Single-Nucleotide Polymorphism in Genes Encoding G Protein Subunits GNB3 and GNAQ Increase the Risk of Cardiovascular Morbidity among Patients Undergoing Renal Replacement Therapy
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
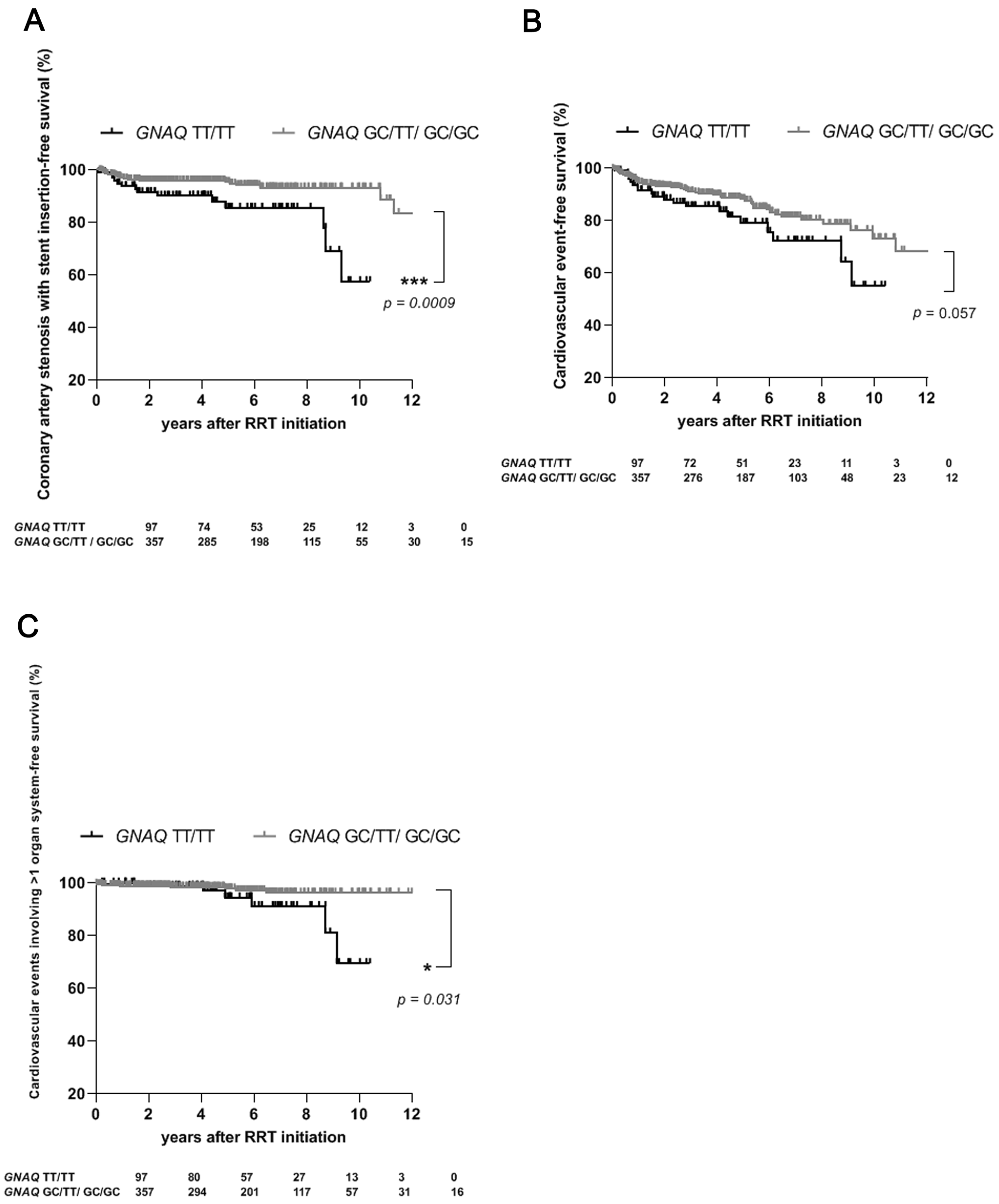
2.2. For Patients Undergoing RRT, the TT/TT Genotype of GNAQ Confers an Increased Risk of the Need for Coronary Artery Stenosis Requiring Stent Insertion and of the Occurrence of Cardiovascular Events, Particularly Those Involving Multiple Organ Systems
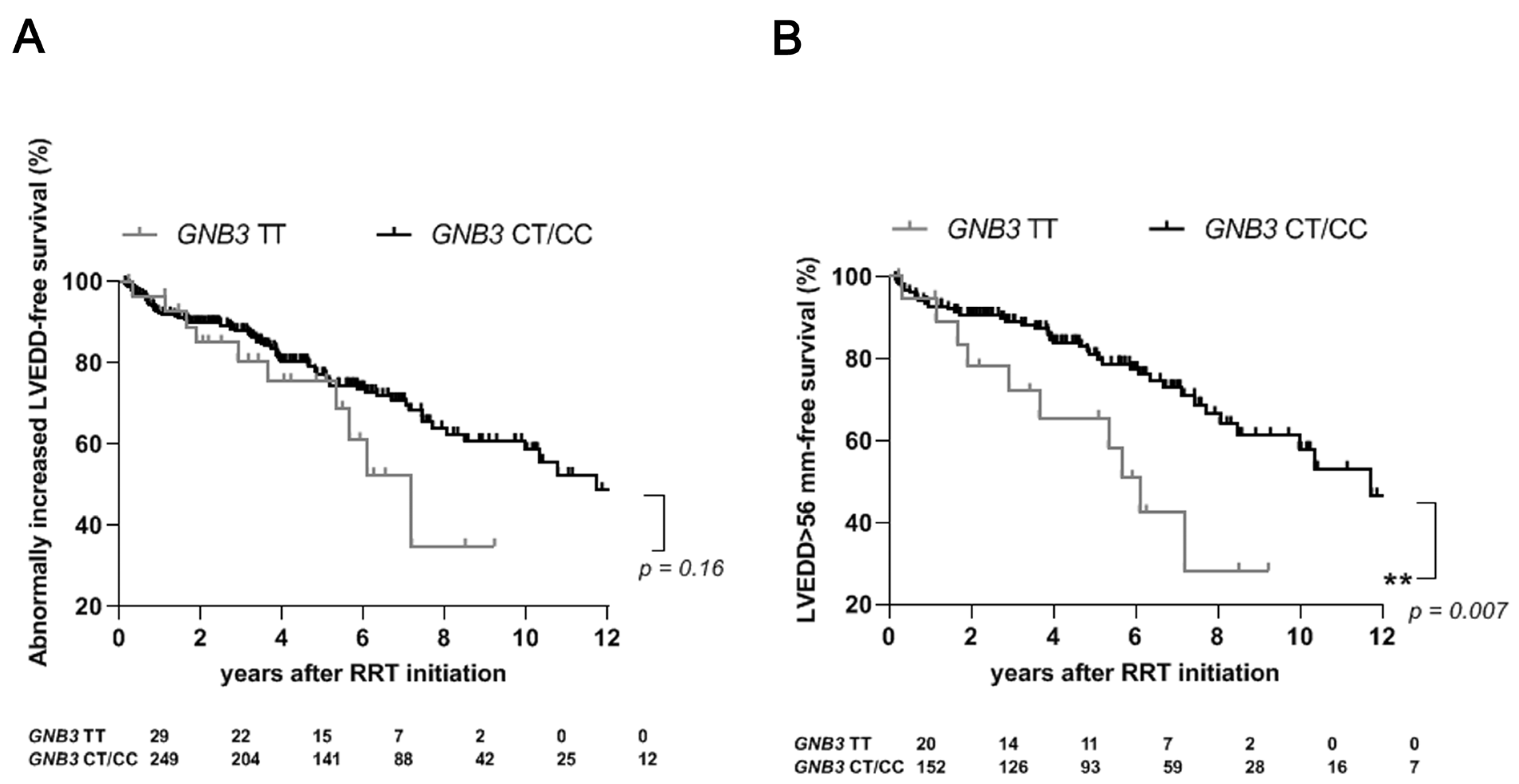
2.3. The TT Genotype of GNB3 Is an Independent Risk Factor for Abnormally Increased LVEDD in Male Dialysis Patients
2.4. The GNAS c.393C > T Polymorphism Is Not Associated with Cardiovascular Events or Abnormal Increase in LVEDD among Patients Undergoing RRT
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Genotyping for GNB3, GNAQ, and GNAS
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Semplicini, A.; Grandi, T.; Sandonà, C.; Cattelan, A.; Ceolotto, G. G-protein b3-subunit gene C825T polymorphism and cardiovascular risk: An updated review. High Blood Press. Cardiovasc. Prev. 2015, 22, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Elefsinioti, A.L.; Bagos, P.G.; Spyropoulos, I.C.; Hamodrakas, S.J. A database for G proteins and their interaction with GPCRs. BMC Bioinform. 2004, 5, 208. [Google Scholar] [CrossRef] [PubMed]
- Siffert, W.; Rosskopf, D.; Siffert, G.; Busch, S.; Moritz, A.; Erbel, R.; Sharma, A.M.; Ritz, E.; Wichmann, H.; Jakobs, K.H.; et al. Association of a human G-protein beta3 subunit variant with hypertension. Nat. Genet. 1998, 18, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Kimura, L.; Suwazono, Y.; Nakagawa, H.; Daimon, M.; Oizumi, T.; Kayama, T.; Kato, T.; Li, L.; Chen, S.; et al. Multivariate meta-analysis of the association of G-protein beta 3 gene (GNB3) haplotypes with cardiovascular phenotypes. Mol. Biol. Rep. 2014, 41, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Poch, E.; González, D.; Gómez-Angelats, E.; Enjuto, M.; Paré, J.C.; Rivera, F.; de La Sierra, A. G-Protein beta(3) subunit gene variant and left ventricular hypertrophy in essential hypertension. Hypertension 2000, 35, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Semplicini, A.; Siffert, W.; Sartori, M.; Monari, A.; Naber, C.; Frigo, G.; Santonastaso, M.; Cozzutti, E.; Winnicki, M.; Palatini, P. G protein beta3 subunit gene 825T allele is associated with increased left ventricular mass in young subjects with mild hypertension. Am. J. Hypertens. 2001, 14, 1191–1195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brand, E.; Wang, J.G.; Herrmann, S.M.; Staessen, J.A. An epidemiological study of blood pressure and metabolic phenotypes in relation to the Gbeta3 C825T polymorphism. J. Hypertens. 2003, 21, 729–737. [Google Scholar] [CrossRef]
- Sartori, M.; Parotto, E.; Ceolotto, G.; Papparella, I.; Lenzini, L.; Calò, L.A.; Semplicini, A. C825T polymorphism of the GNB3 gene codifying the G-protein beta3-subunit and cardiovascular risk. Rev. Ann. Ital. Med. Int. 2004, 19, 240–248. [Google Scholar]
- Peitz, T.; Möhlendick, B.; Siffert, W.; Heinemann, F.M.; Kribben, A.; Eisenberger, U.; Friebus-Kardash, J. GNB3 c.825C > T (rs5443) Polymorphism and Risk of Acute Cardiovascular Events after Renal Allograft Transplant. Int. J. Mol. Sci. 2022, 23, 9783. [Google Scholar] [CrossRef]
- Liggett, S.B.; Kelly, R.J.; Parekh, R.R.; Matkovich, S.J.; Benner, B.J.; Hahn, H.S.; Syed, F.M.; Galvez, A.S.; Case, K.L.; McGuire, N.; et al. A functional polymorphism of the Galphaq (GNAQ) gene is associated with accelerated mortality in African-American heart failure. Hum. Mol. Genet. 2007, 16, 2740–2750. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.H.; Lieb, W.; Erdmann, J.; Savidou, D.; Heusch, G.; Leineweber, K.; Jakob, H.; Hense, H.W.; Löwel, H.; Brockmeyer, N.H.; et al. Characterization of the GNAQ promoter and association of increased Gq expression with cardiac hypertrophy in humans. Eur. Heart J. 2008, 29, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.H.; Klenke, S.; Mitchell, A.; Knüfermann, T.; Jakob, H.; Thielmann, M.; Siffert, W.; Peters, J. GNAQ TT(-695/-694)GC Polymorphism Is Associated with Increased Gq Expression, Vascular Reactivity, and Myocardial Injury after Coronary Artery Bypass Surgery. Anesthesiology 2017, 127, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Möhlendick, B.; Schmid, K.W.; Siffert, W. The GNAS SNP c.393C > T (rs7121) as a marker for disease progression and survival in cancer. Pharmacogenomics 2019, 20, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Frey, U.H.; Eisenhardt, A.; Lümmen, G.; Rübben, H.; Jöckel, K.H.; Schmid, K.W.; Siffert, W. The T393C polymorphism of the G alpha s gene (GNAS1) is a novel prognostic marker in bladder cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hingorani, A.D.; Sharma, P.; Hopper, R.; Dickerson, C.; Trutwein, D.; Lloyd, D.D.; Brown, M.J. Association of the G(s)alpha gene with essential hypertension and response to beta-blockade. Hypertension 1999, 34, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wieneke, H.; Svendsen, J.H.; Lande, J.; Spencker, S.; Martinez, J.G.; Strohmer, B.; Toivonen, L.; Le Marec, H.; Garcia-Fernandez, J.F.; Corrado, D.; et al. Polymorphisms in the GNAS gene as predictors of ventricular tachyarrhythmias and sudden cardiac death: Results from the DISCOVERY Trial and Oregon Sudden Unexpected Death Study. J. Am. Heart Assoc. 2016, 5, e003905. [Google Scholar] [CrossRef]
- Frey, U.H.; Moebus, S.; Mohlenkamp, S.; Kälsch, H.; Bauer, M.; Lehmann, N.; Nöthen, M.; Mühleisen, T.M.; Stang, A.; Erbel, R.; et al. GNB3 gene 825 TT variant predicts hard coronary events in the population-based Heinz Nixdorf Recall study. Atherosclerosis 2014, 237, 437–442. [Google Scholar] [CrossRef]
- Bagos, P.G.; Elefsinioti, A.L.; Nikolopoulos, G.K.; Hamodrakas, S.J. The GNB3 C825T polymorphism and essential hypertension: A meta-analysis of 34 studies including 14,094 cases and 17,760 controls. J. Hypertens. 2007, 25, 487–500. [Google Scholar] [CrossRef]
- Beige, J.; Engeli, S.; Ringel, J.; Offermann, G.; Distler, A.; Sharma, A.M. Donor G protein beta3 subunit 825TT genotype is associated with reduced kidney allograft survival. J. Am. Soc. Nephrol. 1999, 10, 1717–1721. [Google Scholar] [CrossRef]
- Viklický, O.; Hubáček, J.A.; Vítko, Š.; Heemann, U.W.; Vasarhelyi, B.; Kohnle, M.; Teplan, V.; Lácha, J.; Szabó, A.J. The 825C/T polymorphism of the G-protein subunit beta3 does not influence blood pressure and renal function in kidney transplant recipients. Nephrol. Dial. Transplant. 2000, 15, 1663–1666. [Google Scholar]
- Schunkert, H.; Hense, H.W.; Döring, A.; Riegger, G.A.; Siffert, W. Association between a polymorphism in the G protein beta3 subunit gene and lower renin and elevated diastolic blood pressure levels. Hypertension 1998, 32, 510–513. [Google Scholar] [CrossRef]
- Mahmood, M.S.; Sajadeen Urfy Mian, Z.; Afzal, A.; Frossard, P.M. G-protein beta-3 subunit gene 825C > T dimorphism is associated with left ventricular hypertrophy but not essential hypertension. Med. Sci. Monit. 2005, 11, CR6–CR69. [Google Scholar] [PubMed]
- Shlyakhto, E.V.; Shwartz, E.I.; Nefedova, Y.B.; Zukova, A.V.; Tatyana AVinnic, T.A.; Konrady, A.O. Lack of association of G-protein subunit gene C825T polymorphism with left ventricular hypertrophy in essential hypertension. Med. Sci. Monit. 2002, 8, CR337–CR340. [Google Scholar] [PubMed]
- Gbadoe, M.K.; Berdouzi, N.; Aldasoro Aguiñano, A.A.; Ndiaye, N.C.; Sophie Visvikis-Siest, S. Cardiovascular diseases-related GNB3 C825T polymorphism has a significant sex-specific effect on serum soluble E-selectin levels. J. Inflamm. 2016, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Von Beckerath, N.; Schusterschitz, Y.; Koch, W.; Griesser, K.; Mehilli, J.; Gorchakova, O.; Schömig, A.; Kastrati, A. G protein beta 3 subunit 825T allele carriage and risk of coronary artery disease. Atherosclerosis 2003, 167, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Klenke, S.; Nils Lehmann, N.; Erbel, R.; Jöckel, K.H.; Siffert, W.; Frey, U.H.; Peters, J. Genetic variations in G-protein signal pathways influence progression of coronary artery calcification: Results from the Heinz Nixdorf Recall study. Atherosclerosis 2020, 310, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Voora, D.; Horton, J.; Shah, S.H.; Shaw, L.K.; Newby, L.K. Polymorphisms associated with in vitro aspirin resistance are not associated with clinical outcomes in patients with coronary artery disease who report regular aspirin use. Am. Heart J. 2011, 162, 166–172.e1. [Google Scholar] [CrossRef]
- Tobin, M.D.; Braund, P.S.; Burton, P.R.; Thompson, J.R.; Steeds, R.; Channer, K.; Cheng, S.; Lindpaintner, K.; Samani, N.J. Genotypes and haplotypes predisposing to myocardial infarction: A multilocus case-control study. Eur. Heart J. 2004, 25, 459–467. [Google Scholar] [CrossRef]
- Renner, W.; Hoffmann, M.M.; Grunbacher, G.; Winkelmann, B.R.; Boehm, B.O.; Marz, W. G-protein beta3 subunit (GNB3) gene polymorphisms and cardiovascular disease: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis 2007, 192, 108–112. [Google Scholar] [CrossRef]
- Plat, A.W.; Stoffers, H.E.; Klungel, O.H.; van Schayck, C.P.; de Leeuw, P.W.; Soomers, F.L.; Schiffers, P.M.; Kester, A.D.M.; Kroon, A.A. The contribution of six polymorphisms to cardiovascular risk in a Dutch high-risk primary care population: The HIPPOCRATES project. J. Hum. Hypertens. 2009, 23, 659–667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorn, G.W., II; Brown, J.H. Gq signaling in cardiac adaptation and maladaptation. Trends Cardiovasc. Med. 1999, 9, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.L.; Thomas, W.G. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Mestril, R.; Samali, A. Losing heart: The role of apoptosis in heart disease—A novel therapeutic target? FASEB J. 2002, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Wettschureck, N.; Rutten, H.; Zywietz, A.; Gehring, D.; Wilkie, T.M.; Chen, J.; Chien, K.R.; Offermanns, S. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat. Med. 2001, 7, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Rapacciuolo, A.; Naga Prasad, S.V.; Takaoka, H.; Thomas, S.A.; Koch, W.J.; Rockman, H.A. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 2002, 105, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef] [PubMed]
- Howes, A.L.; Miyamoto, S.; Adams, J.W.; Woodcock, E.A.; Brown, J.H. Galphaq expression activates EGFR and induces Akt mediated cardiomyocyte survival: Dissociation from Galphaq mediated hypertrophy. J. Mol. Cell. Cardiol. 2006, 40, 597–604. [Google Scholar] [CrossRef]
- Harkness, A.; Ring, L.; Daniel, X.A.; Oxborough, D.; Robinson, S.; Sharma, V. Education Committee of the British Society of Echocardiography Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G1–G18. [Google Scholar] [CrossRef]
| RR (95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographic Characteristics | All Patients n = 454 | GNB3 TT n = 45 | GNB3 CT/CC n = 409 | GNAQ TT/TT n = 97 | GNAQ GC/TT/GC/GC n = 357 | GNAS TT n = 118 | GNAS CT/CC n = 336 | ||||||
| Age in years, median (range) | 51 (18–81) | 47 (22–70) | 52 (18–63) | 0.08 | 53 (21–75) | 50 (18–81) | 0.14 | 50 (21–75) | 51 (18–81) | 0.19 | |||
| Women, n (%) | 168 (37) | 16 (36) | 152 (37) | 0.96 (0.6–1.4) | 0.83 | 45 (46) | 123 (34) | 1.35 (1.0–1.7) | 0.03 | 40 (34) | 128 (38) | 0.89 (0.7–1.2) | 0.42 |
| Men, n (%) | 286 (63) | 29 (64) | 257 (63) | 1.03 (0.8–1.3) | 0.83 | 52 (54) | 234 (66) | 0.82 (0.7–1.0) | 0.03 | 78 (66) | 208 (62) | 1.07 (0.9–1.2) | 0.42 |
| Previous renal transplants, n (%) | 72 (16) | 6 (13) | 66 (16) | 0.83 (0.4–1.7) | 0.63 | 15 (15) | 57 (16) | 0.97 (0.6–1.6) | 0.90 | 19 (16) | 53 (16) | 1.0 (0.6–1.6) | 0.93 |
| Time on dialysis (days), median (range) | 1637 (50–7068) | 1541 (82–5135) | 1649 (50–7068) | 0.36 | 1586 (85–4581) | 1650 (50–7068) | 0.72 | 1691 (114–7068) | 1627 (50–6243) | 0.43 | |||
| Peritoneal dialysis patients, n (%) | 96 (21) | 9 (20) | 87 (21) | 0.94 (0.5–1.7) | 0.84 | 27 (28) | 69 (19) | 1.44 (0.97–2.) | 0.07 | 27 (23) | 69 (21) | 1.10 (0.8–1.6) | 0.59 |
| Obesity (grade 1–3), n (%) | 115 (25) | 13 (29) | 102 (25) | 1.16 (0.7–1.8) | 0.56 | 31 (32) | 84 (24) | 1.36 (0.9–1.9) | 0.09 | 31 (32) | 84 (24) | 1.40 (0.9–1.9) | 0.09 |
| Diabetes mellitus type I, n (%) | 13 (3) | 2 (4) | 11 (3) | 1.65 (0.4–6.2) | 0.50 | 3 (3) | 10 (3) | 1.10 (0.3–3.6) | 0.88 | 4 (3) | 9 (3) | 1.30 (0.4–3.8) | 0.69 |
| Development of diabetic glomerulosclerosis among type I diabetics, n (%) | 12 (92) | 2 (100) | 10 (91) | 1.10 (0.4–10.6) | 0.66 | 3 (100) | 9 (90) | 1.10 (0.5–4.8) | 0.57 | 4 (100) | 8 (89) | 1.13 (0.6–3.4) | 0.49 |
| Diabetes mellitus type II, n (%) | 55 (12) | 6 (13) | 49 (12) | 1.11 (0.5–2.3) | 0.79 | 8 (8) | 47 (13) | 0.63 (0.3–1.2) | 0.19 | 10 (8) | 45 (13) | 0.63 (0.3–1.2) | 0.16 |
| insulin-dependent diabetes mellitus type II, n (%) | 19 (4) | 2 (4) | 17 (4) | 1.07 (0.3–3.9) | 0.93 | 6 (6) | 13 (4) | 1.70 (0.7–4.2) | 0.27 | 5 (3) | 15 (4) | 0.76 (0.3–2.1) | 0.62 |
| Development of diabetic glomerulosclerosis among type II diabetics, n (%) | 14 (25) | 3 (50) | 11 (22) | 2.23 (0.8–4.9) | 0.14 | 2 (25) | 12 (26) | 0.98 (0.3–2.8) | 0.98 | 2 (20) | 12 (27) | 0.75 (0.2–2.3) | 0.66 |
| Diabetes mellitus type I-III, n (%) | 69 (15) | 8 (18) | 61 (15) | 1.19 (0.6–2.2) | 0.61 | 11 (11) | 58 (16) | 0.70 (0.4–1.2) | 0.23 | 14 (12) | 55 (16) | 0.72 (0.4–1.2) | 0.24 |
| Development of diabetic glomerulosclerosis among type I-III diabetics, n (%) | 27 (39) | 5 (63) | 22 (36) | 1.73 (0.8–2.9) | 0.15 | 5 (45) | 22 (38) | 1.20 (0.5–2.2) | 0.64 | 6 (43) | 21 (38) | 1.12 (0.5–2.1) | 0.75 |
| Therapy with lipid-lowering agents, n (%) | 130 (29) | 17 (38) | 113 (28) | 1.37 (0.9–2.0) | 0.15 | 32 (33) | 98 (27) | 1.20 (0.9–1.7) | 0.29 | 30 (25) | 100 (30) | 0.85 (0.6–1.2) | 0.37 |
| Antihypertensive therapy with ≥5 drugs, n (%) | 42 (10) | 4 (9) | 38 (10) | 0.98 (0.4–2.4) | 0.96 | 9 (9) | 33 (10) | 0.98 (0.5–1.9) | 0.96 | 9 (8) | 33 (10) | 0.79 (0.4–1.6) | 0.52 |
| Causes of renal failure | |||||||||||||
| 1. Diabetic glomerulosclerosis, n (%) | 27 (6) | 5 (11) | 22 (5) | 2.10 (0.8–4.9) | 0.12 | 5 (11) | 22 (5) | 0.85 (0.3–2.1) | 0.71 | 6 (5) | 21 (6) | 0.81 (0.3–1.9) | 0.65 |
| due to diabetes mellitus type I, n (%) | 12 (3) | 2 (4) | 10 (2) | 1.80 (0.5–6.9) | 0.43 | 3 (3) | 9 (3) | 1.23 (0.4–4.1) | 0.76 | 4 (3) | 8 (2) | 1.42 (0.5–4.3) | 0.56 |
| due to diabetes mellitus type II, n (%) | 14 (3) | 3 (7) | 11 (3) | 2.50 (0.8–7.7) | 0.14 | 2 (2) | 12 (3) | 0.61 (0.2–2.4) | 0.51 | 2 (2) | 12 (4) | 0.48 (0.1–1.8) | 0.31 |
| 2. Chronic glomerulonephritis, n (%) | 161 (35) | 18 (40) | 143 (35) | 1.14 (0.8–1.6) | 0.50 | 40 (41) | 121 (34) | 1.22 (0.9–1.6) | 0.18 | 46 (39) | 115 (34) | 1.14 (0.9–1.5) | 0.35 |
| 3. Nephrosclerosis, n (%) | 53 (12) | 5 (11) | 48 (12) | 0.95 (0.4–2.1) | 0.90 | 10 (3) | 43 (12) | 0.86 (0.4–1.6) | 0.64 | 10 (8) | 43 (13) | 0.66 (0.3–1.2) | 0.21 |
| 4. Polycystic kidney disease, n (%) | 60 (13) | 4 (9) | 56 (14) | 0.65 (0.3–1.6) | 0.37 | 13 (13) | 47 (13) | 1.02 (0.6–1.8) | 0.95 | 17 (14) | 43 (13) | 1.13 (0.7–1.9) | 0.66 |
| 5. Tubulointerstitial nephritis, n (%) | 15 (3) | 3 (7) | 12 (3) | 2.27 (0.7–7.0) | 0.18 | 5 (5) | 10 (3) | 1.84 (0.6–5.0) | 0.25 | 4 (3) | 11 (3) | 1.04 (0.4–3.0) | 0.95 |
| 6. Congenital anomalies, n (%) | 32 (7) | 3 (7) | 29 (7) | 0.94 (0.3–2.7) | 0.92 | 4 (4) | 28 (8) | 0.53 (0.2–1.4) | 0.20 | 9 (8) | 23 (7) | 1.11 (0.5–2.3) | 0.78 |
| 7. Autoimmune disease, n (%) | 13 (3) | 1 (2) | 12 (3) | 0.76 (0.1–4.3) | 0.79 | 5 (5) | 8 (2) | 2.30 (0.8–6.5) | 0.13 | 6 (5) | 7 (2) | 2.44 (0.9–6.8) | 0.09 |
| 8. Amyloidosis, n (%) | 6 (1) | 0 (0) | 6 (1) | 0.0 (0.0–5.5) | 0.41 | 3 (3) | 3 (1) | 3.68 (0.9–15.7) | 0.09 | 1 (1) | 5 (1) | 0.57 (0.1–3.6) | 0,60 |
| 9. Reflux nephropathy/recurrent pyelonephritis, n (%) | 25 (6) | 0 (0) | 25 (6) | 0.0 (0.0–1.3) | 0.09 | 4 (4) | 21 (6) | 0.70 (0.3–1.9) | 0.50 | 9 (8) | 16 (5) | 1.60 (0.7–3.4) | 0.24 |
| 10. HUS, n (%) | 7 (2) | 1 (2) | 6 (1) | 1.51 (0.2–9.1) | 0.70 | 1 (1) | 6 (2) | 0.61 (0.1–3.8) | 0.65 | 1 (1) | 6 (2) | 0.48 (0.1–2.9) | 0.48 |
| 11. Other, n (%) | 55 (12) | 5 (11) | 55 (13) | 0.91 (0.4–2.0) | 0.83 | 7 (7) | 48 (13) | 0.54 (0.3–1.1) | 0.10 | 9 (8) | 46 (14) | 0.56 (0.3–1.1) | 0.08 |
| RR (95% CI) | p Value | p Value after Bonferroni Correction | p Value after Benjamini and Hochberg Correction | RR (95% CI) | p value | p Value after Bonferroni Correction | p Value after Benjamini and Hochberg Correction | RR (95% CI) | p value | p Value after Bonferroni Correction | p Value after Benjamini and Hochberg Correction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Events During Dialysis | All Patients n = 454 | GNB3 TT n = 45 | GNB3 CT/CC n = 409 | GNAQ TT/TT n = 97 | GNAQ GC/TT/GC/GC n = 357 | GNAS TT n = 118 | GNAS CT/CC n = 336 | ||||||||||||
| Cardiovascular event, n (%) | 69 (15) | 5 (11) | 64 (16) | 0.71 (0.3–1.6) | 0.42 | 1.0 | 0.79 | 20 (21) | 49 (14) | 1.50 (0.9–2.4) | 0.09 | 1.0 | 0.38 | 19 (16) | 50 (15) | 1.08 (0.7–1.7) | 0.75 | 1.0 | 0.89 |
| Cardiovascular event involving >1 organ system, n (%) | 14 (3) | 1 (2) | 13 (3) | 0.70 (0.1–3.9) | 0.73 | 1.0 | 0.84 | 6 (6) | 8 (2) | 2.76 (1.0–7.4) | 0.05 | 0.85 | 0.38 | 3 (3) | 11 (3) | 0.78 (0.2–2.5) | 0.69 | 1.0 | 0.89 |
| Acute PAOD, n (%) | 18 (4) | 2 (4) | 16 (4) | 1.14 (0.3–4.1) | 0.86 | 1.0 | 0.86 | 6 (6) | 12 (3) | 1.84 (0.7–4.6) | 0.21 | 1.0 | 0.62 | 6 (5) | 12 (4) | 1.42 (0.6–3.6) | 0.47 | 1.0 | 0.87 |
| Transient ischemic attack, n (%) | 4 (1) | 1 (2) | 3 (1) | 3.03 (0.4–20.4) | 0.31 | 1.0 | 0.79 | 1 (1) | 3 (1) | 1.23 (0.2–8.4) | 0.86 | 1.0 | 0.94 | 2 (2) | 2 (1) | 2.85 (0.5–16.0) | 0.27 | 1.0 | 0.87 |
| Stroke, n (%) | 13 (3) | 1 (2) | 12 (3) | 0.76 (0.1–4.3) | 0.79 | 1.0 | 0.84 | 3 (3) | 10 (3) | 1.10 (0.3–3.6) | 0.88 | 1.0 | 0.94 | 4 (3) | 9 (3) | 1.27 (0.4–3.8) | 0.69 | 1.0 | 0.89 |
| Carotid artery stenosis, n (%) | 4 (1) | 0 (0) | 4 (1) | 0.0 (0.0–8.4) | 0.51 | 1.0 | 0.84 | 0 (0) | 4 (1) | 0.0 (0.0–3.5) | 0.30 | 1.0 | 0.70 | 1 (1) | 3 (1) | 1.0 (0.1–6.5) | 0.96 | 1.0 | 0.96 |
| Myocardial infarction, n (%) | 25 (5) | 0 (0) | 25 (6) | 0.0 (0.0–1.3) | 0.09 | 1.0 | 0.68 | 7 (7) | 18 (5) | 1.43 (0.6–3.2) | 0.41 | 1.0 | 0.70 | 5 (4) | 20 (6) | 0.71 (0.3–1.8) | 0.48 | 1.0 | 0.87 |
| Coronary artery stenosis requiring stent insertion, n (%) | 34 (7) | 1 (2) | 33 (8) | 0.28 (0.05–1.5) | 0.16 | 1.0 | 0.68 | 14 (14) | 20 (6) | 2.58 (1.4–4.8) | 0.003 | 0.05 | 0.05 | 7 (6) | 27 (8) | 0.74 (0.3–1.6) | 0.46 | 1.0 | 0.87 |
| Heart valve intervention, n (%) | 16 (4) | 1 (2) | 15 (4) | 0.61 (0.1–3.4) | 0.62 | 1.0 | 0.84 | 4 (4) | 12 (3) | 1.23 (0.4–3.5) | 0.72 | 1.0 | 0.94 | 7 (6) | 9 (3) | 2.21 (0.9–5.6) | 0.10 | 1.0 | 0.87 |
| Coronary artery bypass grafting, n (%) | 14 (3) | 1 (2) | 13 (3) | 0.70 (0.1–3.9) | 0.73 | 1.0 | 0.84 | 3 (3) | 11 (3) | 1.0 (0.3–3.2) | 0.99 | 1.0 | 0.99 | 5 (4) | 9 (3) | 1.58 (0.6–4.4) | 0.40 | 1.0 | 0.87 |
| MACE (cardiovascular death, stroke, myocardial infarction), n (%) | 36 (8) | 1 (2) | 35 (9) | 0.26 (0.1–1.4) | 0.14 | 1.0 | 0.68 | 10 (10) | 26 (7) | 1.42 (0.7–2.8) | 0.33 | 1.0 | 0.70 | 8 (7) | 28 (8) | 0.81 (0.4–1.7) | 0.59 | 1.0 | 0.89 |
| New onset of chronic cardiovascular disease during dialysis | |||||||||||||||||||
| Coronary artery disease, n (%) | 60 (13) | 5 (11) | 55 (13) | 0.83 (0.3–1.8) | 0.66 | 1.0 | 0,84 | 18 (19) | 42 (12) | 1.58 (0.9–2.6) | 0.08 | 1.0 | 0.38 | 13 (11) | 47 (14 | 0.79 (0.4–1.4) | 0.41 | 1.0 | 0.87 |
| Coronary artery disease of ≥2 vessels, n (%) | 29 (6) | 0 (0) | 29 (7) | 0.0 (0.0–1.1) | 0.07 | 1.0 | 0.68 | 8 (8) | 21 (6) | 1.40 (0.6–3.0) | 0.40 | 1.0 | 0.70 | 7 (6) | 22 (7) | 0.91 (0.4–2.0) | 0.81 | 1.0 | 0.89 |
| Heart failure, n (%) | 68 (15) | 6 (13) | 62 (15) | 0.88 (0.4–1.8) | 0.75 | 1.0 | 0.84 | 13 (13) | 55 (15) | 0.87 (0.5–1.5) | 0.62 | 1.0 | 0.88 | 17 (14) | 51 (15) | 0.95 (0.6–1.6) | 0.84 | 1.0 | 0.89 |
| Echocardiographic parameters of left ventricle | |||||||||||||||||||
| LVEDD male > 56 mm/female > 51 mm (278/454), n (%) | 78 (28) | 10 (34) | 68 (27) | 1.26 (0.7–2.0) | 0.42 | 1.0 | 0.79 | 17 (29) | 61 (28) | 1.06 (0.7–1.6) | 0.81 | 1.0 | 0.94 | 17 (23) | 61 (30) | 0.77 (0.5–1.2) | 0.26 | 1.0 | 0.87 |
| IVSd male > 12 mm/female > 11 mm (299/454), n (%) | 146 (49) | 11 (41) | 135 (50) | 0.82 (0.5–1.2) | 0.38 | 1.0 | 0.79 | 33 (56) | 113 (47) | 1.19 (0.9–1.5) | 0.22 | 1.0 | 0.62 | 37 (46) | 109 (50) | 0.91 (0.7–1.2) | 0.51 | 1.0 | 0.87 |
| LVPWd male 12 mm/female > 12 mm (208/454), n (%) | 74 (36) | 10 (45) | 64 (34) | 1.32 (0.8–2.0) | 0.31 | 1.0 | 0.79 | 13 (32) | 61 (37) | 0.87 (0.5–1.4) | 0.56 | 1.0 | 0.87 | 19 (32) | 55 (37) | 0.85 (0.5–1.3) | 0.45 | 1.0 | 0.87 |
| Multivariable Cox regression considering only significantly different variables of the univariate analysis | Multivariable Cox regression considering all variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate RR (95% CI) | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| Variable | Coronary Artery Stenosis Requiring Stent Insertion n = 34 | No Coronary Artery Stenosis n = 420 | ||||||||
| Age in years, median | 59 | 50 | <0.0001 | 1.07 | 1.0–1.1 | 0.001 | 1.07 | 1.0–1.1 | 0.001 | |
| Males, n (%) | 27 (79) | 259 (62) | 1.29 (1.0–1.5) | 0.04 | 1.89 | 0.8–4.6 | 0.16 | 2.00 | 0.8–4.9 | 0.13 |
| Previous renal transplants, n (%) | 8 (24) | 64 (15) | 1.54 (0.8–2.8) | 0.20 | 1.23 | 0.4–3.5 | 0.69 | |||
| Time on dialysis (days), median | 2424 | 1596 | 0.001 | 1.00 | 1.0–1.0 | 0.92 | 1.00 | 1.0–1.0 | 0.98 | |
| Peritoneal dialysis patients, n (%) | 4 (12) | 92 (22) | 0.54 (0.2–1.2) | 0.16 | 1.03 | 0.3–3.3 | 0.96 | |||
| Obesity, n (%) | 10 (29) | 105 (25) | 1.18 (0.7–1.9) | 0.57 | 0.58 | 0.2–1.6 | 0.28 | |||
| Diabetes mellitus type I-III, n (%) | 11 (32) | 58 (14) | 2.34 (1.3–3.8) | 0.004 | 4.74 | 2.0–11.3 | 0.0005 | 6.08 | 2.3–15.9 | 0.0002 |
| LVEDD male > 56 mm/female > 51 mm (278/454), n (%) | 12 (44) | 66 (26) | 1.69 (1.0–2.6) | 0.05 | 2.06 | 1.0–4.5 | 0.07 | 2.14 | 0.9–4.9 | 0.07 |
| GNAQ TT/TT genotype | 14 (41) | 83 (20) | 2.08 (1.3–3.1) | 0.003 | 4.29 | 1.8–10.1 | 0.001 | 4.46 | 1.9–10.5 | 0.001 |
| Multivariable Cox Regression Considering Only Significantly Different Variables of the Univariate Analysis | Multivariable Cox Regression Considering All Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate RR (95% CI) | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| Variable | Cardiovascular Event n = 69 | No Events n = 385 | ||||||||
| Age in years, median | 57 | 49 | <0.0001 | 1.05 | 1.0–1.1 | 0.005 | 1.05 | 1.0–1.1 | 0.001 | |
| Males, n (%) | 51 (74) | 235 (61) | 1.21 (1.0–1.4) | 0.04 | 1.60 | 0.9–2.9 | 0.13 | 1.71 | 0.9–3.2 | 0.09 |
| Previous renal transplants, n (%) | 14 (20) | 58 (15) | 1.35 (0.8–2.2) | 0.27 | 1.57 | 0.8–3.2 | 0.21 | |||
| Time on dialysis (days), median | 2260 | 1551 | <0.0001 | 1.00 | 1.0–1.0 | 0.23 | 1.00 | 1.0–1.0 | 0.27 | |
| Peritoneal dialysis patients, n (%) | 14 (20) | 82 (21) | 0.95 (0.6–1.5) | 0.85 | 1.55 | 0.8–3.2 | 0.24 | |||
| Obesity, n (%) | 20 (29) | 95 (25) | 1.17 (0.8–1.7) | 0.45 | 0.78 | 0.4–1.6 | 0.47 | |||
| Diabetes mellitus type I-III, n (%) | 23 (33) | 46 (12) | 2.79 (1.8–4.2) | <0.0001 | 3.16 | 1.7–5.8 | 0.002 | 3.79 | 1.9–7.5 | 0.0001 |
| LVEDD male > 56 mm/female > 51 mm (278/454), n (%) | 20 (38) | 58 (26) | 1.5 (0.98–2.2) | 0.06 | 1.52 | 0.9–2.7 | 0.15 | 1.54 | 0.9–2.8 | 0.15 |
| GNAQ TT/TT genotype | 20 (29) | 77 (20) | 1.45 (0.9–2.2) | 0.09 | 1.93 | 1.0–3.6 | 0.04 | 1.93 | 1.0–3.6 | 0.04 |
| Multivariable Cox Regression Considering Only Significantly Different Variables of the Univariate Analysis | Multivariable Cox Regression Considering All Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate RR (95% CI) | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| Variable | Cardiovascular Events Involving >1 Organ System n = 14 | Without Involvement of >1 Organ System n = 440 | ||||||||
| Age in years, median | 58 | 51 | 0.005 | 1.07 | 1.0–1.2 | 0.06 | 1.10 | 1.0–1.2 | 0.04 | |
| Males, n (%) | 10 (71) | 276 (63) | 1.14 (0.7–1.4) | 0.51 | 0.65 | 0.2–2.5 | 0.53 | |||
| Previous renal transplants, n (%) | 5 (36) | 67 (15) | 2.35 (1.1–4.3) | 0.04 | 1.45 | 0.4–5.4 | 0.58 | 2.71 | 0.6–13.5 | 0.22 |
| Time on dialysis (days), median | 3505 | 1596 | <0.0001 | 1.00 | 1.0–1.0 | 0.001 | 1.00 | 1.0–1.0 | 0.002 | |
| Peritoneal dialysis patients, n (%) | 1 (7) | 95 (22) | 0.33 (0.1–1.5) | 0.19 | 0.00 | 0.0–10.1 | 0.97 | |||
| Obesity, n (%) | 6 (43) | 109 (25) | 1.73 (0.8–2.8) | 0.13 | 5.73 | 1.3–25.9 | 0.02 | |||
| Diabetes mellitus type I-III, n (%) | 4 (29) | 65 (15) | 1.93 (0.8–3.9) | 0.16 | 2.87 | 0.5–15.4 | 0.22 | |||
| LVEDD male >56 mm/female > 51 mm(278/454), n (%) | 7 (64) | 71 (27) | 2.39 (1.3–3.5) | 0.007 | 4.50 | 1.2–17.2 | 0.03 | 4.25 | 1.0–19.1 | 0.06 |
| GNAQ TT/TT genotype | 6 (43) | 91 (21) | 2.07 (1.0- 3.4) | 0.046 | 4.77 | 1.4–16.8 | 0.02 | 4.91 | 1.2–19.9 | 0.03 |
| RR (95% CI) | p Value | p Value after Bonferroni Correction | p Value after Benjamini and Hochberg Correction | RR (95% CI) | p Value | p Value after Bonferroni Correction | p Value after Benjamini and Hochberg correction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Events During Dialysis | All Men n = 286 | GNB3 TT n = 29 | GNB3 CT/CC n = 257 | All Women n = 168 | GNB3 TT n = 16 | GNB3 CT/CC n = 152 | ||||||||
| Cardiovascular event, n (%) | 51 (18) | 4 (14) | 47 (18) | 0.75 (0.3–1.8) | 0.55 | 1.0 | 0.87 | 18 (11) | 1 (6) | 17 (11) | 0.56 (0.1–2.7) | 0.54 | 1.0 | 0.79 |
| Cardiovascular event involving >1 organ system, n (%) | 10 (3) | 1 (3) | 9 (4) | 0.99 (0.2–5.5) | 0.99 | 1.0 | 0.99 | 4 (2) | 0 (0) | 4 (3) | 0.0 (0.0–8.1) | 0.51 | 1.0 | 0.79 |
| Acute PAOD, n (%) | 13 (5) | 2 (7) | 11 (4) | 1.61 (0.4–5.9) | 0.52 | 1.0 | 0.87 | 5 (3) | 0 (0) | 5 (3) | 0.0 (0.0–6.4) | 0.46 | 1.0 | 0.79 |
| Transient ischemic attack, n (%) | 1 (0.3) | 0 (0) | 1 (0.4) | 0.0 (0.0–33.1) | 0.74 | 1.0 | 0.94 | 3 (2) | 1 (6) | 2 (1) | 4.75 (0.6–33.7) | 0.16 | 1.0 | 0.79 |
| Stroke, n (%) | 8 (3) | 1 (3) | 7 (3) | 1.11 (0.2–6.3) | 0.92 | 1.0 | 0.98 | 4 (2) | 0 (0) | 4 (3) | 0.0 (0.0–8.1) | 0.51 | 1.0 | 0.79 |
| Carotid artery stenosis, n (%) | 3 (1) | 0 (0) | 3 (1) | 0.0 (0.0–10.7) | 0.56 | 1.0 | 0.87 | 1 (1) | 0 (0) | 1 (1) | 0.0 (0.0–34.8) | 0.75 | 1.0 | 0.79 |
| Myocardial infarction, n (%) | 19 (7) | 0 (0) | 19 (7) | 0.0 (0.0–1.6) | 0.13 | 1.0 | 0.68 | 6 (4) | 0 (0) | 6 (4) | 0.0 (0.0–5.3) | 0.42 | 1.0 | 0.79 |
| Coronary artery stenosis requiring stent insertion, n (%) | 27 (9) | 1 (3) | 26 (10) | 0.34 (0.1–1.8) | 0.24 | 1.0 | 0.68 | 7 (4) | 0 (0) | 7 (5) | 0.0 (0.0–4.5) | 0.38 | 1.0 | 0.79 |
| Heart valve intervention, n (%) | 13 (5) | 1 (3) | 12 (5) | 0.74 (0.1–4.0) | 0.77 | 1.0 | 0.94 | 3 (2) | 0 (0) | 3 (2) | 0.0 (0.0–11.0) | 0.57 | 1.0 | 0.79 |
| Coronary artery bypass grafting, n (%) | 12 (4) | 1 (3) | 11 (4) | 0.81 (0.1–4.4) | 0.83 | 1.0 | 0.94 | 2 (1) | 0 (0) | 2 (1) | 0.0 (0.0–16.9) | 0.64 | 1.0 | 0.79 |
| MACE (cardiovasacular death, stroke, myocardial infarction), n (%) | 26 (9) | 1 (4) | 25 (10) | 0.35 (0.1–1.8) | 0.26 | 1.0 | 0.68 | 10 (6) | 0 (0) | 10 (7) | 0.0 (0.0–3.1) | 0.29 | 1.0 | 0.79 |
| New onset of chronic cardiovascular disease during dialysis | ||||||||||||||
| Coronary artery disease, n (%) | 46 (16) | 4 (14) | 42 (16) | 0.84 (0.3–2.0) | 0.72 | 1.0 | 0.93 | 14 (8) | 1 (6) | 13 (9) | 0.73 (0.1–3.7) | 0.75 | 1.0 | 0.79 |
| Coronary artery disease of ≥2 vessels, n (%) | 24 (8) | 0 (0) | 24 (9) | 0.0 (0.0–1.3) | 0.09 | 1.0 | 0.68 | 5 (3) | 0 (0) | 5 (3) | 0.0 (0.0–6.4) | 0.46 | 1.0 | 0.79 |
| Heart failure, n (%) | 52 (18) | 3 (10) | 49 (19) | 0.54 (0.2–1.4) | 0.25 | 1.0 | 0.68 | 16 (10) | 3 (19) | 13 (9) | 2.19 (0.7–6.0) | 0.19 | 1.0 | 0.79 |
| Echocardiographic parameters of left ventricle | ||||||||||||||
| LVEDD male >56 mm/female > 51 mm(278/454), n (%) | 49 (17) | 10 (50) | 39 (26) | 1.95 (1.1–3.1) | 0.02 | 0.32 | 0.34 | 29 (27) | 0 (0) | 29 (30) | 0.0 (0.0–1.0) | 0.05 | 0.80 | 0.79 |
| IVSd male > 12 mm/female > 11 mm (299/454), n (%) | 98 (34) | 7 (41) | 91 (51) | 0.80 (0.4–1.3) | 0.42 | 1.0 | 0.87 | 48 (46) | 4 (40) | 44 (46) | 0.86 (0.4–1.6) | 0.70 | 1.0 | 0.79 |
| LVPWd male 12 mm/female > 12 mm (208/454), n (%) | 58 (20) | 8 (57) | 50 (42) | 1.36 (0.8–2.0) | 0.28 | 1.0 | 0.68 | 16 (8) | 2 (25) | 14 (21) | 1.20 (0.3–3.4) | 0.79 | 1.0 | 0.79 |
| Multivariable Cox Regression Considering All Variables | |||||||
|---|---|---|---|---|---|---|---|
| Univariate RR (95% CI) | p Value | HR | 95% CI | p Value | |||
| Variable | LVEDD > 56 mm n = 49 | LVEDD ≤ 56 mm n = 123 | |||||
| Age in years, median | 54 | 49 | 0.19 | 1.01 | 1.0–1.0 | 0.44 | |
| Previous renal transplants, n (%) | 8 (16) | 18 (15) | 1.12 (0.5–2.3) | 0.78 | 0.90 | 0.4–2.0 | 0.79 |
| Time on dialysis (days), median | 1488 | 1834 | 0.17 | 1.00 | 1.0–1.0 | 0.00001 | |
| Peritoneal dialysis patients, n (%) | 8 (16) | 24 (20) | 0.84 (0.4–1.7) | 0.63 | 0.93 | 0.4–2.1 | 0.86 |
| Obesity, n (%) | 15 (31) | 32 (26) | 1.18 (0.7–1.9) | 0.54 | 1.22 | 0.6–2.4 | 0.57 |
| Diabetes mellitus type I–III, n (%) | 8 (16) | 17 (14) | 1.18 (0.5–2.5) | 0.67 | 1.05 | 0.4–2.5 | 0.92 |
| Coronary artery disease, n (%) | 10 (20) | 22 (18) | 1.14 (0.6–2.2) | 0.70 | 0.92 | 0.4–2.0 | 0.83 |
| GNB3 TT genotype | 10 (20) | 10 (8) | 2.51 (1.1–5.5) | 0.02 | 2.35 | 1.2–4.8 | 0.02 |
| RR (95% CI) | p Value | RR (95% CI) | p Value | RR (95% CI) | p Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Patients n = 435 | GNB3 TT n = 43 | GNB3 CT/CC n = 392 | All Men n = 274 | GNB3 TT n = 29 | GNB3 CT/CC n = 245 | All Women n = 161 | GNB3 TT n = 14 | GNB3 CT/CC n = 147 | |||||||
| Reduction of antihypertensive therapy from ≥5 to ≤5 drugs, n (%) | 25 (6) | 2 (5) | 23 (6) | 0.79 (0.2–2.8) | 0.75 | 16 (6) | 2 (7) | 14 (6) | 1.21 (0.3–4.3) | 0.8 | 9 (6) | 0 (0) | 9 (6) | 0.0 (0.0–3.8) | 0.34 |
| No change in antihypertensive therapy with ≥5 drugs, n (%) | 17 (4) | 2 (5) | 15 (4) | 1.22 (0.3–4.5) | 0.79 | 13 (5) | 1 (3) | 12 (5) | 0.71 (0.1–3.9) | 0.73 | 4 (2) | 1 (7) | 3 (2) | 3.5 (0.5–22.0) | 0.24 |
| Enchantment of antihypertensive therapy from ≤5 to ≥5 drugs, n (%) | 45 (10) | 9 (21) | 36 (9) | 2.3 (1.2–4.2) | 0.02 | 27 (10) | 2 (7) | 25 (10) | 0.68 (0.2–2.3) | 0.57 | 18 (11) | 7 (50) | 11 (7) | 6.68 (3.0–13.7) | 0.0001 |
| No change in antihypertensive therapy with ≤5 drugs, n (%) | 348 (80) | 30 (70) | 318 (81) | 0.86 (0.7–1.0) | 0.08 | 218 (80) | 24 (83) | 194 (79) | 1.05 (0.8–1.2) | 0.65 | 130 (81) | 6 (43) | 124 (84) | 0.51 (0.3–0.8) | 0.0002 |
| Gene | rsID | Position | 5′-3′Primer | 3′-5′ Primer | Annealing Temperature (°C) | Restriction Enzyme |
|---|---|---|---|---|---|---|
| GNAS | rs7121 | c.393C > T | TGT GGC CGC CAT GAG CAA | TAA GGC CAC ACA AGT CGG GGT | 64 °C | BseGI |
| GNAQ | rs72466452 | g.78032143delinsAA | AGG GTG CGG GAG CAG TAG GCG | CCT CCT GGA AGG CTT TCC TGG G | 62 °C | PdiI |
| GNB3 | rs5443 | c.825C > T | GCT GCC CAG GTC TGA TCC C | TGG GGA GGG TCC TTC CAG C | 66 °C | BseDI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birkner, S.; Möhlendick, B.; Wilde, B.; Schoenfelder, K.; Boss, K.; Siffert, W.; Kribben, A.; Friebus-Kardash, J. Single-Nucleotide Polymorphism in Genes Encoding G Protein Subunits GNB3 and GNAQ Increase the Risk of Cardiovascular Morbidity among Patients Undergoing Renal Replacement Therapy. Int. J. Mol. Sci. 2023, 24, 15260. https://doi.org/10.3390/ijms242015260
Birkner S, Möhlendick B, Wilde B, Schoenfelder K, Boss K, Siffert W, Kribben A, Friebus-Kardash J. Single-Nucleotide Polymorphism in Genes Encoding G Protein Subunits GNB3 and GNAQ Increase the Risk of Cardiovascular Morbidity among Patients Undergoing Renal Replacement Therapy. International Journal of Molecular Sciences. 2023; 24(20):15260. https://doi.org/10.3390/ijms242015260
Chicago/Turabian StyleBirkner, Simon, Birte Möhlendick, Benjamin Wilde, Kristina Schoenfelder, Kristina Boss, Winfried Siffert, Andreas Kribben, and Justa Friebus-Kardash. 2023. "Single-Nucleotide Polymorphism in Genes Encoding G Protein Subunits GNB3 and GNAQ Increase the Risk of Cardiovascular Morbidity among Patients Undergoing Renal Replacement Therapy" International Journal of Molecular Sciences 24, no. 20: 15260. https://doi.org/10.3390/ijms242015260
APA StyleBirkner, S., Möhlendick, B., Wilde, B., Schoenfelder, K., Boss, K., Siffert, W., Kribben, A., & Friebus-Kardash, J. (2023). Single-Nucleotide Polymorphism in Genes Encoding G Protein Subunits GNB3 and GNAQ Increase the Risk of Cardiovascular Morbidity among Patients Undergoing Renal Replacement Therapy. International Journal of Molecular Sciences, 24(20), 15260. https://doi.org/10.3390/ijms242015260





