Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone
Abstract
:1. Introduction
2. Results
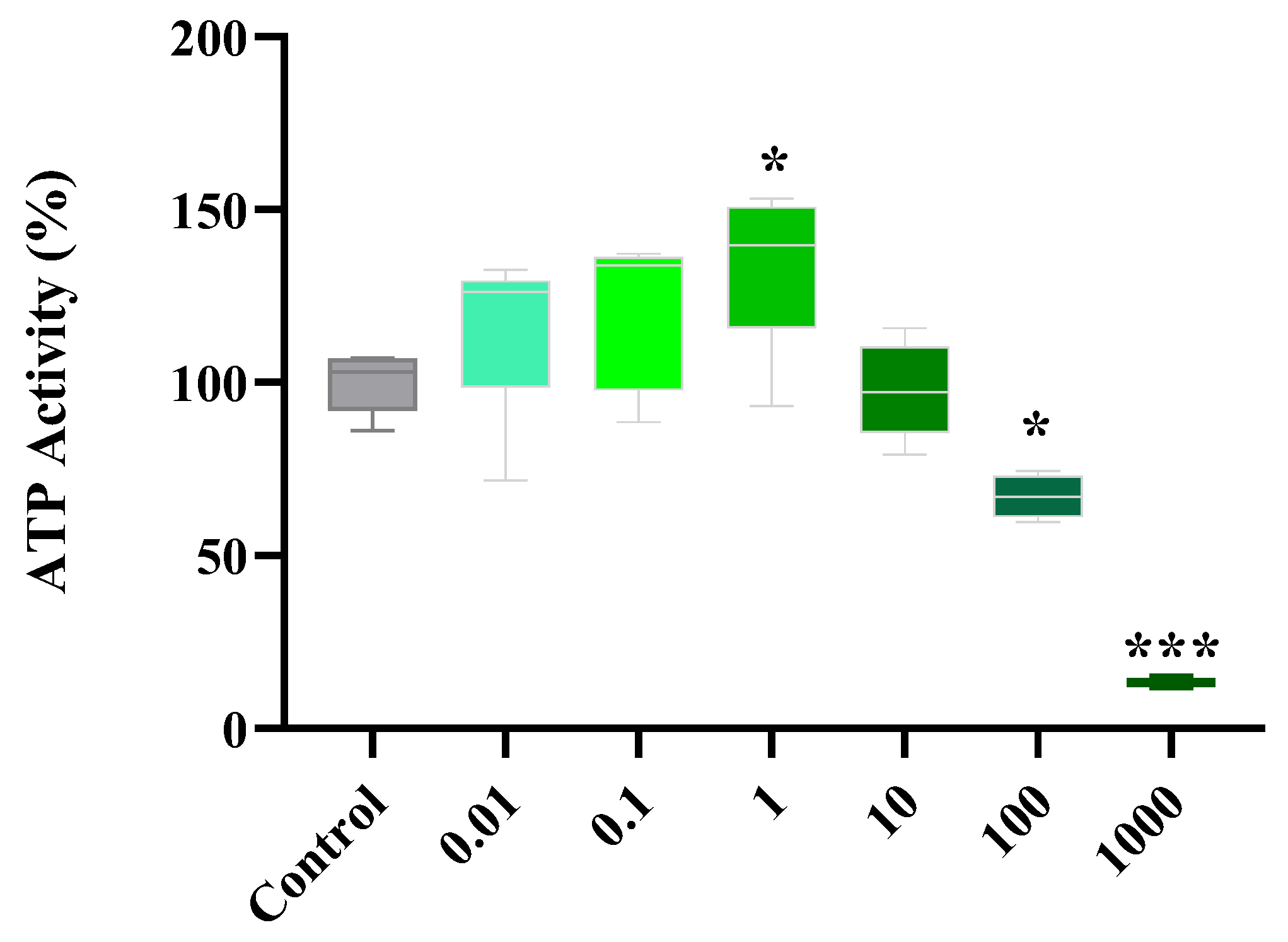
2.1. Dose Response of Flavonoids
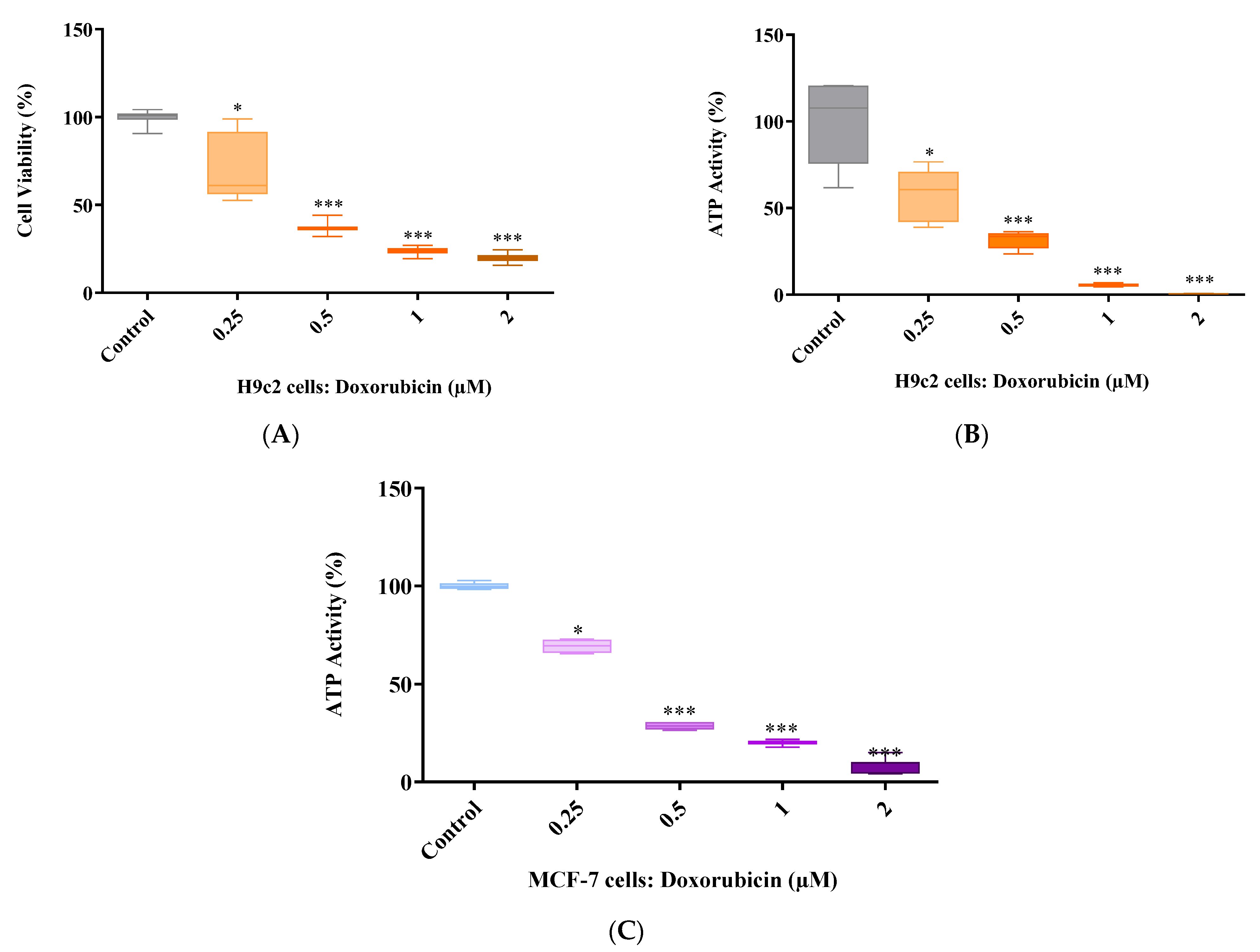
2.2. Doxorubicin Dose Response
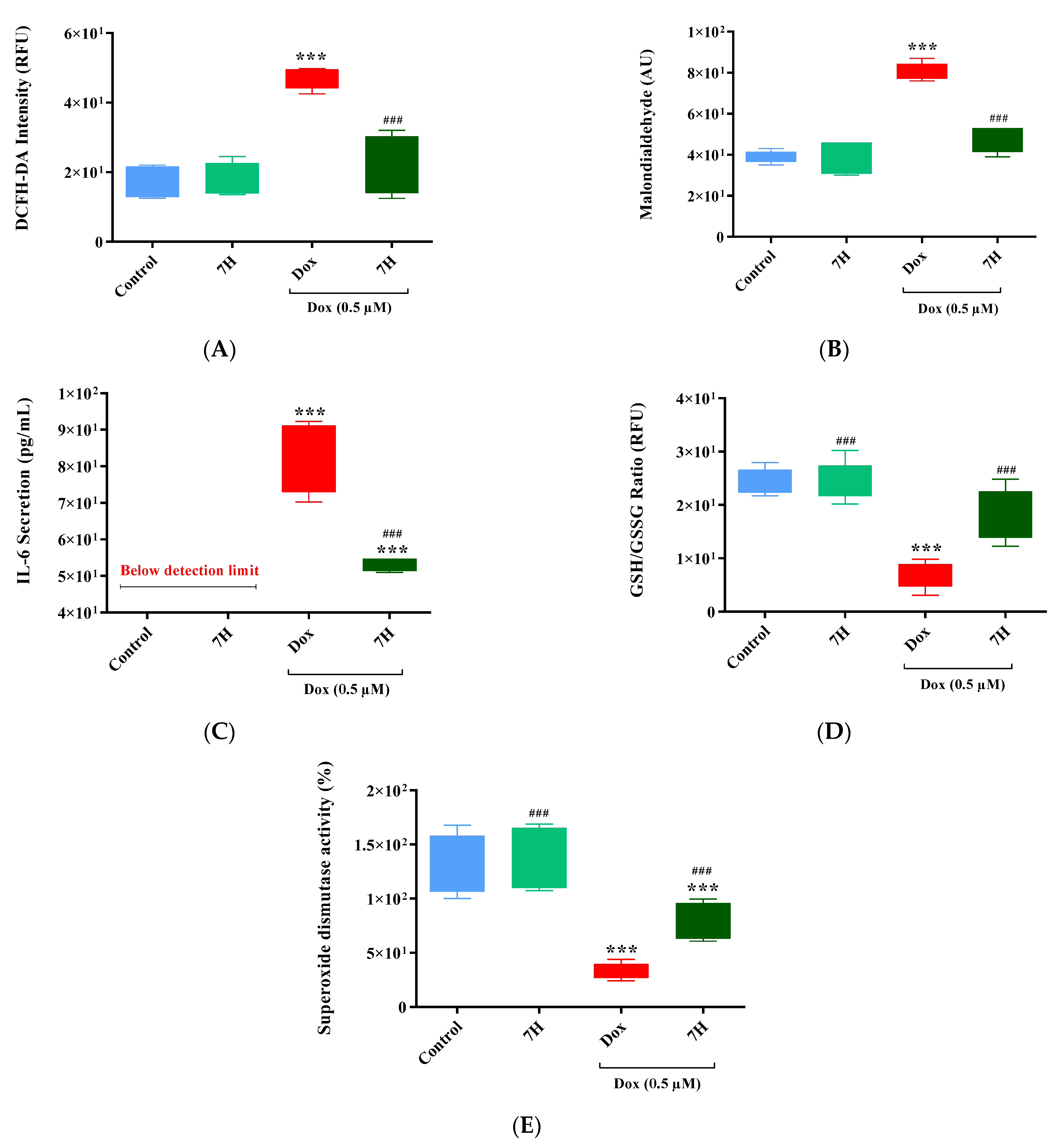
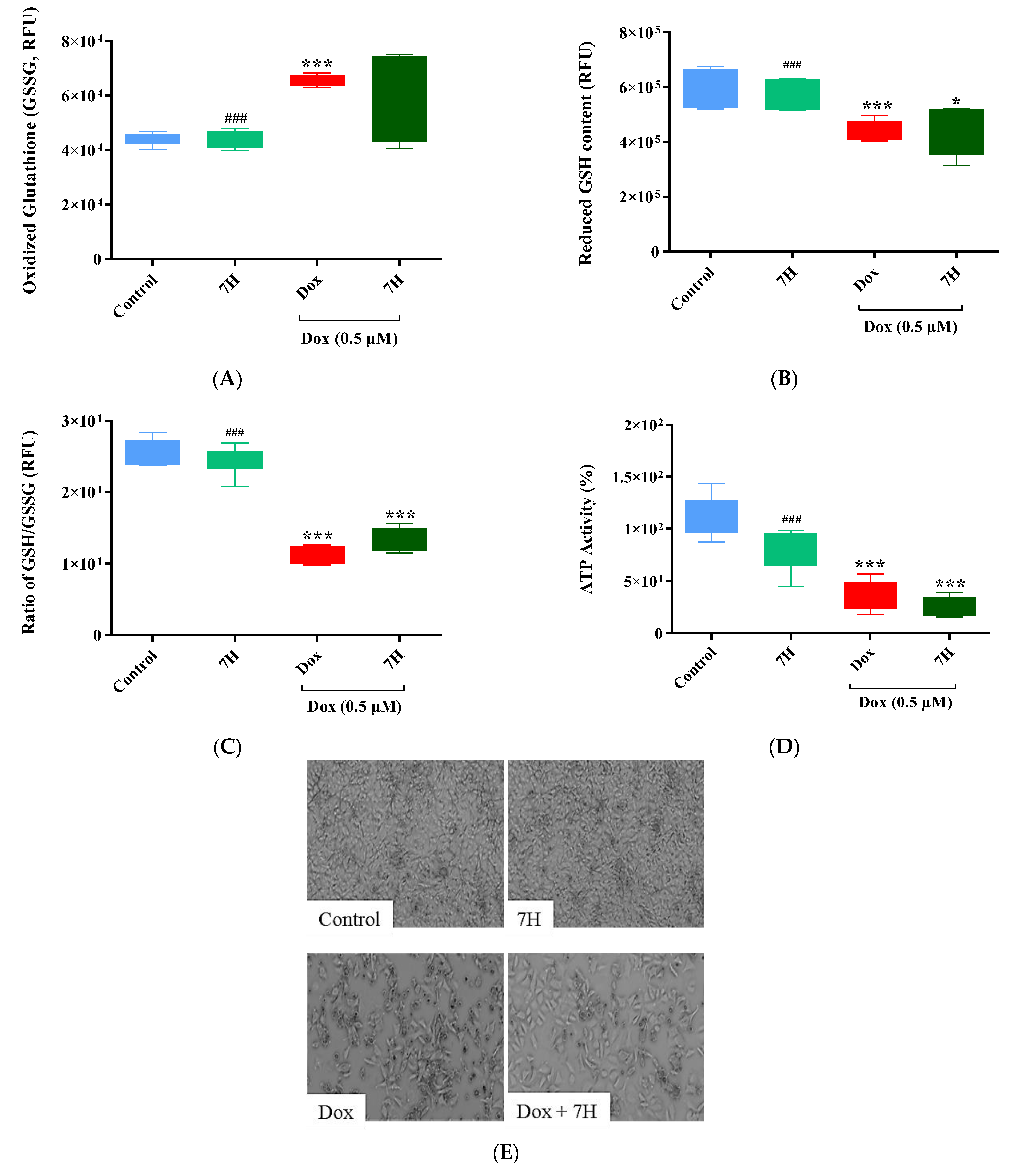
2.3. Doxorubicin-Induced Oxidative Stress in Cardiac Cells
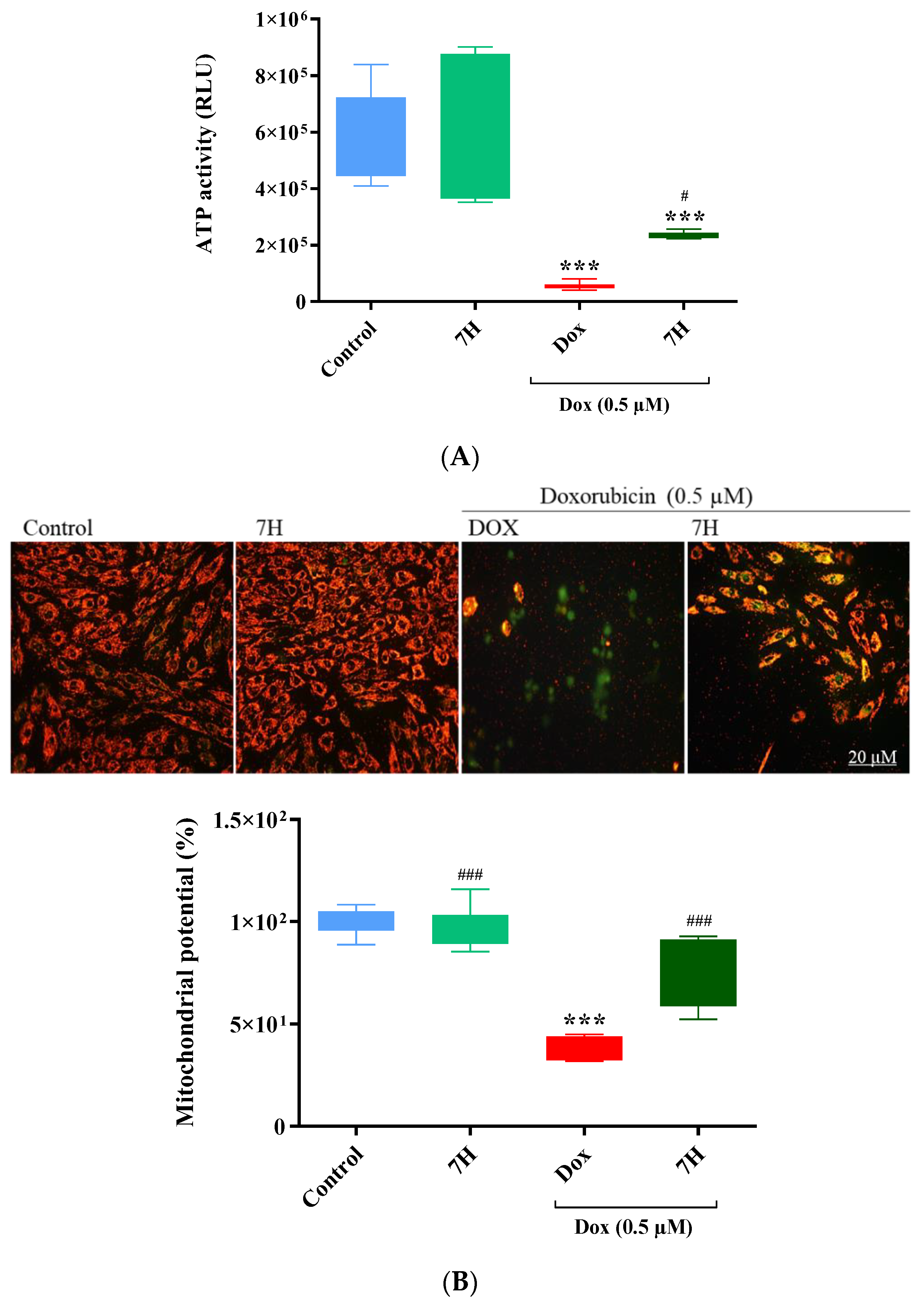
2.4. Mitochondrial Bioenergetics of Cardiac Cells
2.5. Cardiac ATP Activity and Mitochondrial Membrane Potential
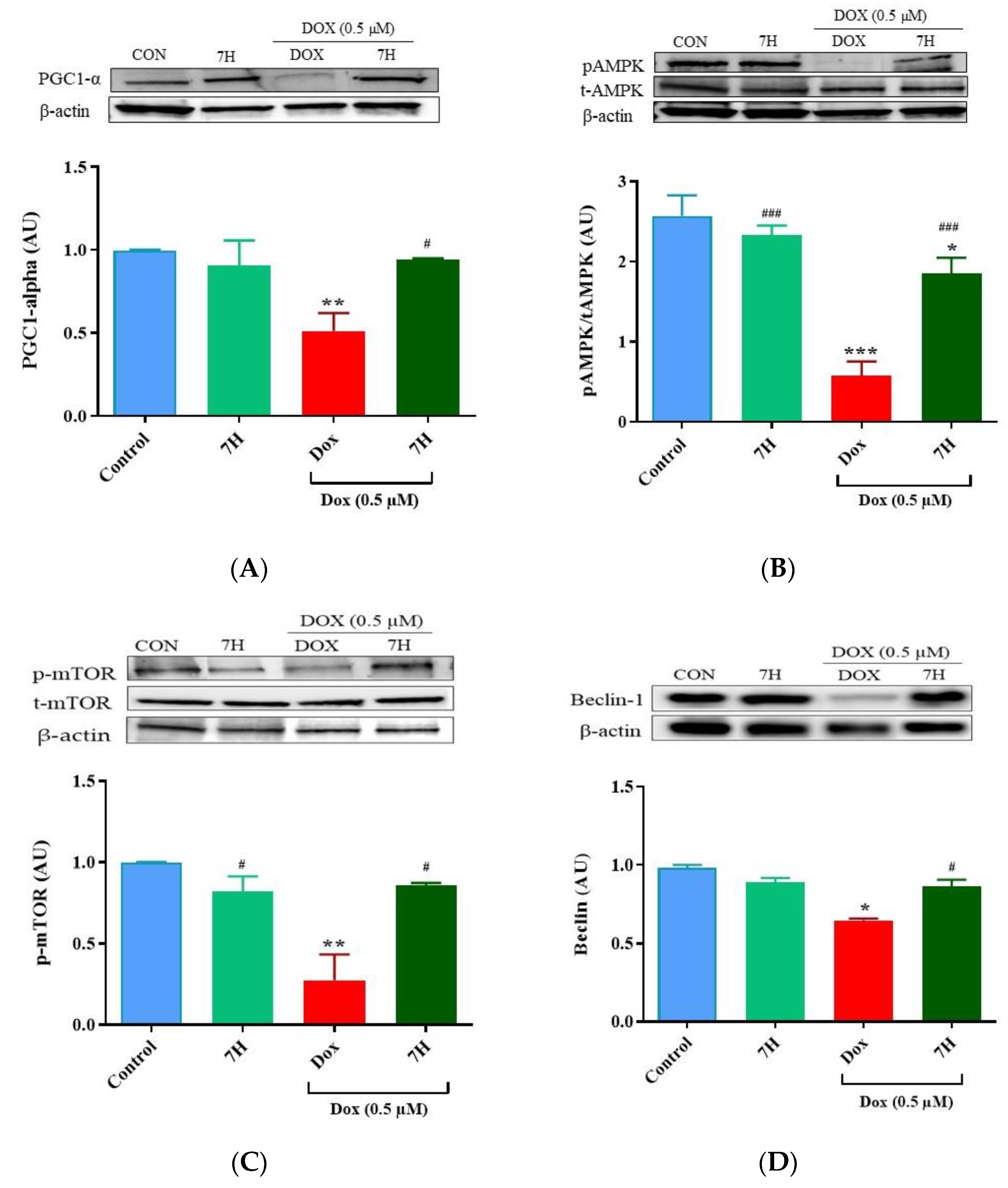
2.6. Autophagy and Mitochondrial Proteins
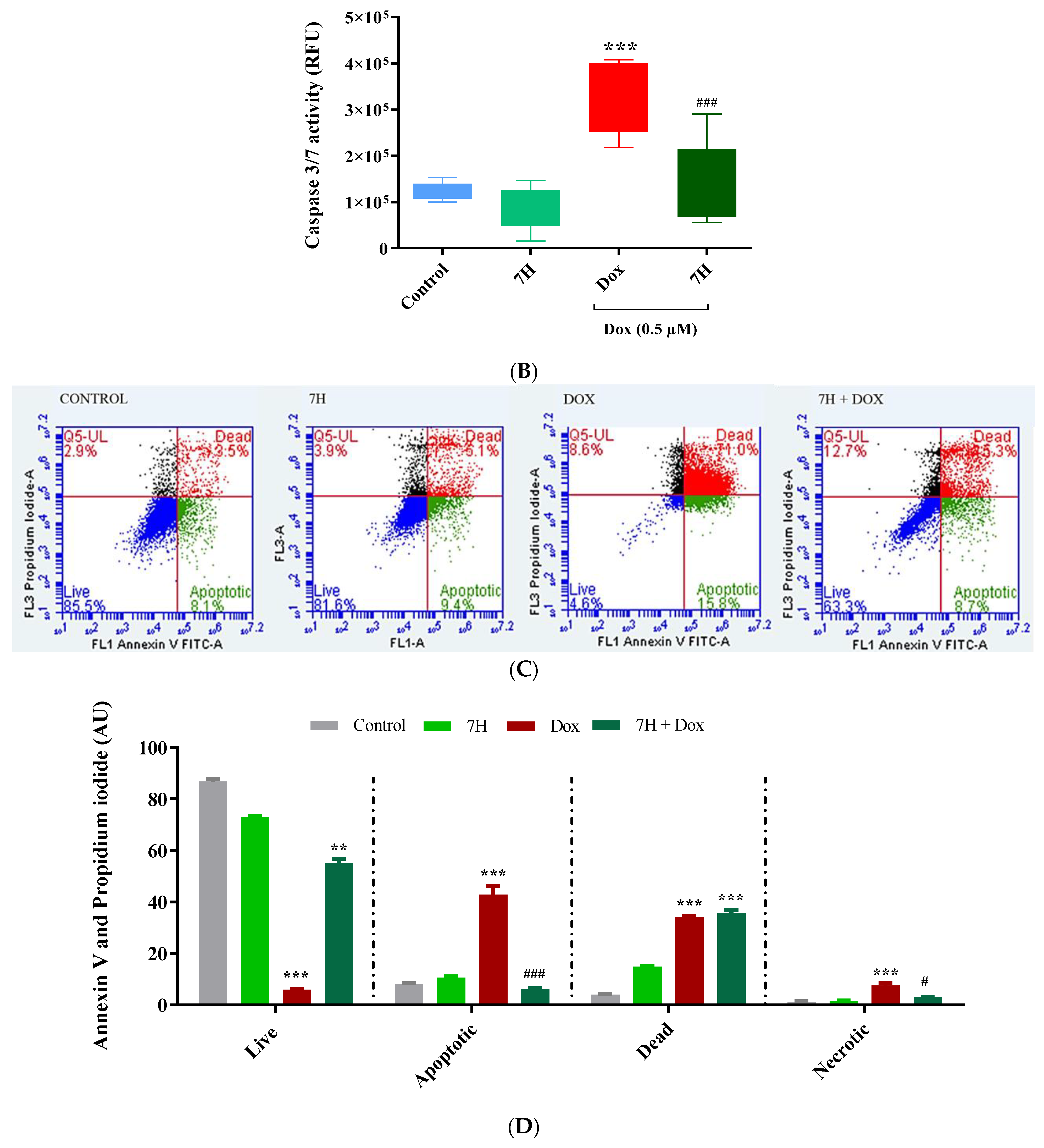
2.7. Doxorubicin-Induced Apoptosis in Cardiac Cells
2.8. Breast Cancer Cells: Antioxidant Activity and Caspase 3/7
3. Discussion
4. Methods
4.1. Materials and Reagents
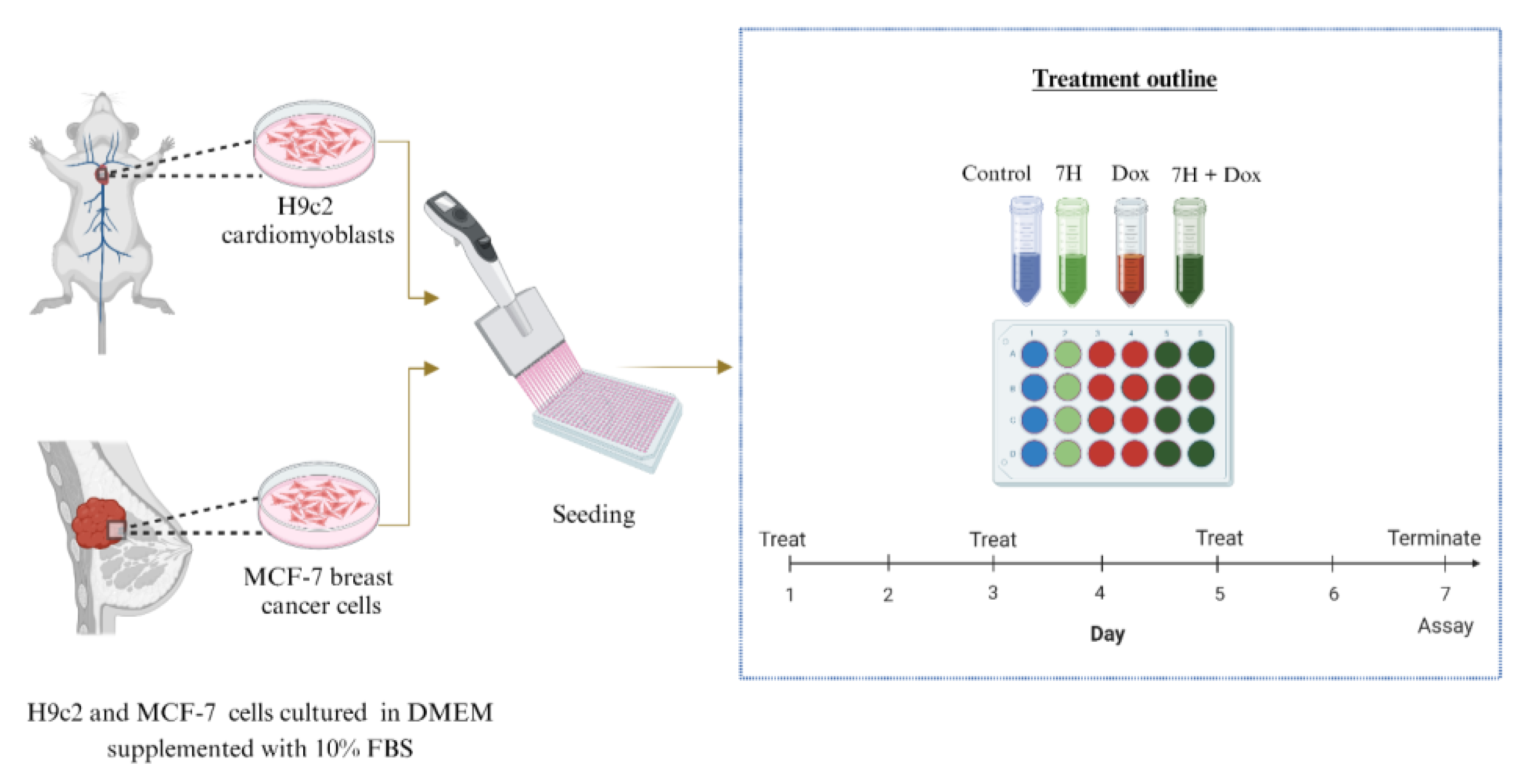
4.2. In Vitro Models
4.3. Dose Response
4.4. Oxidative Stress
4.5. Inflammatory Cytokines
4.6. Endogenous Antioxidant Levels
4.7. Mitochondrial Bioenergetics
4.8. Mitochondrial Membrane Potential (MMP)
4.9. Evaluating Cell Death
4.10. Western Blot Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonadonna, G.; Monfardini, S.; De Lena, M.; Fossati-Bellani, F.; Beretta, G. Phase I and Preliminary Phase II Evaluation of Adriamycin (NSC 123127). Cancer Res. 1970, 30, 2572–2582. [Google Scholar] [PubMed]
- Christidi, E.; Huang, H.; Shafaattalab, S.; Maillet, A.; Lin, E.; Huang, K.; Laksman, Z.; Davis, M.K.; Tibbits, G.F.; Brunham, L.R. Variation in RARG increases susceptibility to doxorubicin-induced cardiotoxicity in patient specific induced pluripotent stem cell-derived cardiomyocytes. Sci. Rep. 2020, 10, 10363. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Wu, F.; Lu, W.-J.; Bai, R.; Ke, B.; Liu, T.; Li, L.; Lan, F.; Cui, M. Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIα to IIβ in human stem cell derived cardiomyocytes. J. Cell Mol. Med. 2019, 23, 4627–4639. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Miller, T.L.; Lipsitz, S.R.; Neuberg, D.S.; Dahlberg, S.E.; Colan, S.D.; Silverman, L.B.; Henkel, J.M.; Franco, V.I.; Cushman, L.L.; et al. Continuous Versus Bolus Infusion of Doxorubicin in Children With ALL: Long-term Cardiac Outcomes. Pediatrics 2012, 130, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Sirangelo, I.; Sapio, L.; Ragone, A.; Naviglio, S.; Iannuzzi, C.; Barone, D.; Giordano, A.; Borriello, M. Vanillin Prevents Doxorubicin-Induced Apoptosis and Oxidative Stress in Rat H9c2 Cardiomyocytes. Nutrients 2020, 12, 2317. [Google Scholar] [CrossRef]
- Visscher, H.; Ross, C.; Rassekh, S.; Barhdadi, A.; Dubé, M.-P.; Al-Saloos, H.; Sandor, G.; Caron, H.; Dalen, E.; Kremer, L.; et al. Pharmacogenomic Prediction of Anthracycline-Induced Cardiotoxicity in Children. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 30, 1422–1428. [Google Scholar] [CrossRef]
- Harishkumar, R.; Christopher, J.G.; Ravindran, R.; Selvaraj, C.I. Nuciferine Attenuates Doxorubicin-Induced Cardiotoxicity: An In Vitro and In Vivo Study. Cardiovasc. Toxicol. 2021, 21, 947–963. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, X.; Wang, E.; Jia, X.; Wang, G.; Wen, J. Irigenin treatment alleviates doxorubicin (DOX)-induced cardiotoxicity by suppressing apoptosis, inflammation and oxidative stress via the increase of miR-425. Biomed. Pharmacother. 2020, 125, 109784. [Google Scholar] [CrossRef]
- Choi, J.-S.; Piao, Y.-J.; Kang, K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharm. Res. 2011, 34, 607–613. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z.-P. Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur. J. Pharmacol. 2020, 881, 173078. [Google Scholar] [CrossRef]
- Prasanna, P.L.; Renu, K.; Valsala Gopalakrishnan, A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020, 250, 117599. [Google Scholar] [CrossRef] [PubMed]
- Wallace Kendall, B.; Sardão Vilma, A.; Oliveira Paulo, J. Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Wenningmann, N.; Knapp, M.; Ande, A.; Vaidya, T.R.; Ait-Oudhia, S. Insights into Doxorubicin-induced Cardiotoxicity: Molecular Mechanisms, Preventive Strategies, and Early Monitoring. Mol. Pharmacol. 2019, 96, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Endo, S.; Miyagi, N.; Abe, N.; Miura, T.; Nishinaka, T.; Terada, T.; Oyama, M.; Goda, H.; El-Kabbani, O.; et al. Structure–activity relationship of flavonoids as potent inhibitors of carbonyl reductase 1 (CBR1). Fitoterapia 2015, 101, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, J.; Zhao, L.; Li, S.; Li, K. Quercetin attenuates the cardiotoxicity of doxorubicin–cyclophosphamide regimen and potentiates its chemotherapeutic effect against triple-negative breast cancer. Phytother. Res. 2022, 36, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Peluso, J.; Chalhoub, S.; Gillet, Y.I.; Benkirane-Jessel, N.; Rochel, N.; Fuhrmann, G.; Ubeaud-Sequier, G. Quercetin potentializes the respective cytotoxic activity of gemcitabine or doxorubicin on 3D culture of AsPC-1 or HepG2 cells, through the inhibition of HIF-1α and MDR1. PLoS ONE 2020, 15, e0240676. [Google Scholar] [CrossRef]
- Awasthi, M.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Molecular docking and 3D-QSAR-based virtual screening of flavonoids as potential aromatase inhibitors against estrogen-dependent breast cancer. J. Biomol. Struct. Dyn. 2015, 33, 804–819. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Du, B.; Miao, X.; Bai, Y.; Xin, Y.; Tan, Y.; Cui, W.; Liu, B.; Cui, T.; et al. Therapeutic effect of MG-132 on diabetic cardiomyopathy is associated with its suppression of proteasomal activities: Roles of Nrf2 and NF-κB. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H567–H578. [Google Scholar] [CrossRef]
- Adams, B.; Mapanga, R.F.; Essop, M.F. Partial inhibition of the ubiquitin–proteasome system ameliorates cardiac dysfunction following ischemia–reperfusion in the presence of high glucose. Cardiovasc. Diabetol. 2015, 14, 94. [Google Scholar] [CrossRef]
- Sangweni, N.F.; Moremane, M.; Riedel, S.; van Vuuren, D.; Huisamen, B.; Mabasa, L.; Barry, R.; Johnson, R. The Prophylactic Effect of Pinocembrin Against Doxorubicin-Induced Cardiotoxicity in an In Vitro H9c2 Cell Model. Front. Pharmacol. 2020, 11, 1172. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Wei, W.; Zhang, N.; Wu, H.; Ma, Z.; Li, L.; Deng, W.; Tang, Q. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm. Sin. B 2019, 9, 690–701. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Qin, D.; Yue, R.; Deng, P.; Wang, X.; Zheng, Z.; Lv, M.; Zhang, Y.; Pu, J.; Xu, J.; Liang, Y.; et al. 8-Formylophiopogonanone B antagonizes doxorubicin-induced cardiotoxicity by suppressing heme oxygenase-1-dependent myocardial inflammation and fibrosis. Biomed. Pharmacother. 2021, 140, 111779. [Google Scholar] [CrossRef]
- Rong, J.; Li, L.; Jing, L.; Fang, H.; Peng, S. JAK2/STAT3 Pathway Mediates Protection of Metallothionein Against Doxorubicin-Induced Cytotoxicity in Mouse Cardiomyocytes. Int. J. Toxicol. 2016, 35, 317–326. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, X.; Luo, Y.; You, J.; Yin, D.; Xu, Q.; He, H.; He, M. Quercetin protects cardiomyocytes against doxorubicin-induced toxicity by suppressing oxidative stress and improving mitochondrial function via 14-3-3γ. Toxicol. Mech. Methods 2019, 29, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Nur, E.; Verwijs, M.; de Waart, D.R.; Schnog, J.-J.B.; Otten, H.-M.; Brandjes, D.P.; Biemond, B.J.; Elferink, R.P.J.O.; CURAMA Study Group. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. Biochim. Biophys. Acta 2011, 1812, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fan, S.; Shu, L.; Huang, W.; Xie, L.; Bi, C.; Yu, H.; Wang, Y.; Li, Y. Exploration the Mechanism of Doxorubicin-Induced Heart Failure in Rats by Integration of Proteomics and Metabolomics Data. Front. Pharmacol. 2020, 11, 600561. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.M.; Zavorka Thomas, M.; Magdy, T.; Eisenmann, E.D.; Uddin, M.E.; DiGiacomo, D.F.; Pan, A.; Keiser, M.; Otter, M.; Xia, S.H.; et al. Targeting OCT3 attenuates doxorubicin-induced cardiac injury. Proc. Natl. Acad. Sci. USA 2021, 118, e2020168118. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Asgary, S.; Mohammadi, P.; Hozeifi, S.; Hoseinzadeh-Chahkandak, F.; Xu, S.; Farzaei, M.H. Natural AMPK Activators in Cardiovascular Disease Prevention. Front. Pharmacol. 2022, 12, 738420. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Sangweni, N.F.; Dludla, P.V.; Chellan, N.; Mabasa, L.; Sharma, J.R.; Johnson, R. The Implication of Low Dose Dimethyl Sulfoxide on Mitochondrial Function and Oxidative Damage in Cultured Cardiac and Cancer Cells. Molecules 2021, 26, 7305. [Google Scholar] [CrossRef] [PubMed]




| Metabolic Parameters (mpH/min/µg Protein) | Treatment | |||
|---|---|---|---|---|
| Control | 7H | DOX | Dox + 7H | |
| Basal respiration | 48.1 ± 0.9 | 51.8 ± 2.1 | 15.4 ± 0.2 ** | 21 ± 0.7 * |
| Maximal respiration | 116.3 ± 5.2 | 145.6 ± 5.7 | 16.7 ± 1.7 *** | 40.5 ± 2.3 ***, ## |
| ATP-linked respiration | 44.6 ± 0.9 | 46.9 ± 1.6 | 12.5 ± 0.2 *** | 20.25 ± 0.6 ** |
| Spare respiration | 58.2 ± 1.0 | 93.83 ± 4.1 | 6.9 ± 0.7 *** | 19.6 ± 2.9 ***, # |
| State apparent | 2.6 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 | 2.0 ± 0.1 |
| Coupling efficiency | 90.0 ± 1.6 | 83.9 ± 5.5 | 44.6 ± 0.9 *** | 94.8 ± 1.1 ### |
| Respiratory control | 47.9 ± 6.2 | 32.7 ± 5.0 | 12.7 ± 2.7 *** | 32.9 ± 6.2 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangweni, N.F.; Gabuza, K.; van Aarde, R.; Mabasa, L.; van Vuuren, D.; Huisamen, B.; Barry, R.; Johnson, R. Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone. Int. J. Mol. Sci. 2023, 24, 15395. https://doi.org/10.3390/ijms242015395
Sangweni NF, Gabuza K, van Aarde R, Mabasa L, van Vuuren D, Huisamen B, Barry R, Johnson R. Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone. International Journal of Molecular Sciences. 2023; 24(20):15395. https://doi.org/10.3390/ijms242015395
Chicago/Turabian StyleSangweni, Nonhlakanipho F., Kwazi Gabuza, Ruzayda van Aarde, Lawrence Mabasa, Derick van Vuuren, Barbara Huisamen, Reenen Barry, and Rabia Johnson. 2023. "Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone" International Journal of Molecular Sciences 24, no. 20: 15395. https://doi.org/10.3390/ijms242015395
APA StyleSangweni, N. F., Gabuza, K., van Aarde, R., Mabasa, L., van Vuuren, D., Huisamen, B., Barry, R., & Johnson, R. (2023). Doxorubicin-Induced Cardiomyopathy: A Preliminary Study on the Cardioprotective Benefits of 7-Hydroxyflavanone. International Journal of Molecular Sciences, 24(20), 15395. https://doi.org/10.3390/ijms242015395






