Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research
Abstract
1. Introduction
2. Sources and Search Criteria
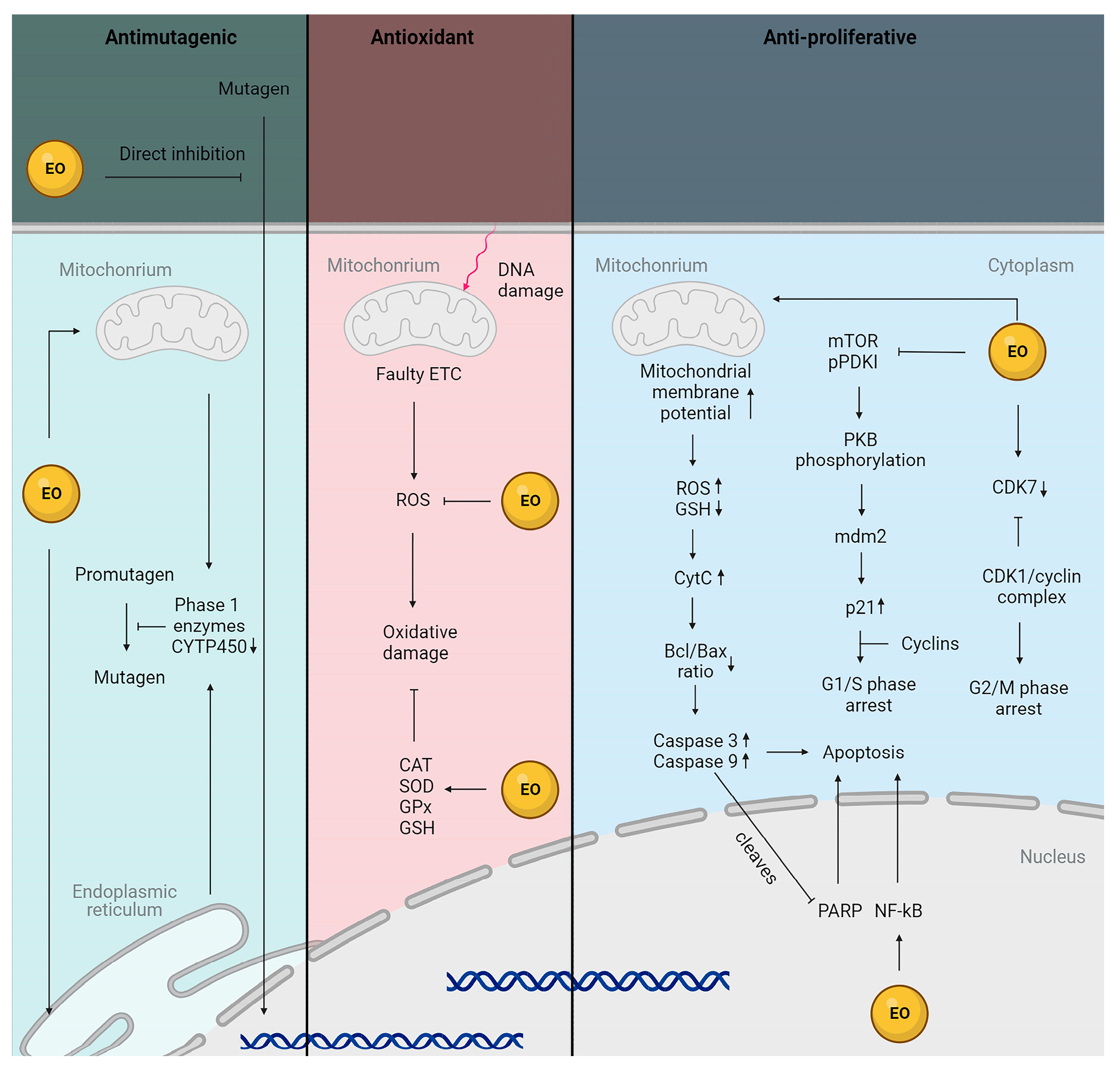
3. Antioxidant Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
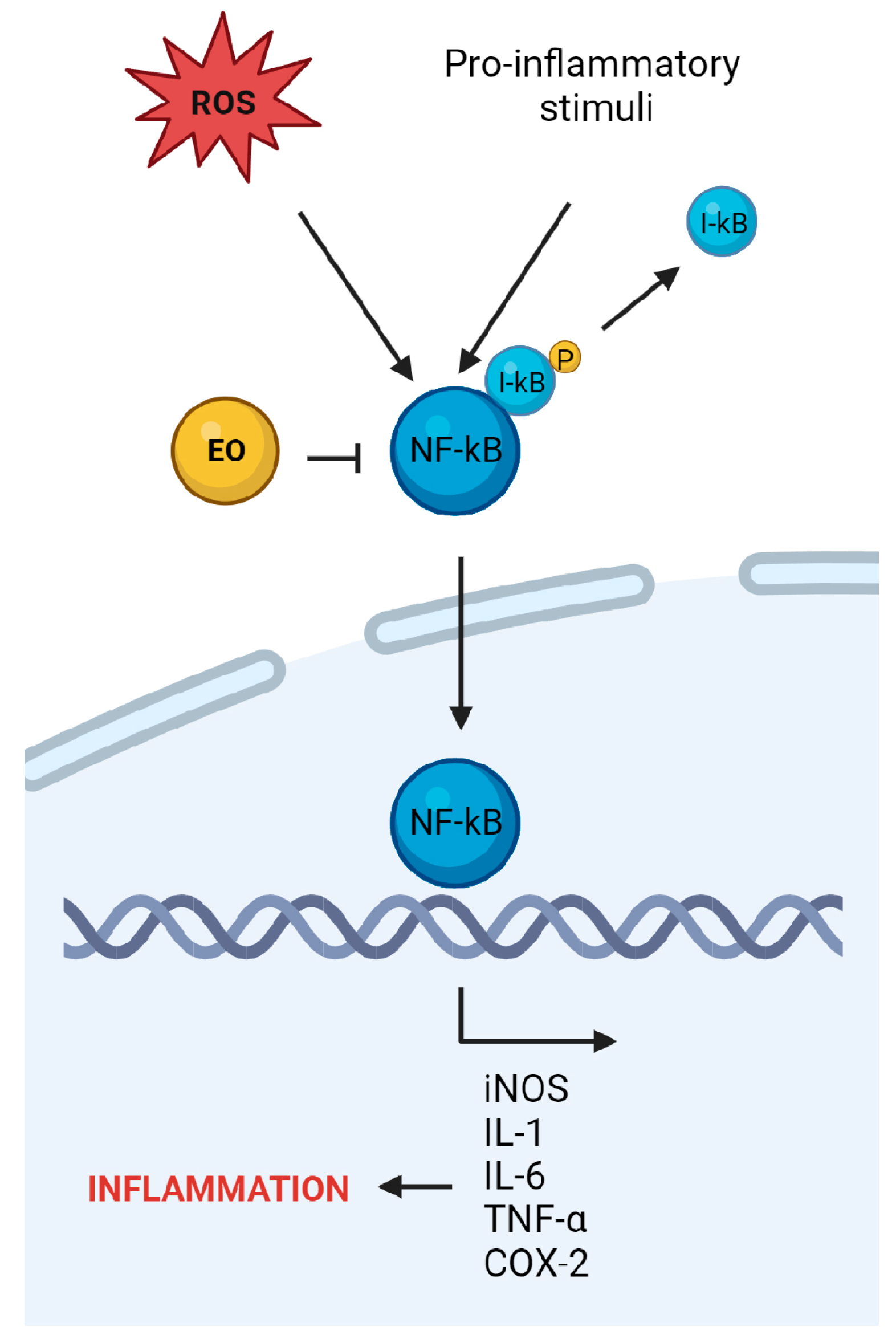
4. Anti-Inflammatory Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
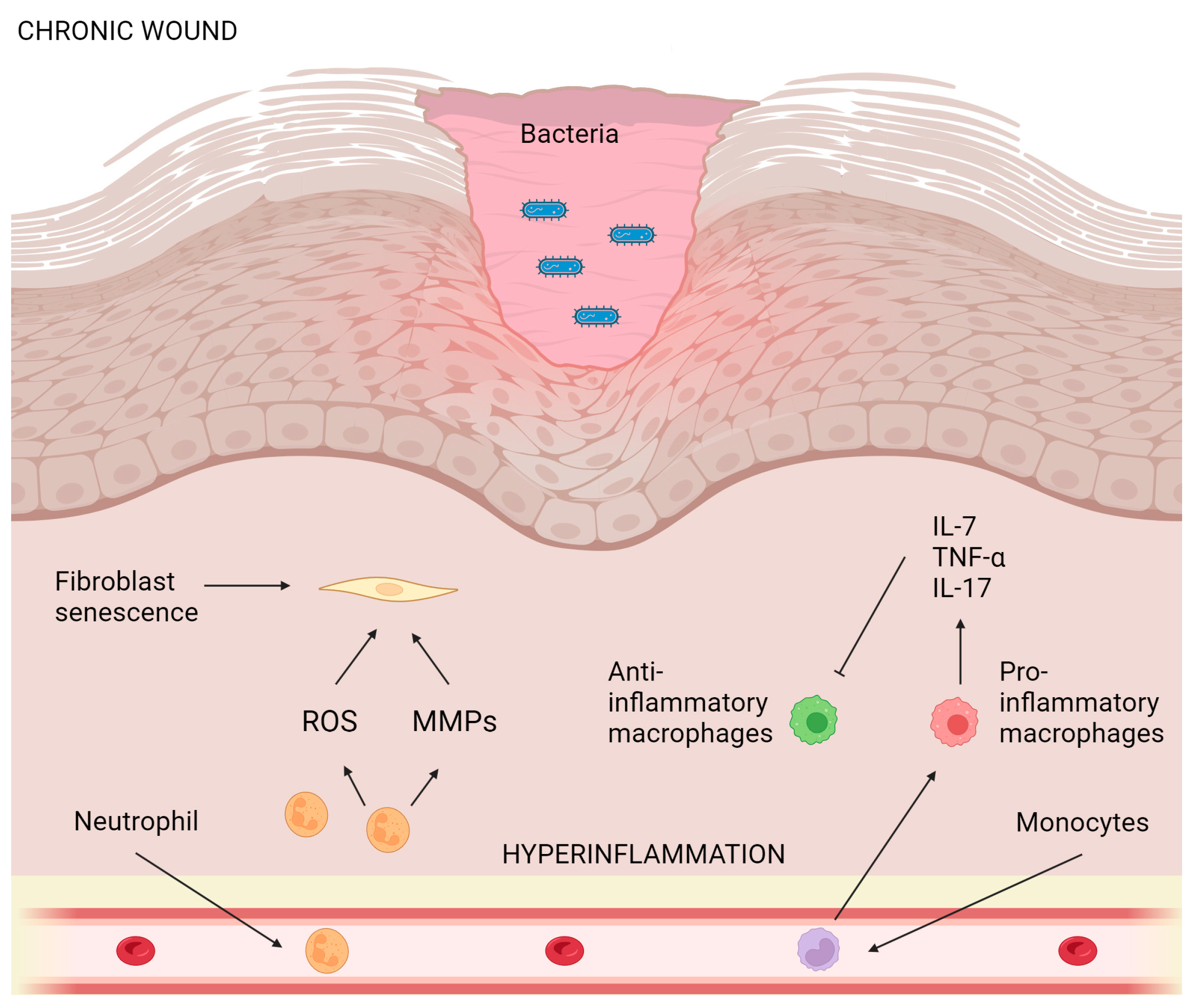
5. Wound-Healing Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
6. Anti-Aging Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
7. Anti-Melanogenic Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
8. Anti-Cancer Potential of Essential Oils from Plants Belonging to the Lamiaceae Family
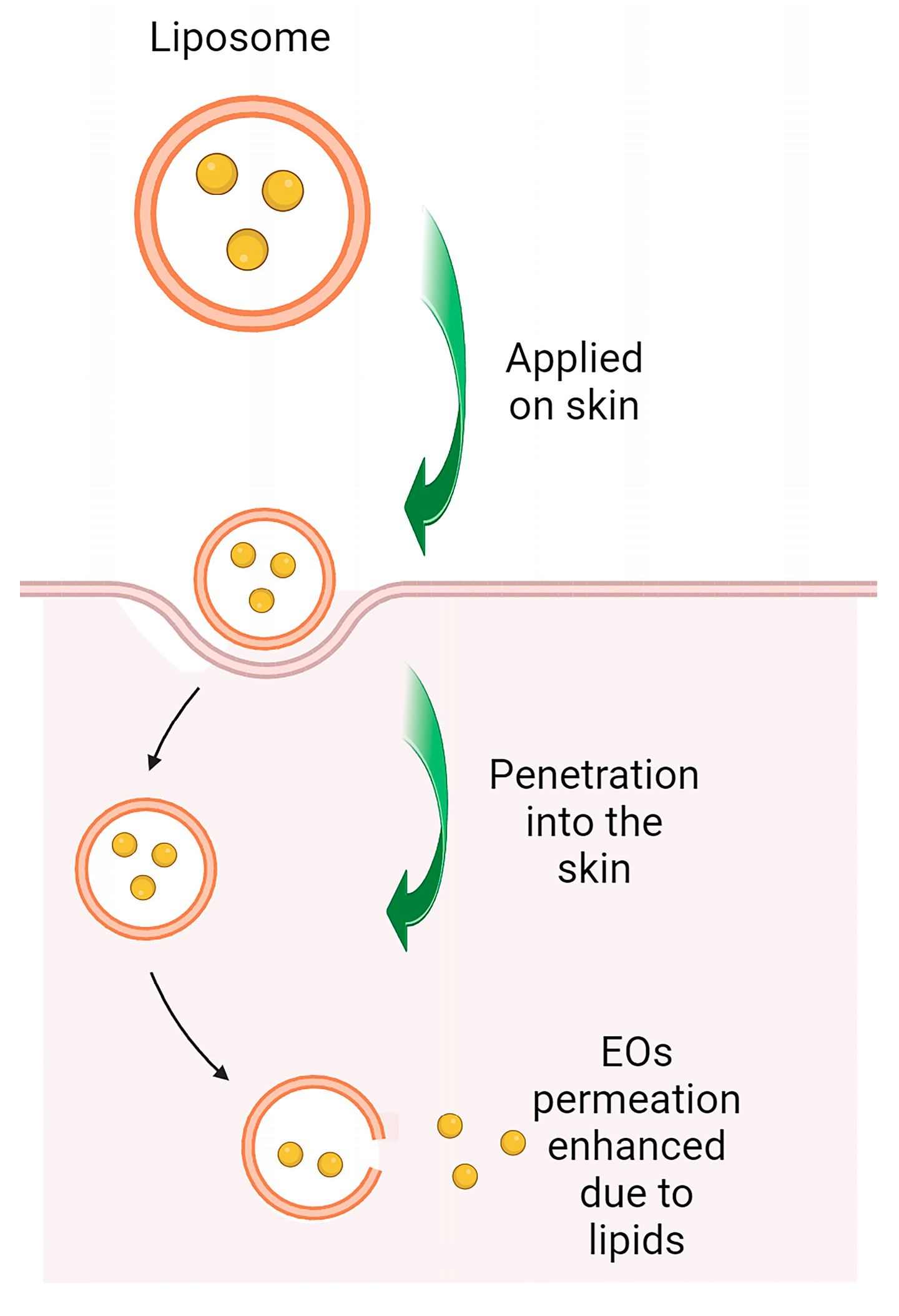
9. Nanotechnology as a Strategy for Precise Delivery of Lamiaceae Essential Oils in Skin Diseases
10. Patented Compositions of Essential Oils and Their Role in Skin Lesions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, F.; Chen, Y.P.; Salmaki, Y.; Drew, B.T.; Wilson, T.C.; Scheen, A.C.; Celep, F.; Bräuchler, C.; Bendiksby, M.; Wang, Q.; et al. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol. 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef]
- Sim, L.Y.; Rani, N.Z.A.; Husain, K. Lamiaceae: An insight on their anti-allergic potential and its mechanisms of action. Front. Pharmacol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Mamadalieva, N.; Akramov, D.; Ovidi, E.; Tiezzi, A.; Nahar, L.; Azimova, S.; Sarker, S. Aromatic Medicinal Plants of the Lamiaceae Family from Uzbekistan: Ethnopharmacology, Essential Oils Composition, and Biological Activities. Medicines 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Ramos Da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; De Jesus Pereira Franco, C.; Oliveira Dos Anjos, T.; Cascaes, M.M.; Almeida Da Costa, W.; Helena De Aguiar Andrade, E.; Santana De Oliveira, M. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid. Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Ćustić, M.H.; Satovic, Z. Medicinal plants of the family lamiaceae as functional foods-A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Bunse, M.; Daniels, R.; Gründemann, C.; Heilmann, J.; Kammerer, D.R.; Keusgen, M.; Lindequist, U.; Melzig, M.F.; Morlock, G.E.; Schulz, H.; et al. Essential Oils as Multicomponent Mixtures and Their Potential for Human Health and Well-Being. Front. Pharmacol. 2022, 13, 956541. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2022, 12, e1900434. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Kumar Mahawer, S.; Himani; Arya, S.; Kumar, R.; Prakash, O. Extractions Methods and Biological Applications of Essential Oils; Intech Open: London, UK, 2022. [Google Scholar]
- Kar, S.; Gupta, P.; Gupta, J. Essential oils: Biological activity beyond aromatherapy. Nat. Prod. Sci. 2018, 24, 139–147. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.F.A.; Seleiman, M.F.; Mohamed, I.A.A.; Taha, R.S.; Wasonga, D.O.; Battaglia, M.L. Activity of Essential Oils and Plant Extracts as Biofungicides for Suppression of Soil-Borne Fungi Associated with Root Rot and Wilt of Marigold (Calendula officinalis L.). Horticulturae 2023, 9, 222. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Hu, H.; Zhong, W.; Cao, R.; Xu, Y.; Li, R. Chemical Composition and Antifungal, Anti-Inflammatory, Antiviral, and Larvicidal Activities of the Essential Oils of Zanthoxylum acanthopodium DC. from China and Myanmar. Molecules 2022, 27, 5243. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Yusoff, K.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Membrane disruption properties of essential oils-a double-edged sword? Processes 2021, 9, 595. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Essential oils of lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- De Cássia Da Silveira E Sá, R.; Andrade, L.N.; De Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Kar, S.; Subbaram, S.; Carrico, P.M.; Melendez, J.A. Redox-control of matrix metalloproteinase-1: A critical link between free radicals, matrix remodeling and degenerative disease. Respir. Physiol. Neurobiol. 2010, 174, 299–306. [Google Scholar] [CrossRef]
- Monboisse, J.C.; Borel, J.P. Oxidative damage to collagen. EXS 1992, 62, 323–327. [Google Scholar] [CrossRef]
- Hayashi, A.; Ryu, A.; Suzuki, T.; Kawada, A.; Tajima, S. In vitro degradation of tropoelastin by reactive oxygen species. Arch. Dermatol. Res. 1998, 290, 497–500. [Google Scholar] [CrossRef]
- Umeda, H.; Nakamura, F.; Suyama, K. Oxodesmosine and isooxodesmosine, candidates of oxidative metabolic intermediates of pyridinium cross-links in elastin. Arch. Biochem. Biophys. 2001, 385, 209–219. [Google Scholar] [CrossRef]
- Xuan, Y.; Yang, Y.; Xiang, L.; Zhang, C. The Role of Oxidative Stress in the Pathogenesis of Vitiligo: A Culprit for Melanocyte Death. Oxid. Med. Cell. Longev. 2022, 2022, 8498472. [Google Scholar] [CrossRef] [PubMed]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Mathachan, S.R.; Khurana, A.; Gautam, R.K.; Kulhari, A.; Sharma, L.; Sardana, K. Does oxidative stress correlate with disease activity and severity in vitiligo? An analytical study. J. Cosmet. Dermatol. 2021, 20, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mal, J.; Mehndiratta, V.; Chander, R.; Patra, S.K. Study of oxidative stress in vitiligo. Indian J. Clin. Biochem. 2011, 26, 78–81. [Google Scholar] [CrossRef][Green Version]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Rübsam, K.; Gibbons, N.C.J.; Maitland, D.J.; Chavan, B.; Zothner, C.; Rokos, H.; Wood, J.M. Methionine sulfoxide reductases A and B are deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J. Investig. Dermatol. 2008, 128, 808–815. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, C.Y.; Li, K.; Wang, T.; Zhang, B.; Gao, T.W. Decreased methionine sulphoxide reductase A expression renders melanocytes more sensitive to oxidative stress: A possible cause for melanocyte loss in vitiligo. Br. J. Dermatol. 2009, 161, 504–509. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Kavanagh, R.; Kanto, H.; Terzieva, S.; Hauser, J.; Kobayashi, N.; Schwemberger, S.; Cornelius, J.; Babcock, G.; Shertzer, H.G.; et al. α-melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005, 65, 4292–4299. [Google Scholar] [CrossRef]
- Kadekaro, A.L.; Leachman, S.; Kavanagh, R.J.; Swope, V.; Cassidy, P.; Supp, D.; Sartor, M.; Schwemberger, S.; Babcock, G.; Wakamatsu, K.; et al. Melanocortin 1 receptor genotype: An important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010, 24, 3850–3860. [Google Scholar] [CrossRef]
- Spencer, J.D.; Gibbons, N.C.J.; Rokos, H.; Peters, E.M.J.; Wood, J.M.; Schallreuter, K.U. Oxidative stress via hydrogen peroxide affects proopiomelanocortin peptides directly in the epidermis of patients with vitiligo. J. Investig. Dermatol. 2007, 127, 411–420. [Google Scholar] [CrossRef]
- Narendhirakannan, R.T.; Hannah, M.A.C. Oxidative stress and skin cancer: An overview. Indian J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed]
- Venza, I.; Venza, M.; Visalli, M.; Lentini, G.; Teti, D.; D’Alcontres, F.S. ROS as Regulators of Cellular Processes in Melanoma. Oxid. Med. Cell. Longev. 2021, 2021, 1208690. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell. Signal. 2002, 14, 879–897. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Pospíšil, P.; Kruk, J. Plant-derived antioxidants in disease prevention 2018. Oxid. Med. Cell. Longev. 2018, 2018, 2068370. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Agents, A. Natural Product Communications Essential Oils from Lamiaceae Species as Promising. Nat. Prod. Commun. 2007, 2, 1934578X0700200416. [Google Scholar]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Ruckemann-Dziurdzińska, K.; Roszkiewicz, J.; Nowicki, R.J. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postep. Dermatol. Alergol. 2014, 31, 84–91. [Google Scholar] [CrossRef]
- Deng, Y.; Chang, C.; Lu, Q. The Inflammatory Response in Psoriasis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Lai, C.C.; Lai, C.P.; Chao, W.W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crops Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Aoe, M.; Ueno-Iio, T.; Shibakura, M.; Shinohata, R.; Usui, S.; Arao, Y.; Ikeda, S.; Miyahara, N.; Tanimoto, M.; Kataoka, M. Lavender essential oil and its main constituents inhibit the expression of tnf-α-induced cell adhesion molecules in endothelial cells. Acta Med. Okayama 2017, 71, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-inflammatory effect of lavender (Lavandula angustifolia Mill.) essential oil prepared during different plant phenophases on THP-1 macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Côté, H.; Pichette, A.; St-Gelais, A.; Legault, J. The biological activity of monarda Didyma l. Essential oil and its effect as a diet supplement in mice and broiler chicken. Molecules 2021, 26, 3368. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ge, Y.; Luo, Z.; Zhou, Y.; Zhang, X.; Zhang, J.; Fu, Q. Evaluation of the chemical composition, antioxidant and anti-inflammatory activities of distillate and residue fractions of sweet basil essential oil. J. Food Sci. Technol. 2017, 54, 1882–1890. [Google Scholar] [CrossRef]
- Manaharan, T.; Thirugnanasampandan, R.; Jayakumar, R.; Ramya, G.; Ramnath, G.; Kanthimathi, M.S. Antimetastatic and anti-inflammatory potentials of essential oil from edible ocimum sanctum leaves. Sci. World J. 2014, 2014, 239508. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Suganya, P.; Jeyaprakash, K.; Mallavarapu, G.R.; Murugan, R. Comparison of the chemical composition, tyrosinase inhibitory and anti-inflammatory activities of the essential oils of Pogostemon plectranthoides from India. Ind. Crops Prod. 2015, 69, 300–307. [Google Scholar] [CrossRef]
- Lorenzo-Leal, A.C.; Palou, E.; López-Malo, A.; Bach, H. Antimicrobial, Cytotoxic, and Anti-Inflammatory Activities of Pimenta dioica and Rosmarinus officinalis Essential Oils. Biomed Res. Int. 2019, 2019, 1639726. [Google Scholar] [CrossRef] [PubMed]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed. Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- Tosun, A.; Khan, S.; Kim, Y.S.; Calín-Sánchez, Á.; Hysenaj, X.; Carbonell-Barrachina, Á.A. Essential oil composition and anti-inflammatory activity of Salvia officinalis L. (Lamiaceae) in murin macrophages. Trop. J. Pharm. Res. 2014, 13, 937–942. [Google Scholar] [CrossRef]
- Roxo, M.; Zuzarte, M.; Gonçalves, M.J.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Antifungal and anti-inflammatory potential of the endangered aromatic plant Thymus albicans. Sci. Rep. 2020, 10, 18859. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Alves-Silva, J.M.; Alves, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. New insights on the anti-inflammatory potential and safety profile of Thymus carnosus and Thymus camphoratus essential oils and their main compounds. J. Ethnopharmacol. 2018, 225, 10–17. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cabral, C.; Évora, L.; Ferreira, I.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arab. J. Chem. 2019, 12, 3236–3243. [Google Scholar] [CrossRef]
- Sun, J.; Sun, P.; Kang, C.; Zhang, L.; Guo, L.; Kou, Y. Chemical composition and biological activities of essential oils from six lamiaceae folk medicinal plants. Front. Plant Sci. 2022, 13, 919294. [Google Scholar] [CrossRef]
- Simões, R.R.; Coelho, I.d.S.; Junqueira, S.C.; Pigatto, G.R.; Salvador, M.J.; Santos, A.R.S.; de Faria, F.M. Oral treatment with essential oil of Hyptis spicigera Lam. (Lamiaceae) reduces acute pain and inflammation in mice: Potential interactions with transient receptor potential (TRP) ion channels. J. Ethnopharmacol. 2017, 200, 8–15. [Google Scholar] [CrossRef]
- da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; da Silva Melo, D.A.; Donadio, M.V.F.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.B.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Du, Z.; Zheng, Y.; Liang, X.; Huang, G.; Zhang, Q.; Liu, Z.; Zhang, K.; Zheng, X.; Lin, L.; et al. Phytochemical composition and bioactivities of essential oils from six Lamiaceae species. Ind. Crops Prod. 2019, 133, 357–364. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A new eucalyptol-rich lavender (Lavandula stoechas L.) essential oil: Emerging potential for therapy against inflammation and cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef] [PubMed]
- Arantes, S.; Candeias, F.; Lopes, O.; Lima, M.; Pereira, M.; Tinoco, T.; Cruz-Morais, J.; Martins, M.R. Pharmacological and toxicological studies of essential oil of Lavandula stoechas subsp. Luisieri. Planta Med. 2016, 82, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Bounihi, A.; Hajjaj, G.; Alnamer, R.; Cherrah, Y.; Zellou, A. In vivo potential anti-inflammatory activity of melissa officinalis l. essential oil. Adv. Pharmacol. Sci. 2013, 2013, 101759. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I.; Schmidt, T.J. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Oliveira Brito Pereira Bezerra Martins, A.; Cesário, F.R.A.S.; Ferreira e Castro, F.; de Albuquerque, T.R.; Martins Fernandes, M.N.; Fernandes da Silva, B.A.; Quintans Júnior, L.J.; da Costa, J.G.M.; Melo Coutinho, H.D.; et al. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chem. Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef]
- De Lima, V.T.; Vieira, M.C.; Kassuya, C.A.L.; Cardoso, C.A.L.; Alves, J.M.; Foglio, M.A.; De Carvalho, J.E.; Formagio, A.S.N. Chemical composition and free radical-scavenging, anticancer and anti-inflammatory activities of the essential oil from Ocimum kilimandscharicum. Phytomedicine 2014, 21, 1298–1302. [Google Scholar] [CrossRef]
- Piva, R.C.; Verdan, M.H.; Branquinho, L.S.; Kassuya, C.A.L.; Cardoso, C.A.L. Anti-inflammatory activity and chemical composition of aqueous extract and essential oil from leaves of Ocimum selloi Benth. J. Ethnopharmacol. 2021, 275, 114136. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, N.N.; Ouassou, H.; Sheikh, R.A.; Assaggaf, H.; Bakrim, S.; Abdallah, E.M.; Alshahrani, M.M.; Al Awadh, A.A.; Lee, L.H.; et al. Chemical Composition and Antioxidant, Antimicrobial, and Anti-Inflammatory Properties of Origanum compactum Benth Essential Oils from Two Regions: In Vitro and In Vivo Evidence and In Silico Molecular Investigations. Molecules 2022, 27, 7329. [Google Scholar] [CrossRef]
- Barreto, R.S.S.; Quintans, J.S.S.; Amarante, R.K.L.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.M.; Duarte, M.C.; Coutinho, H.D.M.; Menezes, I.R.A.; et al. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-α-bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef]
- El Ouahdani, K.; Es-Safi, I.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bari, A.; Bousta, D. Thymus algeriensis and artemisia herba-alba essential oils: Chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, O.; Adli, H.E.D.; Halla, N.; Kahloula, K. Phytochem & biosub journal. Phytochem. Biosub. J. 2014, 8, 221–231. [Google Scholar]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical composition and anti-inflammatory activity of algerian thymus vulgaris essential oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Darwish, N.H.E.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In vitro antifungal and topical anti-inflammatory properties of essential oil from wild-growing thymus vulgaris (Lamiaceae) used for medicinal purposes in algeria: A new source of carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Sheikh, S.; Kafshdooz, E.; Sonboli, A. Accelerative effect of topical Zataria multiflora essential oil against infected wound model by modulating inflammation, angiogenesis, and collagen biosynthesis. Pharm. Biol. 2021, 59, 1–10. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. Wound healing: An overview. Plast. Reconstr. Surg. 2006, 117, 1e-S–32e-S. [Google Scholar] [CrossRef]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef] [PubMed]
- Chabane, S.; Boudjelal, A.; Napoli, E.; Benkhaled, A.; Ruberto, G. Phytochemical composition, antioxidant and wound healing activities of Teucrium polium subsp. capitatum (L.) Briq. essential oil. J. Essent. Oil Res. 2021, 33, 143–151. [Google Scholar] [CrossRef]
- Napoli, E.; Boudjelal, A.; Benkhaled, A.; Chabane, S.; Gentile, D.; Ruberto, G. Chemical composition, safety and efficacy of Pistacia vera L. oleoresin essential oils in experimental wounds. J. Essent. Oil Res. 2021, 33, 464–470. [Google Scholar] [CrossRef]
- Modarresi, M.; Farahpour, M.R.; Baradaran, B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology 2019, 27, 531–537. [Google Scholar] [CrossRef]
- Stulberg, D.L.; Penrod, M.A.; Blatny, R.A. Common bacterial skin infections. Am. Fam. Physician 2002, 66, 119–124. [Google Scholar]
- Ibrahim, F.; Khan, T.; Pujalte, G.G.A. Bacterial Skin Infections. Prim. Care Clin. Off. Pract. 2015, 42, 485–499. [Google Scholar] [CrossRef]
- Yasin, Z.A.M.; Ibrahim, F.; Rashid, N.N.; Razif, M.F.M.; Yusof, R. The Importance of Some Plant Extracts as Skin Anti-aging Resources: A Review. Curr. Pharm. Biotechnol. 2017, 18, 864–876. [Google Scholar] [CrossRef]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement. Altern. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical Compositions and Anti-Skin-Ageing Activities of Origanum vulgare L. Essential oil from tropical and Mediterranean region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N.; et al. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Lin, R.F.; Feng, X.X.; Li, C.W.; Zhang, X.J.; Yu, X.T.; Zhou, J.Y.; Zhang, X.; Xie, Y.L.; Su, Z.R.; Zhan, J.Y.X. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J. Ethnopharmacol. 2014, 154, 408–418. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Ancans, J.; Tobin, D.J.; Hoogduijn, M.J.; Smit, N.P.; Wakamatsu, K.; Thody, A.J. Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells. Exp. Cell Res. 2001, 268, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ohbayashi, N.; Fukuda, M. Recent advances in understanding the molecular basis of melanogenesis in melanocytes. F1000 Res. 2020, 9, 608. [Google Scholar] [CrossRef]
- Nordlund, J.J. The Melanocyte and the Epidermal Melanin Unit: An Expanded Concept. Dermatol. Clin. 2007, 25, 271–281. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael-Jubeli, R.; Bakar, J.; Dakroub, H.; Rizk, T.; Baillet-Guffroy, A.; Lteif, R.; Tfayli, A. Origanum essential oils reduce the level of melanin in B16-F1 melanocytes. Eur. J. Dermatol. 2019, 29, 596–602. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Scharf, D.R.; Simionatto, E.L.; Carvalho, J.E.; Salvador, M.J.; Santos, É.P.; Stefanello, M.É.A. Chemical composition and cytotoxic activity of the essential oils of Cantinoa stricta (Benth.) Harley & J.F.B. Pastore (Lamiaceae). Rec. Nat. Prod. 2015, 10, 257–261. [Google Scholar]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Iannarelli, R.; Papa, F.; Maggi, F. Antioxidant activity and cytotoxicity on tumour cells of the essential oil from Cedronella canariensis var. canariensis (L.) Webb & Berthel. (Lamiaceae). Nat. Prod. Res. 2015, 29, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, M.H.O.; Morgan, J.M.S.; Cesca, K.; Flach, A.; de Moura, N.F. Cytotoxic Activity of Cunila angustifolia Essential Oil. Chem. Biodivers. 2020, 17, e1900656. [Google Scholar] [CrossRef] [PubMed]
- Andrew, B.M.; Rex, G.C.; Kim, O.; Juan, A.F.S.; Luis, V.E.; Ballantines, F.A.; Jose, V.M.; Dany, R.A. Evaluation of essential oils from 22 Guatemalan medicinal plants for in vitro activity against cancer and established cell lines. J. Med. Plants Res. 2018, 12, 42–49. [Google Scholar] [CrossRef]
- Zarlaha, A.; Kourkoumelis, N.; Stanojkovic, T.P.; Kovala-Demertzi, D. Cytotoxic activity of essential oil and extracts of Ocimum Basilicum against human carcinoma cells. Molecular docking study of isoeugenol as a potent cox and lox inhibitor. Dig. J. Nanomater. Biostruct. 2014, 9, 907–917. [Google Scholar]
- Kumar, V.; Shriram, V.; Bhagat, R.; Khare, T.; Kapse, S.; Kadoo, N. Phytochemical profile, anti-oxidant, anti-inflammatory, and anti-proliferative activities of Pogostemon deccanensis essential oils. 3 Biotech. 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Alexa, E.; Sumalan, R.M.; Danciu, C.; Obistioiu, D.; Negrea, M.; Poiana, M.A.; Rus, C.; Radulov, I.; Pop, G.; Dehelean, C. Synergistic antifungal, allelopatic and anti-proliferative potential of Salvia officinalis L., and Thymus vulgaris L. Essential oils. Molecules 2018, 23, 185. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Formisano, C.; Rigano, D.; Canzoneri, M.; Bruno, M.; Senatore, F. Comparison of essential oil components and in vitro anticancer activity in wild and cultivated Salvia verbenaca. Nat. Prod. Res. 2015, 29, 1630–1640. [Google Scholar] [CrossRef]
- Popovici, R.; Vaduva, D.; Pinzaru, I.; Dehelean, C.; Farcas, C.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Maleci Bini, L.; Giuliani, C.; et al. Phytochemical analysis, biological activity, and secretory structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [CrossRef]
- Bendif, H.; Boudjeniba, M.; Miara, M.D.; Biqiku, L.; Bramucci, M.; Lupidi, G.; Quassinti, L.; Vitali, L.A.; Maggi, F. Essential Oil of Thymus munbyanus subsp. coloratus from Algeria: Chemotypification and in vitro Biological Activities. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Arulselvi, P.I. Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. J. Herbs Spices Med. Plants 2018, 24, 37–51. [Google Scholar] [CrossRef]
- Liang, X.; Xu, Z.; Grice, J.; Zvyagin, A.; Roberts, M.; Liu, X. Penetration of Nanoparticles into Human Skin. Curr. Pharm. Des. 2013, 19, 6353–6366. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. 2022, 61, e202107960. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Devel. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol. 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Maibach, H.I. Skin penetration of nanoparticles. In Emerging Nanotechnologies in Immunology: The Design, Applications and Toxicology of Nanopharmaceuticals and Nanovaccines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–88. ISBN 9780323400169. [Google Scholar]
- Patzelt, A.; Mak, W.C.; Jung, S.; Knorr, F.; Meinke, M.C.; Richter, H.; Rühl, E.; Cheung, K.Y.; Tran, N.B.N.N.; Lademann, J. Do nanoparticles have a future in dermal drug delivery? J. Control. Release 2017, 246, 174–182. [Google Scholar] [CrossRef]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles skin absorption: New aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Carbone, C.; Teixeira, M.D.C.; Sousa, M.D.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrimazole-loaded mediterranean essential oils NLC: A synergic treatment of Candida skin infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Tomou, E.M.; Stojković, D.; Ćirić, A.; Bilia, A.R.; Skaltsa, H. Nanovesicles loaded with origanum onites and satureja thymbra essential oils and their activity against food-borne pathogens and spoilage microorganisms. Molecules 2021, 26, 2124. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Vina, N. Indikasi Keharaman Operasi Plastik Dalam Perspektif Hukum Islam; Vrije University: Amsterdam, The Netherlands, 2020; Volume 1, ISBN 9788578110796. [Google Scholar]
- Badea, M.L.; Iconaru, S.L.; Groza, A.; Chifiriuc, M.C.; Beuran, M.; Predoi, D. Peppermint essential oil-doped hydroxyapatite nanoparticles with antimicrobial properties. Molecules 2019, 24, 2169. [Google Scholar] [CrossRef]
- Carbone, C.; Caddeo, C.; Grimaudo, M.A.; Manno, D.E.; Serra, A.; Musumeci, T. Ferulic Acid-NLC with Lavandula Essential Oil: A Possible Strategy for Wound-Healing? Nanomaterials 2020, 10, 898. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Rafiq, M.; Jadhav, A.H.; Srinivasappa, P.M.; Abdal-hay, A.; Sultan, P.; Rather, S.; Macossay, J.; et al. Polyurethane and cellulose acetate micro-nanofibers containing rosemary essential oil, and decorated with silver nanoparticles for wound healing application. Int. J. Biol. Macromol. 2023, 226, 690–705. [Google Scholar] [CrossRef]
- Valizadeh, A.; Khaleghi, A.A.; Roozitalab, G.; Osanloo, M. High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol. Toxicol. 2021, 22, 52. [Google Scholar] [CrossRef]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Rashidipour, M.; Ashrafi, B.; Nikbakht, M.R.; Veiskarami, S.; Taherikalani, M.; Soroush, S. Encapsulation of Satureja khuzistanica jamzad essential oil in chitosan nanoparticles with enhanced antibacterial and anticancer activities. Prep. Biochem. Biotechnol. 2021, 51, 971–978. [Google Scholar] [CrossRef]
- Benedec, D.; Oniga, I.; Cuibus, F.; Sevastre, B.; Stiufiuc, G.; Duma, M.; Hanganu, D.; Iacovita, C.; Stiufiuc, R.; Lucaciu, C.M. Origanum vulgare mediated green synthesis of biocompatible gold nanoparticles simultaneously possessing plasmonic, antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 1041–1058. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, Z.; Yang, J.; Zhang, M.; Cai, F.; Lu, P. ZnO nanoparticles stabilized oregano essential oil Pickering emulsion for functional cellulose nanofibrils packaging films with antimicrobial and antioxidant activity. Int. J. Biol. Macromol. 2021, 190, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green Synthesis of Magnetic Nanoparticles Using Satureja hortensis Essential Oil toward Superior Antibacterial/Fungal and Anticancer Performance. Biomed Res. Int. 2021, 8822645. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the antimicrobial activity of oregano oil by encapsulation in chitosan–alginate nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against foodborne pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef] [PubMed]
- Perez, N.; Altube, M.J.; Barbosa, L.R.S.; Romero, E.L.; Perez, A.P. Thymus vulgaris essential oil + tobramycin within nanostructured archaeolipid carriers: A new approach against Pseudomonas aeruginosa biofilms. Phytomedicine 2022, 102, 154179. [Google Scholar] [CrossRef] [PubMed]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Amit Koparde, A.; Chandrashekar Doijad, R.; Shripal Magdum, C. Natural Products in Drug Discovery. Pharmacogn.-Med. Plants 2019, 20, 200–216. [Google Scholar] [CrossRef]
- De Almeida Chaves, D.S.; de Melo, G.O.; Corrêa, M.F.P. A Review of Recent Patents Regarding Antithrombotic Drugs Derived from Natural Products. Stud. Nat. Prod. Chem. 2018, 61, 1–47. [Google Scholar]
- Balbani, A.P.S.; Silva, D.H.S.; Montovani, J.C. Patents of drugs extracted from Brazilian medicinal plants. Expert Opin. Ther. Pat. 2009, 19, 461–473. [Google Scholar] [CrossRef]
- Altuntas, S.; Dereli, T.; Kusiak, A. Forecasting technology success based on patent data. Technol. Forecast. Soc. Change 2015, 96, 202–214. [Google Scholar] [CrossRef]
| Tested Plant | Plant Part | Number of Identified Compounds (the Main Constituents > 5%) | Cell Line | Tested Essential Oil Concentrations | Effects | Ref. |
|---|---|---|---|---|---|---|
| Glechoma hederacea L. | aerial parts | 29 compounds (trans-3-pinanone, β-caryophyllene, 4,5,6,7-tetrahydro-5-isopropenyl-3,6-betadimethyl-6-alpha-vinylbenzofuran) | RAW 264.7 macrophages stimulated with LPS | 5–20 μg/mL | suppress NO production, regulate expression of iNOS, COX-2, and HO-1, and TNF-α | [55] |
| Lavandula angustifolia L. | - | (linalyl acetate, linalool, β-caryophyllen, trans-βocimene, lavandulyl acetate) | Murine brain endothelial bEnd.3 cells stimulated with TNF-α | 0.01% | inhibition of TNF-α-induced NF-κB activation | [56] |
| Lavandula angustifolia L. | whole plant | 71 compounds (linalool, terpinene-4-ol, α-terpineol, linalyl acetate) | THP-1 human monocyte/macrophage stimulated with LPS | 100 μL of DMSO was added to 900 μL of essential oil in a final volume of 1 mL. The emulsions were diluted with phosphate-buffered saline 500-fold | decreased IL-6, IL-1β, and IL-8 expression | [57] |
| Monarda Didyma L. | flowering aerial parts | 20 compounds (1-octen-3-ol, p-cymene, γ-terpinene, thymol methyl ether, carvacrol methyl ether, thymol, carvacrol) | U937 cells stimulated with LPS | 0.5 μL/mL | decreased expression of IL-6 | [58] |
| Ocimum basilicum L. | whole plant | 25 compounds (the distillate fraction contained estragole, methyl eugenol, α-bergamotene, carotol, α-cadinol) | RAW 264.7 macrophages stimulated with LPS | 20 µg/mL | the distillate fraction suppressed the production of NO and iNOS, and expression of TNF-α, IL-1β, and IL-6 | [59] |
| Ocimum sanctum L. | leaves | - | Lymphocytes stimulated with LPS | 250 μg/mL | downregulation of MMP-9 expression | [60] |
| Origanum vulgare L. | - | 32 constituents (carvacrol, thymol, p-cymene) | human keratinocytes NCTC 2544 treated with interferon-gamma (IFN-γ) and histamine (H) | 25 μg/mL | reduction of ROS, ICAM-1, iNOS, and COX-2 | [61] |
| Pogostemon plectranthoides Desf. | leaf | 37 compounds (cyclosativene, caryophyllene oxide, 1-epi-cubenol, eudesma-4(15), 7-dien-1-β-ol, mustakone) | human red blood cell | 62.5–1000 μg/mL | cell membrane stabilization activity | [62] |
| Rosmarinus officinalis L. | whole plant | - | THP-1 human monocyte/macrophage stimulated with LPS | 5 μg/mL | increased level of IL-10 expression | [63] |
| Salvia officinalis L. | aerial parts | 25 compounds, (1,8-cineole, camphor, β-pinene, α-terpineol, α-pinene) | RAW 264.7 macrophages stimulated with LPS | 0.16–1.25 µL/mL | inhibited NO production | [64] |
| Salvia officinalis L. | leaves | 24 compounds (camphene, 1,8-cineole, α-thujone, camphor, bornyl acetate) | RAW 264.7 macrophages stimulated with LPS | 50–500 μg/mL | reduced NO and NF-κB production | [65] |
| Thymus albicans L. | flowering parts | 35 compounds (1,8-cineole, linalool, borneol) | RAW 264.7 macrophages stimulated with LPS | 0.32–0.64 μL/mL | reduced the production of nitrites, an NO-derived sub-product, and iNOS protein levels | [66] |
| Thymus camphoratus L. | flowering aerial parts | 60 compounds (α-pinene, camphene, 1,8-cineole, linalool, borneol) | RAW 264.7 macrophages stimulated with LPS | 0.16–0.32 μL/mL | inhibitory effects towards NO production, inhibiting the expression of iNOS and COX-2 | [67] |
| Thymus zygis L. | aerial parts | 41 compounds (p-cymene, thymol, carvacrol, γ-terpinene, linalool) | RAW 264.7 macrophages stimulated with LPS | 0.08–0.64 µL/mL | inhibition of NO production | [68] |
| Tested Plant | Plant Part | Number of Identified Compounds (the Main Constituents > 5%) | Animals | Tested Essential Oil Concentrations/Types of Administration (Oral/Topical) | Effects | Ref. |
|---|---|---|---|---|---|---|
| Agastache rugosa Gronov. | leaves | 37 compounds (p-allylguaiacol/eugenol, patchouli alcohol, pogostone) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | inhibition of the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Hyptis spicigera Lam. | aerial parts | 14 compounds (α-pinene, β-pinene, 1,8-cineole, β-caryophyllene) | Swiss mice | 1000 mg/kg (oral administration) | temperature of the hind paw was reduced; edema was diminished | [70] |
| Lavandula Augustifolia L. | - | 28 compounds (D-limonene, linalyl acetate, linalool) | Swiss mice | 50 μL/ear (topical treatment)/ 0.6 g/kg (oral treatment) | inhibition of paw edema induced by carrageenan and by croton oil | [71] |
| Lavandula angustifolia L. | leaves and stem | 27 compounds (1,8-cineole, borneol, camphor, limonene, camphene) | Swiss mice | 0.25, 0.5, and 1 mg/ear (topical administration) 75, 100, and 250 mg/kg (oral administration) | topical treatment reduced edema formation, MPO activity, and NO production in croton-oil-induced ear edema model or carrageenan-induced paw edema model/oral treatment reduced edema formation, MPO activity, and NO production | [72] |
| Lavandula angustifolia L. | 24 compounds (D-limonene, α-pinene, linalool, linalyl acetate, isobornyl acetate, benzylacetone) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB, and IL-6 | [73] | |
| Lavandula angustifolia L. | aerial parts | 54 compounds (γ-terpineol, lavandulyl propionate) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | inhibition of the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Lavandula stoechas L. | aerial parts | 21 compounds (β-pinene, 1,8-cineole) | Swiss albino mice | 200 and 20 mg/kg (oral administration) 82 and 410 mg/kg (topical administration) | reduced carrageenan-induced paw edema/ reduced acute ear edema | [74] |
| Lavandula stoechas L. | aerial parts | 28 compounds (1,8-cineole, trans-α-necrodyl acetate, E-caryophyllene, trans-α-necrodol, lavandulol) | Swiss albino mice | 200 mg/kg (oral administration) | inhibition of carrageenan-induced rat paw oedema | [75] |
| Melissa officinalis L. | leaves | (nerol, citral, isopulegol) | Wistar rats | 200, 400 mg/kg (oral administration) | reduction in edema induced by carrageenan | [76] |
| Mentha haplocalyx L. | 32 compounds (p-cymene, D-limonene, γ-terpinene, α-isomenthone, L-menthone, DL-menthol) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB, IL-6, and COX-2 | [73] | |
| Mentha piperita L. | 28 compounds (menthone, isomenthone, menthol, trans-anethole) | Charles River Wistar rats | 125–500 mg/kg (oral administration) | inhibited paw edema induced by carrageenan | [77] | |
| Mentha piperita L. | leaves | 51 compounds (neomenthol, menthol, menthy acetate) | ICR mice | 200, 400 and 800 mg/ear (topically treatment) | inhibition of paw edema induced by croton oil | [78] |
| Mentha spicata L. subsp. crispata | 28 compounds (menthone, menthol, carvone) | Charles River Wistar rats | 125–500 mg/kg (oral administration) | inhibited paw edema induced by carrageenan | [77] | |
| Mentha suaveolens L. | 20 compounds (piperitenone oxide) | Charles River Wistar rats | 1125–500 mg/kg (oral administration) | inhibited paw edema induced by carrageenan | [77] | |
| Ocimum basilicum L. | leaves | 14 compounds (linalool, estragole) | Swiss albino mice | 100 µg/mL (topical administration) | reduced paw edema induced by carrageenan and dextran | [79] |
| Ocimum kilimandscharicum L. | leaves | 45 compounds (limonene, 1,8 cineole, camphor) | Swiss mice | 30 and 100 mg/kg (oral administration) | inhibited carrageenan-induced pleurisy | [80] |
| Ocimum selloi L. | leaves | 9 compounds (methyl chavicol, E-anethole) | Swiss mice | 30–300 mg/kg (oral administration) | significantly prevented paw edema, mechanical hyperalgesia, and cold hyperalgesia after carrageenan model | [81] |
| Origanum compactum L. | aerial parts | 11 compounds (p-cymene, β-pinene, carvacrol, thymol) | Wistar rats | 100 mg/kg (oral administration) | inhibition of paw edema induced by carrageenan | [82] |
| Perilla frutescens (L.) Britton | 20 compounds (linalool, 2-pyrimidinamine, 2-hexanoylfuran, β-caryophyllene) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB, IL-6, and COX-2 | [73] | |
| Perilla frutescens (L.) Britton | leaves | 24 compounds (β-caryophyllene, linalool) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | inhibited the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Pogostemon cablin (Blanco) Benth. | 14 compounds (α-guaiene, α-bulnesene, seychellene, patchouli alcohol) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB and COX-2 | [73] | |
| Pogostemon cablin (Blanco) Benth. | leaves | 35 compounds (p-allylguaiacol/eugenol, patchouli alcohol, pogostone) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | Inhibited the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Rosmarinus offcinalis L. | leaves | 46 compounds (levo verbenone, chavibetol, borneol, (+)-2-bornanone, eucalyptol) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | Inhibited the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Rosmarinus officinalis L. | 23 compounds (D-limonene, α-pinene, linalool) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB, and IL-6 | [73] | |
| Salvia japonica L. | aerial parts | 47 compounds (L-α-pinene, linalool, (+)-2-bornanone, benzyl acetate, triacetin, terpenyl acetate) | Rats with adjuvant arthritis | 100 mg/kg (oral administration) | inhibited the expression of IL-1, IL-6, TNF-α, and COX-2 | [69] |
| Scutellaria baicalensis Georgi. | 44 compounds (o-cymene, curcumene, (Z,E)-α-farnesene, γ-muurolene) | BALB/c mice 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced mice models | 100 µg/mL (topical administration) | decreased the production of TNF-α, NF-κB, IL-6, and COX-2 | [73] | |
| Stachys lavandulifolia Vahl. | aerial parts | (–)-α-bisabolol, bicyclogermacrene | Swiss mice | 25 or 50 mg/kg (oral administration) | reduced pro-inflammatory cytokine IL-1β | [83] |
| Thymus algeriensis Boiss. & Reut. | aerial parts | 9 compounds (borneol, thymol, carvacrol) | rats | 150 mg/kg (oral administration) | inhibited paw edema induced by carrageenan | [84] |
| Thymus fontanesii Boiss. & Reut. | aerial parts | 24 compounds (p-cymene, γ-terpinene, carvacrol) | mice | 50 mg/kg and 100 mg/kg (oral administration) | inhibited paw edema induced by carrageenan | [85] |
| Thymus vulgaris L. | aerial parts | 66 compounds (p-cymene, γ-terpinene, thymol) | Swiss albino mice | 400 mg/kg (oral administration) | reduction in edema induced by carrageenan | [86] |
| Thymus vulgaris L. | - | 25 compounds (p-cymene, γ-terpinene, carvacrol) | Swiss mice | 100, 10 and 2 mg/kg (topical administration) | inhibited paw edema induced by croton oil | [87] |
| Zataria multiflora Boiss. | - | 29 compounds (p-cymene, γ-terpinene, thymol, carvacrol) | BALB/c mice | 1–2% (topical administration) | decreased the expression of IL-1β and TNF-α | [88] |
| Tested Plant | Plant Part | Number of Identified Compounds (the Main Constituents) | Cell Line | Essential Oil Concentrations | Ref. |
|---|---|---|---|---|---|
| Cantinoa stricta (Benth.) Harley & J. F. B. Pastore | flowers | 46 compounds (α-pinene, β-pinene, limonene + β-phellandrene, spathulenol, caryophyllene oxide) | UACC-62 | TGI = 25.19 µg/mL | [111] |
| Cedronella canariensis (L.) Webb & Berthel | aerial parts | 61 compounds (β-pinene, pinocarvone) | A375 | IC50 = 4.3 µg/mL | [112] |
| Cunila angustifolia Benth. | leaves | 17 compounds (menthone, isomenthol, pulegone) | SK-Mel-28 | IC50 = 279.9 µg/mL | [113] |
| Lavandula stoechas L. | aerial parts | 21 compounds (β-pinene, 1,8-cineole) | MV3 | IC50 = 0.06 µL/mL | [74] |
| Mentha piperita L. | aerial parts | - | A-375 | IC50 = 0.4 µL/mL | [114] |
| Ocimum basilicum L. | leaves | linalool and isoeugenol | FemX | IC50 = 96.72 µg/mL | [115] |
| Ocimum basilicum L. | aerial parts | - | A-375 | IC50 = 0.36 µL/mL | [114] |
| Origanum vulgare L. | aerial parts | - | A-375 | IC50 = 0.09 µL/mL | [114] |
| Pogostemon deccanensis Desf. | aerial parts | 47 compounds (ethanone, 1-(2,4,6-trihydroxyphenyl)-, epi-cadinol, benzofuran, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-, trans-) | B16F1 | 2 µg/mL—2.1% survival ratio | [116] |
| Rosmarinus officinalis L. | leaves | - | A-375 | IC50 = 0.24 µL/mL | [114] |
| Salvia aurea L. | aerial parts | 35 compounds (aromadendrene, α-amorphene, caryophyllene oxide, elemenone, aristolone) | M14 | IC50 = 12.5 µg/mL | [117] |
| Salvia aurea L. | aerial parts | 35 compounds (aromadendrene, α-amorphene, caryophyllene oxide, elemenone, aristolone) | A2058 | IC50 = 21.2 µg/mL | [117] |
| Salvia aurea L. | aerial parts | 35 compounds (aromadendrene, α-amorphene, caryophyllene oxide, elemenone, aristolone) | A375 | IC50 = 15.9 µg/mL | [117] |
| Salvia judaica Boiss | aerial parts | 45 compounds (tetradecanoic acid, caryophyllene oxide, α-copaene) | M14 | IC50 = 11.6 µg/mL | [117] |
| Salvia Judaica Boiss | aerial parts | 45 compounds (tetradecanoic acid, caryophyllene oxide, α-copaene) | A2058 | IC50 = 19.4 µg/mL | [117] |
| Salvia Judaica Boiss | aerial parts | 45 compounds (tetradecanoic acid, caryophyllene oxide, α-copaene) | A375 | IC50 = 14.4 µg/mL | [117] |
| Salvia officinalis L. | whole plant | 14 compounds (1,8-cineole, α-thujone, β-thujone, camphor, γ-muurolene) | A375 | IC50 = 10.7 µg/mL | [118] |
| Salvia officinalis L. | whole plant | 10 compounds (α-thujone, β-thujone, γ-elemene, γ-muurolene, sclareol) | M14 | IC50 = 8.2 µg/mL | [118] |
| Salvia officinalis L. | whole plant | 10 compounds (α-thujone, β-thujone, γ-elemene, γ-muurolene, sclareol) | A2058 | IC50 = 11.7 µg/mL | [118] |
| Salvia officinalis L. | aerial parts | 14 compounds (β-pinene, eucalyptol, α-thujone, camphene, p-thymol, caryophyllene) | A375 | 50 µg/mL—39% inhibition ratio | [119] |
| Salvia verbenaca L. | aerial parts | 76 constituents (hexahydrofarnesyl acetone, hexadecanoic acid) | M14 | IC50 = 8.1 µg/mL | [120] |
| Salvia viscosa Jacq. | aerial parts | 31 compounds (β-copaen-4-α-ol, caryophyllene oxide, α-cubebene, carvacrol) | M14 | IC50 = 13.3 µg/mL | [117] |
| Salvia viscosa Jacq. | aerial parts | 31 compounds (β-copaen-4-α-ol, caryophyllene oxide, α-cubebene, carvacrol) | A2058 | IC50 = 23.6 µg/mL | [117] |
| Salvia viscosa Jacq. | aerial parts | 31 compounds (β-copaen-4-α-ol, caryophyllene oxide, α-cubebene, carvacrol) | A375 | IC50 = 16.2 µg/mL | [117] |
| Satureja hortensis L. | aerial parts | 18 compounds ((+)-4-carene, γ-terpinene, o-cymene, thymol, carvacrol) | A375 | IC50 = 22.27 µg/mL | [121] |
| Stachys annua L. | aerial parts | 53 compounds (phytol, germacrene D, spathulenol, bicyclogermacrene) | A375 | IC50 = 37.2 µg/mL | [122] |
| Thymus munbyanus Boiss & Reuth | flowers | 103 compounds (1,8-cineole, camphor, borneol) | A375 | IC50 = 46.95 µg/mL | [123] |
| Thymus vulgaris L. | aerial parts | 8 compounds (γ-terpinene, p-thymol, caryophyllene) | A375 | 50 µg/mL—17.5% inhibition ratio | [119] |
| Tested Plant | Chemical Components of Essential Oils | Type of Nanoparticles | Activities | Effect | References |
|---|---|---|---|---|---|
| Ocimum basilicum | eugenol and caryophyllene | chitosan nanoparticles | antibacterial and antibiofilm activity | Staphylococcus aureus | [135] |
| Thymus sp. | Thymol and carvacrol | chitosan nanoparticles | antimicrobial activity | Staphylococcus aureus | [136] |
| Mentha sp. | Menthol, menthone, menthyl acetate, piperitone, limonene, and 1,8-cineole | hydroxyapatite nanoparticles | antimicrobial activity | Staphylococcus aureus, Pseudomonas aeruginosa, or the fungal strain Candida parapsilosis | [137] |
| Lavendula sp. | - | nanostructured lipid carriers (NLCs) | wound-healing activities | [138] | |
| Rosmarinus officinalis L. | - | silver nanoparticles | antimicrobial and wound-healing activity | Staphylococcus aureus | [139] |
| Zataria multiflora Boiss. | - | solid lipid nanoparticles | anti-cancer | anticancer efficacy of the essential oil against melanoma cancer (A-375) cells with 75 μg/mL | [140] |
| Mentha piperita L. | - | chitosan nanoparticles | antioxidant and antimicrobial activities | enhanced antibacterial activity with MBC values of 0.57 and mg·mL−1 against S. aureus; antioxidant activities were improved by about 2.4-fold in DPPPH test | [141] |
| Satureja khuzistanica Jamzad | carvacrol | chitosan nanoparticles | antibacterial activities | activities on Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis strains | [142] |
| Origanum vulgare L. | - | gold nanoparticles | antioxidant, antimicrobial properties | significant bactericidal and antioxidant activities, the most sensitive microorganisms being S. aureus and C. albicans, better tolerated by normal human dermal fibroblast cells, while the melanoma cancer cells are more sensitive | [143] |
| Origanum vulgare L. | - | ZnO nanoparticles | antioxidant activity | excellent antioxidative properties in DPPH test | [144] |
| Satureja hortensis L. | - | iron nanoparticles (FeNPs) | antimicrobial activity | possessed higher antimicrobial properties against selected pathogenic microorganisms, S. aureus, P. aeruginosa, and C. albicans | [145] |
| Origanum vulgare L. | o-cymene/m-cymene, terpinolene, carvacrol, -terpinene | chitosan—alginate nanoparticles | antimicrobial activity | possessed strong antimicrobial activity against S. aureus, P. aeruginosa, and C. albicans | [146] |
| Thymus capitatus L. and Origanum vulgare L. | carvacrol, thymol | chitosan nanoparticles | antimicrobial activity | exhibited enhanced bactericidal activity against S. aureus | [147] |
| Thymus vulgaris L. | p-cymene, thymol, α-terpineol and linalool | archaeolipids carriers (NAC) | antioxidant, anti-inflammatory, and antibiofilm activity | exhibited enhanced activity against P. aeruginosa | [148] |
| Lavandula angustifolia L. | - | silver nanoparticles | antimicrobial and wound-healing activity | excellent bactericidal properties against S. aureus | [149] |
| The Active Ingredient from the Lamiaceae Family | Application | Patent Number | Year |
|---|---|---|---|
| Mentha camphor oil, Lavandula angustifolia oil | Skin pruritus, allergic dermatitis, eczema | CN106420937A | 2017 |
| Melissa oil | An organic skin moisturizer | US 8,986,752 B1 | 2015 |
| Origanum compactum oil | Treatment of keratoses | US 9,040,103 B2 | 2015 |
| Peppermint oil | Inflammation of skin | US 9,180,146 B2 | 2015 |
| Monarda fistulosa and/or Monarda didyma oil | Inflammation of skin | US 2016/0213727 A1 | 2016 |
| Ocimum americanum oil, Mentha pulegium oil | Cosmetic application | WO2017112998A1 | 2017 |
| Oregano oil, Thyme oil | Bacterial and fungal infections, and oxidative stress | WO2016187422A1 | 2016 |
| Origanum compactum oil | Therapeutic treatment of actinic keratoses | EP2538933B1 | 2016 |
| Origanum compactum oil | Treatment of malign keratosis | EP2538933A2 | 2016 |
| Rosemary oil, peppermint oil | A hand and body skincare cream | US7887853B1 | 2011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, T.; Merecz-Sadowska, A.; Ghorbanpour, M.; Szemraj, J.; Piekarski, J.; Bijak, M.; Śliwiński, T.; Zajdel, R.; Sitarek, P. Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research. Int. J. Mol. Sci. 2023, 24, 15279. https://doi.org/10.3390/ijms242015279
Kowalczyk T, Merecz-Sadowska A, Ghorbanpour M, Szemraj J, Piekarski J, Bijak M, Śliwiński T, Zajdel R, Sitarek P. Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research. International Journal of Molecular Sciences. 2023; 24(20):15279. https://doi.org/10.3390/ijms242015279
Chicago/Turabian StyleKowalczyk, Tomasz, Anna Merecz-Sadowska, Mansour Ghorbanpour, Janusz Szemraj, Janusz Piekarski, Michal Bijak, Tomasz Śliwiński, Radosław Zajdel, and Przemysław Sitarek. 2023. "Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research" International Journal of Molecular Sciences 24, no. 20: 15279. https://doi.org/10.3390/ijms242015279
APA StyleKowalczyk, T., Merecz-Sadowska, A., Ghorbanpour, M., Szemraj, J., Piekarski, J., Bijak, M., Śliwiński, T., Zajdel, R., & Sitarek, P. (2023). Enhanced Natural Strength: Lamiaceae Essential Oils and Nanotechnology in In Vitro and In Vivo Medical Research. International Journal of Molecular Sciences, 24(20), 15279. https://doi.org/10.3390/ijms242015279








