A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP)
Abstract
:1. Introduction
2. Result
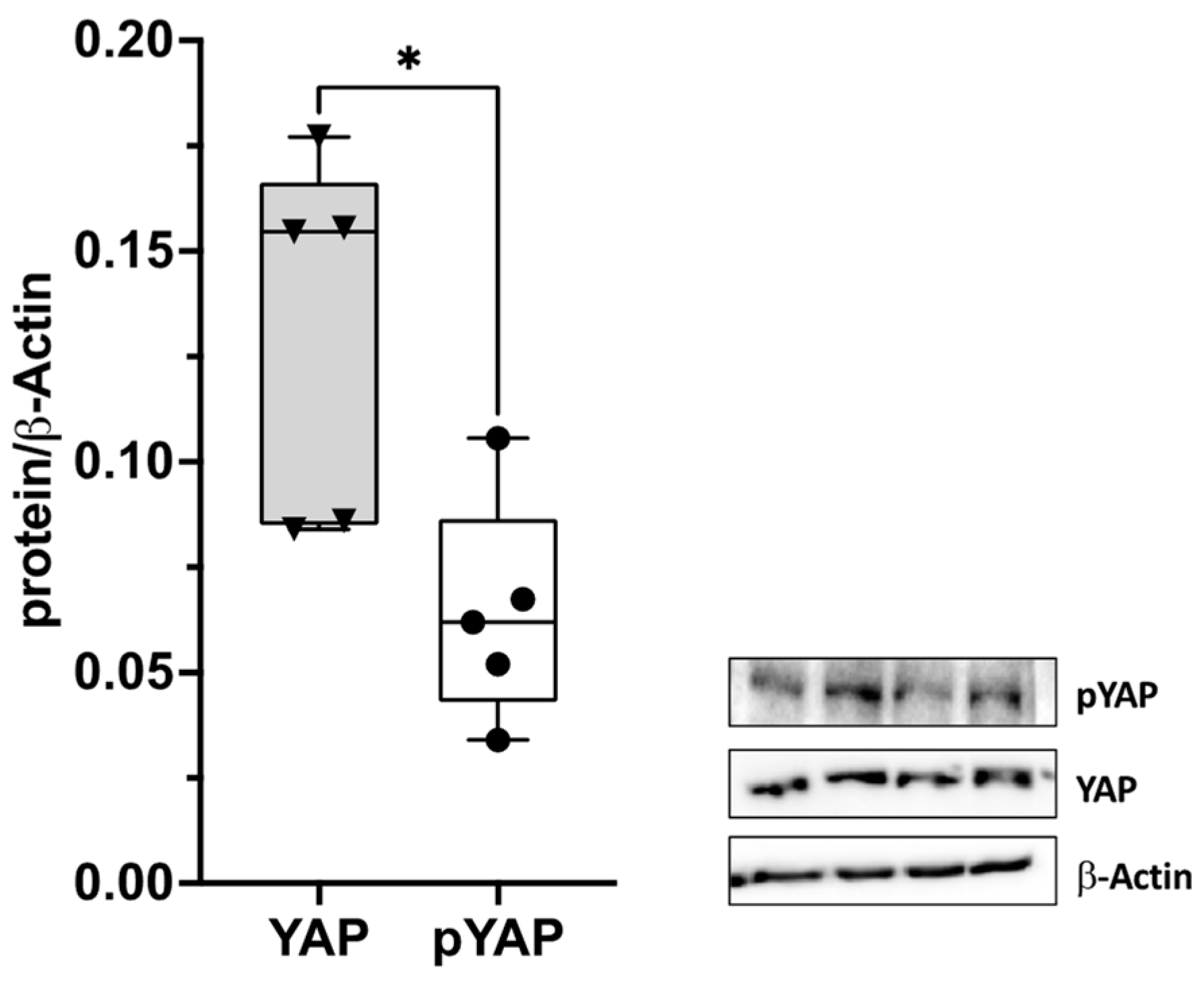
2.1. Yes-Associated Protein (YAP) in the Thoracolumbar Fascia (TLF)
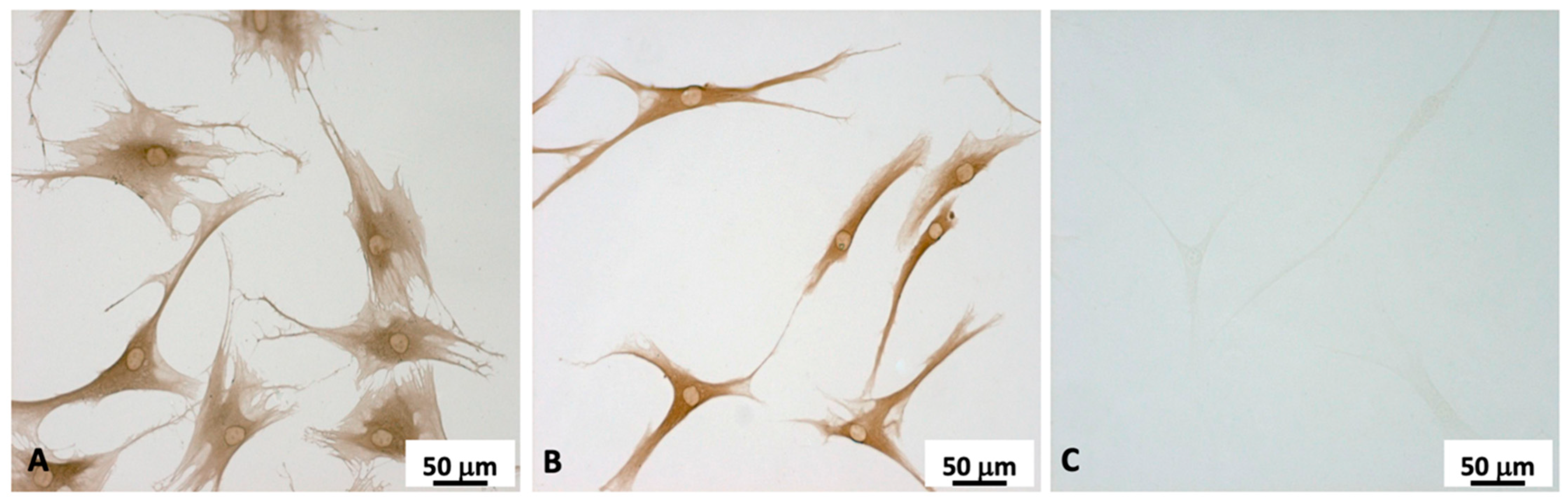
2.2. Fibroblasts Extraction and Characterization
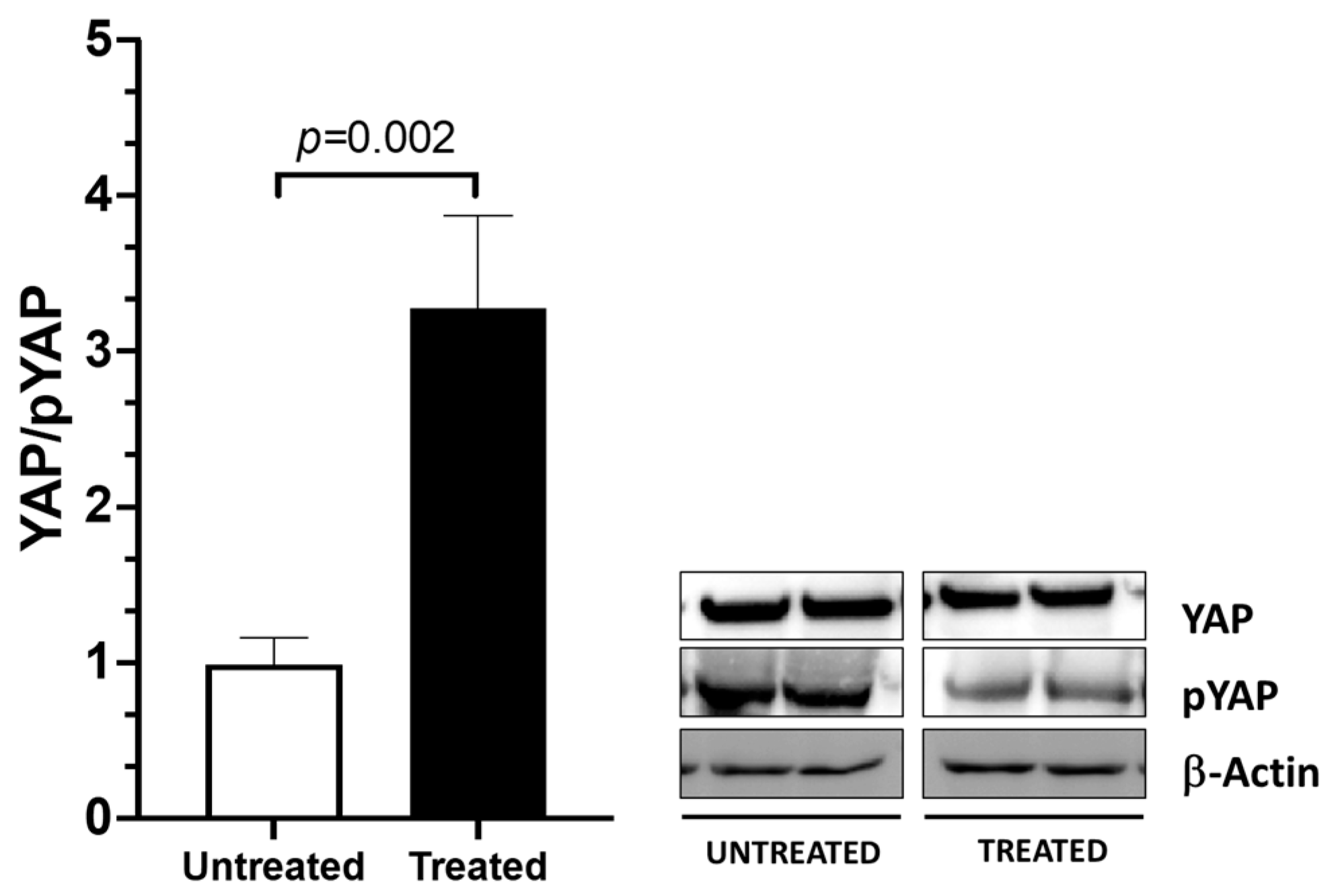
2.3. YAP Activation
2.4. Collagen Type 1 A (COL1A1) and Hyaluronan-Binding Protein 2 (HABP2) Gene Expression after the fESW Treatment
2.5. The fESW Treatment Enhances Fibroblast Proliferation via YAP-Mediated Signaling
3. Discussion
4. Materials and Methods
4.1. Patients and Tissues
4.2. Cell Isolation
4.3. Cell Characterization
4.4. Cell Treatment with Focal Extracorporeal Shockwaves (fESWs)
4.5. Immunoblotting
4.6. RNA Extraction and Real-Time PCR
| Patient Number | Age | Sex |
|---|---|---|
| 1 | 30 | F |
| 2 | 37 | F |
| 3 | 45 | M |
| 4 | 45 | F |
| 5 | 54 | F |
| 6 | 61 | M |
| 7 | 68 | M |
| 8 | 70 | M |
| Gene (Accession Number) | Forward Primer | Reverse Primer |
|---|---|---|
| COL1A1 NM_000088.4 | 5′- TTCTCAGCGTGGGTAAGTGT- 3′ | 3′- TTCTCAGCGTGGGTAAGTGT- 5′ |
| HABP2 NM_001177660.3 | 5′- AATGGCTCTGGTGGGAAAGA- 3′ | 3′- TTCTCAGCGTGGGTAAGTGT- 5′ |
| GAPDH NM_001256799.3 | 5′- TTCTCAGCGTGGGTAAGTGT- 3′ | 3′- TTCTCAGCGTGGGTAAGTGT- 5′ |
4.7. The Cell Proliferation Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef] [PubMed]
- Hoheisel, U.; Rosner, J.; Mense, S. Innervation changes induced by inflammation of the rat thoracolumbar fascia. Neuroscience 2015, 300, 351–359. [Google Scholar] [CrossRef]
- Kondrup, F.; Gaudreault, N.; Venne, G. The deep fascia and its role in chronic pain and pathological conditions: A review. Clin Anat. 2022, 35, 649–659. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Petrelli, L.; Guidolin, D.; De Caro, R.; Stecco, C. A Closer Look at the Cellular and Molecular Components of the Deep/Muscular Fasciae. Int. J. Mol. Sci. 2021, 22, 1411. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Langevin, H.M.; Cornbrooks, C.J.; Taatjes, D.J. Fibroblasts Form a Body-Wide Cellular Network. Histochem. Cell Biol. 2004, 122, 7–15. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M. The Fascia of the Limbs and Back—A Review. J. Anat. 2009, 214, 1–18. [Google Scholar] [CrossRef]
- Lendahl, U.; Muhl, L.; Betsholtz, C. Identification, Discrimination and Heterogeneity of Fibroblasts. Nat. Commun. 2022, 13, 3409. [Google Scholar] [CrossRef]
- Wang, T.; Long, Y.; Ma, L.; Dong, Q.; Li, Y.; Guo, J.; Jin, L.; Di, L.; Zhang, Y.; Wang, L.; et al. Single-Cell RNA-Seq Reveals Cellular Heterogeneity from Deep Fascia in Patients with Acute Compartment Syndrome. Front. Immunol. 2023, 13, 1062479. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.J.; Koenderink, G.H. A Guide to Mechanobiology: Where Biology and Physics Meet. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 3043–3052. [Google Scholar] [CrossRef]
- Frairia, R.; Berta, L. Biological Effects of Extracorporeal Shock Waves on Fibroblasts. A Review. Muscles. Ligaments Tendons J. 2011, 1, 138–147. [Google Scholar]
- Pirri, C.; Fede, C.; Petrelli, L.; De Rose, E.; Biz, C.; Guidolin, D.; De Caro, R.; Stecco, C. Immediate Effects of Extracorporeal Shock Wave Therapy in Fascial Fibroblasts: An In Vitro Study. Biomedicines 2022, 10, 1732. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Petrelli, L.; Guidolin, D.; Fan, C.; De Caro, R.; Stecco, C. Sensitivity of the Fasciae to the Endocannabinoid System: Production of Hyaluronan-Rich Vesicles and Potential Peripheral Effects of Cannabinoids in Fascial Tissue. Int. J. Mol. Sci. 2020, 21, 2936. [Google Scholar] [CrossRef]
- Fede, C.; Pirri, C.; Fan, C.; Albertin, G.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Sensitivity of the Fasciae to Sex Hormone Levels: Modulation of Collagen-I, Collagen-III and Fibrillin Production. PLoS ONE 2019, 14, e0223195. [Google Scholar] [CrossRef]
- Pirri, C.; Caroccia, B.; Angelini, A.; Petrelli, L.; Piazza, M.; Biz, C.; Ruggieri, P.; De Caro, R.; Stecco, C. Evidence of Renin–Angiotensin System Receptors in Deep Fascia: A Role in Extracellular Matrix Remodeling and Fibrogenesis? Biomedicines 2022, 10, 2608. [Google Scholar] [CrossRef]
- Stecco, A.; Cowman, M.; Pirri, N.; Raghavan, P.; Pirri, C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering 2022, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-Wide Association between YAP/TAZ/TEAD and AP-1 at Enhancers Drives Oncogenic Growth. Nat. Cell Biol. 2015, 17, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Tapon, N. Sensing the Local Environment: Actin Architecture and Hippo Signalling. Curr. Opin. Cell Biol. 2014, 31, 74–83. [Google Scholar] [CrossRef]
- Nakajima, H.; Yamamoto, K.; Agarwala, S.; Terai, K.; Fukui, H.; Fukuhara, S.; Ando, K.; Miyazaki, T.; Yokota, Y.; Schmelzer, E.; et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev. Cell 2017, 40, 523–536.e6. [Google Scholar] [CrossRef]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Rege, T.A.; Hagood, J.S. Thy-1 as a Regulator of Cell-cell and Cell-matrix Interactions in Axon Regeneration, Apoptosis, Adhesion, Migration, Cancer, and Fibrosis. FASEB J. 2006, 20, 1045–1054. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Erikss, J.E. Vimentin Coordinates Fibroblast Proliferation and Keratinocyte Differentiation in Wound Healing via TGF-β-Slug Signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [PubMed]
- Calvo, F.; Ege, N.; Grande-Garcia, A.; Hooper, S.; Jenkins, R.P.; Chaudhry, S.I.; Harrington, K.; Williamson, P.; Moeendarbary, E.; Charras, G.; et al. Mechanotransduction and YAP-Dependent Matrix Remodelling Is Required for the Generation and Maintenance of Cancer-Associated Fibroblasts. Nat. Cell Biol. 2013, 15, 637–646. [Google Scholar] [CrossRef]
- Halder, G.; Dupont, S.; Piccolo, S. Transduction of Mechanical and Cytoskeletal Cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 2012, 13, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Lorthongpanich, C.; Thumanu, K.; Tangkiettrakul, K.; Jiamvoraphong, N.; Laowtammathron, C.; Damkham, N.; U-Pratya, Y.; Issaragrisil, S. YAP as a Key Regulator of Adipo-Osteogenic Differentiation in Human MSCs. Stem Cell Res. Ther. 2019, 10, 402. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and Pharmacological Disruption of the TEAD-YAP Complex Suppresses the Oncogenic Activity of YAP. Genes Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, X.; Feng, W.; Yu, Y.; Jeong, K.; Guo, W.; Lu, Y.; Mills, G.B. Verteporfin Inhibits YAP Function through Up-Regulating 14-3-3σ Sequestering YAP in the Cytoplasm. Am. J. Cancer Res. 2016, 6, 27–37. [Google Scholar]
- Pan, D. The Hippo Signaling Pathway in Development and Cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Link, P.A.; Choi, K.M.; Diaz Espinosa, A.M.; Jones, D.L.; Gao, A.Y.; Haak, A.J.; Tschumperlin, D.J. Combined Control of the Fibroblast Contractile Program by YAP and TAZ. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2022, 322, L23–L32. [Google Scholar] [CrossRef]
- Mooring, M.; Fowl, B.H.; Lum, S.Z.; Liu, Y.; Yao, K.; Softic, S.; Kirchner, R.; Bernstein, A.; Singhi, A.D.; Jay, D.G.; et al. Hepatocyte Stress Increases Expression of Yes-Associated Protein and Transcriptional Coactivator with PDZ-Binding Motif in Hepatocytes to Promote Parenchymal Inflammation and Fibrosis. Hepatology 2020, 71, 1813–1830. [Google Scholar] [CrossRef]
- Liang, M.; Yu, M.; Xia, R.; Song, K.; Wang, J.; Luo, J.; Chen, G.; Cheng, J. Yap/Taz Deletion in Gli+ Cell-Derived Myofibroblasts Attenuates Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 3278–3290. [Google Scholar] [CrossRef]
- Mia, M.M.; Cibi, D.M.; Ghani, S.A.B.A.; Singh, A.; Tee, N.; Sivakumar, V.; Bogireddi, H.; Cook, S.A.; Mao, J.; Singh, M.K. Loss of Yap/Taz in Cardiac Fibroblasts Attenuates Adverse Remodelling and Improves Cardiac Function. Cardiovasc. Res. 2022, 118, 1785–1804. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Niederer, D.; Vogt, L.; Banzer, W. Remote effects of lower limb stretching: Preliminary evidence for myofascial connectivity? J. Sports Sci. 2016, 34, 2145–2148. [Google Scholar] [CrossRef]
- Mambetsariev, N.; Mirzapoiazova, T.; Mambetsariev, B.; Sammani, S.; Lennon, F.E.; Garcia, J.G.N.; Singleton, P.A. Hyaluronic Acid Binding Protein 2 Is a Novel Regulator of Vascular Integrity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 483–490. [Google Scholar] [CrossRef]
- Ou, W.; Xu, W.; Liu, F.; Guo, Y.; Huang, Z.; Feng, T.; Liu, C.Y.; Du, P. Increased Expression of Yes-Associated Protein/YAP and Transcriptional Coactivator with PDZ-Binding Motif/TAZ Activates Intestinal Fibroblasts to Promote Intestinal Obstruction in Crohn’s Disease. eBioMedicine 2021, 69, 103452. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. Mechanisms of fibrogenesis. Exp. Biol. Med. 2008, 233, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; McCollum, D. Control of Cellular Responses to Mechanical Cues through YAP/TAZ Regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Caroccia, B.; Angelini, A.; Piazza, M.; Petrelli, L.; Caputo, I.; Montemurro, C.; Ruggieri, P.; De Caro, R.; Stecco, C. A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP). Int. J. Mol. Sci. 2023, 24, 15389. https://doi.org/10.3390/ijms242015389
Pirri C, Caroccia B, Angelini A, Piazza M, Petrelli L, Caputo I, Montemurro C, Ruggieri P, De Caro R, Stecco C. A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP). International Journal of Molecular Sciences. 2023; 24(20):15389. https://doi.org/10.3390/ijms242015389
Chicago/Turabian StylePirri, Carmelo, Brasilina Caroccia, Andrea Angelini, Maria Piazza, Lucia Petrelli, Ilaria Caputo, Chiara Montemurro, Pietro Ruggieri, Raffaele De Caro, and Carla Stecco. 2023. "A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP)" International Journal of Molecular Sciences 24, no. 20: 15389. https://doi.org/10.3390/ijms242015389
APA StylePirri, C., Caroccia, B., Angelini, A., Piazza, M., Petrelli, L., Caputo, I., Montemurro, C., Ruggieri, P., De Caro, R., & Stecco, C. (2023). A New Player in the Mechanobiology of Deep Fascia: Yes-Associated Protein (YAP). International Journal of Molecular Sciences, 24(20), 15389. https://doi.org/10.3390/ijms242015389











