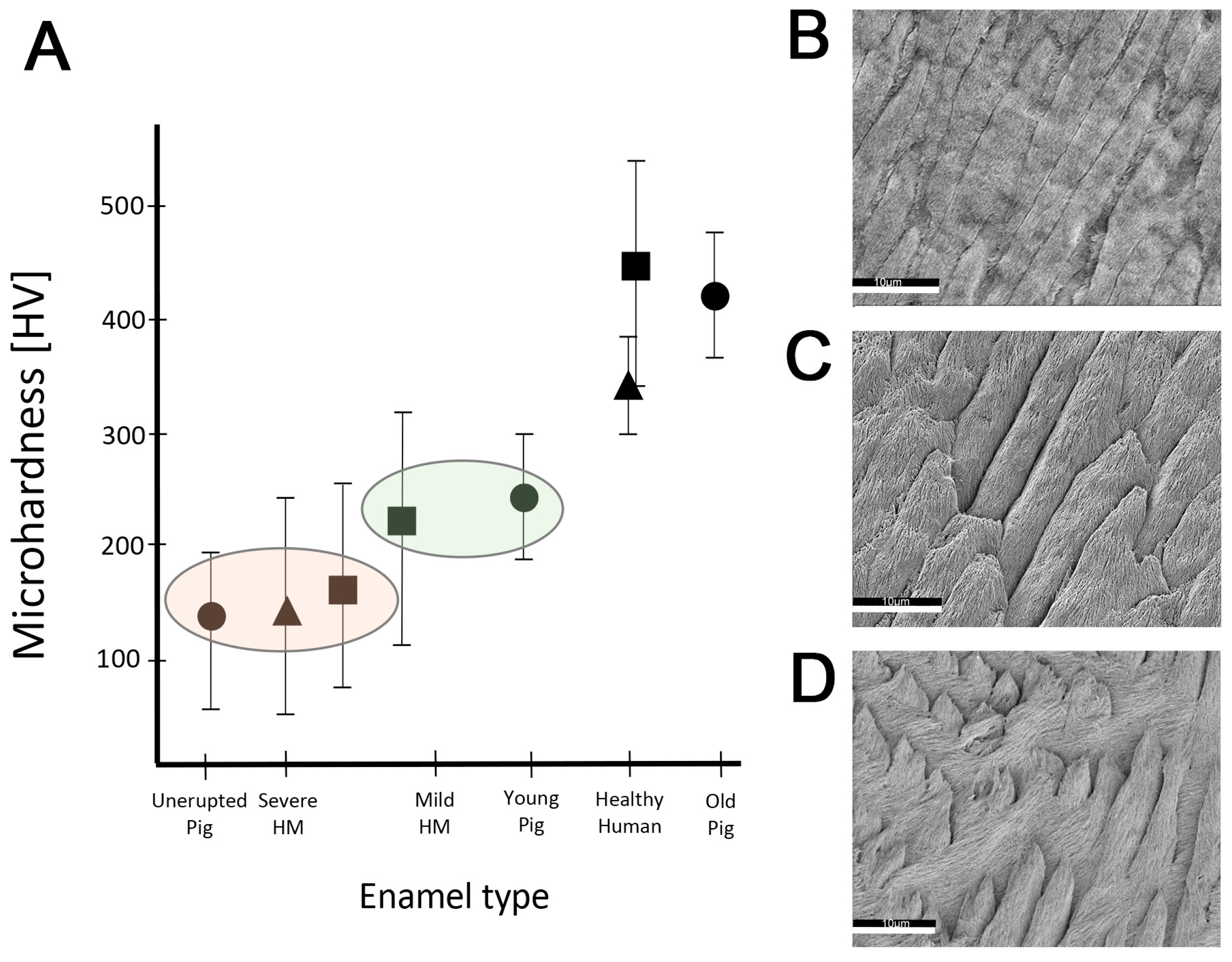
2.3. Hardness and Lose Packing of Enamel Rods Are Similar in Pig Enamel and Human-Demarcated Opacities
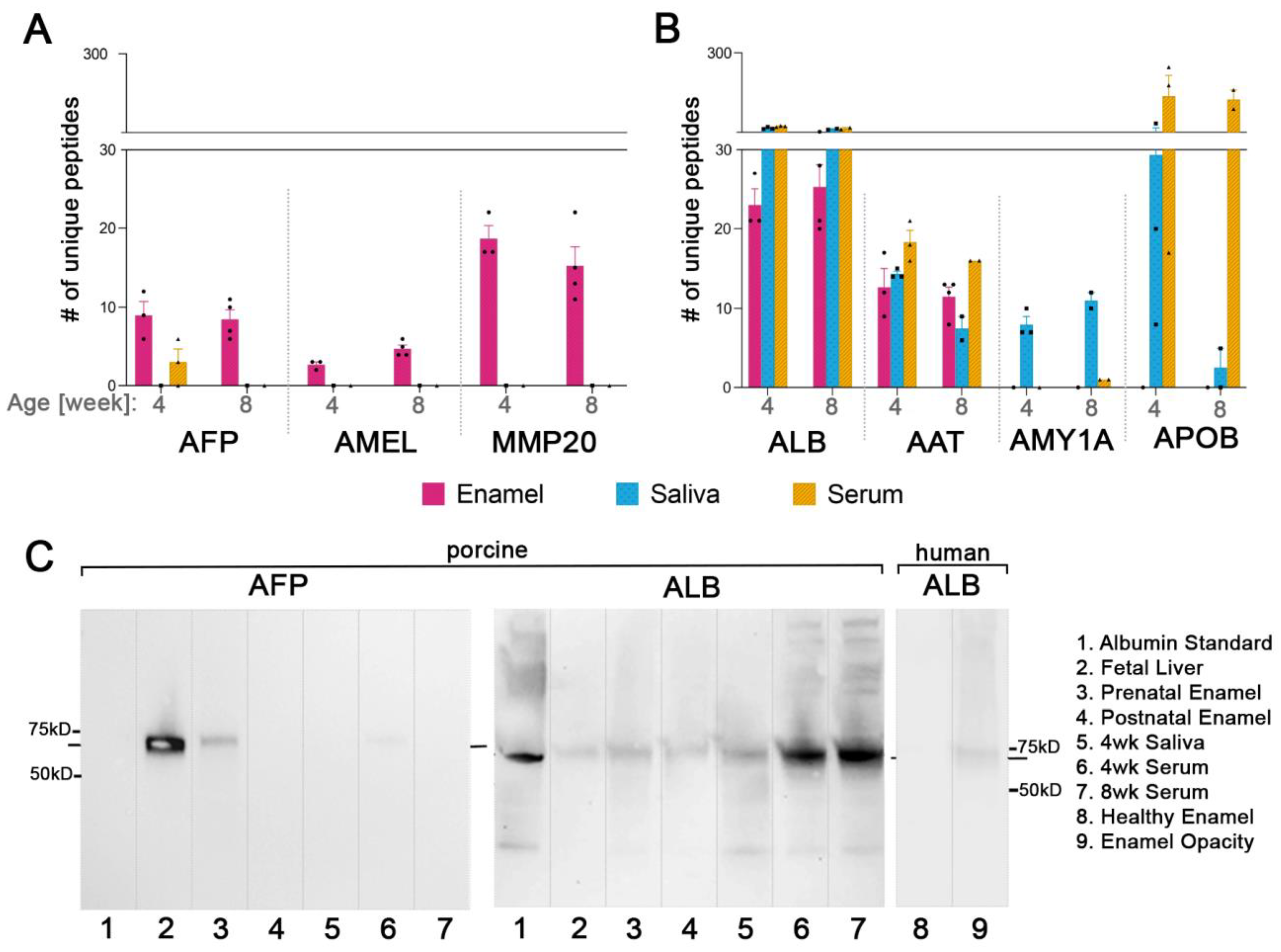
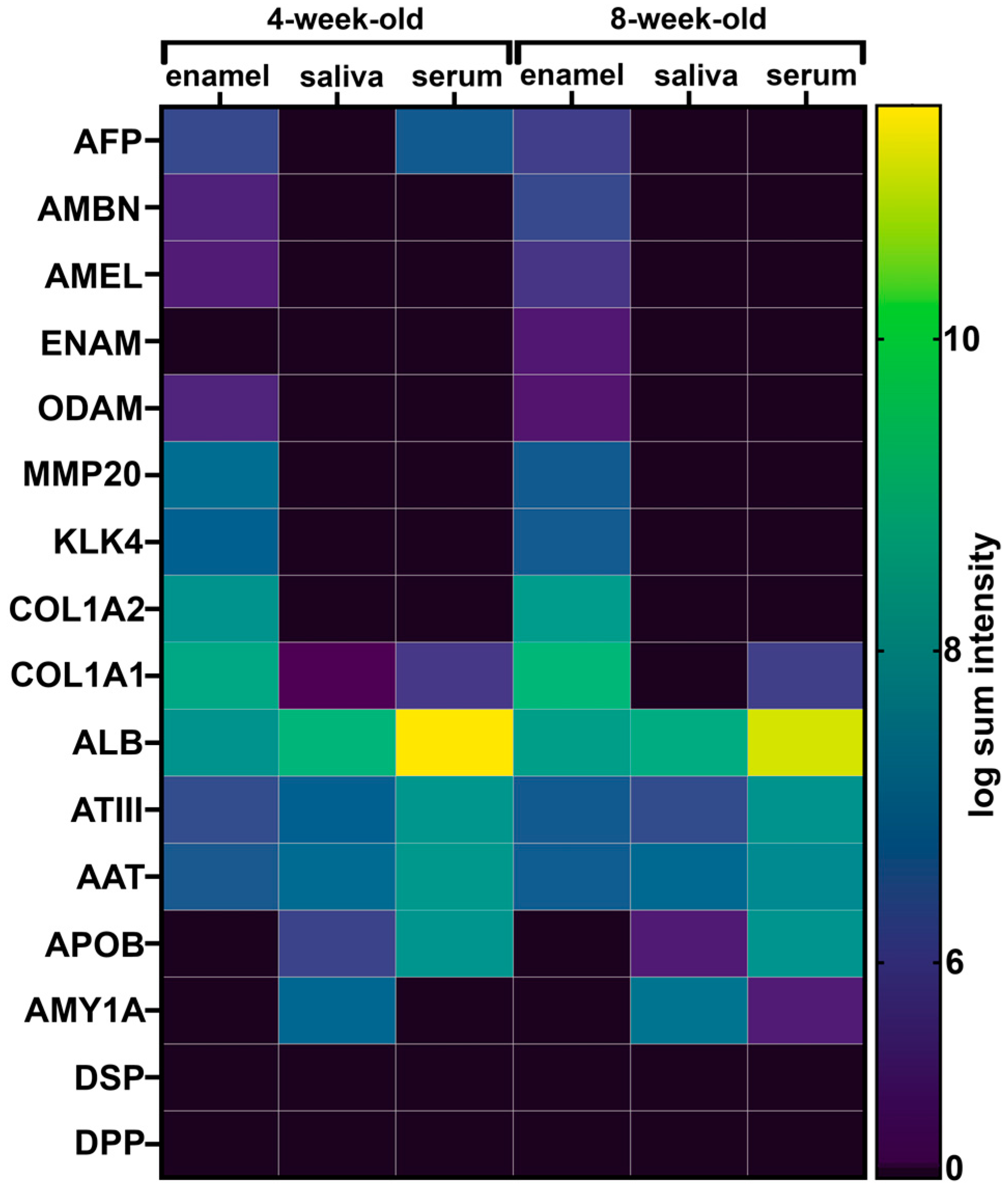
2.4. Fetal Serum Albumin, Alpha-Fetoprotein (AFP), Is Present in Porcine Enamel and Is of Developmental Origin
2.5. Fetal Albumin Is a Time Stamp for Natural Inclusion into Enamel Formed before Birth and Is Not Found in Enamel That Formed after Birth
2.6. The Adult Form of Serum Albumin Occurs Naturally in Enamel and Is Detected in Serum, Saliva, and Enamel That Formed at Different Times
2.7. Common Proteins from Serum and Saliva Are Not Seen Leaking into Enamel as Contamination and Were Not Detected in Erupted Tooth Enamel
2.8. Opaque Pig Enamel Is Not Enriched in Endogenous Enamel Proteins and Enzymes
3. Discussion
3.1. The Similarity in Size between Human and Porcine Teeth Is Important for a Useful Model System and for Testing of Treatment
3.2. The Similarity in Protein Content between Porcine and Hypomineralized Human Enamel Is Key for a Good Animal Model
| Human | Pig | Mouse | Zebrafish | |
|---|---|---|---|---|
| Crown Size (height) | 5–10 mm [4,64] | 5–12 mm [65,66] | Molars: 1–2 mm; Incisor: >1 cm [67] | Continued replacement: <100 microns [68] |
| Duration of crown mineralization before eruption | Primary: 10 to 36 months (incisors to molars) Permanent: 30–78 months (first molar to canines) [69] | Primary: 3–4 months Permanent: 4–12 months [70] | 2.5 weeks [67] Only one set of teeth | 2 weeks [68] Continuous tooth replacement |
| Enamel Mineral Density [g/cc] | Primary: 1.6–2.2 [71] Permanent: 2.1–3.1 [72]; | Primary: 1.6–2.0 [29] Permanent: 2.8–2.9 [28] | Incisor tip: 3.0 [73] | No enamel |
| Hardness [GPa] | Primary: 3.0 [62] Permanent: 3.6–4.1 Hypomineralized: 1.4–2.1 [52,61] | Primary: 3.4 Permanent: 3.9–4.1 [29] | Incisors: 3–4 [74] | No enamel |
| Micro-structure | Decussating Rod/Interrod keyhole pattern [75] | Rods/interrod sheets [55] | Rod/interrod Sheath [76] | No enamel |
| Posteruptive maturation | Little [77,78,79] | Extensive [29] | Little [47] | No enamel |
| Protein matrix composition in healthy, erupted teeth | Healthy: Enamel matrix proteins, no albumin [2] Albumin in enamel opacities [15] | Similar to hypomineralized human enamel with albumin in erupted enamel [60] | Similar to healthy mature human enamel, no albumin [2] | No Enamel |
| Major limitation for use in studies | Limited access to untreated hypomineralized samples | Cost for large animal model in controlled facility | Small sample size | Enameloid, not enamel, small size |
| Major advantage | Ideal sample for studies of etiology and treatment in humans | Similar tooth properties; ease for proteomic, molecular pathway studies; genetic tractability; | Ease of genetic modifications and tractability; molecular pathways. | Ease of genetic, molecular pathways and toxicology studies [80] |
3.3. As in Demarcated Opacities, Pig Enamel Contains Inhibitors of KLK4, the Key Enzyme for Enamel Maturation
3.4. Fetal and Adult Forms of Albumin Contribute to the High Protein Content of Porcine Enamel Based on Proteomics, Immunoblotting, and Immunofluorescence
3.5. The Porcine Model Is Uniquely Suitable to Elucidate the Etiology and Improve Treatment Protocols for MH
4. Materials and Methods
4.1. Saliva Samples
4.2. Blood Samples
4.3. Fetal Liver Tissue Collection
4.4. Tooth Collection, Enamel Sampling, and Protein Extraction
4.5. Protein Digestion and Sequence Analysis by LC-MS/MS
4.6. Western Blot
4.7. Immunofluorescence Imaging
4.8. Microhardness Measurements and SEM Imaging
4.9. Macrophotography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, K.A.; Mountain, R.V.; Pickett, O.R.; Den Besten, P.K.; Bidlack, F.B.; Dunn, E.C. Teeth as Potential New Tools to Measure Early-Life Adversity and Subsequent Mental Health Risk: An Interdisciplinary Review and Conceptual Model. Biol. Psychiatry 2020, 87, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and Its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef] [PubMed]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef]
- Nanci, A.; TenCate, A.R. Ten Cate’s Oral Histology: Development, Structure, and Function, 9th ed.; Elsevier: St. Louis, MO, USA, 2018; ISBN 978-0-323-48524-1. [Google Scholar]
- Schwendicke, F.; Elhennawy, K.; Reda, S.; Bekes, K.; Manton, D.J.; Krois, J. Global burden of molar incisor hypomineralization. J. Dent. 2018, 68, 10–18. [Google Scholar] [CrossRef]
- The D3 Group. Prevalence of Molar Hypomineralisation. Available online: https://www.thed3group.org/prevalence.html (accessed on 12 October 2023).
- Haque Afzal, S.; Wigen, T.I.; Skaare, A.B.; Brusevold, I.J. Molar-incisor hypomineralisation in Norwegian children: Prevalence and associated factors. Eur. J. Oral Sci. 2023, 131, e12930. [Google Scholar] [CrossRef]
- Garot, E.; Denis, A.; Delbos, Y.; Manton, D.; Silva, M.; Rouas, P. Are hypomineralised lesions on second primary molars (HSPM) a predictive sign of molar incisor hypomineralisation (MIH)? A systematic review and a meta-analysis. J. Dent. 2018, 72, 8–13. [Google Scholar] [CrossRef]
- The D3 Group. D3G Specialists Guide for Diagnosing Molar Hypomin. Available online: https://www.thed3group.org/specialist-2.html (accessed on 12 October 2023).
- Weerheijm, K.L.; Jälevik, B.; Alaluusua, S. Molar-incisor hypomineralisation. Caries Res. 2001, 35, 390–391. [Google Scholar] [CrossRef]
- Raposo, F.; de Carvalho Rodrigues, A.C.; Lia, É.N.; Leal, S.C. Prevalence of Hypersensitivity in Teeth Affected by Molar-Incisor Hypomineralization (MIH). Caries Res. 2019, 53, 424–430. [Google Scholar] [CrossRef]
- Bartlett, J.D. Dental enamel development: Proteinases and their enamel matrix substrates. ISRN Dent. 2013, 2013, 684607. [Google Scholar] [CrossRef]
- Chai, H.; Lee, J.J.-W.; Constantino, P.J.; Lucas, P.W.; Lawn, B.R. Remarkable resilience of teeth. Proc. Natl. Acad. Sci. USA 2009, 106, 7289–7293. [Google Scholar] [CrossRef]
- Hubbard, M.J.; Mangum, J.E.; Perez, V.A.; Williams, R. A Breakthrough in Understanding the Pathogenesis of Molar Hypomineralisation: The Mineralisation-Poisoning Model. Front. Physiol. 2021, 12, 802833. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.A.; Mangum, J.E.; Hubbard, M.J. Pathogenesis of Molar Hypomineralisation: Aged Albumin Demarcates Chalky Regions of Hypomineralised Enamel. Front. Physiol. 2020, 11, 579015. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Perez, V.A.; Mangum, J.E.; Hubbard, M.J. Pathogenesis of Molar Hypomineralisation: Hypomineralised 6-Year Molars Contain Traces of Fetal Serum Albumin. Front. Physiol. 2020, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.A.; Monk, B.C.; Swain, M.V.; Drummond, B.K. Protein content of molar-incisor hypomineralisation enamel. J. Dent. 2010, 38, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Mangum, J.E.; Crombie, F.A.; Kilpatrick, N.; Manton, D.J.; Hubbard, M.J. Surface integrity governs the proteome of hypomineralized enamel. J. Dent. Res. 2010, 89, 1160–1165. [Google Scholar] [CrossRef]
- Robinson, C. Enamel maturation: A brief background with implications for some enamel dysplasias. Front. Physiol. 2014, 5, 388. [Google Scholar] [CrossRef]
- Xie, Z.; Kilpatrick, N.M.; Swain, M.V.; Munroe, P.R.; Hoffman, M. Transmission electron microscope characterisation of molar-incisor-hypomineralisation. J. Mater. Sci. Mater. Med. 2008, 19, 3187–3192. [Google Scholar] [CrossRef]
- Uchida, T.; Murakami, C.; Wakida, K.; Dohi, N.; Iwai, Y.; Simmer, J.P.; Fukae, M.; Satoda, T.; Takahashi, O. Sheath proteins: Synthesis, secretion, degradation and fate in forming enamel. Eur. J. Oral Sci. 1998, 106 (Suppl. 1), 308–314. [Google Scholar] [CrossRef]
- Uchida, T.; Tanabe, T.; Fukae, M.; Shimizu, M.; Yamada, M.; Miake, K.; Kobayashi, S. Immunochemical and immunohistochemical studies, using antisera against porcine 25 kDa amelogenin, 89 kDa enamelin and the 13-17 kDa nonamelogenins, on immature enamel of the pig and rat. Histochemistry 1991, 96, 129–138. [Google Scholar] [CrossRef]
- Tanabe, T.; Fukae, M.; Uchida, T.; Shimizu, M. The localization and characterization of proteinases for the initial cleavage of porcine amelogenin. Calcif. Tissue Int. 1992, 51, 213–217. [Google Scholar] [CrossRef]
- Chiba, R.; Okubo, M.; Yamamoto, R.; Saito, M.M.; Kobayashi, S.; Beniash, E.; Yamakoshi, Y. Porcine keratin 75 in developing enamel. J. Oral Biosci. 2019, 61, 163–172. [Google Scholar] [CrossRef]
- Hu, J.C.-C.; Yamakoshi, Y.; Yamakoshi, F.; Krebsbach, P.H.; Simmer, J.P. Proteomics and genetics of dental enamel. Cells Tissues Organs 2005, 181, 219–231. [Google Scholar] [CrossRef]
- Kallistová, A.; Horáček, I.; Šlouf, M.; Skála, R.; Fridrichová, M. Mammalian enamel maturation: Crystallographic changes prior to tooth eruption. PLoS ONE 2017, 12, e0171424. [Google Scholar] [CrossRef]
- Kirkham, J.; Robinson, C.; Weatherell, J.A.; Richards, A.; Fejerskov, O.; Josephsen, K. Maturation in Developing Permanent Porcine Enamel. J. Dent. Res. 1988, 67, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Sova, S.S.; Tjäderhane, L.; Heikkilä, P.A.; Jernvall, J. A microCT Study of Three-Dimensional Patterns of Biomineralization in Pig Molars. Front. Physiol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Depalle, B.; Karaaslan, H.; Obtel, N.; Gil-Bona, A.; Teichmann, M.; Mascarin, G.; Pugach-Gordon, M.; Bidlack, F.B. Rapid post-eruptive maturation of porcine enamel. Front. Physiol. 2023, 14, 1099645. [Google Scholar] [CrossRef]
- Ekambaram, M.; Yiu, C.K.Y. Bonding to hypomineralized enamel—A systematic review. Int. J. Adhes. Adhes. 2016, 69, 27–32. [Google Scholar] [CrossRef]
- Elhennawy, K.; Manton, D.J.; Crombie, F.; Zaslansky, P.; Radlanski, R.J.; Jost-Brinkmann, P.-G.; Schwendicke, F. Structural, mechanical and chemical evaluation of molar-incisor hypomineralization-affected enamel: A systematic review. Arch. Oral Biol. 2017, 83, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Fragelli, C.M.B.; de Souza, J.F.; Bussaneli, D.G.; Jeremias, F.; dos Santos-Pinto, L.; de Cordeiro, R.C.L. Survival of sealants in molars affected by molar-incisor hypomineralization: 18-month follow-up. Braz. Oral Res. 2017, 31, 30. [Google Scholar] [CrossRef]
- Krämer, N.; Bui Khac, N.-H.N.; Lücker, S.; Stachniss, V.; Frankenberger, R. Bonding strategies for MIH-affected enamel and dentin. Dent. Mater. 2018, 34, 331–340. [Google Scholar] [CrossRef]
- Linner, T.; Khazaei, Y.; Bücher, K.; Pfisterer, J.; Hickel, R.; Kühnisch, J. Comparison of four different treatment strategies in teeth with molar-incisor hypomineralization-related enamel breakdown—A retrospective cohort study. Int. J. Paediatr. Dent. 2020, 30, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.A.; Wong, F.; Jälevik, B.; Vierrou, A.M.; Alaluusua, S.; Espelid, I. Best Clinical Practice Guidance for clinicians dealing with children presenting with Molar-Incisor-Hypomineralisation (MIH): An EAPD Policy Document. Eur. Arch. Paediatr. Dent. 2010, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- William, V.; Messer, L.B.; Burrow, M.F. Molar incisor hypomineralization: Review and recommendations for clinical management. Pediatr. Dent. 2006, 28, 224–232. [Google Scholar] [PubMed]
- Chay, P.L.; Manton, D.J.; Palamara, J.E.A. The effect of resin infiltration and oxidative pre-treatment on microshear bond strength of resin composite to hypomineralised enamel. Int. J. Paediatr. Dent. 2014, 24, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Crombie, F.; Manton, D.; Palamara, J.; Reynolds, E. Resin infiltration of developmentally hypomineralised enamel. Int. J. Paediatr. Dent. 2014, 24, 51–55. [Google Scholar] [CrossRef]
- Mathu-Muju, K.; Wright, J.T. Diagnosis and treatment of molar incisor hypomineralization. Compend. Contin. Educ. Dent. 2006, 27, 604–610. [Google Scholar]
- Somani, C.; Taylor, G.D.; Garot, E.; Rouas, P.; Lygidakis, N.A.; Wong, F.S.L. An update of treatment modalities in children and adolescents with teeth affected by molar incisor hypomineralisation (MIH): A systematic review. Eur. Arch. Paediatr. Dent. 2022, 23, 39–64. [Google Scholar] [CrossRef]
- Steffen, R.; Krämer, N.; Bekes, K. The Würzburg MIH concept: The MIH treatment need index (MIH TNI): A new index to assess and plan treatment in patients with molar incisior hypomineralisation (MIH). Eur. Arch. Paediatr. Dent. 2017, 18, 355–361. [Google Scholar] [CrossRef]
- Almuallem, Z.; Busuttil-Naudi, A. Molar incisor hypomineralisation (MIH)—An overview. Br. Dent. J. 2018, 225, 601–609. [Google Scholar] [CrossRef]
- Heitmüller, D.; Thiering, E.; Hoffmann, U.; Heinrich, J.; Manton, D.; Kühnisch, J.; Neumann, C.; Bauer, C.P.; Heinrich-Weltzien, R.; Hickel, R.; et al. Is there a positive relationship between molar incisor hypomineralisations and the presence of dental caries?: Caries in children with MIH. Int. J. Paediatr. Dent. 2013, 23, 116–124. [Google Scholar] [CrossRef]
- Wright, J.T. Diagnosis and treatment of molar-incisor hypomineralization. In Handbook of Clinical Techniques in Pediatric Dentistry; Soxman, J.A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 99–106. ISBN 978-1-118-99819-9. [Google Scholar]
- Duverger, O.; Ohara, T.; Bible, P.W.; Zah, A.; Morasso, M.I. DLX3-Dependent Regulation of Ion Transporters and Carbonic Anhydrases is Crucial for Enamel Mineralization: DLX3 and Enamel PH Regulation. J. Bone Miner. Res. 2017, 32, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Jedeon, K.; De la Dure-Molla, M.; Brookes, S.J.; Loiodice, S.; Marciano, C.; Kirkham, J.; Canivenc-Lavier, M.-C.; Boudalia, S.; Bergès, R.; Harada, H.; et al. Enamel Defects Reflect Perinatal Exposure to Bisphenol A. Am. J. Pathol. 2013, 183, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Lyngstadaas, S.P.; Møinichen, C.B.; Risnes, S. Crown morphology, enamel distribution, and enamel structure in mouse molars. Anat. Rec. 1998, 250, 268–280. [Google Scholar] [CrossRef]
- Suckling, G.; Elliott, D.C.; Thurley, D.C. The production of developmental defects of enamel in the incisor teeth of penned sheep resulting from induced parasitism. Arch. Oral Biol. 1983, 28, 393–399. [Google Scholar] [CrossRef]
- Kierdorf, H.; Witzel, C.; Upex, B.; Dobney, K.; Kierdorf, U. Enamel hypoplasia in molars of sheep and goats, and its relationship to the pattern of tooth crown growth. J. Anat. 2012, 220, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, A.J.; de Teruel-Fernández, J.D.; Alcolea-Rubio, L.A.; Hernández-Fernández, A.; Martínez-Beneyto, Y.; Gispert-Guirado, F. Structural differences in enamel and dentin in human, bovine, porcine, and ovine teeth. Ann. Anat. 2018, 218, 7–17. [Google Scholar] [CrossRef]
- Cooper, W.E.G. A microchemical, microradiographic and histological investigation of amelogenesis in the pig. Arch. Oral Biol. 1968, 13, 27–46. [Google Scholar] [CrossRef]
- Fagrell, T.G.; Dietz, W.; Jälevik, B.; Norén, J.G. Chemical, mechanical and morphological properties of hypomineralized enamel of permanent first molars. Acta Odontol. Scand. 2010, 68, 215–222. [Google Scholar] [CrossRef]
- Jalevik, B.; Dietz, W.; Noren, J.G. Scanning electron micrograph analysis of hypomineralized enamel in permanent first molars. Int. J. Paediatr. Dent. 2005, 15, 233–240. [Google Scholar] [CrossRef]
- Kodaka, T.; Debari, K.; Yamada, M.; Kuroiwa, M. Correlation between Microhardness and Mineral Content in Sound Human Enamel (Short Communication). Caries Res. 1992, 26, 139–141. [Google Scholar] [CrossRef]
- Popowics, T.E.; Rensberger, J.M.; Herring, S.W. Enamel microstructure and microstrain in the fracture of human and pig molar cusps. Arch. Oral Biol. 2004, 49, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Garot, E.; Rouas, P.; Somani, C.; Taylor, G.D.; Wong, F.; Lygidakis, N.A. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2022, 23, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.J.; Mangum, J.E.; Perez, V.A.; Nervo, G.J.; Hall, R.K. Molar Hypomineralisation: A Call to Arms for Enamel Researchers. Front. Physiol. 2017, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.J.; Perez, V.A.; Ganss, B. 100 Years of Chalky Teeth Research: From Pioneering Histopathology to Social Good. Front. Dent. Med. 2021, 1, 632534. [Google Scholar] [CrossRef]
- Rodd, H.D.; Graham, A.; Tajmehr, N.; Timms, L.; Hasmun, N. Molar Incisor Hypomineralisation: Current Knowledge and Practice. Int. Dent. J. 2021, 71, 285–291. [Google Scholar] [CrossRef]
- Green, D.R.; Schulte, F.; Lee, K.-H.; Pugach, M.K.; Hardt, M.; Bidlack, F.B. Mapping the Tooth Enamel Proteome and Amelogenin Phosphorylation Onto Mineralizing Porcine Tooth Crowns. Front. Physiol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Crombie, F.A.; Manton, D.J.; Palamara, J.E.A.; Zalizniak, I.; Cochrane, N.J.; Reynolds, E.C. Characterisation of developmentally hypomineralised human enamel. J. Dent. 2013, 41, 611–618. [Google Scholar] [CrossRef]
- Habelitz, S.; Marshall, S.J.; Marshall, G.W.; Balooch, M. Mechanical properties of human dental enamel on the nanometre scale. Arch. Oral Biol. 2001, 46, 173–183. [Google Scholar] [CrossRef]
- Silverstone, L.M.; Saxton, C.A.; Dogon, I.L.; Fejerskov, O. Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res. 1975, 9, 373–387. [Google Scholar] [CrossRef]
- Volchansky, A.; Cleaton-Jones, P. Clinical crown height (length)—A review of published measurements. J. Clin. Periodontol. 2001, 28, 1085–1090. [Google Scholar] [CrossRef]
- Emken, S.; Witzel, C.; Kierdorf, U.; Frölich, K.; Kierdorf, H. Wild boar versus domestic pig—Deciphering of crown growth in porcine second molars. J. Anat. 2023, 242, 1078–1095. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.L.; Widowski, T.M. Normal profiles for deciduous dental eruption in domestic piglets: Effect of sow, litter, and piglet characteristics. J. Anim. Sci. 2009, 87, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Catón, J.; Tucker, A.S. Current knowledge of tooth development: Patterning and mineralization of the murine dentition. J. Anat. 2009, 214, 502–515. [Google Scholar] [CrossRef]
- Stock, D.W. Zebrafish dentition in comparative context. J. Exp. Zool. Part B Mol. Dev. Evol. 2007, 308, 523–549. [Google Scholar] [CrossRef] [PubMed]
- Logan, W.; Kronfeld, R. Development of the human jaws and surrounding structures from birth to the age of fifteen years. JADA 1933, 20, 379–427. [Google Scholar]
- Tonge, C.H.; McCance, R.A. Normal development of the jaws and teeth in pigs, and the delay and malocclusion produced by calorie deficiencies. J. Anat. 1973, 115, 1. [Google Scholar]
- Elfrink, M.E.; Kalin, K.; van Ruijven, L.J.; ten Cate, J.M.; Veerkamp, J.S. MicroCT study on the enamel mineral density of primary molars. Eur. J. Paediatr. Dent. 2016, 17, 60–64. [Google Scholar] [PubMed]
- He, B.; Huang, S.; Zhang, C.; Jing, J.; Hao, Y.; Xiao, L.; Zhou, X. Mineral densities and elemental content in different layers of healthy human enamel with varying teeth age. Arch. Oral Biol. 2011, 56, 997–1004. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Teepe, J.D.; Hu, Y.; Smith, C.E.; Fajardo, R.J.; Chun, Y.-H.P. Estimating mineral changes in enamel formation by ashing/BSE and microCT. J. Dent. Res. 2014, 93, 256–262. [Google Scholar] [CrossRef]
- Pugach, M.K.; Gibson, C.W. Analysis of enamel development using murine model systems: Approaches and limitations. Front. Physiol. 2014, 5, 313. [Google Scholar] [CrossRef]
- Tafforeau, P.; Zermeno, J.P.; Smith, T.M. Tracking cellular-level enamel growth and structure in 4D with synchrotron imaging. J. Hum. Evol. 2012, 62, 424–428. [Google Scholar] [CrossRef]
- Smith, C.E.; Hu, Y.; Hu, J.C.-C.; Simmer, J.P. Quantitative analysis of the core 2D arrangement and distribution of enamel rods in cross-sections of mandibular mouse incisors. J. Anat. 2019, 234, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Brudevold, F.; Aasenden, R.; Bakhos, Y. A preliminary study of posteruptive maturation of teeth in situ. Caries Res. 1982, 16, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.A.B.; Magalhães, A.C.; Rios, D.; Lima, J.E.O. Cross-Sectional Hardness of Enamel from Human Teeth at Different Posteruptive Ages. Caries Res. 2009, 43, 491–494. [Google Scholar] [CrossRef]
- Lynch, R.J.M. The primary and mixed dentition, post-eruptive enamel maturation and dental caries: A review. Int. Dent. J. 2013, 63 (Suppl. 2), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Samuels, B.D.; Aho, R.; Brinkley, J.F.; Bugacov, A.; Feingold, E.; Fisher, S.; Gonzalez-Reiche, A.S.; Hacia, J.G.; Hallgrimsson, B.; Hansen, K.; et al. FaceBase 3: Analytical tools and FAIR resources for craniofacial and dental research. Development 2020, 147, dev191213. [Google Scholar] [CrossRef]
- Odanaka, H.; Obama, T.; Sawada, N.; Sugano, M.; Itabe, H.; Yamamoto, M. Comparison of protein profiles of the pellicle, gingival crevicular fluid, and saliva: Possible origin of pellicle proteins. Biol. Res. 2020, 53, 3. [Google Scholar] [CrossRef]
- Johnsson, M.; Levine, M.J.; Nancollas, G.H. Hydroxyapatite Binding Domains in Salivary Proteins. Crit. Rev. Oral Biol. Med. 1993, 4, 371–378. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Torres, G.I.; Levine, M.J. Salivary amylase promotes adhesion of oral streptococci to hydroxyapatite. J. Dent. Res. 1995, 74, 1360–1366. [Google Scholar] [CrossRef]
- Heller, D.; Helmerhorst, E.J.; Oppenheim, F.G. Saliva and Serum Protein Exchange at the Tooth Enamel Surface. J. Dent. Res. 2017, 96, 437–443. [Google Scholar] [CrossRef]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A.; Fortin, M.; Ghitescu, L. Endocytotic functions of ameloblasts and odontoblasts: Immunocytochemical and tracer studies on the uptake of plasma proteins. Anat. Rec. 1996, 245, 219–234. [Google Scholar] [CrossRef]
- Pham, C.-D.; Smith, C.E.; Hu, Y.; Hu, J.C.-C.; Simmer, J.P.; Chun, Y.-H.P. Endocytosis and Enamel Formation. Front. Physiol. 2017, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Açil, Y.; Mobasseri, A.E.; Warnke, P.H.; Terheyden, H.; Wiltfang, J.; Springer, I. Detection of Mature Collagen in Human Dental Enamel. Calcif. Tissue Int. 2005, 76, 121–126. [Google Scholar] [CrossRef]
- Goettig, P.; Magdolen, V.; Brandstetter, H. Natural and synthetic inhibitors of kallikrein-related peptidases (KLKs). Biochimie 2010, 92, 1546–1567. [Google Scholar] [CrossRef]
- Cavanagh, M.E.; Cornelis, M.E.; Dziegielewska, K.M.; Luft, A.J.; Lai, P.C.; Lorscheider, F.L.; Saunders, N.R. Proteins in cerebrospinal fluid and plasma of fetal pigs during development. Dev. Neurosci. 1982, 5, 492–502. [Google Scholar] [CrossRef]
- Martin, M.; Tesouro, M.A.; González-Ramón, N.; Piñeiro, A.; Lampreave, F. Major plasma proteins in pig serum during postnatal development. Reprod. Fertil. Dev. 2005, 17, 439. [Google Scholar] [CrossRef]
- Pandya, M.; Liu, H.; Dangaria, S.J.; Zhu, W.; Li, L.L.; Pan, S.; Abufarwa, M.; Davis, R.G.; Guggenheim, S.; Keiderling, T.; et al. Integrative Temporo-Spatial, Mineralogic, Spectroscopic, and Proteomic Analysis of Postnatal Enamel Development in Teeth with Limited Growth. Front. Physiol. 2017, 8, 793. [Google Scholar] [CrossRef]
- Hesselager, M.O.; Codrea, M.C.; Sun, Z.; Deutsch, E.W.; Bennike, T.B.; Stensballe, A.; Bundgaard, L.; Moritz, R.L.; Bendixen, E. The Pig PeptideAtlas: A resource for systems biology in animal production and biomedicine. Proteomics 2016, 16, 634–644. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; The UniProt Consortium; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Seddon, Y.M.; Guy, J.H.; Edwards, S.A. Optimising oral fluid collection from groups of pigs: Effect of housing system and provision of ropes. Vet. J. 2012, 193, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Walz, A.; Odenbreit, S.; Stühler, K.; Wattenberg, A.; Meyer, H.E.; Mahdavi, J.; Borén, T.; Ruhl, S. Identification of glycoprotein receptors within the human salivary proteome for the lectin-like BabA and SabA adhesins of Helicobacter pylori by fluorescence-based 2-D bacterial overlay. Proteomics 2009, 9, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Book, S.A.; Bustad, L.K. The Fetal and Neonatal Pig in Biomedical Research. J. Anim. Sci. 1974, 38, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Castiblanco, G.A.; Rutishauser, D.; Ilag, L.L.; Martignon, S.; Castellanos, J.E.; Mejía, W. Identification of proteins from human permanent erupted enamel. Eur. J. Oral Sci. 2015, 123, 390–395. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. ISBN 978-1-58829-343-5. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Yang, X.; Vidunas, A.J.; Beniash, E. Optimizing Immunostaining of Enamel Matrix: Application of Sudan Black B and Minimization of False Positives from Normal Sera and IgGs. Front. Physiol. 2017, 8, 239. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. FIJI: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Robertson, A.J.; Toumba, K.J. Cross-polarized photography in the study of enamel defects in dental paediatrics. J. Audiov. Media Med. 1999, 22, 63–70. [Google Scholar] [CrossRef] [PubMed]


| Age | Sample | Total Proteins Identified (>2 Peptides) | Common Proteins per Age (>2 Peptides) |
|---|---|---|---|
| Enamel of 4-week-old pigs | i | 342 | 203 |
| ii | 237 | ||
| iii | 433 | ||
| Enamel of 8-week-old pigs | I | 217 | 109 |
| II | 216 | ||
| III | 409 | ||
| IV | 184 |
| Peptides of AFP | Match to AFP Seq at Peptide No | Enamel | Serum | Saliva | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 Week | 8 Week | 4 Week | 8 Week | 4 Week | 8 Week | |||||||||||||
| i | ii | iii | I | II | III | IV | i | ii | iii | I | II | i | ii | iii | I | II | ||
| K.DVLTVIEK.S | 69 | |||||||||||||||||
| R.RHPFLYAPTILSLAAQYDK.I | 169 | |||||||||||||||||
| R.HPFLYAPTILSLAAQYDK.I | 170 | |||||||||||||||||
| R.DFNQLSSR.E | 339 | |||||||||||||||||
| R.FTYEYSR.R | 355 | |||||||||||||||||
| K.LAVPVILR.V | 366 | |||||||||||||||||
| K.GYQELLEK.C | 377 | |||||||||||||||||
| K.YIQESQALAK.R | 405 | |||||||||||||||||
| K.LGEYYLQNAFLVAYTK.K | 423 | |||||||||||||||||
| K.KAPQLTPPELMALTR.K | 439 | |||||||||||||||||
| K.KAPQLTPPELM*ALTR.K | 439* | |||||||||||||||||
| K.APQLTPPELMALTR.K | 440 | |||||||||||||||||
| K.QQFLINLVK.Q | 551 | |||||||||||||||||
| K.QKPQITEEQLEAVIADFSGLLEK.C | 560 | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Bona, A.; Karaaslan, H.; Depalle, B.; Sulyanto, R.; Bidlack, F.B. Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization. Int. J. Mol. Sci. 2023, 24, 15577. https://doi.org/10.3390/ijms242115577
Gil-Bona A, Karaaslan H, Depalle B, Sulyanto R, Bidlack FB. Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization. International Journal of Molecular Sciences. 2023; 24(21):15577. https://doi.org/10.3390/ijms242115577
Chicago/Turabian StyleGil-Bona, Ana, Hakan Karaaslan, Baptiste Depalle, Rosalyn Sulyanto, and Felicitas B. Bidlack. 2023. "Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization" International Journal of Molecular Sciences 24, no. 21: 15577. https://doi.org/10.3390/ijms242115577
APA StyleGil-Bona, A., Karaaslan, H., Depalle, B., Sulyanto, R., & Bidlack, F. B. (2023). Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization. International Journal of Molecular Sciences, 24(21), 15577. https://doi.org/10.3390/ijms242115577